GnRH induces expression of miR-132 and miR-212, which reduce expression of p250RhoGAP, leading to Rac activation, changes in cellular morphology and increased migration.
Abstract
GnRH is central to the regulation of reproductive function. It acts on pituitary gonadotropes to stimulate LH and FSH synthesis and secretion. We had previously presented evidence for translational control of LHβ synthesis; therefore we investigated whether micro-RNAs might play a role in GnRH regulation in LβT2 cells. We show here that GnRH strongly induces the AK006051 gene transcript that encodes two micro-RNAs, miR-132 and miR-212, within the first intron. We show furthermore that the AK006051 promoter region is highly GnRH responsive. We verify that the p250Rho GTPase activating protein (GAP) is a target of miR-132/212 and show that GnRH treatment leads to a decrease in mRNA and protein expression. This reduction is blocked by an anti-miR to miR-132/212 and mimicked by a pre-miR-132. GnRH inhibits p250RhoGAP expression through a miR-132/212 response element within the 3′-untranslated region. The loss of p250RhoGAP expression leads to activation of Rac and marked increases in both the number and length of neurite-like processes extending from the cell. Knockdown of p250RhoGAP by small interfering RNA induces the same morphological changes observed with GnRH treatment. In addition, loss of p250RhoGAP causes an increase in cellular motility. Our findings suggest a novel pathway regulating long-term changes in cellular motility and process formation via the GnRH induction of miR-132/212 with the subsequent down-regulation of p250RhoGAP.
The integration and precise coordination of hormones along the hypothalamic-pituitary-gonadal axis are essential for sexual maturation and reproductive function in mammals. The hypothalamic decapeptide GnRH stimulates the synthesis and secretion of the pituitary gonadotropins LH and FSH, which then regulate the production of gonadal steroids and gametogenesis (1, 2). GnRH is released in a pulsatile fashion that is essential for pituitary gonadotrope function and causes pulsatile release of LH into the circulation (3). The pulse amplitude and frequency of GnRH release greatly increases before ovulation and is essential for inducing the LH surge. GnRH effects in the gonadotrope cell are mediated by a specific receptor (GnRH-R) that is a member of the G protein-coupled receptor family (4). Many studies have investigated the signaling pathways downstream of GnRH-R activation that lead to induction of gonadotropin gene expression and secretion (2, 5–9). Most of these studies have been performed in primary pituitary cultures as well as immortalized gonadotrope cell lines, such as the αT3 and LβT2 cells (10, 11). These cells are sensitive to GnRH pulses and respond by altering gene expression and LH and FSH secretion accordingly.
Although transcriptional regulation in the pituitary gonadotrope has been extensively studied (12, 13), not much is known about posttranscriptional regulation of mRNA stability and translation. Micro-RNAs (miRNAs) are single-stranded RNA molecules of about 21–23 nucleotides that regulate gene expression posttranscriptionally by targeting the 3′-untranslated region (3′-UTR) of specific mRNAs (14–16). The miRNA sequences are encoded in a stem-loop structure in the primary transcript that is cleaved in the nucleus by the ribonuclease III enzyme Drosha to form the precursor miRNA (pre-miRNA), which is subsequently exported to the cytoplasm by the exportin pathway (15, 17, 18). The pre-miRNA then is cleaved by another ribonuclease III enzyme Dicer and the mature miRNA strand incorporated into the RISC complex (14–16, 19). Mature miRNA are partially complementary to sequences, known as miRNA recognition elements (MREs), located in the 3′-UTR of mRNAs (20). The first seven nucleotides of the miRNA after the initial adenine are termed the seed sequence and specifies initial mRNA targeting whereas the remaining miRNA sequence is thought to stabilize the miRNA-target complex (19, 20). Annealing of miRNA to its target sequences can inhibit translation either by blocking protein translation machinery or by sequestering the mRNA transcript away from ribosomal interaction. miRNAs can also trigger mRNA degradation in a similar process to RNA interference.
Numerous studies have shown that miRNAs regulate development, differentiation, and the normal functioning of tissues (21, 22), yet the role of miRNAs in the reproductive system is not known. GnRH alters the expression of miRNAs in the LβT2 immortalized gonadotrope cells, but nothing is known about their function (23). Because GnRH alters translation of LHβ and other genes in these cells, we wanted to test the hypothesis that this translational regulation is mediated by alterations in miRNA expression (24). We show here that GnRH induces the expression of multiple miRNAs. We focus on two of these miRNAs, miR-132 and miR-212, which are encoded by the same gene, and show that this gene is induced by GnRH. Furthermore, we show that the p250RhoGAP protein is a downstream target of miR132/212 that is involved in morphological changes and migration of LβT2 cells.
Results
GnRH regulates miRNA expression in LβT2 cells
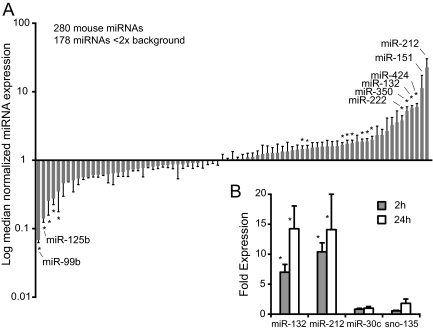
To define the alterations in miRNA expression in gonadotropes after GnRH treatment, we performed miRNA expression profiling. LβT2 cells were treated with 100 nm GnRH for 2 h after which total RNA was extracted and profiled in duplicate on NCODE arrays that contain 280 mouse miRNAs. We chose a short 2 h treatment with a maximal dose of GnRH to highlight genes that are likely direct targets of GnRH signaling. Of the 280 mouse miRNAs, only 85 were detected above background (Fig. 1A and Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Most miRNAs did not change in expression, but a number were significantly up- or down-regulated. Among the most highly up-regulated miRNAs, miR-212 expression was induced 41-fold and miR-132 was induced 10-fold. Of the miRNAs that we observed to change with GnRH, only miR-132 has been reported previously (23). The array results were verified using Taqman assays specific for the mature forms of miR-132 and miR-212 (Fig. 1B). We also measured miR-30c and sno-135 expression as controls and verified that they did not change with GnRH treatment for 2 or 24 h.
Fig. 1.
