A reduction in extracellular glucose inhibits the firing activity of GnRH neurons; this inhibition is attenuated by androgens and requires activation of AMP-activated protein kinase.
Abstract
GnRH neurons integrate steroidal and metabolic cues to regulate fertility centrally. Central glucoprivation reduces LH secretion, which is governed by GnRH release, suggesting GnRH neuron activity is modulated by glucose availability. Here we tested whether GnRH neurons can sense changes in extracellular glucose, and whether glucosensing is altered by the steroids dihydrotestosterone (DHT) and/or estradiol (E). Extracellular recordings were made from GnRH neurons in brain slices from ovariectomized (OVX) mice ± DHT and/or E implants. Firing rate was reduced by a switch from 4.5 to 0.2 mm glucose in cells from OVX, OVX+E, and OVX+DHT+E mice, but not OVX+DHT mice. This suggests that androgens reduce the sensitivity of GnRH neurons to changes in extracellular glucose, but E mitigates this effect. Next we investigated potential mechanisms. In the presence of the ATP-sensitive potassium channel antagonist tolbutamide, glucosensing persisted. In contrast, glucosensing was attenuated in the presence of compound C, an antagonist of AMP-activated protein kinase (AMPK), suggesting a role for AMPK in glucosensing. The AMPK activator N1-(b-d-ribofuranosyl)-5-aminoimidazole-4-carboxamide (AICAR) mimicked the effect of low glucose and was less effective in cells from DHT-treated mice. The effect of DHT to diminish responses to low glucose and AICAR was abolished by blockade of fast synaptic transmission. Both AICAR and low glucose activated a current with a reversal potential near −50 mV, suggesting a nonspecific cation current. These studies indicate that glucosensing is one mechanism by which GnRH neurons sense fuel availability and point to a novel role for AMPK in the central regulation of fertility.
GnRH neurons form the final common pathway in the central regulation of fertility. GnRH is secreted in discrete pulses, each of which elicits a corresponding pulse of LH release from the pituitary (1). The frequency and amplitude of GnRH secretion change in response to steroids across the female reproductive cycle, leading to a surge of GnRH and LH at midcycle that is required for ovulation (2). GnRH neurons are also regulated by metabolic cues, and interactions among steroidal and metabolic signals may be important for the regulation of reproduction under both normal and pathophysiological states (3, 4).
Numerous studies have explored the link between metabolism and fertility, but the specific metabolic signals involved and mechanisms by which they are conveyed to the reproductive system have not been fully elucidated. The majority of existing evidence for glucose regulation of GnRH release is derived from studies of experimental glucoprivation induced by fasting or intracerebroventricular infusion of insulin or glucose antimetabolites (2-deoxyglucose or 2-thioglucose). A reduction in central glucose availability causes a suppression of LH levels and LH pulse frequency (5), as well as a reduction in pulses of electrical activity in the preoptic area thought to correspond with GnRH release (6). In insulin-induced hypoglycemic rats, restoration of glucose, but not other metabolic substrates, restores LH pulsatility (7). In steroid-primed ovariectomized (OVX) rats as a model of the GnRH/LH surge, caudal hindbrain infusion of 2-thioglucose both prevents the LH surge and reduces c-fos expression in GnRH neurons (8), suggesting an inhibition of GnRH neuronal activity. Importantly, these studies implicate a central site in the sensing of low glucose availability by the GnRH-LH axis. That glucoprivic agents were infused into brain regions devoid of GnRH neurons suggests that other neuronal populations might relay these signals synaptically. One study suggests direct sensing of glucose by GnRH neurons (9). GnRH neuron firing rate was reduced by a switch from high to low glucose in high K+ and high Mg2+/low Ca2+ solution; this divalent cation combination inhibits presynaptic vesicle release. This study further demonstrated the expression of an estradiol (E)-potentiated ATP-sensitive potassium (KATP) current in a subset of GnRH neurons; however, neither the contribution of this current to glucosensing nor the steroid dependence of the response to diminished glucose was assessed.
Here we examined glucosensing in GnRH neurons, its dependence on steroids and fast synaptic transmission, and possible underlying mechanisms. We sought to determine whether GnRH neuron-firing activity could be altered by changes in extracellular glucose within the physiological range. These experiments were performed in defined animal models treated with E and/or the nonaromatizable androgen dihydrotestosterone (DHT). The interaction between steroids and glucosensing is relevant to normal reproduction, because steroid levels change across the female cycle. It is also of importance to reproductive disorders such as polycystic ovary syndrome (PCOS), the leading cause of infertility in women, which is characterized by both elevated androgens and poor blood glucose control (10).
Results
GnRH neurons respond to changes in glucose concentration within the physiological range
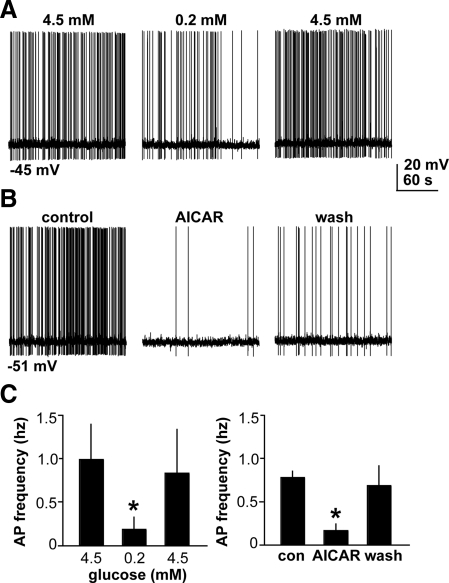
We first tested whether GnRH neurons in brain slices from OVX mice could respond to a reduction in extracellular glucose from 4.5 mm to 0.2 mm. These represent brain extracellular glucose concentrations after peripheral administration of a bolus of glucose or insulin, respectively (11), and are the approximate ends of the normal physiological range. Targeted extracellular recordings, which preserve the recorded cell's native level of ATP and glucose, were used to monitor firing activity. To observe a potential suppression of activity in response to lowered glucose, only cells that were actively firing during a control period were tested. Acute exposure to low glucose resulted in a decrease in firing that was not immediately reversed upon restoration of 4.5 mm glucose (n = 11, P < 0.05; Fig. 1A). Firing rate was suppressed by greater than half in 82% (nine of 11) of cells. Although GnRH neurons spontaneously change their firing rate and undergo periods of quiescence (12), in cells in which the glucose concentration was maintained at 4.5 mm for a period of 30 min, the firing rate on average did not change (n = 6, P = 0.98; Fig. 1B). This indicates that the decrease in firing rate in treated cells is due to low glucose exposure.
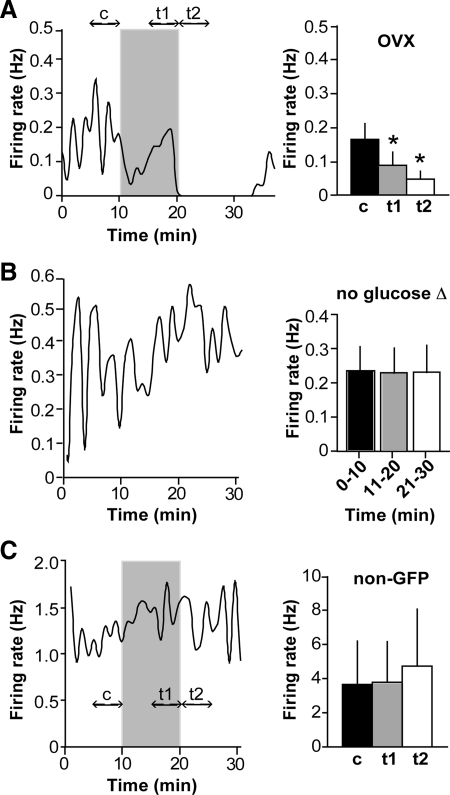
Fig. 1.
