Next generation sequencing reveals abnormal microRNA expression in endometriomas.
Abstract
Endometriosis is a common disease seen by gynecologists. Clinical features involve pelvic pain and unexplained infertility. Although endometriosis is pathologically characterized by endometrial tissue outside the normal uterine location, endometriosis is otherwise not easily explained. Endometriomas, endometriotic cysts of the ovary, typically cause pain and distortion of pelvic anatomy. To begin to understand the pathogenesis of endometriomas, we describe the first transcriptome-microRNAome analysis of endometriomas and eutopic endometrium using next-generation sequencing technology. Using this approach, we generated a total of more than 54 million independent small RNA reads from our 19 clinical samples. At the microRNA level, we found 10 microRNA that were up-regulated (miR-202, 193a-3p, 29c, 708, 509-3-5p, 574-3p, 193a-5p, 485-3p, 100, and 720) and 12 microRNA that were down-regulated (miR-504, 141, 429, 203, 10a, 200b, 873, 200c, 200a, 449b, 375, and 34c-5p) in endometriomas compared with endometrium. Using in silico prediction algorithms, we correlated these microRNA with their corresponding differentially expressed mRNA targets. To validate the functional roles of microRNA, we manipulated levels of miR-29c in an in vitro system of primary cultures of human endometrial stromal fibroblasts. Extracellular matrix genes that were potential targets of miR-29c in silico were significantly down-regulated using this biological in vitro system. In vitro functional studies using luciferase reporter constructs further confirmed that miR-29c directly affects specific extracellular matrix genes that are dysregulated in endometriomas. Thus, miR-29c and other abnormally regulated microRNA appear to play important roles in the pathophysiology of uterine function and dysfunction.
Endometriosis is defined as endometrial tissue, including glands and stroma, located in an ectopic location, outside the uterine cavity. Endometriomas are endometriotic cysts of the ovary, also known as chocolate cysts (1). Most endometriomas are resistant to current medical therapy and the mainstay of treatment is surgical. Up to 16% of women of reproductive age undergoing hysterectomy have endometriosis (2). Up to 40% of women presenting with infertility will have endometriosis (3).
MicroRNA are small, single-stranded noncoding RNA molecules approximately 22 nucleotides in length that are transcribed from microRNA loci (4). MicroRNA are thought to function as repressors of gene function through mRNA cleavage and translational repression (4). Multiple studies suggest that microRNA play roles in both benign and malignant disease of the human reproductive tract. Abnormal expression levels of microRNA have been observed in multiple human reproductive tract diseases including preeclampsia (5), endometrioid endometrial adenocarcinoma (6), uterine leiomyomata (7–10), ovarian adenocarcinoma (11–13), and endometriosis (7, 14–18). Furthermore, novel microRNA have been discovered in the female reproductive tract by our own group using next-generation sequencing (19). Importantly, microRNA profiles in endometrial epithelium from women without disease change based on phase of menstrual cycle (20). Additionally, studies suggest that specific microRNA play a role in human reproductive tract diseases. For example, miR-31 represses cell cycle regulator gene E2F2 in serous ovarian cancer. Manipulations of miR-31 expression affect proliferation and apoptosis of serous ovarian cancer cell lines (12). In benign reproductive disease, loss of let-7 binding sites in the high mobility group AT-hook 2 3′ untranslated region (UTR) is associated with higher mRNA levels of high mobility group AT-hook 2, which has been associated with uterine leiomyomata (21). Additionally, studies suggest that miR-21 plays a role in uterine leiomyoma growth in vitro (22). These studies suggest that microRNA play important roles in diseases of the female reproductive tract. Although many profiling studies have been performed, few studies have confirmed functional roles of microRNA in the female reproductive tract.
Studies to date on microRNA expression in endometriosis have used advanced microarray technology (16–18). Although these studies support the hypothesis that microRNA are involved in endometriosis, these analyses have profiled only a fraction of the 939 currently known human microRNA (miRBase 15.0, April 2010) (23) because microarray technology is limited to the probes on the array designed to identify microRNA that have been identified at any given time. We report here the first use of next-generation sequencing for extensive microRNA profiling of pathologically normal endometrium from women without endometriosis and endometriomas. Furthermore, transcriptome profiling and in silico microRNA targeting predictions suggest that multiple biologically important pathways are dysregulated in endometriomas potentially through functional microRNA. Additionally, we show that in vitro manipulation of miR-29c in primary cultures of human endometrial stromal fibroblasts affects gene expression of miR-29c targets. Lastly, we show direct in vitro functional effects of miR-29c on specific gene targets involving the extracellular matrix. These data suggests that microRNA play a functional role in the pathogenesis of endometriosis.
Results
Clinical demographics
After obtaining permission from the Institutional Review Board for Baylor College of Medicine and its affiliated institutions, specimens were collected from women undergoing clinically indicated gynecologic surgery for removal of an endometriotic mass and/or other benign indications. All women had written informed consent. Specimens of endometrium were obtained from the uterine fundus at the time of hysterectomy at a distance from any uterine leiomyomata. Specimens of endometriomas were full-thickness 1-cm biopsies of the cyst wall and were isolated away from areas of obvious ovarian cortex. All specimens were preserved in RNAlater (Ambion, Carlsbad, CA) and frozen at −80 C.
Clinical demographics for both endometriosis and control patient groups are detailed in Table 1. The most common indication for hysterectomy among our control group was pelvic pain and/or vaginal bleeding secondary to uterine leiomyomata. As expected, the population of women undergoing surgery for endometriomas is younger (median age 34 yr; range 20–48 yr) than our nonendometriosis control population (median age 45 yr; range 39–48 yr). None of the nonendometriosis control group had a history of infertility, although two had never attempted to become pregnant. Five of the women in the endometriosis group had given birth to live-born children, whereas three had never attempted pregnancy. Only two of the endometriosis group had a history of infertility. The majority of the patients in both groups designated themselves as Latina, consistent with the population served by the institutions at which these specimens were collected. For the endometriosis group, two of 10 had a known family history of endometriosis (ID no. 7 and no. 14). None of the nonendometriosis control group had a family history of endometriosis. From our nonendometriosis control group, eight of nine had uterine leiomyomas. For the endometriosis group, six of 10 had unilateral or bilateral adnexal surgery (either salpingo-oophorectomy or ovarian cystectomy) without hysterectomy, two of 10 had a prior hysterectomy, and two of 10 had hysterectomy for uterine leiomyomata with adnexal surgery at time of sample collection. Thus, our patient population shows a certain amount of ethnic diversity with controlled pathologic homogeneity (endometriosis vs. nonendometriosis). Our samples for the validation studies showed similar characteristics (Table 1).