GnRH regulates miRNA expression in LβT2 cells. A, Cells were stimulated with 100 nm GnRH for 2 h and then harvested and total RNA extracted. miRNAs were profiled on NCODE microarrays in quadruplicate. Data were median normalized and miRNAs with expression less than 2× background were excluded from further analysis leaving 85 genes with detectable expression. miRNAs are ranked by log median normalized expression relative to untreated cells. Statistically significant changes were indicated by asterisks (P < 0.05). B, Cells were stimulated with 10 nm GnRH for 2 or 24 h. miRNA expression was profiled by QPCR using specific Taqman assays. Data were analyzed with REST. Significant changes are indicated by asterisks (P < 0.05).
miR-132 and miR-212 are encoded by expressed sequence tag AK006051 and are coordinately regulated
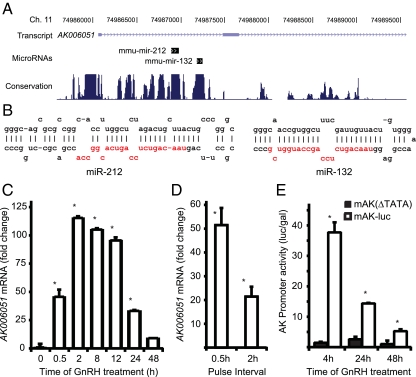
Both miR-132 and miR-212 are localized to the first intron of a noncoding mouse expressed sequence tag AK006051 (Fig. 2A). The intron sequence covering the two miRNA precursors is highly conserved across species, as is the upstream promoter, and is predicted to form a strong stem loop structure covering each miRNA (Fig. 2B). The entire intron is located within a CpG island, and the gene has multiple CRE consensus sequences directly upstream, indicating that it may be highly regulated. To test whether miR-132 and miR-212 were induced as a result of increase in expression of the AK006051 gene, we measured AK006051 mRNA expression by quantitative PCR (QPCR) analysis. Treatment of LβT2 cells with 10 nm GnRH for increasing times caused a time-dependent increase in AK006051 expression (Fig. 2C). In these and subsequent experiments, we chose to use 10 nm GnRH to study more physiological responses. Robust expression was observed as early as 30 min after GnRH treatment and was maximal at 2 h but declined at 24–48 h.
Fig. 2.
Gene encoding miR-132 and miR-212 is induced by GnRH. A, Diagram of genomic locus miR-132 and miR-212 (adapted from UCSC genome browser). Position of miR-132 and miR-212 in the first intron of transcript AK006051. Species conservation is shown below. B, Predicted structure of precursors to miR-132 and miR-212. C, Time course of expression of AK006051. Cells were stimulated with 10 nm GnRH for different times and expression was measured by QPCR. Data are presented as fold change over basal. D, AK006051 is induced by fast GnRH pulse frequencies. Cells were treated with 10 nm GnRH for 2 min every 30 or 120 min for 6 h as described in Materials and Methods. AK006051 expression was measured by QPCR as before. E, AK promoter is induced by GnRH. mAK-luc or mAK(ΔTATA) was transfected into LβT2 cells and stimulated with 10 nm GnRH for 4, 24, or 48 h. Luciferase activity was measured and normalized to cotransfected TK-LacZ and is presented relative to untreated cells. Asterisks indicate statistical significance (P < 0.05) vs. basal; # indicates significance (P < 0.05) vs. GnRH treated.
Because pulsatile GnRH stimulation is necessary for gonadotrope function in vivo and LβT2 cells exhibit pulse sensitivity with respect to LH and FSH expression and secretion (25), we investigated whether AK006051 is differentially regulated by GnRH pulse frequency (Fig. 2D). We stimulated cells with 10 nm GnRH for 2 min at 30-min and 120-min intervals for 6 h. For all treatment conditions the media on all cells were changed every 30 min to avoid effects due to secretion of autocrine factors. AK006051 mRNA was measured by QPCR as before. Whereas there is significant induction of AK006051 at the lower pulse frequency, expression is more than doubled at the high-pulse frequency showing that AK006051 mRNA is differentially up-regulated by high-GnRH pulse frequency.
To verify that changes in promoter activity are responsible for the increase in AK006051 mRNA expression, we cloned the AK006051 promoter region and constructed a reporter plasmid −1.3 kb mAK-luc containing 1.3 kb of sequence upstream of the transcriptional start site (Fig. 2E). We also constructed the control reporter −1.3 kb mAK(delTATA)-luc in which 200 bp containing the TATA box were deleted. These reporter plasmids were transfected into LβT2 cells. Treatment with 10 nm GnRH for 4 h caused a 40-fold increase in luciferase activity. Promoter induction had declined to 14-fold at 24 h and 5-fold at 48 h. The control reporter lacking the TATA box was not induced by GnRH (Fig. 2E). Therefore, the promoter region alone is sufficient for the strong induction of AK006051 transcription.
Degradation of p250RhoGAP requires GnRH induction of miR-132/212
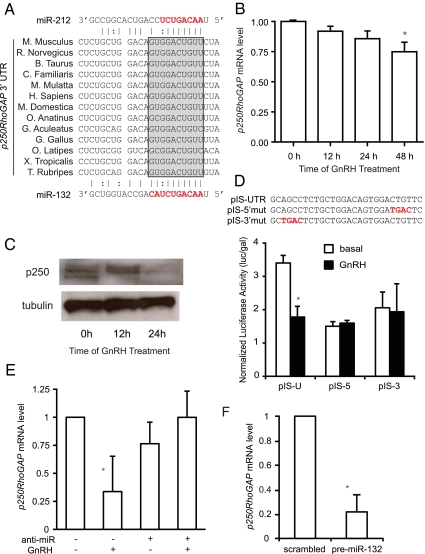
The seed sequences of miR-132 and miR-212 are identical, and the mature miRNAs only differ by four nucleotides so the miRNAs likely target similar mRNAs. We used a number of in silico algorithms to identify potential miR-132 and miR-212 targets and looked for targets that were predicted by multiple methods. Among the potential targets, p250RhoGAP (also known as RICS, Grit, and p200RhoGAP) was predicted by multiple algorithms (23). The seed sequences of both miR-132 and miR-212 are complementary to a MRE that is conserved across 13 species (Fig. 3A). p250RhoGAP is a guanosine triphosphatase (GTPase)-activating protein (GAP) for the Rho subfamily of small GTPases (21–25 kDa) and has been shown to be a bona fide target for miR-132/212 in neurons (26); therefore, we tested whether GnRH would alter p250RhoGAP mRNA and/or protein expression in LβT2 cells. Both the mRNA and protein levels of p250RhoGAP are reduced under GnRH stimulation (Fig. 3, B and C). The p250RhoGAP protein appears to be more sensitive to GnRH than the mRNA, suggesting that miR132/212 repress translation as well as down-regulating mRNA levels.