GnRH neurons are sensitive to a physiological reduction in extracellular glucose concentration. A, Representative plot of firing rate over time in a GnRH neuron from an OVX mouse (left) and summary (n = 11 cells, right). Firing events are binned in 60-sec intervals. Glucose concentration was switched from 4.5 to 0.2 mm; shaded region indicates period of 0.2 mm treatment. Double-headed arrows indicate time intervals in which frequency was averaged for analysis, designated c for control period, t1 for low glucose, and t2 for post-low glucose. Mean ± sem firing rates for c (black bars), t1 (gray bars), and t2 (white bars) are shown in the bar graph. B, Representative plot of firing rate over time in a cell from an OVX mouse in which the glucose concentration was held constant at 4.5 mm (left), and summary (n = 6 cells, right). There was no change in average firing rate over time, in contrast to cells in which the glucose concentration was reduced. C, Representative plot of firing rate over time in a non-GFP-expressing neuron in the preoptic area (left), and summary (n = 5, right). Time of 0.2 mm glucose treatment is indicated by shading. Low glucose failed to affect firing in five of five non-GFP (non-GnRH) neurons. *, P < 0.05.
In most cells, the firing rate continued to decline upon restoration of 4.5 mm glucose. To determine whether this was a delayed effect of low glucose or caused by the acute increase in glucose, some cells were incubated in low glucose for 15 rather than 10 min (n = 3; data not shown). The firing rate continued to decline after the initial 10 min (by an average of 80.9 ± 13.6%) and did not decline when 4.5 mm glucose was restored (mean change of −5.5 ± 9.6%). This suggests that the low firing rate in the early part of the glucose restoration period is a continuation of the response to low glucose.
Activity suppression by low glucose is at least partly specific to GnRH neurons
It has been suggested that all neurons throughout the brain can be silenced by glucose deprivation (13). To determine whether the observed response is widespread, or specific to a neuronal subpopulation that includes GnRH neurons, non-green fluorescent protein (GFP)-expressing neurons in the preoptic area were recorded under the same treatment protocol. Of immunofluorescently identified GnRH neurons, 84–94% express GFP in this transgenic model (14). Further, some non-GFP cells studied exhibited markedly high mean firing frequencies uncharacteristic of GnRH neurons (>15 Hz), indicating that they were probably not GnRH neurons. None of the non-GFP neurons recorded were inhibited by low glucose (Fig. 1C; n = 5, P = 0.3), indicating that the observed response is not a ubiquitous neuroprotective phenomenon and is at least partly specific to GnRH neurons.
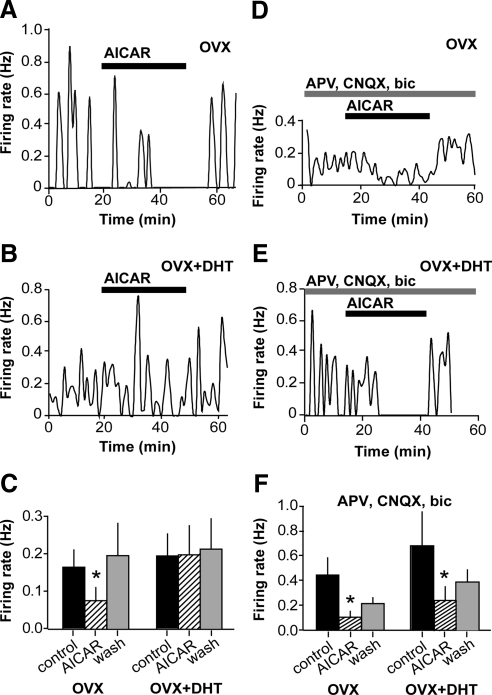
DHT inhibits the response to low glucose but is counteracted by E
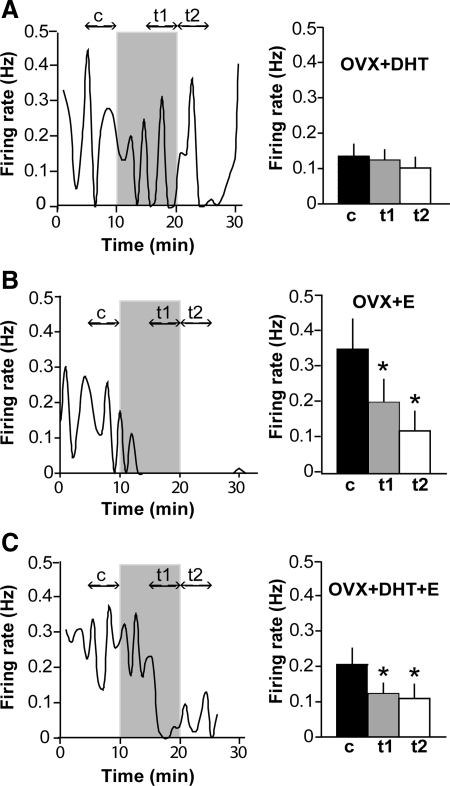
To test whether steroids alter the response to a decrease in extracellular glucose, the low glucose challenge was repeated in GnRH neurons in brain slices from OVX mice treated with DHT (OVX+DHT), E (OVX+E), or both (OVX+E+DHT) via sc SILASTIC (Dow Corning Corp., Midland, MI) implants. Hormone levels achieved with these treatments have been previously published (15–17); the E replacement is a low physiological level, whereas the DHT replacement elevates circulating androgens in female mice to a level below that which restores seminal vesicle weight in a castrated male mouse. This submale level mimics the elevation in androgen in women with PCOS. Similar to GnRH neurons from OVX, GnRH neurons from OVX+E (n = 13) and OVX+E+DHT (n = 10) mice responded to 0.2 mm glucose with a decrease in firing rate (P < 0.05; Fig. 2, B and C). This effect was not observed, however, in the majority of cells from mice treated with DHT alone, and on average low glucose had no effect in this group (n = 12, P > 0.05; Fig. 2A). Percent responders (defined as >50% inhibition of firing rate) in OVX+E, OVX+E+DHT, and OVX+DHT were 64% (10 of 13), 70% (seven of 10), and 25% (three of 12), respectively. Basal firing rate among cells was higher in OVX+E compared with OVX and OVX+DHT (one-way ANOVA, P < 0.05), which may reflect the fact that recordings were made in the afternoon, when E can have a positive feedback effect (17). However, because a proportion of cells in all groups were completely quiescent at the time recordings were initiated, basal firing rate here reflects only the average among active cells, as opposed to overall averages as previously studied. There was a negative correlation between basal firing rate and percent change in firing rate in response to low glucose (Pearson r = −0.3, R2 = 0.09; P < 0.05). Cells with basal firing rate less than 0.1 Hz were less likely to be inhibited by low glucose, suggesting that the lack of response in the DHT group may be due to a basement effect.
Fig. 2.
DHT inhibits the response to low glucose but is counteracted by E. A, Representative plot of firing rate over time in a GnRH neuron from an OVX+DHT mouse and summary (n = 12 cells, right). See Fig. 1 legend for details. Low glucose failed to inhibit firing in cells from mice treated with DHT. B, Representative plot of firing rate over time in a GnRH neuron from an OVX+E mouse and summary (n = 14 cells, right). Low glucose inhibited firing in GnRH neurons from mice treated with E. C, Representative plot of firing rate over time in a GnRH neuron from an OVX+DHT+E mouse and summary (n = 10 cells, right). In the presence of both DHT and E, GnRH neuron firing activity was inhibited by low glucose. *, P < 0.05.