Table 1.
Study participant characteristics
| Patient ID | Cycle phase | Age | Parity | Other pathological diagnosis | Ethnicity | Medications | Infertility | Indication for surgery | Location of endometriosis |
|---|---|---|---|---|---|---|---|---|---|
| Endometriosis samples for transcriptome-microRNAome anaylsis | |||||||||
| M1 | Proliferative | 20 | 0 | None | Latina | None | n/a | Pain | Endometrioma |
| 2 | Secretory | 42 | 2 | Uterine leiomyomas | Latina | None | No | Pain/bleeding | Endometrioma |
| M2 | Prior hysterectomy | 37 | 2 | Uterine leiomyomas | African American | Hydrochlorothizide | No | Pain | Endometrioma |
| M3 | Prior hysterectomy | 40 | 2 | Uterine leiomyomas | African American | None | No | Pain | Endometrioma |
| 6 | Proliferative | 25 | 1 | None | Latina | None | No | Pain | Endometrioma |
| 7 | Proliferative | 23 | 0 | None | Caucasian | None | n/a | Pain | Endometrioma |
| 9 | Proliferative | 43 | 2 | None | Latina | None | No | Pain | Endometrioma |
| M4 | Proliferative | 23 | 0 | None | Latina | None | n/a | Pain | Endometrioma |
| 14 | Proliferative | 30 | 0 | None | Caucasian | None | Yes | Pain/infertility | Endometrioma |
| 19 | Interval phase | 48 | 0 | None | Latina | None | Yes | Pain | Endometrioma |
| Nonendometriosis control samples for transcriptome-microRNAome anaylsis | |||||||||
| 1 | Proliferative | 48 | 0 | Uterine leiomyomas | Latina | None | n/a | Pain/bleeding | None |
| 3 | Proliferative | 45 | 2 | Uterine leiomyomas | Latina | None | No | Pain/bleeding | None |
| 4 | Secretory | 45 | 2 | None | Latina | None | No | Pelvic organ prolapse | None |
| 12 | Interval phase | 40 | 3 | Uterine leiomyomas | African American | None | No | Pain | None |
| 18 | Proliferative | 39 | 0 | Uterine leiomyomas | Latina | None | n/a | Pain | None |
| 20 | Interval phase | 48 | 2 | Uterine leiomyomas | Latina | None | No | Pain/bleeding | None |
| 24 | Proliferative | 42 | 2 | Uterine leiomyomas | African American | None | No | Pain | None |
| 25 | Proliferative | 39 | 2 | Uterine leiomyomas | African American | None | No | Pain | None |
| 32 | Proliferative | 47 | 3 | Uterine leiomyomas | Latina | None | No | Pain | None |
| Endometriosis samples for validation studies | |||||||||
| 19753 | Prior hysterectomy | 45 | 2 | Uterine leiomyomas | Latina | None | No | Pain | Endometrioma |
| 44 | Proliferative | 30 | 0 | None | Asian | None | n/a | Pain | Endometrioma |
| 54 | Proliferative | 28 | 1 | None | Latina | None | Yes | Pain/infertility | Endometrioma |
| M6 | Prior hysterectomy | 37 | 3 | Uterine leiomyomas | Latina | None | No | Pain | Endometrioma |
| 62 | Proliferative | 25 | 0 | None | Asian | None | n/a | Pain | Endometrioma |
| 75 | Proliferative | 32 | 0 | None | Asian | None | yes | Pain/infertility | Endometrioma |
| 107 | Proliferative | 29 | 3 | None | Latina | None | No | Pain | Endometrioma |
| M8 | Prior hysterectomy | 48 | 3 | Uterine leiomyomas | Latina | None | No | Pain | Endometrioma |
| Nonendometriosis control samples for validation studies | |||||||||
| 39 | Proliferative | 48 | 5 | Uterine leiomyomas | African-American | None | No | Pain | None |
| 56 | Inactive | 56 | 6 | Uterine leiomyomas | African-American | Hydrochlorothizide | No | Pelvic organ prolapse | None |
| 64 | Proliferative | 44 | 3 | Uterine leiomyomas | Latina | None | No | Bleeding | None |
| 66 | Proliferative | 37 | 0 | Uterine leiomyomas | African-American | None | n/a | Pain/bleeding | None |
| 93 | Proliferative | 38 | 5 | Uterine leiomyomas | Latina | None | No | Pain/bleeding | None |
| 94 | Proliferative | 37 | 1 | Uterine leiomyomas | African-American | None | No | Pain | None |
| 103 | Proliferative | 22 | 0 | None | Asian | None | n/a | Pain | None |
| 105 | Proliferative | 49 | 4 | None | Latina | None | No | Bleeding | None |
| 110 | Secretory | 40 | 3 | Uterine leiomyomas | latina | None | No | Bleeding | None |
| 112 | Proliferative | 42 | 4 | Uterine leiomyomas | Latina | None | No | Pain/bleeding | None |
| 113 | Proliferative | 47 | 3 | Uterine leiomyomas | Latina | None | No | Pain | None |
n/a, Never attempted pregnancy.