Fig. 3.
p250RhoGAP is a target of miR-132 and miR-212. A, Alignment of MRE in 3′-UTR of p250RhoGAP from 13 species. Complementarity with miR-212 is shown above and with miR-132 below the alignment. Solid lines indicate Watson-Crick pairing; dotted lines indicate U-G pairing. The shaded box indicates seed sequence complementarity. B, Reduction of p250RhoGAP mRNA by GnRH. Cells were treated with GnRH for increasing times, and p250RhoGAP mRNA levels were measured by QPCR. Data were analyzed by REST. C, Reduction of p250RhoGAP protein by GnRH. Cells were treated with 10 nm GnRH for 12 and 24 h, and protein level was measured by Western blot. D, p250RhoGAP MRE confers GnRH-dependent repression. Sequence of MRE sequence from p250RhoGAP 3′-UTR cloned into pIS-luc as well as two mutants. Plasmids were transfected into LβT2 cells with TK-luc and then stimulated with 10 nm GnRH for 24 h. Luciferase activity was measured and corrected for β-galactosidase activity. E, An anti-miR blocks the repression of p250RhoGAP mRNA by GnRH. Cells were transfected with the dual miR-132/212 anti-miR or control anti-miR, and then starved and treated with 10 nm GnRH for 24 h. p250RhoGAP mRNA levels were measured by QPCR. F, A pre-miR-132 reduces p250RhoGAP expression. Cells were electroporated with the pre-miR-132 or scrambled pre-miR and harvested after 24 h. p250RhoGAP mRNA levels were measured by QPCR. Asterisks indicate statistical significance vs. control (P < 0.05).
To test whether the MRE identified in the 3′-UTR of p250RhoGAP is responsible for the reduction in p250RhoGAP, we constructed a reporter plasmid containing the potential MRE (27). The reporter plasmid pIS-UTR was constructed by cloning the MRE of p250RhoGAP into the pIS luciferase plasmid downstream of the luciferase-coding sequence (Fig. 3D). We also constructed two mutants pIS-5′mut and pIS-3′mut that have mutations in the seed sequence region or the region complementary to the 3′-end of the miRNA, respectively. The plasmids were transfected into LβT2 cells then stimulated with 10 nm GnRH for 24 h because this was the time where we saw greatest suppression of p250RhoGAP protein. GnRH stimulation caused a 50% decrease in luciferase activity of pIS-UTR but there was no change in the two mutants, suggesting that complementarity is required throughout the miRNA not just in the seed sequence (see Fig. 5D). Basal luciferase activity was reduced in the two mutants, possibly by effects on plasmid transfection or expression, but importantly there was no repression by GnRH. These findings confirm that the MRE of p250RhoGAP can confer GnRH down-regulation of protein expression on a heterologous gene.
Fig. 5.
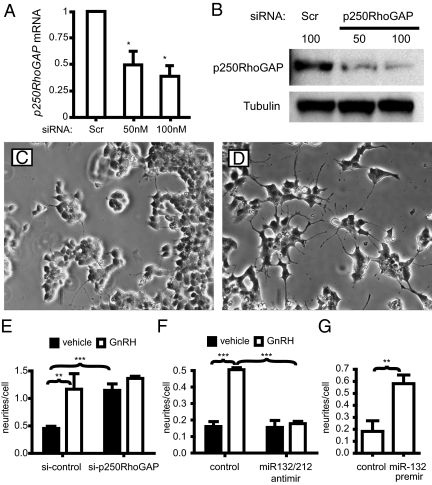
Knockdown of p250RhoGAP stimulates neurite-like outgrowths. A, Cells were electroporated with increasing concentrations of siRNA against p250RhoGAP. p250RhoGAP mRNA expression was measured by QPCR. B, p250RhoGAP protein expression measured by Western blot. C, Cells electroporated with control siRNA. D, Cells were electroporated with siRNA against p250RhoGAP. E, Quantification of outgrowth number and length. Neurite-like outgrowth number and length were quantified with Image J as before. F, Anti-mir132/212 prevents the increase in outgrowths with GnRH. Cells were treated with 10 nm GnRH for 24 h and then neurite number and length were measured as before. G, Pre-miR-132 increases neurite-like outgrowths. Cells were electroporated with the pre-miR-132 or a scrambled control for 24 h after which neurite length and number were measured. Asterisks indicate statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Scr, Scrambled.
To confirm that miR-132 and miR-212 are responsible for the decrease in p250RhoGAP, we electroporated cells with an anti-miR. Introduction of anti-miRs to either miR-132 or miR-212 did not prevent the down-regulation of p250RhoGAP (data not shown) so we designed a degenerate anti-miR to block both miR-132 and miR-212 using locked nucleic acids. This dual anti-miR prevented the GnRH-induced decrease in p250RhoGAP mRNA compared with a scrambled control (Fig. 3E) showing that miR-132 and miR-212 are required for down-regulation. To test whether induction of the miRNAs is sufficient for down-regulation, we electroporated cells with a precursor RNA to miR-132 (pre-miR132) and saw a significant decrease in p250RhoGAP mRNA after 24 h compared with a control pre-miR (Fig. 3F). Therefore, induction of miR-132 and -212 is both necessary and sufficient for down-regulation of p250RhoGAP expression.
GnRH regulates neurite-like outgrowths via miR-132/212 and p250RhoGAP
The Rho family members RhoA, Rac1, and Cdc42 are small GTPases that regulate actin cytoskeleton rearrangements including stress fiber, lamellapodia, and filopodia formation. They also regulate migration, cell growth, cell survival, and vesicle trafficking. The Rho GTPases cycle between active and inactive forms in response to three regulators (28–30). Guanine nucleotide exchange factors (RhoGEFs) promote exchange of GDP for GTP, thereby activating signaling. GTPase-activating proteins (GAPs) increase the inherent GTPase activity of Rho family members, thereby deactivating signaling. Guanine nucleotide dissociation inhibitors stabilize the GDP-bound form of Rho family members preventing activation. A previous study has confirmed p250RhoGAP as a regulator of dendritic plasticity in hippocampal neurons (26). p250RhoGAP can also promote proliferation by interacting with another GAP, p120RasGAP, to activate the ras family of small GTPases and as a result allowing ERK activation (31). Therefore, we were interested in whether GnRH altered cellular morphology and migration of LβT2 cells.