GnRH neurons respond to low glucose when fast synaptic transmission is blocked
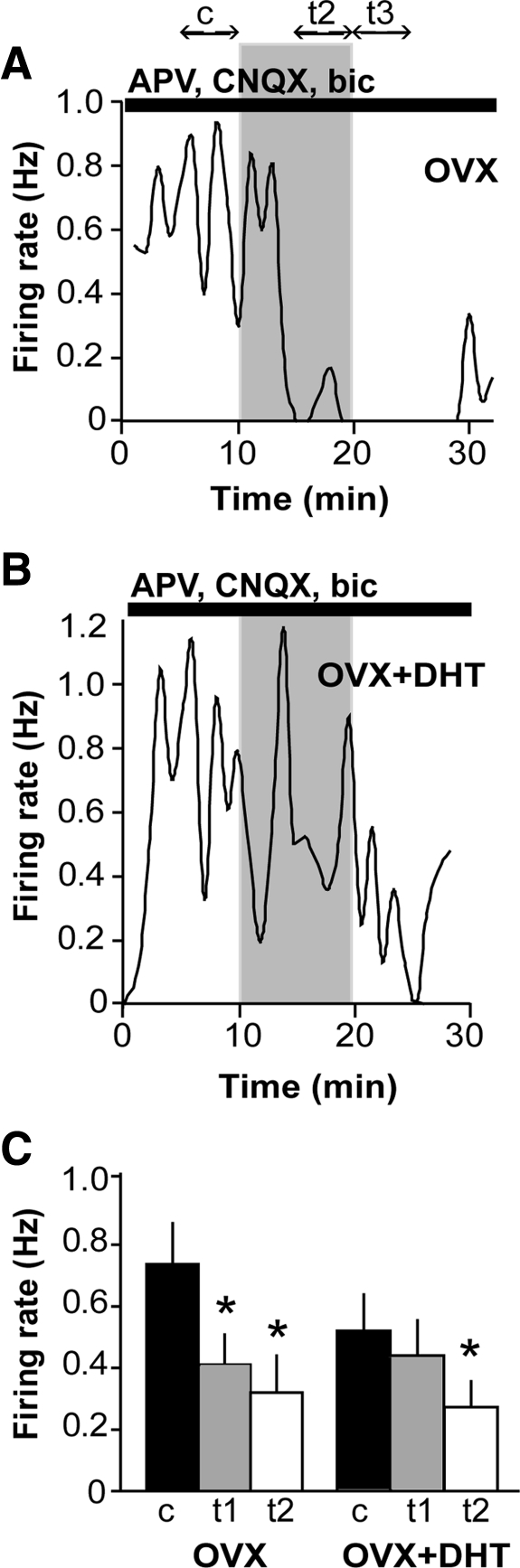
Numerous studies suggest a role for afferent neurons in conveying glucose signals to GnRH neurons. γ-Aminobutyric acid (GABA) neurons, which form a major input to GnRH neurons (18–20), have been show to relay metabolic signals to GnRH neurons (21, 22). To test whether fast synaptic transmission mediated via ionotropic GABA and/or glutamatergic receptors mediated the response to low glucose, the low glucose challenge was repeated in the presence of antagonists of GABAA, N-methyl-d-aspartate, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors [bicuculline, (2R)-amino-5-phosphonovaleric acid (APV), and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), respectively]. This treatment not only blocked receptors directly on GnRH neurons but also throughout the neuronal network maintained in the brain slice. Blockers increased the basal firing rate among recorded (active) cells (P < 0.0005, OVX; P < 0.005, OVX+DHT). An excitatory effect of blockers has been observed previously (23) and may reflect neuromodulatory influences including increased excitatory and/or reduced inhibitory neuromodulatory input via the network, although it could also reflect a potential effect of bicuculline to block small-conductance calcium-activated potassium channels (24). In the presence of these receptor blockers, 83% (10 of 12) of GnRH neurons were inhibited by low glucose (n = 12, P < 0.05; Fig. 3A), similar to the percentage in the absence of blockers (82%, Fig. 1). This indicated that fast GABA and glutamatergic transmission were not required for the response of GnRH neurons to low glucose, suggesting that glucose was either sensed directly or conveyed via neuromodulatory inputs. Additionally, the inhibitory effect of low glucose was more pronounced (two-way ANOVA OVX vs. OVX+blockers; P = 0.005 for interaction).
Fig. 3.
Glucosensing persists in OVX and is unmasked in OVX+DHT cells when fast synaptic transmission is blocked. A, Representative plot of firing rate over time in a GnRH neuron from an OVX mouse in APV, CNQX, and bicuculline (bic). See Fig. 1 legend for details. B, Representative plot of firing rate over time in a cell from an OVX+DHT mouse in the presence of synaptic blockers. C, Summary of effects of low glucose on GnRH neurons from OVX and OVX+DHT mice in APV, CNQX, and bic (n = 13 per group). Low glucose had an inhibitory effect in OVX cells at both times analyzed. The effect of low glucose was significant in OVX+DHT cells only at the latter time point. *, P < 0.05.
Blocking fast synaptic transmission unmasks glucosensing in GnRH neurons from DHT-treated mice
Because DHT increases GABAergic transmission to GnRH neurons (15), and androgen receptors have not been detected on GnRH neurons (25), we postulated that an intrinsic response might be masked by the altered fast synaptic transmission in DHT-treated mice (15). We therefore repeated the low-glucose challenge in cells from OVX+DHT mice in the presence of APV, CNQX, and bicuculline. With fast synaptic transmission blocked, there was a tendency for a greater proportion of cells from DHT-treated mice to be inhibited by low glucose (six of 11 or 55% compared with 25% in the absence of blockers; P = 0.1 using Fisher's exact test), and on average there was a significant decrease in firing during the second post-low-glucose analysis interval (n = 11, P < 0.05; Fig. 3B). The observation that the change was only evident in this latter time period suggests a possible difference in the latency of the response between OVX and OVX+DHT cells. However, there was no statistical difference in overall response between OVX and OVX+DHT in the presence of blockers (two-way ANOVA; P = 0.27 for interaction).
KATP channels are not required for glucosensing
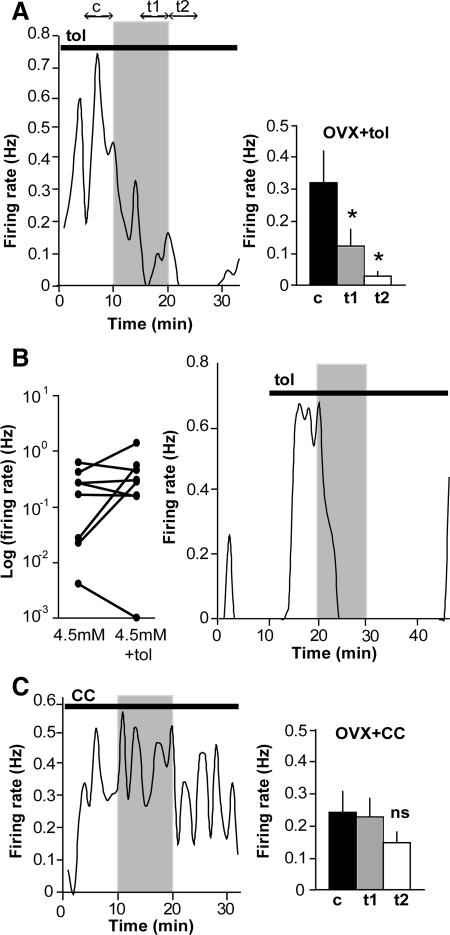
KATP channels, which play an important role in glucosensing in the pancreas and in brain regions that regulate feeding behavior and peripheral glucose homeostasis (26, 27), have been demonstrated to influence GnRH neuronal activity in an estrogen-sensitive manner (9, 28). Zhang et al. (9) showed that E potentiated KATP current in GnRH neurons, but this study did not assess the effect of E on glucosensing or demonstrate a role for KATP current in glucosensing. We hypothesized that KATP channels mediate the response to low glucose in GnRH neurons. To test their involvement, exposure to low glucose was repeated in the presence of the sulfonylurea tolbutamide at 200 μm, a concentration that blocks more than or equal to 95% of KATP current (29). The basal firing rate tended to be higher in tolbutamide-treated than untreated cells (n = 11 in each condition; P = 0.1). In contrast to our hypothesis, GnRH neurons maintained their sensitivity to low glucose when KATP channels were blocked (n = 11, P < 0.05; Fig. 4A), indicating that KATP channels are not required for glucosensing in GnRH neurons. In 100% of tested cells the firing rate was suppressed by more than 50%. In a separate experiment, only three of eight cells acutely treated with tolbutamide responded with an increase in firing rate (P > 0.2, n = 8; Fig. 4B). This suggests that in cells from OVX mice, only a fraction have functional and/or open KATP channels under these recording conditions, that membrane changes are insufficiently large to alter firing rate, or that effects on inhibitory presynaptic neurons mask the direct effect of tolbutamide. Regardless, KATP channel activation does not appear to be required for the inhibitory response of GnRH neurons to low glucose, indicating the involvement of one or more other mechanisms. The right portion of Fig. 4B shows a cell that is excited by acute application of tolbutamide and is subsequently treated with 0.2 mm glucose (also containing tolbutamide). This cell is still clearly inhibited by low glucose, indicating that even in cells that are regulated by KATP channels, another mechanism mediates the response to low glucose.