Endometriomas have dysregulated microRNA expression
Our work in ovarian cancer (11, 12) showed that microRNA play a functional role in ovarian cancer. Thus, we hypothesized that microRNA may also play a significant role in endometriomas. Using Illumina next-generation sequencing (San Diego, CA) to identify and quantify microRNA present in nonendometriosis control endometrium and endometriomas with our validated bioinformatics pipeline (11, 12, 19), we obtained more than 54 million usable small RNA reads for an average of 2.8 million usable reads per clinical sample. Examination of individual microRNA expression compared with expression of all microRNA revealed that the let-7 family members were highly abundant in both nonendometriosis control endometrium and endometriomas (listed in bold in Table 2). MiR-29c is the 25th most abundant microRNA in endometriomas at 0.36% but is the 58th most abundant microRNA in nonendometriosis control endometrium at 0.04% (Table 2). More than 65% of the sequences from both the nonendometriosis control group and endometrioma group mapped to known microRNA from miRBase 15.0. Previous work from our group has shown that some of the sequence reads that do not map on known microRNA precursors represent novel microRNA (19).
Table 2.
Top 30 microRNA expressed in endometriomas and corresponding abundance in nonendometriosis control endometrium
| Unique miRNA | Relative abundance (%) |
|
|---|---|---|
| Endometrioma | Control endometrium | |
| hsa-let-7f | 20.40 | 23.66 |
| hsa-let-7a | 14.39 | 21.52 |
| hsa-mir-199a-3p | 9.21 | 6.43 |
| hsa-mir-199b-3p | 9.21 | 6.43 |
| hsa-let-7b | 8.19 | 10.90 |
| hsa-mir-21 | 6.10 | 2.29 |
| hsa-let-7c | 5.45 | 5.52 |
| hsa-let-7g | 3.70 | 4.01 |
| hsa-mir-143 | 2.53 | 2.95 |
| hsa-mir-103 | 1.71 | 1.40 |
| hsa-mir-140-3p | 1.39 | 1.10 |
| hsa-mir-29a | 1.17 | 0.64 |
| hsa-mir-101 | 1.17 | 0.69 |
| hsa-let-7e | 0.88 | 1.20 |
| hsa-mir-320a | 0.83 | 0.56 |
| hsa-let-7i | 0.78 | 0.62 |
| hsa-mir-26a | 0.75 | 0.31 |
| hsa-mir-1 | 0.72 | 1.79 |
| hsa-mir-145 | 0.66 | 0.40 |
| hsa-let-7d | 0.62 | 0.60 |
| hsa-mir-424 | 0.45 | 0.11 |
| hsa-mir-451 | 0.41 | 0.11 |
| hsa-mir-107 | 0.38 | 0.32 |
| hsa-mir-191 | 0.38 | 0.32 |
| hsa-mir-29c | 0.36 | 0.04 |
| hsa-mir-25 | 0.34 | 0.29 |
| hsa-mir-221 | 0.32 | 0.29 |
| hsa-mir-99a | 0.30 | 0.18 |
| hsa-mir-152 | 0.27 | 0.20 |
| hsa-mir-23a | 0.26 | 0.16 |
Bold face indicates the most abundant microRNA family, the let-7 family.
Of 939 known human microRNA from miRBase 15.0, we found 240 individual microRNA expressed in at least one sample of nonendometriosis control endometrium and 286 individual microRNA expressed in at least one sample of endometrioma. Thus, there is a limited subset of microRNA expressed in endometrium and endometriomas.
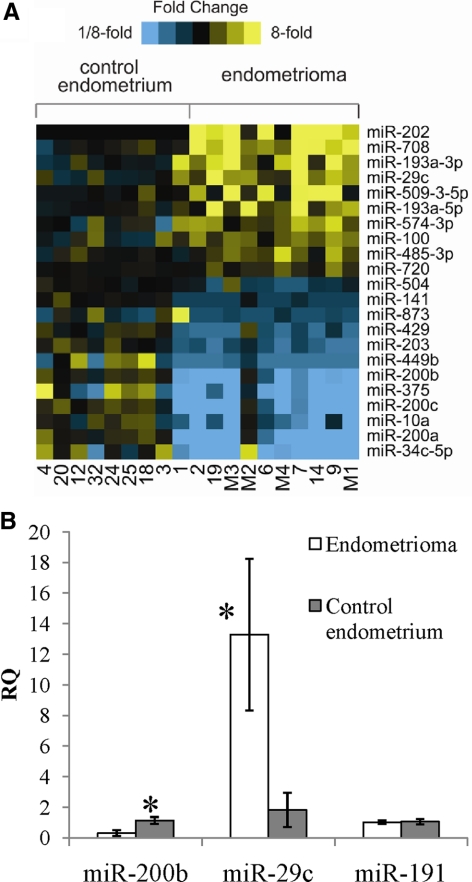
Comparison of individual microRNA expression levels between the nonendometriosis control group and the endometrioma group (fold change >1.5, P < 0.01) revealed 10 microRNA up-regulated and 12 microRNA down-regulated in endometriomas (Fig. 1A). We saw a similar trend in microRNA profiles when comparing eutopic endometrium and ectopic endometriomas from the same patient (data not shown) although our sample size was not powered for this analysis (n = 2). Expression of miR-29c, miR-200b, and miR-191 were validated using independent samples (Fig. 1B), and miR-29c was seen to exhibit increased expression in endometriomas. This high expression is not thought to be from contamination of endometriomas with surrounding ovarian cortex as premenopausal ovarian tissue has little to no expression of miR-29c by TaqMan assay (data not shown), and our next-generation sequencing data for short-term cultures of normal ovarian surface epithelium show only approximately 300 copies of miR-29c per sample (11, 12). MiR-200b exhibits decreased expression in endometriomas. MiR-191 was not changed in the two groups either by next-generation sequencing or TaqMan mature microRNA expression assays.
Fig. 1.
Expression profiling of microRNAs in nonendometriosis control endometrium and endometriomas. A, Heat map representation of transcripts overexpressed (yellow) and underexpressed (blue) in control endometrium vs. endometriomas (P < 0.01, fold change >1.5). Rows, microRNAs; columns, profiled samples. B, RQ QPCR of microRNAs in endometrioma compared with nonendometriosis control endometrium. *, P < 0.05.