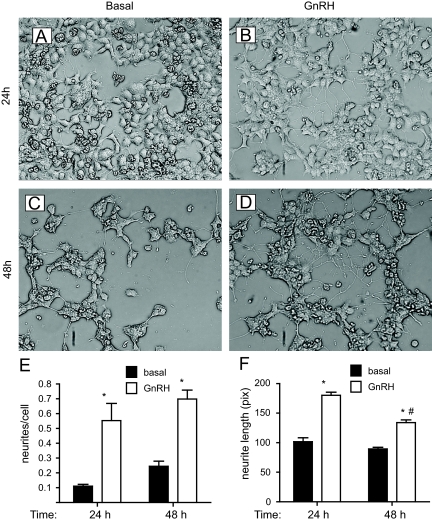
LβT2 cells were plated on Matrigel because extracellular matrix is required for differentiation and the support of neurite outgrowth by neurons and other cells in vivo and in vitro (32). Initially, we tested whether GnRH would cause morphological changes. In serum-free medium, the LβT2 cells show a low number of cellular processes (Fig. 4, A and C). These processes resemble the neurite outgrowths seen during differentiation of PC12 cells although gonadotropes are not considered neurons. The formation of a more elaborate network of neurite-like processes is seen in LβT2 cells stimulated with GnRH for 24 and 48 h (Fig. 4, B and D). GnRH-treated cells exhibit a robust increase in the occurrence, length, and elaboration of processes among cells. Quantification of the number and length of these processes shows that GnRH increases both the number and length of the neurite-like projection (Fig. 4, E and F).
Fig. 4.
GnRH causes neurite-like outgrowths in LβT2 cells. A–D, LβT2 cells stimulated with vehicle (A and C) or 10 nm GnRH (B and D) for 24 h (A and B) or 48 h (C and D). E, Number of neurite-like processes. Number of processes and number of cells were counted per field of cells. Data are shown as number of processes per cell. F, Length of processes. Length of processes was measured in pixels using Image J. Asterisks indicate statistical significance vs. basal (P < 0.05); # indicates significance vs. 24-h GnRH treated.
To determine whether the reduction in p250RhoGAP is sufficient to trigger neurite-like outgrowth, we knocked down p250RhoGAP by small interfering RNA (siRNA). Electroporation of a siRNA against p250RhoGAP caused a 60% decrease in mRNA expression and an 80% decrease in protein expression (Fig. 5A). We then assessed the effect of the knockdown on cellular morphology. Electroporation of the negative control scrambled siRNA did not alter cellular morphology, and cells appear similar to untreated cells (Fig. 5C). Knockdown of p250RhoGAP altered cellular morphology similar to GnRH-treated cells (Fig. 5D), demonstrating that a reduction in p250RhoGAP is sufficient to trigger neurite-like outgrowth. Quantification of the number and length of processes indicated that p250RhoGAP knockdown increased the number of processes to levels seen with GnRH stimulation (Fig. 5E). Stimulation of si-p250RhoGAP-transfected cells with GnRH did not further change morphology (data not shown) or number of neurite-like processes over si-p250RhoGAP alone, indicating that p250RhoGAP is the main regulator of neurite-like processes in gonadotropes.
To verify that the induction of miR-132 and miR-212 is necessary and sufficient for these morphological changes, we electroporated cells with the anti-miR. Introduction of the anti-miR completely blocked the ability of GnRH to increase neurite-like number (Fig. 5F). Electroporation of the pre-miR-132 alone caused an increase in neurite-like process number similar to GnRH treatment or the si-p250RhoGAP (Fig. 5G). These findings demonstrate that induction of the miR-132 and miR-212 and the concomitant decrease in p250RhoGAP are necessary and sufficient for morphological changes and neurite-like outgrowth in LβT2 cells.
GnRH induction of miR132 and miR-212 causes an increase in migration
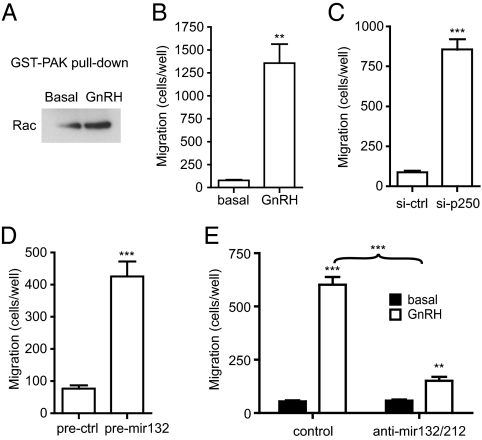
As discussed earlier, p250RhoGAP is a GAP for Rho family members. The miRNA-mediated reduction in p250RhoGAP should therefore increase the activity of Rho, Rac, and Cdc42. Rho activation was assessed using a pulldown assay with a glutathione-S-transferase (GST) fusion protein containing the Rho-binding domain of p21-activated kinase (PAK). This domain binds to the GTP-bound form of Rho, Rac, and Cdc42. Treatment of LβT2 cells with GnRH causes an increase in Rho precipitation by the GST-PAK fusion protein (Fig. 6A), indicating that GnRH activates Rho family members. Because these GTPases are involved in cellular migration, we measured migration in a modified Boyden chamber. Addition of 10 nm GnRH causes an increase in the number of cells that migrate through an 8-μm porous membrane (Fig. 6B). This increase is mimicked by electroporation of the siRNA against p250RhoGAP (Fig. 6C) or the pre-miR-132 (Fig. 6D). Lastly, electroporation of the anti-miR against miR-132/212 drastically reduces the GnRH-induced increase in migration (Fig. 6E). Taken together these results indicate that induction of miR-132 and miR-212 and the concomitant decrease in p250RhoGAP upon GnRH treatment are necessary and sufficient for an increase in cell motility.
Fig. 6.
GnRH increases motility via miR-132 and miR-212. A, GnRH activates Rac. Cells were treated with 10 nm GnRH for 24 h after which GTP-bound Rac was precipitated using GST-PAK1. B, GnRH increases cell motility. Cells were plated in transwell inserts, and 10 nm GnRH was added to the lower well for 24 h. Cells on the underside of the membrane were fixed, stained, and counted. C, Cells were electroporated with siRNA against p250RhoGAP or control siRNA, and motility was measured in the transwell assay. D, Cells were electroporated with pre-miR-132 or control pre-miR, and motility was measured in the transwell assay. E, Cells were electroporated with anti-miR against miR-132/212 or control anti-miR, and motility was measured in the transwell assay. Asterisks indicate statistical significance (**, P < 0.01; ***, P < 0.001). ctrl, Control.