Fig. 4.
AMPK antagonism, but not KATP channel antagonism, attenuates glucosensing in GnRH neurons. A, Representative plot of firing rate over time in a GnRH neuron from an OVX mouse in the presence of 200 μm tolbutamide (tol) and summary (n = 11 cells, right). See Fig. 1 legend for details. Low glucose inhibited GnRH neuron firing despite blockade of KATP channels by tolbutamide. B, Plot of firing rate over time in an OVX cell that is acutely stimulated by tolbutamide and subsequently inhibited by low glucose application. Black line indicates time of tolbutamide application. C, Representative plot of firing rate over time in a GnRH neuron from an OVX mouse in the presence of 20–40 μm CC and summary (n = 15, right). Low glucose failed to reduce firing activity when AMPK was blocked by CC. *, P < 0.05.
The response to low glucose is attenuated when AMP-activated protein kinase (AMPK) is blocked
We next tested the hypothesis that the response to glucose was mediated by AMPK, a cellular energy sensor that is activated by increases in the intracellular AMP/ATP ratio (30). To test this, AMPK was blocked using the antagonist compound C (CC) (31). Either 20 or 40 μm CC was used, but no difference in response was observed between cells treated with these concentrations, and the data were combined for analysis. In the presence of CC, the majority of GnRH neurons (eight of 14 or 57%) failed to respond to low glucose (n =14, P > 0.05; Fig. 4C). On average, a small decrement in firing rate occurred at the onset of washout, but this decrease was not statistically significant and not as robust as that in GnRH neurons in the absence of CC. Thus, AMPK antagonism attenuated the response to low glucose, indicating a potential mechanistic role for AMPK.
GnRH neurons are inhibited by an AMPK activator
To test the hypothesis that low glucose inhibits GnRH neurons via AMPK activation, we pharmacologically activated AMPK by acute application of N1-(b-d-ribofuranosyl)-5-aminoimidazole-4-carboxamide (AICAR). AICAR inhibited firing in GnRH neurons from OVX mice (n =7, P < 0.05; Fig. 5A,C). The latency of the response to AICAR was longer than that of low glucose, likely reflecting the fact that AICAR must be converted to 5-amino-4-imidazolecarboxamide ribotide inside the cell; it is 5-amino-4-imidazolecarboxamide ribotide that subsequently activates AMPK (32). The response to AICAR was maintained in the presence of blockers of fast synaptic transmission (APV, CNQX, bicuculline; n = 4, P < 0.05; Fig. 5, D and F). Similar to AICAR, 2 mm metformin also inhibited firing activity of GnRH neurons (n = 6, control, 0.51 ± 0.18 Hz; metformin, 0.31 ± 0.14 Hz; P < 0.05, data not shown), supporting an inhibitory role for AMPK in GnRH neuronal regulation.
Fig. 5.
AICAR inhibits firing activity of GnRH neurons from OVX mice and in cells from OVX+DHT mice when fast synaptic transmission is blocked. A, Representative plot of firing rate over time in a GnRH neuron from an OVX mouse. Events are binned in 60-sec intervals. Black bar indicates time of 2 mm AICAR application. B, Representative plot of firing rate over time in a GnRH neuron from an OVX+DHT mouse. C, Summary of effect of acute AICAR application. Mean + sem firing rate is shown for control period (black bars), AICAR (striped bars), and washout (wash, gray bars). AICAR reversibly decreased firing rate in cells from OVX (n = 7) but not OVX+DHT (n = 9) mice. D and E, Firing rate over time in cells from OVX (D) and OVX+DHT (E) mice in the presence of APV, CNQX, and bicuculline (bic). Acute AICAR (black bar) reversibly inhibited firing in both groups in the presence of blockers of receptors for fast synaptic transmission. F, Summary of effect of acute AICAR application in the presence of blockers of fast synaptic transmission. *, P < 0.05.
AICAR fails to inhibit GnRH neurons from DHT-treated mice
Because DHT rendered GnRH neurons less sensitive to low glucose, and the above data suggested that AMPK is an important mediator of the response to low glucose, we hypothesized that GnRH neurons from DHT-treated mice would be less sensitive to the inhibitory effects of AICAR. In fact, the majority of GnRH neurons tested (seven of nine) were not inhibited by AICAR, and overall AICAR had no effect on the firing rate in cells from this group (P = 0.61; Fig. 5, B and C). However, similar to low glucose, AICAR inhibited firing in cells from DHT mice in the presence of blockers of fast synaptic transmission (n = 8, P < 0.05; Fig. 5, E and F). Together these findings suggest that the insensitivity of GnRH neurons from DHT-treated mice to low glucose is due to alterations in the presynaptic network.
AICAR and low glucose activate a similar current in GnRH neurons
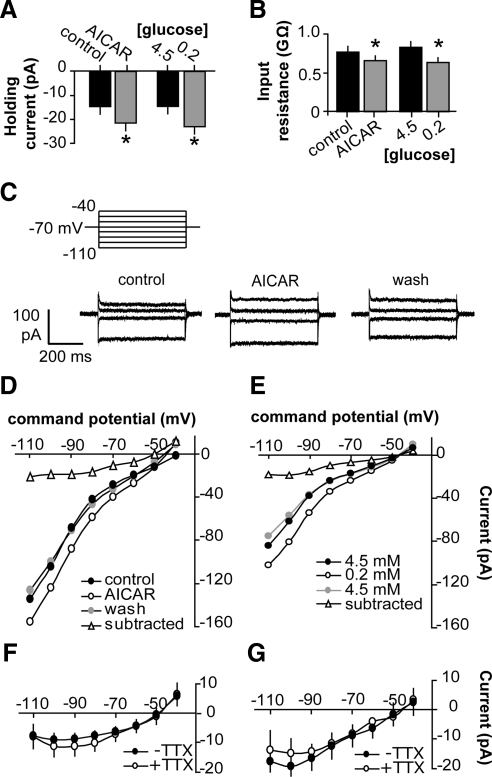
Whole-cell recordings were employed to identify the membrane changes induced by AICAR or low glucose to generate neuronal inhibition. In voltage-clamp mode at a holding potential of −70mV, AICAR (n = 19) activated a net inward current of 6.9 ± 1.5 pA (P < 0.001; Fig. 6A) and reduced input resistance from 0.77 ± 0.08 to 0.66 ± 0.06 GΩ (P < 0.05; Fig. 6B), indicating an increase in one or more conductances. Similarly, low glucose (n = 8) activated a net inward current of −8.5 ± 2.0 pA (P < 0.01; Fig. 6A) and reduced input resistance from 0.84 ± 0.07 to 0.64 ± 0.07 GΩ (P < 0.05; Fig. 6B). These effects were at least partially reversible upon washout, assessed in a subset of cells (P < 0.05 AICAR vs. washout, n = 6; P < 0.01 low glucose vs. glucose restoration, n = 6). Steady-state current-voltage relationships revealed that both AICAR and low glucose activated a current that reversed around −50 mV (Fig. 6, C–G); the current was similar in the presence and absence of tetrodotoxin (TTX; Fig. 6, F and G). The reversal potential of −50 mV indicated either a nonspecific cationic or chloride conductance. To assess the latter, in a subset of cells we used an internal solution with a chloride concentration of 140 mm (compared with 20 mm in standard internal). However, changing [Cl-]in failed to alter the magnitude or reversal potential of the AICAR-activated current (140 mm Cl− n = 3, Vrev = −56 ± 5.7 mV, current at −90mV = −12 ± 3.2 pA; 20 mm Cl− n = 19, Vrev = −51.9±2.3 mV, current at −90mV = −10.3 ± 2.1 pA), suggesting AICAR does not activate a chloride conductance. An antagonist of the hyperpolarization-activated cation current Ih, ZD7288 (30 μm), also had no effect (n = 2, Vrev = −47.5 ± 2.5 mV, current at −90mV = −11.8±2.4 pA).