Genes differentially expressed in endometriomas are predicted targets of differentially expressed microRNA in endometriomas
MicroRNA are thought to function as posttranscriptional repressors, either by destabilization of mRNA transcripts or repression of translation (24). MicroRNA function appears to be defined by the seed sequence, a seven-base run of nucleotides typically at positions 2–8 of the microRNA. MicroRNA seed sequences are important for specificity of binding to complementary regions on the 3′ UTR of target genes, leading to repression (24). Thus, if a microRNA is up-regulated, its functional targets that are repressed through mRNA decay should be down-regulated due to increased microRNA binding to their 3′ UTRs. Conversely, if a microRNA is down-regulated, its functional target mRNA should be up-regulated. To examine whether these predictions hold true, we explored the potential functional effects of our differentially expressed microRNA in endometriomas using mRNA gene profiling data from the same tissues samples (Table 1).
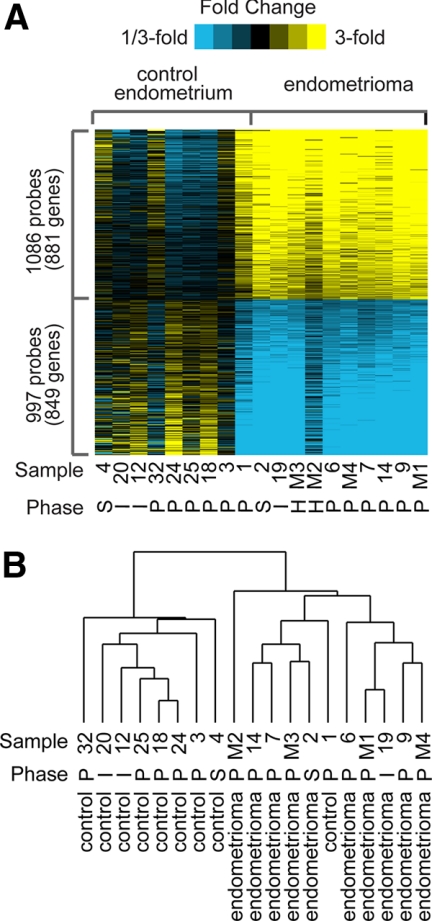
Gene expression profiles from nine independent nonendometriosis control (eutopic endometrium) and 10 independent endometrioma samples were compared (fold change >1.5, P < 0.01) to identify differentially expressed genes (Fig. 2A and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). From the nine nonendometriosis eutopic control samples, six were proliferative phase, one was secretory phase, and two were interval phase endometrium, as dated by surgical pathology and consistent with patient provided last menstrual period. We found more than 1700 differentially expressed gene products when endometriomas were compared with normal eutopic endometrium. These results are displayed in Fig. 2A, in which the heat map represents 1086 probes or 881 unique genes up-regulated and 997 probes or 849 unique genes down-regulated in endometriomas. Because our collection of endometrioma samples was from a mostly Latina patient population, we wanted to determine whether our differentially expressed gene set was similar to published data sets. We compared our data set with three publicly available and previously published data sets (25–27). Although these data sets were comparing eutopic endometrium and ectopic endometrium from the same patient and used different tissues types [i.e. peritoneal implants (25, 27), ovarian implants (26), or a combination of peritoneal implants and cyst wall from endometriomas (25)], we found that our differentially expressed gene list correlated well with these published data sets (Supplemental Fig. 2).
Fig. 2.
Expression profiling of genes in nonendometriosis control endometrium and endometriomas. A, Heat map representation of transcripts overexpressed (yellow) and underexpressed (blue) in control endometrium vs. endometriomas (P < 0.01, fold change >1.5). Menstrual cycle phase is indicated by S, secretory; I, interval; P, proliferative; or H, prior hysterectomy. Rows, Transcripts; columns, profiled samples. B, Hierarchical clustering of control endometrium and endometriomas. Menstrual cycle phase is indicated.
Further supporting that our data set is representative of endometriosis, gene ontology enrichment analysis showed an increase in inflammatory response genes (Supplemental Fig. 3). Four of the top five up-regulated gene ontology terms involved inflammation (immune response, immune system process, defense response, and inflammatory response), which is consistent with published data (26, 28–30) and clinical correlation of the inflammatory nature of endometriosis (31, 32). Although we did not use paired endometrium from the same patient in the same menstrual cycle phase, we have a representative data set, consistent with previous studies. Thus, our choice of nonendometriosis endometrium is a reasonable control.
Hierarchical clustering of our data shown in Fig. 2B suggests that endometriomas cluster separately from nonendometriosis control endometrium, as expected. Additionally, samples did not cluster based on phase of the menstrual cycle, although we did not have enough samples to study cycle-correlated genes in depth. Oddly, sample no. 1 from the nonendometriosis endometrium has a similar molecular profile to endometriomas as seen in the heat map and clusters with them.
Although progesterone resistance is a hallmark of endometriosis endometrium (33), endometriotic tissues are still considered estrogen responsive (34). However, recently a study showed that in a xenograft model of endometriosis, estrogen treatment did not increase the size of endometriotic lesions (35). In humans, endometriomas do not respond clinically to hormonal manipulations, which squelch endogenous estrogen production such as GHRH analogs (36). The preferred treatment is usually surgical with preference given to complete removal of cyst rather than drainage alone (37). To examine estrogen-responsive genes, we compared our differentially expressed gene list to a list of genes with estrogen receptor binding sites in mammary tissue (38). Of note, not all genes with estrogen receptor binding sites have a change in gene expression in response to estrogen. Statistical analysis revealed the set of genes low in endometriomas are enriched for genes with estrogen receptor binding sites in their promoter regions using mammary gland tissue (Supplemental Fig. 2).