Discussion
The data in this study suggest a novel pathway used by GnRH to regulate cytoskeletal rearrangements and cell motility. We demonstrate that GnRH induces expression of the AK006051 RNA and that the AK006051 gene promoter is responsive to GnRH. The first intron of the AK006051 gene encodes miR-132 and miR-212. Increases in the mature spliced mRNA from the AK006051 gene are paralleled by increases in mature mir-132 and miR-212, suggesting that excision of the precursor miRNAs is an efficient process. We do not know whether splicing of the first intron is required before excision of the precursor miRNAs by Drosha or whether they occur in parallel. The mature miRNAs then target other mRNAs for degradation or translational inhibition. We verified that p250RhoGAP is a target for miR-132/212 in LβT2 cells. The MRE in the p250RhoGAP 3′-UTR is able to confer GnRH-induced suppression to a heterologous gene, and an anti-miR that blocks both miR-132 and miR-212 prevents the reduction in p250RhoGAP, whereas a pre-miR-132 reduces p250RhoGAP. The reduction in p250RhoGAP then activates the Rho family of small GTPases and leads to cytoskeletal rearrangements, outgrowth of neurite-like process, and increases in cell motility. The morphological changes and increased motility are prevented by the anti-miR and mimicked by the pre-miR, suggesting both necessity and sufficiency.
Although the transcription factors regulating AK006051 expression have not been studied in pituitary cells, a prior publication using cultured cortical neurons showed that miR-132/212 expression is controlled by brain-derived neurotrophic factor (BDNF) activation of ERK signaling via MSK1/2 and CREB (33). The induction was completely lost in MSK1/2 null cells but only partially lost in CREB S133A mutant cells. A reporter construct containing 3 kb of upstream sequence and 1 kb of exon 1, intron 1, and exon 2 was highly induced by BDNF but the response was lost with just the promoter alone, even though the CRE in the promoter did contribute to the overall induction. In contrast, we find that the AK006051 promoter alone is sufficient for strong induction, which may reflect a difference between the primary cortical neurons and the immortalized gonadotropes. Another group showed that dominant-negative A-CREB impairs the induction of miR-132 by BDNF in cortical neurons and showed that CREB binds to three CRE sequences directly upstream of miR-212 and miR-132 in intron 1 by chromatin immunoprecipitation (34). We cannot rule out a contribution from the intronic CREs but our data would suggest that the promoter element may be sufficient in some cells. The expression of miR-132 is highly regulated and has been studied most extensively in the brain. As well as being induced by BDNF, miR-132 is induced by long-term potentiation, bicuculline, pilocarpine-induced seizures, contextual fear conditioning, odor-exposure, and cocaine injection (26, 35, 36). Interestingly, dexamethasone treatment reduced the BDNF induction of miR-132 in cortical neurons, suggesting a role in inflammation (37). Expression is not limited to the brain, however, because miR-132 is induced by lipopolysaccharide in splenocytes, viral infection of lymphatic endothelial cells, nutritional stress in preadipocytes, or human chorionic gonadotropin in ovarian granulosa cells (38–41).
A number of targets for miR-132 have also been identified. The first target to be experimentally verified was p250RhoGAP in cortical neurons (34). Unlike our findings in gonadotrope cells, miR-132 was solely responsible for decreased p250RhoGAP expression because an anti-miR to miR-132 prevented the decrease. Additionally, the repression was thought to be mainly translational because no changes in p250RhoGAP mRNA were observed. Other targets have since been identified and verified including SirT1, acetylcholinesterase, methyl-CpG-binding protein 2, the transcriptional activator p300, p120RasGAP, and the glutamate receptors NR2A, NR2B, and GluR1 (35, 38, 39, 41–43). In gonadotropes, both miR-132 and miR-212 contribute to repression because only a dual-specificity anti-miR blocks the reduction of p250RhoGAP by GnRH. The mRNA encoding p250RhoGAP is also reduced by GnRH treatment in LβT2 cells although not as dramatically as the protein. The role of miR-132 in translational repression in the brain is strengthened by the finding that miR-132 is associated with the fragile-X mental retardation protein, which functions as a translational repressor (44). As fragile-X mental retardation protein is enriched in brain, this may explain why miR-132 is a stronger translational repressor in this tissue.
As mentioned earlier, the p250RhoGAP protein is a GAP for the Rho/Rac/cdc42 family of small GTPases. The role of p250RhoGAP in pituitary cells has not been reported, but this protein has been studied in neurons. Reduction of p250RhoGAP by introduction of a pre-miR-132 or p250RhoGAP siRNA increases dendritic length and branching and increases spine density via kalirin7-Rac1 signaling in hippocampal neurons (45). It also increases neurite outgrowth from cortical neurons in culture (26, 34). The p250RhoGAP gene has been disrupted in mice causing elevated cdc42 activity in neurons and leading to elongated neurites in cerebellar granule and hippocampal neurons (46). Interestingly, the p250RhoGAP-null animals were fertile and had normal litter size, indicating the gene is not essential for reproduction. The p250RhoGAP protein may also be involved in other signaling pathways because it has been found associated with the nerve growth factor receptor TrkA, the adapter proteins N-Shc, Nck, Gab2, Crk, and CrkL, the tyrosine kinase Fyn, and the ras-specific p120RasGAP (31, 47–49). This last interaction leads to increased ras activation, suggesting a role in proliferation as well as motility (31). Interestingly, a recent paper reported that p120RasGAP is itself a target for miR-132 in endothelial cells, suggesting miRNA regulation of this pathway at multiple levels (43).
We show here that increases in miR-132/212 and decreases in p250RhoGAP together increase neurite-like outgrowths from LβT2 pituitary gonadotropes and increase motility. In a previous study, Navratil et al. (50) reported that GnRH induces acute actin cytoskeletal rearrangements in αT3–1 and LβT2 cells and cellular process outgrowth in primary ovine pituitary cells as well as in pituitary slice cultures, and the authors suggested that individual gonadotropes are migrating to the portal vasculature and forming processes to secrete LH and FSH. This suggestion is intriguing given our observation that the AK006051 gene encoding miR-132 and miR-212 is selectively induced by high-frequency GnRH pulses. It is possible that high-frequency GnRH stimulation triggers pituitary gonadotropes to establish an intercellular communication network and move toward the portal vasculature to facilitate the LH surge. Alternatively, the migratory response may have a developmental role in the organization of the anterior pituitary. Whatever its biological role, the underlying mechanism regulating process formation and motility is likely different in the Navratil study because the effects are seen within minutes of GnRH exposure and are likely due to acute changes in signaling whereas our effects require the longer term induction of miRNA expression and subsequent suppression of p250RhoGAP protein expression. The lack of a fertility phenotype in the p250RhoGAP −/− mice might be expected, because the gonadotropes should have increased motility and process formation, so the mice would be sensitive to GnRH and constantly primed for the surge. A more informative model might be a p250RhoGAP transgenic or a knockout of the AK006051 gene. Further studies will be needed to test the biological role for these cytoskeletal rearrangements and how miR-132/212 may play a role in the global regulation of reproduction.