Fig. 6.
Both AICAR and low glucose activate a nonspecific cation current in GnRH neurons. A, AICAR and low glucose increased holding current at −70mV. *, P < 0.05 by paired t test. B, AICAR and low glucose decreased input resistance. *, P < 0.05 by paired t test. C (top), Step protocol used to generate current-voltage data. C (bottom), representative traces before (control), during AICAR, and after 10 min of washout (wash) (every other step shown for clarity). AICAR generated a current with a reversal potential of around −50 mV that was at least partially reversible upon washout (wash). D and E, Representative steady-state current-voltage relationships before (closed circles) and after (open circles) AICAR (D) or low glucose (E) and subtracted current (open triangles). Washout current-voltage plot is shown in gray. G, Mean ± sem AICAR-induced (subtracted) current in cells with (n =11) and without (n = 8) TTX. F, Mean ± sem low-glucose-induced (subtracted) current in cells with (n = 4) and without (n = 4) TTX. The current was similar in the presence and absence of TTX. ms, Millisecond.
Because the whole-cell configuration could potentially alter intracellular ion concentrations or dialyze out critical cell components, we verified that inhibitory effects of AICAR or low glucose persisted in the whole-cell configuration using current-clamp recordings. DC current (0–30 pA) was injected to stimulate tonic firing. AICAR reduced the mean firing rate from 0.77 ± 0.07 to 0.16 ± 0.07 Hz (Fig. 7; n = 4, P < 0.05). Low glucose also reduced the mean firing rate from 0.98 ± 0.4 to 0.18 ± 0.14 Hz (Fig. 7; n = 5, P < 0.05).
Fig. 7.
Inhibitory effects of low glucose and AICAR persist in the whole-cell configuration. Traces (3 min) before and after low glucose (A) or AICAR (B). C, Mean ± sem action potential (AP) frequency before and after low glucose (n = 5) or AICAR (n = 4), and after 15–25 min of washout (wash). *, P < 0.05. s, Second; con, control.
Discussion
Numerous studies have indicated a role for glucose in the central regulation of fertility. Here we demonstrated that GnRH neurons are sensitive to changes in extracellular glucose within the physiological range. Further, we have demonstrated a role for AMPK in glucosensing, establishing AMPK as a novel regulator of GnRH neuron activity. Androgen-induced changes in fast synaptic transmission appear to render GnRH neurons less sensitive to AMPK and thereby low glucose. This finding may have implications for fertility disorders such as PCOS in which elevated circulating androgen and glucose levels are often present (33).
Because they are inhibited by low glucose (and hence fire more in high glucose), GnRH neurons could be classified as glucose excited (34). However, they appear to employ different mechanisms than the classical hypothalamic glucose-excited neurons involved in control of food intake and energy expenditure, which utilize, at least in part, KATP channels and glucokinase (34). Although GnRH neurons express functional KATP channels and glucokinase (9), glucosensing persisted in 100% of tested neurons when KATP channels were blocked. This suggests that KATP channels are not the primary mediators of glucosensing in GnRH neurons, and these channels may have other functions in this cell type. For example, KATP channels were recently shown to be one mediator of rapid effects of E on GnRH neurons (35). The hypothesis that KATP channels do not mediate glucoprivic suppression of the reproductive axis is supported by the recent observation that the suppression of LH secretion by fasting is not reversed by tolbutamide or by genetic deletion of the sulfonylurea-1 subunit of the KATP channel (36). Although we have not definitively linked the changes in GnRH neuron activity observed here with changes in GnRH and thereby LH secretion, neuronal activity is linked with hormone release, and future physiological studies in vivo could test this postulate.
In contrast to KATP blockade, antagonism of AMPK markedly attenuated inhibition of firing activity by low glucose in GnRH neurons, suggesting that it is an important mediator of glucosensing in this cell type. Accordingly, pharmacological activation of AMPK inhibited GnRH neurons, similar to its effect in GT1–7 immortalized GnRH neurons, and activated a similar current as low glucose. AMPK may play a role in glucosensing in other types of neurons. In agouti-related peptide and proopiomelanocortin neurons, selective genetic deletion of the AMPK α2 subunit abolished glucosensing (37). Hypothalamic AMPK plays a role in the counterregulatory response to hypoglycemia (38, 39). In glucose-inhibited neurons of the ventromedial hypothalamus, activation of AMPK by low glucose is excitatory due to closure of cystic fibrosis transmembrane regulator chloride channels (40). Due to high intracellular chloride maintained by GnRH neurons (41), closure of chloride channels would be inhibitory. However, a 7-fold increase in intracellular Cl− failed to shift reversal potential of the AICAR-induced current in GnRH neurons, indicating it is not carried by chloride. In GT1–7 cells, AICAR was shown to block the hyperpolarization-activated cation current, Ih (42). However, Ih is unlikely to be the identity of the current in this study because it is only found in about 40% of GnRH neurons, and AICAR or glucose induced a current in nearly all cells tested, including those not exhibiting Ih (which would be activated by the step protocol used in this study). Further, the Ih blocker ZD7288 had no effect on the AICAR-sensitive current.
A large body of work suggests that distal brain regions relay information about glucose availability to GnRH neurons. Although these brain areas likely affect the response of GnRH neurons in vivo, their influence in this study was minimized by the use of coronal brain sections. Hindbrain neurons are thought to be particularly important for monitoring glucose availability and conveying this information to GnRH neurons (8, 43); however, connections between this brain area and GnRH neurons are severed in coronal slices. Also disrupted were synaptic connections to areas controlling feeding (ventromedial, lateral hypothalamus) and CRH neurons that mediate stress responses (paraventricular nucleus), the latter of which have been implicated in nutritional suppression of fertility in vivo (44). Further, GABAA receptor activation is an important component of hindbrain-mediated glucoprivic suppression of the GnRH-LH axis (45), but the response here was enhanced rather than blocked when fast synaptic transmission was blocked. It is possible that synaptic terminals that remain intact in the brain slice may continue to influence GnRH neuron activity. Isolated terminals can generate postsynaptic currents in isolated neurons (46), and terminal-level regulation of distal inputs could influence cellular responses. The AICAR-induced current was similar in the presence and absence of TTX, suggesting that action potential-evoked neurotransmitter or neuromodulator release by presynaptic neurons does not contribute to this current; it is possible that AICAR may alter spontaneous presynaptic vesicle release. Another possibility is that nonneuronal cells, such as glia, contribute to the observed responses. AMPK is expressed in astrocytes where it induces morphological and functional changes in response to energy depletion (47, 48). The existing model of glial-dependent glucosensing involves closure of neuronal KATP channels by glial-generated lactate (49). This mechanism is unlikely because the effect in GnRH neurons is KATP independent; however, alternative mechanisms of glial-GnRH neuron communication to activate AMPK may exist. Although our data do not definitively indicate AMPK signaling within the GnRH neuron, they strongly suggest this possibility.