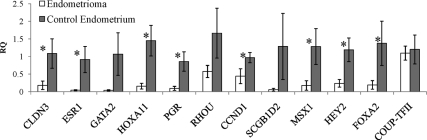
To focus on actual estrogen-responsive genes in the endometrium, we compared our differentially expressed gene list to a set of genes that respond to exogenous estrogen on the endometrium (39). Similarly, we found that the set of genes low in endometriomas are enriched for genes that respond to estrogen in the endometrium (Supplemental Fig. 2). Using independent samples of endometriomas and nonendometriosis endometrium (Table 1) validated these results (Fig. 3). Cyclin D1 (CCND1), secretoglobin, family 1D, member 2 (SCGB1D2), msh homeobox 1, and hairy/enhancer-of-split related with YRPW motif 2 (HEY2) were all expressed at significantly lower levels in independent samples of endometriomas compared with nonendometriosis control endometrium. CCND1 is likely involved in proliferation of the endometrium during the proliferative phase of the menstrual cycle in response to estrogen (40). SCGB1D2 is a member of the uteroglobin family and is expressed in most tissues but has higher levels in steroid hormone responsive tissues such as the prostate (41). The role of estrogen in regulation of this gene is still relatively unknown. In breast cancer cell lines, HEY2 expression is induced with treatment of cells with exogenous estradiol (38). msh homeobox 1 is critical for epithelial stroma interactions in uterine development and is expressed at relatively high levels in the uterus (42). SCGB1D2, HEY2, and CCND1 contain estrogen receptor binding sites and are estrogen responsive (38–40).
Fig. 3.
QPCR analysis of steroid hormone-responsive genes. RQ of estrogen- and progesterone-responsive genes in nonendometriosis control and endometriomas. *, P < 0.05.
Additionally, when we looked at a set of progesterone responsive genes in the mouse (43), we found that our down-regulated set of genes was enriched (Supplemental Fig. 2). Using independent samples, we were able to confirm the low expression of progesterone responsive genes (Fig. 3). Claudin 3 (CLDN3), estrogen receptor α (ESR1), and progesterone receptor (PGR) are progesterone-responsive genes (43) and contain estrogen receptor binding sites (38). Homeobox A11 (HOXA11), progesterone receptor, and Ras homology family member U (RHOU) are estrogen responsive (39) and progesterone responsive (43). Thus, endometriomas may have resistance to both progesterone and estrogen.
To identify potential functional microRNA-mRNA pairs, we used our previously published SigTerms software (44) and TargetScan 5.1 (www.targetscan.org), an on-line publically available algorithm for microRNA targeting. From the 10 microRNA that were up-regulated, we found that only five of the microRNA had predicted mRNA targets that were also differentially expressed (Supplemental Fig. 4). For genes that were up-regulated, we looked for microRNA that were down-regulated. For our 12 microRNA that were down-regulated, we found that all 12 of them had functional targets in TargetScan (Supplemental Fig. 5).
MiR-29c affects expression of target genes in vitro
To examine potential pathways that were affected by changes in gene expression based on our differentially expressed microRNA target genes, we performed pathway analysis using the Ingenuity Pathway Analysis program (Ingenuity Systems, Inc., Redwood, CA). This analysis revealed that our predicted target genes that were also dysregulated in endometriomas were involved in a top network of cellular development, connective tissue development and function, cellular growth and proliferation (Supplemental Fig. 6), centering around TGFβ and mitogen-activated kinase 1 (ERK). Previous work has shown a role for TGFβ signaling through MAPK/ERK to regulate c-fos, c-jun, and fibronection expression in endometrial epithelial and stromal cells (45). Previously published pathway analysis of genes targeted by microRNA in endometriosis by Ohlsson Teague et al. (17) has delineated similar networks focusing on c-jun. Thus, the differentially expressed microRNA have the potential to target important biologically important pathways in endometriomas.
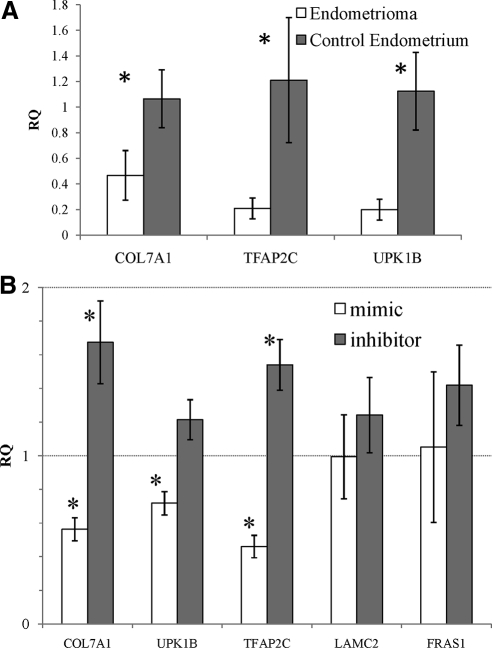
MiR-29c was predicted to target 29 genes. First, we validated the expression of miR-29c mRNA targets in independent samples of nonendometriosis control endometrium and endometriomas (Fig. 4A). Then we undertook a candidate gene approach using an in vitro system instead of a global genome-wide gene expression change. David Functional Analysis (46, 47) revealed that these 29 genes were involved in extracellular matrix and biological adhesion. Because endometriosis is a known disease of severe biological adhesions (48), we focused our attention on the role of miR-29c expression on these potential targets for miR-29c. First, we created primary cultures of human endometrial stromal fibroblasts (HESF) from eutopic endometrium of nonendometriosis control women (49). To validate the effect of miR-29c on target gene expression, we transfected these primary stromal cells with the Dharmacon microRNA mimic and inhibitor for miR-29c (Lafayette, CO) or appropriate nontargeting control.
Fig. 4.
QPCR analysis of genes targeted by miR-29c. A, RQ of miR-29c target genes in nonendometriosis control endometrium and endometriomas in independent samples. B, RQ of gene expression with transfection of miR-29c mimic compared with control mimic construct and RQ of gene expression with transfection of miR-29c hairpin inhibitor compared with control hairpin inhibitor. *, P < 0.05.