Materials and Methods
Materials
GnRH was from Dr. A. F. Parlow (National Hormone and Pituitary Program, Harbor UCLA Medical Centre, Torrance, CA). Activin A was obtained from R&D systems (Minneapolis, MN). Antibodies to p250RhoGAP were a gift from Dr. Tadashi Yamamoto (University of Tokyo, Tokyo, Japan), and antibodies to Rac were from Cell Signaling Technology (Beverly, MA). β-Tubulin and horseradish peroxidase-linked secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Cell culture reagents were purchased from Life Technologies (Carlsbad, CA). Growth factor-reduced Matrigel was purchased from BD Biosciences (San Jose, CA). All other reagents were purchased from either Sigma (St. Louis, MO) or Fischer Scientific (Pittsburgh, PA). GST-PAK1-PBD fusion protein was a gift of Dr. Celine DerMardirossian (The Scripps Research Institute, La Jolla, CA). Plasmid pIS-luc was obtained from Addgene (Cambridge, MA).
Cell culture and hormone treatment
LβT2 cells, a gift from Dr. Pam Mellon [University of California San Diego (UCSD)], at passages 13–19 were cultured in monolayers with DMEM containing 10% fetal bovine serum, penicillin/streptomycin, and Gluta-max in a humidified 10% CO2 atmosphere at 37 C. Cells were plated at 1 × 106 cells/ml in triplicate in 12- and six-well plates or 6-cm dishes coated with poly-l-lysine (20 μg/ml) or Matrigel (1:150 dilution). After 24 h, cells were starved in DMEM containing 0.5% fetal bovine serum, penicillin/streptomycin, and Gluta-max for an additional 24 h. Cells were then stimulated with 10 nm GnRH for 0.5–48 h in starvation media. For pulse frequency experiments, cells were washed with starving media plus 2 ng/ml activin A every 30 min and pulse treated with starvation media containing 10 nm GnRH for 2 min every 30 or 120 min.
Microarrays and QPCR
Total RNA was purified with RNA-Bee (Tel-Test, Friendswood, TX) according to manufacturer's protocol. For the microarray study, the mRNAs were enriched from total RNA using the rRNA reduction kit (Invitrogen) and subsequently labeled using the NCODE labeling kit (Invitrogen). RNA from unstimulated cells was labeled with Cy3 and RNA from GnRH-stimulated cells was labeled with Cy5. Labeled probes were hybridized to NCODE miRNA microarrays, washed, and scanned on a Molecular Devices Genepix 4000B scanner (Molecular Devices, Sunnyvale, CA) by the Veterans Affairs/UCSD Genechip microarray Core (GEO accession number GSE27398). miRNAs with signals less than 2× background were excluded. Signals for the remaining miRNAs were median normalized and analyzed using Prism (GraphPad Software, Inc., San Diego, CA) using a Z-test. For QPCR, first-strand cDNA synthesis was performed using the cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Samples for QPCR were run in 20-μl triplicate reactions on a MJ Research Chromo4 instrument using iTaq SYBR Green Master Mix (Bio-Rad Laboratories, Hercules, CA). Sequence-specific primers for AK006051, p250RhoGAP, β-actin, and glyceraldehyde-3-phosphate dehydrogenase were designed using the Universal Probe Library Assay Design Center (Roche, Indianapolis, IN). Mature miRNA expression was quantitated using Taqman Micro-RNA Assays (Applied Biosystems) for miR-132 and miR-212 as well as to controls miR-30c and sno-135, the expression of which does not change with GnRH treatment. Gene expression levels were calculated after normalization to the housekeeping genes, glyceraldehyde-3-phosphate dehydrogenase and/or β-actin, using the ΔΔCt method and expressed as fold mRNA expression levels with respect to nontreated cells. Genomic localization of miRNAs was derived from the UCSC and Ensembl genome browsers. miRNA targets were predicted using the Miranda, TargetScan, and Mirbase websites.
Western blot
LβT2 cells were washed twice with cold PBS and lysed with 3× radioimmunoprecipitation assay buffer. Lysates were sonicated and then centrifuged for 20 min at 14,000 rpm, 4 C. Total protein was quantified from the supernatants using Bio-Rad's DC Protein Assay, and 25–35 ng protein per sample were loaded with SDS loading buffer onto 4–12% gradient Bis-Tris Criterion gels (Bio-Rad Laboratories) before electrophoresis. Proteins were electrotransferred to polyvinylidene difluoride membranes and blocked in Tris-buffered saline (TBS)-Tween 5% BSA for 1 h at room temperature. The polyclonal primary antibodies were incubated overnight at 4 C in TBS-Tween, 5% BSA, and 0.2% sodium azide. Membranes were washed three times in TBS-Tween and then incubated with horseradish peroxidase-conjugated secondary antibodies in TBS-Tween/5% BSA for 60 min at room temperature and then washed three times in TBS-Tween. Proteins were detected by enhanced chemiluminescence (SuperSignal; Pierce Chemical Co., Rockford, IL).
Pre-mir and anti-mir transfection, siRNA knockdown, and reporter assays
LβT2 cells were transfected by electroporation using the Microporator (Invitrogen) using parameters optimized according to manufacturer's protocol with pre-mir-132, pre-miR control, predesigned siRNA against p250RhoGAP, or scrambled siRNA control (Ambion, Inc., Austin, TX). An anti-mir was designed against miR-132/212 comprising a locked nucleic acid oligo with the following sequence: CTGKAGACTGTTA (Exiqon).
An AK006051 promoter luciferase reporter plasmid, pAK-1.3-luc, was constructed by amplifying the region 1.3 kb upstream of the AK006051 transcriptional start site. The AK006051 promoter region was inserted into the pAP1-luc reporter vector after removal of the AP1 promoter region by restriction digest. A control reporter, pAK-.2-1.3-luc, was also constructed in which the first 200 bp upstream of the transcriptional start site containing the TATA box was deleted.