Detection of metabolic cues by the reproductive system is particularly critical for females, for whom reproduction and subsequent lactation are exceptionally energetically expensive. In this regard, the reproductive effects of negative energy balance are sexually dimorphic, with males being less sensitive to suppressive effects of caloric restriction on fertility (50) and less sensitive to glucoprivic suppression of LH (5). These observations are consistent with the effect of androgen to prevent the suppression of GnRH neuron activity by low glucose. The present findings are also relevant to PCOS, in which women have high androgens and, due to impaired glucose disposal, elevated blood glucose levels. Increased glucose alone may enhance activity of GnRH neurons, contributing to hyperactivity of the GnRH-LH axis in this disorder. Hyperandrogenemia in PCOS may compound this effect by reducing AMPK-mediated inhibition. However, the effect of androgen may be mitigated by E, also present in women with PCOS. Local changes in steroid levels due to central synthesis could also affect this response (51).
Androgen appears to inhibit glucosensing in part through an effect on presynaptic neurotransmission. Preliminary observations from our laboratory indicate that DHT increases GABAergic transmission to GnRH neurons from female mice (our unpublished data); enhanced GABAergic signaling may mask the direct effects of low glucose. Although blockade of fast synaptic transmission enabled glucosensing in cells from OVX+DHT mice, the latency of the response differed in comparison with cells from OVX mice. In contrast, responses to AICAR were similar in cells from OVX and OVX+DHT mice during fast synaptic blockade. Combined, these data suggest possible differences in kinetics of glucose metabolism upstream of AMPK in cells from DHT-treated mice. It is also possible that DHT may alter the range of glucose sensitivity, and that firing is already suppressed at 4.5 mm glucose, suggesting a basement effect. Subpopulations of glucose-excited neurons in the ventromedial hypothalamus exhibit different ranges of glucose sensitivity (52); those responding to glucose concentration changes in higher ranges are termed “high glucose excited,” and the disparity has been attributed to differences in underlying mechanisms (52). Insulin has been shown to blunt the response of glucose-excited neurons in the ventromedial hypothalamus to low glucose (53), raising the possibility that androgen itself or androgen-induced changes in circulating factors may similarly alter the response in GnRH neurons. For example, leptin and melanocortin have been shown to suppress neuronal AMPK activation (54), whereas adiponectin (55) and ghrelin (56) enhance it.
The finding that low glucose or AICAR activated a nonspecific cation current (i.e. one that passes multiple types of ions) was surprising, given that activation of this type of current is typically excitatory. One possible explanation for these findings is that this current generates shunting inhibition. Opening of a channel with a reversal potential of −50 mV will tend to drive the membrane toward −50 mV; however, because this potential is relatively close to the resting potential, little membrane potential change is actually observed. The reduction in membrane resistance caused by the opening of channels will increase the current required to depolarize the cell to action potential threshold; this produces a shunt to changes in membrane potential. Further studies are needed to clarify the mechanisms by which the observed current generates neuronal inhibition.
Overall, these studies have provided novel insight into the question of how GnRH neurons sense low fuel availability and support the idea that AMPK is critical for GnRH neuronal glucosensing. The regulation of GnRH neuron activity by AMPK has implications for the effects of other metabolic cues that are known to interact with this kinase, suggesting novel avenues for future research.
Materials and Methods
Animals
Studies were performed in 2- to 4-month-old female GnRH-GFP mice (14). Mice were housed on a 14-h light, 10-h dark cycle, with lights off at 1630 h, and were maintained on Harlan 2916 rodent chow (Harlan, Bartonsville, IL) and water ad libitum. All procedures were approved by the Animal Care and Use Committee of the University of Virginia and were conducted within the guidelines of the National Research Council's Guide for the Care and Use of Laboratory Animals. Mice were OVX under isoflurane (Abbott Laboratories, North Chicago, IL) anesthesia. Postoperative analgesia was provided by a long-acting local anesthetic (0.25% bupivacaine; 7.5 μl/site; Abbott Laboratories) directly to the surgical site. At the time of surgery, some mice received sc SILASTIC (Dow Corning, Midland, MI) capsules containing 400 μg DHT (OVX+DHT) or 0.625 μg E (OVX+E) in sesame oil. OVX+E+DHT mice received both capsules. Recordings were performed 8–12 d after surgery.
Brain slice preparation and recordings
All chemicals were from Sigma Chemical Co. (St. Louis, MO) unless noted. Brain slices were prepared using modifications of a previously described method (57). Solutions were bubbled with a 95% O2-5% CO2 mixture throughout the experiments and for at least 15 min before exposure to the tissue. The brain was rapidly removed and placed in ice-cold, high-sucrose saline solution containing 250 mm sucrose, 3.5 mm KCl, 26 mm NaHCO3, 10 mm glucose, 1.25 mm Na2HPO4, 1.2 mm MgSO4, and 2.5 mm MgCl2. Coronal 300-μm brain slices were cut with a Vibratome 3000 (Technical Products International, Inc., St. Louis, MO). Slices were incubated for 30 min at 30–32 C in a solution of 50% high-sucrose saline and 50% artificial cerebrospinal fluid (ACSF) containing 135 mm NaCl, 26 mm NaHCO3, 3.5 mm KCl, 10 mm glucose, 1.3 mm Na2HPO4, 1.2 mm MgSO4, and 2.5 mm CaCl2 (pH 7.4). Slices were then transferred to a solution of 100% ACSF at room temperature and kept at least 30 min and no more than 6 h before recording.
For recording, individual brain slices were placed in a recording chamber continuously superfused with oxygenated ASCF solution with glucose adjusted to 4.5 mm and maintained at 28–30 C using an in-line heater (Warner Instruments, Hamden, CT). Slices were incubated in this solution for 15–20 min before the start of recording. Cells were visualized with an upright fluorescent microscope with infrared differential interference contrast (Olympus Corp., Lake Success, NY). GnRH neurons were identified by brief illumination at 470 nm to visualize the GFP signal. Recording pipettes were pulled from borosilicate glass capillaries (1.65-mm outer diameter; 1.12-mm inner diameter; World Precision Instruments, Inc., Sarasota, FL) using a Flaming/Brown P-97 (Sutter Instrument, Novato, CA) and had resistances from 1.5 to 2.5 mΩ when filled with HEPES. Pipettes were placed in contact with a GnRH neuron using an MP-285 micromanipulator (Sutter Instrument). Current and voltage traces were obtained using an EPC-10 amplifier controlled by PatchMaster (HEKA Instruments, Inc., Bellmore, NY).
Experimental design
To study the effects of alterations in extracellular glucose on firing activity of GnRH neurons, targeted extracellular recordings were used. This type of recording is minimally invasive and does not alter the intracellular milieu; thus glucose and ion concentrations as well as the cell response to synaptic inputs that remain within the brain slice are maintained. Recording pipettes were filled with HEPES-buffered solution containing 150 mmNaCl, 10 mm HEPES, 10 mmglucose, 2.5 mmCaCl2, 1.3 mmMgCl2, and 3.5 mm KCl. Initial resistances ranged from 6 to 30 mΩ and either remained stable or increased during recording up to as high as 50 mΩ. Recordings were made in voltage-clamp mode with a pipette holding potential of 0 mV; at low-seal resistance, the amplifier potential does not influence the cell. In this type of recording, action currents are detected, which reflect changes in the action potential firing rate. The phrases “firing rate” and/or “firing activity” will be used to refer to these events.