We examined the impact of overexpressing or inhibiting miR-29c on the expression of specific genes that are predicted targets for miR-29c and compared these results with the impact of a nontargeting control. Using independent cultures of these cells from eight independent nonendometriosis control subjects, we found that specific target gene expression was decreased with the miR-29c mimic and increased with the miR-29c inhibitor compared with nontargeting control (Fig. 4B). Laminin-γ2 (LAMC2), transcription factor AP-2γ (TFAP2C), uroplakin 1B (UPK1B), and Frazier syndrome 1 (FRAS1) have one potential miR-29abc family binding site within each of their 3′ UTRs. Collagen type VII A1 (COL7A1) has two predicted miR-29abc family binding sites within its 3′ UTRs. Endogenous gene expression was statistically significantly changed from nontargeting control for both mimic and inhibitor experiments for COL7A1 and TFAP2C. Uroplakin 1B showed a statistically significant decrease in endogenous gene expression with mimic and showed a trend toward increased endogenous gene expression with the inhibitor. Laminin-γ2 and Frazier syndrome 1 did not show a difference with either mimic or inhibitors (Fig. 4B). In nasopharyngeal carcinomas, miR-29c is down-regulated with a reciprocal up-regulation of other extracellular matrix genes such as collagens IIIA1, VA1, XVA1, and laminin γ1. Mimic experiments showed that the targeted down-regulation of the extracellular matrix genes was cell line specific (50). Thus, we have shown that miR-29c affects the additional extracellular matrix gene expression in the endometrial stromal cells.
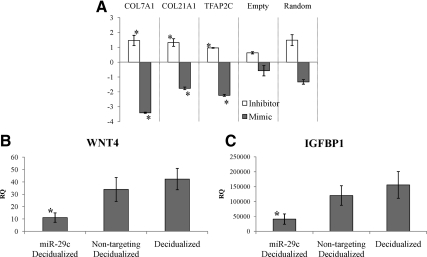
Although these data suggest a functional effect of miR-29c on target gene expression, this change in gene expression may not be from direct targeting. Therefore, we used luciferase constructs containing the entire 3′ UTR of a subset of these genes and cotransfected with miR-29c mimics and inhibitors and nontargeting controls to assess a direct effect. We used human embryonic kidney (HEK) 293T cells because the primary cultures of human endometrial stromal fibroblasts were resistant to transfection with multiple different constructs (i.e. mimic/inhibitor and luciferase construct). Using this in vitro system, we showed a significant decrease in luciferase activity in HEK293T miR-29c target constructs containing the 3′ UTRs of COL3A, a known direct target of miR-29c (50) (data not shown) and COL7A1, COL21A1, and TFAP2C compared with control (Fig. 5A). The transfection of miR-29c hairpin inhibitors did not have a significant effect (Fig. 5A) because miR-29c is typically not expressed in HEK293T cells (data not shown). Thus, miR-29c has an effect on gene expression through direct targeting of the 3′ UTR of these extracellular matrix genes.
Fig. 5.
Biological effects of miR-29c in vitro. A, Luciferase activity repression with miR-29c mimics in HEK293T. RQ of luciferase activity with cotransfection of 3′ UTR constructs and miR-29c mimic compared with control mimic and luciferase activity with cotransfection of 3′ UTR constructs and miR-29c hairpin inhibitor compared with nontargeting hairpin inhibitor control. Empty, luciferase parent vector only; random, random 3′ UTR construct in parent luciferase vector. B and C, RQ of WNT4 (B) and IGFBP1 (C) in HESF overexpressing miR-29c and undergoing in vitro decidualization compared with nondecidualized cells. *, P < 0.05.
Although these data suggest a molecular effect of miR-29c on target gene expression, we examined the biologic effect of miR-29c in uterine function. One of the functions of the uterine endometrium is to prepare for implantation and pregnancy. Previous work has shown that HESF from women with endometriosis have a blunted decidualization response compared with HESF from women without endometriosis (49, 51). To examine the biologic function of miR-29c on these cell lines, we overexpressed miR-29c or nontargeting controls and then decidualized the cells in vitro. We found that overexpression of miR-29c leads to a blunted decidualization response (49, 52) as measured by IGF binding protein (IGFBP)-1 and WNT4 expression levels (Fig. 5, B and C). In silico algorithms do not report that miR-29c targets the 3′ UTR of IGFBP1 or WNT4. Although this affect did not abolish molecular decidualization completely, miR-29c has a biological effect in endometrial cells.
Importantly, Kuokkanen et al. (20) have suggested that because miR-29c is up-regulated in the midsecretory endometrium, miR-29c is a progesterone-responsive microRNA. Our data suggest that by gene expression profile, our endometriomas are progesterone resistant but have increased expression of miR-29c. When we treated either HESF or endometrial epithelial cell lines with progesterone in vitro, we did not see a change in expression of miR-29c compared with vehicle alone (Supplemental Fig. 7). Progesterone is not the only regulatory molecule in the midsecretory endometrium. The regulation of miR-29c expression is an area of further study.
Discussion
In the present study, we identified microRNA that play a functional role in endometriosis, endometrium in an ectopic location. We used next-generation sequencing to profile microRNA in endometriosis and specifically in ovarian endometriomas. We profiled the transcriptome of these same samples to narrow down genes that were functionally targeted by microRNA important in endometriosis. Using this approach, we were able to validate the function of miR-29c in endometriomas. Endometriosis is endometrium in an ectopic location. Our differentially expressed microRNA in endometriomas target mRNA that are involved in diverse biological functions, in particular cellular movement. The targeting of extracellular matrix genes by miR-29c fits with the clinical nature of endometriomas and the biological pathway of cellular movement. Although miR-29c was shown to target some extra cellular matrix genes, the exact targets and specifically the endogenous genes repressed is cell line specific (50). Therefore, it is unfortunate that we could not show a direct effect of miR-29c on 3′ UTR luciferase constructs in the biologically important system of primary culture of human endometrial stromal fibroblasts. However, we were able to show a decrease in endogenous target gene expression. Although we did not examine miR-29c target protein expression specifically, destabilization of mRNA is the predominant biological effect of microRNA as recently published (53).
We did attempt to validate the function of other microRNA in this system. The miR-200bc/429 family was significantly down-regulated in endometriomas with a significant number of predicted target mRNA up-regulated (i.e. 147). David Bioinformatics Functional Analysis (46, 47) revealed that these predicted target genes were involved in the multicellular organismal process and development. When we used the Dharmacon miR200c inhibitor to down-regulate miR-200c levels in endometrial stromal fibroblasts, we did not see an increase in predicted target gene expression. However, all three members of the miR-200bc/429 family were significantly down-regulated in endometriomas, and therefore, inhibition of one of the family members may allow other members to be present and continue to repress target gene expression.