One copy of the miR-132 and miR-212 target site in the 3′-UTR of p250RhoGAP was cloned into the pIS vector containing a multiple cloning site downstream of Firefly Luciferase open reading frame. Two mutants were also constructed in which the UTR region complementary to either the 5′- or 3′-region of miR-132/212. Inserts were constructed by Klenow fill-in with SacI, SpeI, and XbaI restriction sites. Luciferase plasmids were cotransfected with concentrations of 500 ng per 5 × 105 cells along with 25 ng TK-lacz. Luciferase and β-galactosidase activity were measured by the Veritas Microplate Luminometer (Turner BioSystems, Mountain View, CA) using luciferin and a Galacto-light assay kit (Applied Biosystems).
Image analysis
Cells were visualized under bright-field microscopy at ×200 magnification. Five fields of view per treatment condition were analyzed using ImageJ (51) with the NeuroJ macros (52). Ends of neurites were manually entered and then the neurite lengths, given in pixel units, were traced automatically by the software. Outgrowth length and number were calculated as the ratio of measurements of GnRH-treated vs. nontreated and normalized to total cell counts in each field of view.
Cell motility (modified Boyden chamber assay)
Transwells were coated with growth factor-reduced Matrigel (1:150 dilution). Cells grown to 80–90% confluence were washed twice in PBS and incubated in starving media 24 h before harvesting with trypsin. After centrifugation, cells were resuspended in starvation media, and 1 × 105 cells were seeded into the upper chamber of each transwell 10 nm GnRH. Lower chambers contained starvation media with 10 nm GnRH. After 24 h, cells were fixed in cold 100% MeOH for 7 min and washed three times in PBS. Cells that did not migrate to the underside of the membrane were scraped off using a cotton swab. Migrated cells were stained with hematoxylin and eosin, and visualized at ×200 magnification. Five fields of view per membrane were counted.
Statistical analysis
Data are shown as mean ± sem of at least three separate experiments. Individual comparisons between groups were analyzed by modified t test, and comparisons between multiple groups were analyzed by one-way ANOVA or two-way ANOVA followed by Tukey posttest comparisons (Prism 4.0, GraphPad Software). P ≤ 0.05 was considered to be statistically significant. Statistical analysis of QPCR data was performed using the program relative expression software tool (REST) (53) using 2000 random permutations of the data.
Acknowledgments
This work was supported by grant National Institutes of Health Grant HD047400, by National Institute of Child Health and Human Development/National Institutes of Health through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction Research, and by a Veterans Affairs Merit Review Award.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BDNF
- Brain-derived neurotrophic factor
- CRE
- cAMP response element
- CREB
- CRE-binding protein
- GAP
- GTPase-activating protein
- GST
- glutathione-S-transferase
- GTPase
- guanosine triphosphatase
- miRNAs
- micro-RNAs
- MRE
- miRNA recognition element
- PAK
- p21-activated kinase
- QPCR
- quantitative PCR
- REST
- relative expression software tool
- siRNA
- small interfering RNA
- TBS
- Tris-buffered saline
- UTR
- untranslated region.
References
- 1. Conn PM, Crowley WF., Jr 1994. Gonadotropin-releasing hormone and its analogs. Annu Rev Med 45:391–405 [DOI] [PubMed] [Google Scholar]
- 2. Kaiser UB, Conn PM, Chin WW. 1997. Feb Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev 18:46–70 [DOI] [PubMed] [Google Scholar]
- 3. Knobil E. 1974. On the control of gonadotropin secretion in the rhesus monkey. Recent Prog Horm Res 30:1–46 [DOI] [PubMed] [Google Scholar]
- 4. Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJG. 2002. /8/23 Involvement of both Gq/11 and Gs proteins in gonadotropin-releasing hormone receptor-mediated signaling in Lβ T2 cells. J Biol Chem 277:32099–32108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shacham S, Cheifetz MN, Lewy H, Ashkenazi IE, Becker OM, Seger R, Naor Z. 1999. Mechanism of GnRH receptor signaling: from the membrane to the nucleus. Ann Endocrinol (Paris) 60:79–88 [PubMed] [Google Scholar]
- 6. Ando H, Hew CL, Urano A. 2001. Jun Signal transduction pathways and transcription factors involved in the gonadotropin-releasing hormone-stimulated gonadotropin subunit gene expression. Comp Biochem Physiol B Biochem Mol Biol 129:525–532 [DOI] [PubMed] [Google Scholar]
- 7. Song SB, Rhee M, Roberson MS, Maurer RA, Kim KE. 2003. Jan 31 Gonadotropin-releasing hormone-induced stimulation of the rat secretogranin II promoter involves activation of CREB. Mol Cell Endocrinol 199:29–36 [DOI] [PubMed] [Google Scholar]
- 8. Stanislaus D, Janovick JA, Brothers S, Conn PM. 1997. Jun Regulation of G(q/11)α by the gonadotropin-releasing hormone receptor. Mol Endocrinol 11:738–746 [DOI] [PubMed] [Google Scholar]
- 9. Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. 2002. Mar GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 Cells. Mol Endocrinol 16:419–434 [DOI] [PubMed] [Google Scholar]
- 10. Alarid ET, Holley S, Hayakawa M, Mellon PL. 1998. Discrete stages of anterior pituitary differentiation recapitulated in immortalized cell lines. Mol Cell Endocrinol 140:25–30 [DOI] [PubMed] [Google Scholar]
- 11. Dalkin AC, Burger LL, Aylor KW, Haisenleder DJ, Workman LJ, Cho S, Marshall JC. 2001. /1/1 Regulation of gonadotropin subunit gene transcription by gonadotropin-releasing hormone: measurement of primary transcript ribonucleic acids by quantitative reverse transcription-polymerase chain reaction assays. Endocrinology 142:139–146 [DOI] [PubMed] [Google Scholar]
- 12. Salisbury TB, Binder AK, Nilson JH. 2008. Welcoming β-catenin to the gonadotropin-releasing hormone transcriptional network in gonadotropes. Mol Endocrinol 22:1295–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fink MY, Pincas H, Choi SG, Nudelman G, Sealfon SC. 2010. Research resource: gonadotropin-releasing hormone receptor-mediated signaling network in LβT2 cells: a pathway-based web-accessible knowledgebase. Mol Endocrinol 24:1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297 [DOI] [PubMed] [Google Scholar]
- 15. Lee Y, Jeon K, Lee JT, Kim S, Kim VN. 2002. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J 21:4663–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cullen BR. 2004. Transcription and processing of human microRNA precursors. Mol Cell 16:861–865 [DOI] [PubMed] [Google Scholar]