After 15–20 min acclimation to 4.5 mm glucose and establishment of a stable recording, firing rate was monitored for 10 min in 4.5 mm glucose, for 10 min after a switch to 0.2 mm glucose, and for 10 min after a return to 4.5 mm glucose. If cells failed to fire within 10 min after restoration of 4.5 mm glucose, recording was continued until the cell fired, or KCl (15 mm) was applied to verify cell viability. In a subset of recordings, tolbutamide (200 μm), CC (20 or 40 μm), or the combination of APV, CNQX, and bicuculline (each at 20 μm) was used. Drugs were present in both the 4.5 mm and 0.2 mm glucose solutions. Cells that were quiescent or failed to achieve a stable firing pattern during the 10-min control period were not used for extracellular experiments. Changes in solution osmolarity due to the reduction in glucose (4.3 mOsm) were not compensated. At these low concentrations, glucose transport is not saturated (58); glucose is essentially freely membrane permeable and thus osmotically inactive (59).
To examine the effect of pharmacological activation of AMPK, N1-(b-d-ribofuranosyl)-5-aminoimidazole-4-carboxamide (AICAR, 2 mm) was bath applied to cells for 30 min after a 15- to 20-min control recording period. For analysis, firing rate during the last 10 min of AICAR exposure was quantified. The AMPK activator metformin was also tested. After a control recording period, 2 mm metformin was applied to cells for 20 min.
Whole-cell recordings
To examine the effects of AICAR on intrinsic properties of GnRH neurons, whole-cell recordings were used. Pipettes were filled with a solution containing the following: 125 mm K gluconate, 20 mm KCl, 10 mm HEPES, 5 mm EGTA, 4.0 mm MgATP, 0.4 mm NaGTP, 1.0 mm CaCl2, pH 7.2, 300 mOsm. The extracellular solution contained ACSF with 4.5 mm glucose and APV, CNQX, bicuculline, and in some instances 0.5 μm TTX to block persistent and fast sodium currents. Input resistance, series resistance, and membrane capacitance were continually measured, and only recordings with stable input resistance more than 500 mΩ, series resistance less than 20 mΩ, and stable membrane capacitance were used for analysis. Cells were held at −60 mV between step protocols. After attaining the whole-cell configuration, cells were allowed to stabilize for at least 5 min. A step protocol (shown in Fig. 6B) was run under control conditions. AICAR (2 mm) was then bath applied for 15 min. The protocol was run intermittently during this time to monitor current changes. The data obtained at 15 min were used to determine the AICAR-induced current, because a clear suppression of firing was observed by this time point in extracellular recordings in the presence of fast synaptic blockers. Membrane potentials were corrected for 10 mV of liquid junction potential (60).
To assess the effect of low glucose on whole-cell current, the same protocol as above was used. After cell stabilization (≥ 5min), the protocol was run two to four times in 4.5 mm glucose, and then after 10 min in 0.2 mm glucose. If the cell remained stable, the protocol was repeated after restoration of 4.5 mm glucose for 10–20 min. Two traces in each condition were averaged for analysis.
Analysis and statistics
For each recording, mean firing rate (in Hz) was determined for each 5-min interval of recording time. The 5 min immediately before low glucose exposure was used as the control period for analysis. The last 5 min of low-glucose perfusion and the 5 min of the washout period were designated time 1 (t1) and time 2 (t2) after low glucose, respectively. One-way repeated measures ANOVA with Dunnett's Multiple Comparison test was used to compare treatment vs. control frequencies. To compare the effect of low glucose among groups, a two-way ANOVA with Bonferroni's post hoc test was used. Two-tailed paired t test was used to compare firing frequencies pre- and post-AICAR. Fisher's exact test was used to compare percent responders among groups. For all tests, significance was set at P < 0.05, and data are reported as mean ± sem.
Acknowledgments
We thank Debra Fisher (University of Virginia) and Laura Burger (University of Michigan) for expert technical assistance, and Jessica Kennett (University of Virginia) and Pei-San Tsai (University of Colorado) for helpful editorial comments.
This work was supported by National Institute of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants U54 HD28934 and F31 NS62646.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages:
Ligands: 17β-estradiol | Dihydrotestosterone.
Footnotes
- ACSF
- Artificial cerebrospinal fluid
- AMPK
- AMP-activated protein kinase
- APV
- D(-)2-amino-5-phosphonovaleric acid
- CC
- compound C
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione
- DHT
- dihydrotestosterone
- E
- estradiol
- GABA
- γ-aminobutyric acid
- GFP
- green fluorescent protein
- KATP
- ATP-sensitive potassium
- OVX
- ovariectomized
- PCOS
- polycystic ovary syndrome
- TTX
- tetrodotoxin.
References
- 1. Levine JE, Pau KY, Ramirez VD, Jackson GL. 1982. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology 111:1449–1455 [DOI] [PubMed] [Google Scholar]
- 2. Caraty A, Evans NP, Fabre-Nys CJ, Karsch EJ. 1995. The preovulatory gonadotrophin-releasing hormone surge: a neuroendocrine signal for ovulation. J Reprod Fertil(Suppl 49):245–255 [PubMed] [Google Scholar]
- 3. Wade GN, Schneider JE, Li HY. 1996. Control of fertility by metabolic cues. Am J Physiol 270:E1–E19 [DOI] [PubMed] [Google Scholar]
- 4. Wade GN, Schneider JE. 1992. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev 16:235–272 [DOI] [PubMed] [Google Scholar]
- 5. Nagatani S, Bucholtz DC, Murahashi K, Estacio MA, Tsukamura H, Foster DL, Maeda KI. 1996. Reduction of glucose availability suppresses pulsatile luteinizing hormone release in female and male rats. Endocrinology 137:1166–1170 [DOI] [PubMed] [Google Scholar]
- 6. Ohkura S, Ichimaru T, Itoh F, Matsuyama S, Okamura H. 2004. Further evidence for the role of glucose as a metabolic regulator of hypothalamic gonadotropin-releasing hormone pulse generator activity in goats. Endocrinology 145:3239–3246 [DOI] [PubMed] [Google Scholar]
- 7. He D, Funabashi T, Sano A, Uemura T, Minaguchi H, Kimura F. 1999. Effects of glucose and related substrates on the recovery of the electrical activity of gonadotropin-releasing hormone pulse generator which is decreased by insulin-induced hypoglycemia in the estrogen-primed ovariectomized rat. Brain Res 820:71–76 [DOI] [PubMed] [Google Scholar]
- 8. Briski KP, Sylvester PW. 1998. Role of endogenous opiates in glucoprivic inhibition of the luteinizing hormone surge and fos expression by preoptic gonadotropin-releasing hormone neurones in ovariectomized steroid-primed female rats. J Neuroendocrinol 10:769–776 [DOI] [PubMed] [Google Scholar]
- 9. Zhang C, Bosch MA, Levine JE, Ronnekleiv OK, Kelly MJ. 2007. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27:10153–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunaif A, Thomas A. 2001. Current concepts in the polycystic ovary syndrome. Annu Rev Med 52:401–419 [DOI] [PubMed] [Google Scholar]
- 11. Silver IA, Erecińska M. 1994. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci 14:5068–5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pielecka J, Quaynor SD, Moenter SM. 2006. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 147:1474–1479 [DOI] [PubMed] [Google Scholar]
- 13. Mobbs CV, Kow LM, Yang XJ. 2001. Brain glucose-sensing mechanisms: ubiquitous silencing by aglycemia vs. hypothalamic neuroendocrine responses. Am J Physiol Endocrinol Metab 281:E649–E654 [DOI] [PubMed] [Google Scholar]
- 14. Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. 2000. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- 15. Sullivan SD, Moenter SM. 2005. GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod 72:33–41 [DOI] [PubMed] [Google Scholar]
- 16. DeFazio RA, Moenter SM. 2002. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2255–2265 [DOI] [PubMed] [Google Scholar]
- 17. Christian CA, Mobley JL, Moenter SM. 2005. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102:15682–15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leranth C, MacLusky NJ, Sakamoto H, Shanabrough M, Naftolin F. 1985. Glutamic acid decarboxylase-containing axons synapse on LHRH neurons in the rat medial preoptic area. Neuroendocrinology 40:536–539 [DOI] [PubMed] [Google Scholar]