The down-regulation of genes with estrogen receptor binding sites and estrogen-responsive genes was surprising. Although the genes with estrogen receptor binding sites are not always induced with estrogen treatment using a breast cancer cell line (38), some of them are. Recently a xenograft mouse model of endometriosis has shown that endometriotic lesions do not change in size with estrogen treatment (35). Recently Aghajanova et al. (54) has shown that HESF from women with endometriosis have a delayed early response to estrogen. Furthermore, the lack of estrogen responsiveness fits with the clinical nature of endometriomas. Most women with endometriomas do not have significant relief of symptoms with hormonal manipulations but do have significant relief after aggressive surgery (36, 37). The down-regulation of estrogen-responsive genes may represent important biological differences between eutopic endometrium and endometriosis.
Although not all of our samples were dated by menstrual cycle phase due to a history of previous hysterectomy, our gene profiling data set correlated well with high-quality data sets of others (25–27). Our control samples were mostly proliferative. Additionally, our validation studies were performed using independent samples mainly from nonendometriosis control endometrium from the proliferative phase of the menstrual cycle. Furthermore, other groups have suggested that endometriotic lesions do not have variations in gene expression based on menstrual cycle phase (26). Although the perfect control for our experiments is not known, we believe that we have done our best to control for all potential confounders. We realize that gene expression profiles are distinctly different through the menstrual cycle (33, 55). However, due to the limited number of samples from the secretory phase of the menstrual cycle, we were not able to examine differences in gene or microRNA expression based on cycle phase.
Other studies have profiled microRNA in normal endometrium (20, 56, 57), eutopic endometrium of women with endometriosis (16, 18), or endometriotic implants (15, 17, 18). However, these previously published studies used different anatomical locations of endometriosis, different control samples for comparison, and different platforms for microRNA expression profiling than our study. Our study is the first to use next-generation sequencing to generate large amounts of data points for both eutopic endometrium and endometriomas. To date, 939 microRNA have been identified in human (23). Unlike previous studies that are based on a limited subset of microRNA (15–18, 20, 56, 57), our platform is not limited to known microRNA as indicated by our work showing novel microRNA in female reproductive tissues, including endometrium and endometriomas (19).
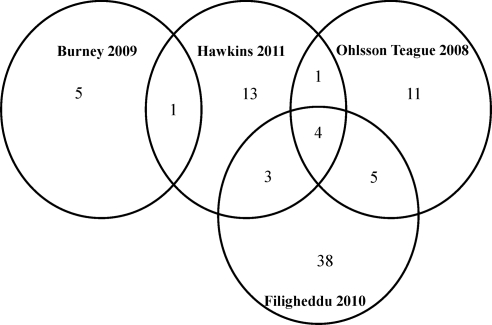
Additionally, our study was the first study to use gene expression profiling on the same samples to determine functional targets for these microRNA. In the studies looking at ectopic implants to endometrium, miR-29c, miR-100, miR-200a, and miR-200b were all differentially expressed in published data sets (15, 17, 18) and ours. A Venn diagram of the microRNA differently expressed in these other studies compared with ours shows that nine of 22 of our differentially expressed microRNA overlap with other studies (15–17), leaving 13 microRNA that are unique to our study (Fig. 6). This difference in microRNA expression patterns may stem from anatomic location of ectopic endometriosis as peritoneal lesions, rectovaginal lesions, and ovarian endometriosis have distinct molecular profiles (58–60). For example, our data set compares endometriomas to nonendometriosis control endometrium, whereas other studies compared peritoneal lesions (17) or endometriomas to eutopic endometrium of patients with endometriosis (15). The difference in anatomic location of endometriosis [peritoneal lesion (17)]or control group [eutopic endometrium from women with endometriosis (15, 17)] could account for this difference in microRNA expression. Interestingly, our most down-regulated microRNA, miR-34c-5p, was also significantly down-regulated in the early secretory endometrium of women with endometriosis (16). Although our samples are whole tissues containing both epithelium and stroma, miR193a, miR-29c, miR-203, and miR200c, which are differentially expressed in our analysis, were all down-regulated in the epithelium of late proliferative endometrium (20).
Fig. 6.
Venn diagram of overlap of differentially expressed microRNA in multiple studies.
In conclusion, our study has taken a new approach to the enigmatic disease endometriosis. We have used traditional whole genome-wide gene expression arrays coupled with the next-generation sequencing technology to identify microRNA that play a functional role in endometriosis. Our in silico analysis along with in vitro validation has shown that microRNA are important in the pathogenesis of endometriosis.
Materials and Methods
Institutional review board approval, collection of tissues, and creation of cell lines
All tissues collected for this study were collected with Baylor College of Medicine, Institutional Review Board (H-21138) approval. Briefly, patients undergoing surgery at Ben Taub General Hospital (Houston, TX) or St. Luke's Episcopal Hospital (Houston, TX) for endometriomas, suspected endometriosis, pelvic pain, abnormal uterine bleeding, pelvic organ prolapse, or uterine leiomyomas were approached for participation. After written informed consent, the patients underwent scheduled surgical procedure. Tissues were collected either as cyst wall of endometrioma or endometrial curettage of hysterectomy specimen and placed directly into RNALater (Ambion, Austin, TX) and frozen at −80 C. Samples were designated as endometriomas or nonendometriosis control endometrium based on surgical pathology reports. All women had regular menstrual cycles and were free of hormones for at least 30 d before surgery. Adenomyosis samples were excluded from study in either endometrioma or nonendometriosis control group due to the potential for confounding. Phase of the menstrual cycle is indicated for each sample. Menstrual cycle phase was determined by patient provided last menstrual period and confirmed by pathology. The phase of the menstrual cycle was not known for many women in the endometriosis group because these women had already had a hysterectomy. Primary cultures of HESF were created, grown, and decidualized as described (49, 61). HEK293T cells were grown according to American Type Culture Collection (ATCC.org).