- 17. Kim VN. 2004. MicroRNA precursors in motion: exportin-5 mediates their nuclear export. Trends Cell Biol 14:156–159 [DOI] [PubMed] [Google Scholar]
- 18. Yi R, Qin Y, Macara IG, Cullen BR. 2003. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev 17:3011–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hutvagner G, Simard MJ. 2008. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 9:22–32 [DOI] [PubMed] [Google Scholar]
- 20. Lewis BP, Burge CB, Bartel DP. 2005. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20 [DOI] [PubMed] [Google Scholar]
- 21. Chen CZ, Li L, Lodish HF, Bartel DP. 2004. MicroRNAs modulate hematopoietic lineage differentiation. Science 303:83–86 [DOI] [PubMed] [Google Scholar]
- 22. Wienholds E, Plasterk RH. 2005. MicroRNA function in animal development. FEBS Lett 579:5911–5922 [DOI] [PubMed] [Google Scholar]
- 23. Yuen T, Ruf F, Chu T, Sealfon SC. 2009. Microtranscriptome regulation by gonadotropin-releasing hormone. Mol Cell Endocrinol 302:12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen KA, Santos SJ, Kreidel MK, Diaz AL, Rey R, Lawson MA. 2004. Acute regulation of translation initiation by gonadotropin-releasing hormone in the gonadotrope cell line LβT2. Mol Endocrinol 18:1301–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. 1997. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 138:1224–1231 [DOI] [PubMed] [Google Scholar]
- 26. Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, Impey S. 2008. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci USA 105:9093–9098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yekta S, Shih IH, Bartel DP. 2004. MicroRNA-directed cleavage of HOXB8 mRNA. Science 304:594–596 [DOI] [PubMed] [Google Scholar]
- 28. Van Aelst L, D'Souza-Schorey C. 1997. Rho GTPases and signaling networks. Genes Dev 11:2295–2322 [DOI] [PubMed] [Google Scholar]
- 29. Bishop AL, Hall A. 2000. Rho GTPases and their effector proteins. Biochem J 348:241–255 [PMC free article] [PubMed] [Google Scholar]
- 30. Takai Y, Sasaki T, Matozaki T. 2001. Small GTP-binding proteins. Physiol Rev 81:153–208 [DOI] [PubMed] [Google Scholar]
- 31. Shang X, Moon SY, Zheng Y. 2007. p200 RhoGAP promotes cell proliferation by mediating cross-talk between Ras and Rho signaling pathways. J Biol Chem 282:8801–8811 [DOI] [PubMed] [Google Scholar]
- 32. Manthorpe M, Engvall E, Ruoslahti E, Longo FM, Davis GE, Varon S. 1983. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol 97:1882–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Remenyi J, Hunter CJ, Cole C, Ando H, Impey S, Monk CE, Martin KJ, Barton GJ, Hutvagner G, Arthur JS. 2010. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem J 428:281–291 [DOI] [PubMed] [Google Scholar]
- 34. Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S. 2005. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA 102:16426–16431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wibrand K, Panja D, Tiron A, Ofte ML, Skaftnesmo KO, Lee CS, Pena JT, Tuschl T, Bramham CR. 2010. Differential regulation of mature and precursor microRNA expression by NMDA and metabotropic glutamate receptor activation during LTP in the adult dentate gyrus in vivo. Eur J Neurosci 31:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR. 2010. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 20:492–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawashima H, Numakawa T, Kumamaru E, Adachi N, Mizuno H, Ninomiya M, Kunugi H, Hashido K. 2010. Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience 165:1301–1311 [DOI] [PubMed] [Google Scholar]
- 38. Strum JC, Johnson JH, Ward J, Xie H, Feild J, Hester A, Alford A, Waters KM. 2009. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol 23:1876–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RS, Gotch F, Boshoff C. 2010. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol 12:513–519 [DOI] [PubMed] [Google Scholar]
- 40. Fiedler SD, Carletti MZ, Hong X, Christenson LK. 2008. Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells. Biol Reprod 79:1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, Soreq H. 2009. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 31:965–973 [DOI] [PubMed] [Google Scholar]
- 42. Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, Mann DA. 2010. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology 138:705–714, 714.e1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN, Lapinski PE, King PD, Weis SM, Cheresh DA. 2010. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med 16:909–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M. 2010. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65:373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Impey S, Davare M, Lasiek A, Fortin D, Ando H, Varlamova O, Obrietan K, Soderling TR, Goodman RH, Wayman GA. 2010. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci 43:146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nasu-Nishimura Y, Hayashi T, Ohishi T, Okabe T, Ohwada S, Hasegawa Y, Senda T, Toyoshima C, Nakamura T, Akiyama T. 2006. Role of the Rho GTPase-activating protein RICS in neurite outgrowth. Genes Cells 11:607–614 [DOI] [PubMed] [Google Scholar]
- 47. Nakamura T, Komiya M, Sone K, Hirose E, Gotoh N, Morii H, Ohta Y, Mori N. 2002. Grit, a GTPase-activating protein for the Rho family, regulates neurite extension through association with the TrkA receptor and N-Shc and CrkL/Crk adapter molecules. Mol Cell Biol 22:8721–8734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao C, Ma H, Bossy-Wetzel E, Lipton SA, Zhang Z, Feng GS. 2003. GC-GAP, a Rho family GTPase-activating protein that interacts with signaling adapters Gab1 and Gab2. J Biol Chem 278:34641–34653 [DOI] [PubMed] [Google Scholar]
- 49. Taniguchi S, Liu H, Nakazawa T, Yokoyama K, Tezuka T, Yamamoto T. 2003. p250GAP, a neural RhoGAP protein, is associated with and phosphorylated by Fyn. Biochem Biophys Res Commun 306:151–155 [DOI] [PubMed] [Google Scholar]
- 50. Navratil AM, Knoll JG, Whitesell JD, Tobet SA, Clay CM. 2007. Neuroendocrine plasticity in the anterior pituitary: gonadotropin-releasing hormone-mediated movement in vitro and in vivo. Endocrinology 148:1736–1744 [DOI] [PubMed] [Google Scholar]
- 51. Abramoff MD, Magelhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics International 11:36–42 [Google Scholar]
- 52. Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. 2004. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58:167–176 [DOI] [PubMed] [Google Scholar]
- 53. Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]