- 19. Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. 2003. Seasonal plasticity within the GnRH system of the ewe: changes in identified GnRH inputs and in glial association. Endocrinology 144:3663–3676 [DOI] [PubMed] [Google Scholar]
- 20. Sim JA, Skynner MJ, Pape JR, Herbison AE. 2000. Late postnatal reorganization of GABA(A) receptor signalling in native GnRH neurons. Eur J Neurosci 12:3497–3504 [DOI] [PubMed] [Google Scholar]
- 21. Sullivan SD, Moenter SM. 2004. γ-Aminobutyric acid neurons integrate and rapidly transmit permissive and inhibitory metabolic cues to gonadotropin-releasing hormone neurons. Endocrinology 145:1194–1202 [DOI] [PubMed] [Google Scholar]
- 22. Sullivan SD, DeFazio RA, Moenter SM. 2003. Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. J Neurosci 23:8578–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Christian CA, Moenter SM. 2008. Critical roles for fast synaptic transmission in mediating estradiol negative and positive feedback in the neural control of ovulation. Endocrinology 149:5500–5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. 1999. Bicuculline block of small-conductance calcium-activated potassium channels. Pflugers Arch 438:314–321 [DOI] [PubMed] [Google Scholar]
- 25. Herbison AE, Skinner DC, Robinson JE, King IS. 1996. Androgen receptor-immunoreactive cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinology 63:120–131 [DOI] [PubMed] [Google Scholar]
- 26. Miki T, Nagashima K, Seino S. 1999. The structure and function of the ATP-sensitive K+ channel in insulin-secreting pancreatic β-cells. J Mol Endocrinol 22:113–123 [DOI] [PubMed] [Google Scholar]
- 27. Rother E, Könner AC, Brüning JC. 2008. Neurocircuits integrating hormone and nutrient signaling in control of glucose metabolism. Am J Physiol Endocrinol Metab 294:E810–E816 [DOI] [PubMed] [Google Scholar]
- 28. Huang W, Acosta-Martínez M, Levine JE. 2008. Ovarian steroids stimulate adenosine triphosphate-sensitive potassium (KATP) channel subunit gene expression and confer responsiveness of the gonadotropin-releasing hormone pulse generator to KATP channel modulation. Endocrinology 149:2423–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liss B, Bruns R, Roeper J. 1999. Alternative sulfonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurons. EMBO J 18:833–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winder WW, Hardie DG. 1999. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277:E1–E10 [DOI] [PubMed] [Google Scholar]
- 31. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 1995. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229:558–565 [DOI] [PubMed] [Google Scholar]
- 33. Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. 2007. Glucose intolerance in polycystic ovary syndrome–a position statement of the Androgen Excess Society. J Clin Endocrinol Metab 92:4546–4556 [DOI] [PubMed] [Google Scholar]
- 34. Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. 2004. Neuronal glucosensing: what do we know after 50 years? Diabetes 53:2521–2528 [DOI] [PubMed] [Google Scholar]
- 35. Zhang C, Kelly MJ, Ronnekleiv OK. 2010. 17β-Estradiol rapidly increases K(ATP) activity in GnRH via a protein kinase signaling pathway. Endocrinology 151:4477–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang W, Acosta-Martínez M, Horton TH, Levine JE. 2008. Fasting-induced suppression of LH secretion does not require activation of ATP-sensitive potassium channels. Am J Physiol Endocrinol Metab 295:E1439–E1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. 2007. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117:2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. 2004. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes 53:1953–1958 [DOI] [PubMed] [Google Scholar]
- 39. McCrimmon RJ, Shaw M, Fan X, Cheng H, Ding Y, Vella MC, Zhou L, McNay EC, Sherwin RS. 2008. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes 57:444–450 [DOI] [PubMed] [Google Scholar]
- 40. Murphy BA, Fakira KA, Song Z, Beuve A, Routh VH. 2009. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol Cell Physiol 297:C750–C758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DeFazio RA, Heger S, Ojeda SR, Moenter SM. 2002. Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2872–2891 [DOI] [PubMed] [Google Scholar]
- 42. Wen JP, Lv WS, Yang J, Nie AF, Cheng XB, Yang Y, Ge Y, Li XY, Ning G. 2008. Globular adiponectin inhibits GnRH secretion from GT1–7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem Biophys Res Commun 371:756–761 [DOI] [PubMed] [Google Scholar]
- 43. Murahashi K, Bucholtz DC, Nagatani S, Tsukahara S, Tsukamura H, Foster DL, Maeda KI. 1996. Suppression of luteinizing hormone pulses by restriction of glucose availability is mediated by sensors in the brain stem. Endocrinology 137:1171–1176 [DOI] [PubMed] [Google Scholar]
- 44. Maeda K, Cagampang FR, Coen CW, Tsukamura H. 1994. Involvement of the catecholaminergic input to the paraventricular nucleus and of corticotropin-releasing hormone in the fasting-induced suppression of luteinizing hormone release in female rats. Endocrinology 134:1718–1722 [DOI] [PubMed] [Google Scholar]
- 45. Singh SR, Briski KP. 2005. Central GABAA but not GABAB receptors mediate suppressive effects of caudal hindbrain glucoprivation on the luteinizing hormone surge in steroid-primed, ovariectomized female rats. J Neuroendocrinol 17:407–412 [DOI] [PubMed] [Google Scholar]
- 46. Turrigiano G, Abbott LF, Marder E. 1994. Activity-dependent changes in the intrinsic properties of cultured neurons. Science 264:974–977 [DOI] [PubMed] [Google Scholar]
- 47. Favero CB, Mandell JW. 2007. A pharmacological activator of AMP-activated protein kinase (AMPK) induces astrocyte stellation. Brain Res 1168:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blázquez C, Woods A, de Ceballos ML, Carling D, Guzmán M. 1999. The AMP-activated protein kinase is involved in the regulation of ketone body production by astrocytes. J Neurochem 73:1674–1682 [DOI] [PubMed] [Google Scholar]
- 49. Ainscow EK, Mirshamsi S, Tang T, Ashford ML, Rutter GA. 2002. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K(+) channels. J Physiol 544:429–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, Becker KG, Wood W, Duffy K, Vinayakumar P, Maudsley S, Mattson MP. 2007. Sex-dependent metabolic, neuroendocrine and cognitive responses to dietary energy restriction and excess. Endocrinology 148:4318–4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schumacher M, Akwa Y, Guennoun R, Robert F, Labombarda F, Desarnaud F, Robel P, De Nicola AF, Baulieu EE. 2000. Steroid synthesis and metabolism in the nervous system: trophic and protective effects. J Neurocytol 29:307–326 [DOI] [PubMed] [Google Scholar]
- 52. Silver IA, Erecińska M. 1998. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol 79:1733–1745 [DOI] [PubMed] [Google Scholar]
- 53. Cotero VE, Routh VH. 2009. Insulin blunts the response of glucose-excited neurons in the ventrolateral-ventromedial hypothalamic nucleus to decreased glucose. Am J Physiol Endocrinol Metab 296:E1101–E1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB. 2004. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574 [DOI] [PubMed] [Google Scholar]
- 55. Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. 2007. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6:55–68 [DOI] [PubMed] [Google Scholar]
- 56. Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. 2004. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279:12005–12008 [DOI] [PubMed] [Google Scholar]
- 57. Nunemaker CS, DeFazio RA, Moenter SM. 2002. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143:2284–2292 [DOI] [PubMed] [Google Scholar]
- 58. Heidenrich KA, Gilmore PR, Garvey WT. 1989. Glucose transport in primary cultured neurons. J Neurosci Res 22:397–407 [DOI] [PubMed] [Google Scholar]
- 59. Rosen AS, Andrew RD. 1991. Glucose concentration inversely alters neocortical slice excitability through an osmotic effect. Brain Res 555:58–64 [DOI] [PubMed] [Google Scholar]
- 60. Barry PH. 1994. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51:107–116 [DOI] [PubMed] [Google Scholar]