RNA extraction, gene expression profiling, and small RNA sequencing
Total RNA was isolated from 50–100 mg of frozen tissue or a 10-cm dish of primary cultures of human endometrial stromal fibroblasts at 80% confluence using the mirVana kit (Applied Biosystems, Foster City, CA). RNA quality control was performed using the 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). High-quality RNA was subjected to Illumina's Human WG-6 version 2.0 BeadChips (Illumina) at Texas Children's Cancer Genomics and Proteomics Core Laboratory. The array platform consists of 48,701 probes of 20,091 unique genes. Expression data were quantile normalized. Array data sets have been deposited into the Gene Expression Omnibus (GSE23339). Small RNA library creation was performed using the DGE-small RNA sample prep kit (Illumina) according to the manufacturer's protocol. Purified cDNA was quantified using the Quant-iT PicoGreen dsDNA kit (Invitrogen, Carlsbad, CA) and diluted to 10 nm for sequencing on the Illumina 1G genome analyzer at the University of Houston. Data output was analyzed by our previous analysis pipeline (11, 12, 19). Briefly, unique reads with a minimum read count of 10 were aligned to human microRNA sequences from miRBase version 15.0. Counts of each unique read were tabulated, normalized to total usable reads, and had 40 counts added. Differentially expressed gene and microRNAs were identified by Student's t test on log-transformed data and fold change (ratio of averages of endometriomas to nonendometriosis control samples). Java TreeView (62) represented expression values as color maps.
Quantitative PCR for mRNA and microRNA
RNA was treated with Turbo deoxyribonuclease (DNAse; Applied Biosystems) according to the manufacturer's protocol. DNAse-treated RNA (1000 ng) was reverse transcribed in a 50-μl reaction using 250 U Superscript III reverse transcriptase (Invitrogen) and random primers (Invitrogen). Samples were diluted to 100 μl, and 5 μl was used for each quantitative PCR (QPCR) reaction. QPCR was performed on the ABI One Step Plus using either predesigned TaqMan gene expression assays (Applied Biosystems) or custom primers designed using Primer Express software (Applied Biosystems) for SYBR Green. TaqMan assays and custom primers are listed in Supplemental Fig. 8. Expression of human ribosomal gene L19 was used as an endogenous control. TaqMan PCR was performed using TaqMan universal PCR master mix (Applied Biosystems), and PCR with custom primers was performed using SYBR Green PCR master mix (Applied Biosystems) in 10 μl. The reaction conditions were as follows: 2 min at 50 C, 10 min at 95 C, followed by 40 cycles of 15 sec at 95 C (denaturation) and 1 min at 60 C (annealing/extension). Each sample was analyzed in duplicate or triplicate and a nontemplate control (nuclease free water) sample was included on each plate for each primer-probe set. All custom primers had efficiency of 85–110%. All SYBR Green runs had dissociation curves to detect potential primer-dimers. The relative quantity (RQ) of transcript was calculated using the 2−ΔΔCT method (63) and plotted as mean ± sem. Student's t test was used to generate P values for statistical significance. Transcript levels of microRNAs that were differentially expressed were measured by QPCR. Again, six independent samples in each group were used for comparison. Total RNA (25 ng) was reverse transcribed in a reaction volume of 15 μl using the TaqMan microRNA reverse transcription kit (Applied Biosystems). Mature microRNA expression was carried out using cDNA diluted 1:15 and TaqMan mature microRNA assays (Applied Biosystems). U6 snRNA was used as endogenous control. Assays were run using 2 μl of cDNA and run on a One Step Plus as above using TaqMan universal PCR master mix (Applied Biosystems).
microRNA target prediction
microRNA predicted to target differentially expressed mRNA were identified using TargetScanHuman (release 5.1) (www.targetscan.org), PicTar (http://pictar.mdc-berlin.de/), and miRanda (September 2008) (http://www.microrna.org/microrna/home.do). The retrieval of the putative miRNA-mRNA pairs was facilitated by SigTerms software (44, 64–66).
microRNA target validation
After selection of candidate microRNAs and their target mRNA, primary cultures of HESF and HEK293T cells were transfected with Dharmacon mimics and inhibitors using Lipofectamine 2000 (Invitrogen). After optimization of conditions, 100 nm of inhibitor and 150 nm of mimic and 100 nm of hairpin inhibitor no. 1 and 150 nm of control mimic no. 1 were transfected into endometrial stromal fibroblasts and RNA was isolated after 48 h. HEK293T cells were cotransfected with 100 ng of 3′ UTR-luciferase constructs containing the entire 3′ UTR of COL7A1, COL21A, TFAP2C, random UTR no. 1, or empty luciferase construct (SwitchGearGenomics, Menlo Park, CA) and 50 nm of mimic or inhibitor. The media were changed after 4–6 h. Cells were harvested 48 h later in lysis buffer. Luciferase activity was detected using the Steady Glo kit (Promega, Madison, WI). The RNA was isolated as above with the mirVana kit (Applied Biosystems). RNA was DNAse treated and cDNA was created as above. Nontargeting inhibitors and mimics (Dharmacon) and empty vector and random 3′ UTR-luciferase constructs were used as a negative control. Mock transfected was used as a reference.
Acknowledgments
We thank Drs. William Gibbons and Michael Heard for supplying tissue samples for this work, Ruihong Chen and Limei Ran for technical support for tissue culture, and Paola A. Arias for technical support of RNA isolation.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreement U54HD0077495 (to S.M.H., C.J.C., D.Y.H., P.H.G., and M.M.M.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research; the Women's Reproductive Health Research Program 5K12HD050128 (to S.M.H.); the Herman L. and LeNan Gardner Research Fund in Obstetrics and Gynecology (to S.M.H.); and the Caroline Wiess Law Fund for Molecular Medicine (to S.M.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CCND1
- Cyclin D1
- COL7A1
- collagen type VII A1
- DNAse
- deoxyribonuclease
- HEK
- human embryonic kidney
- HESF
- human endometrial stromal fibroblast
- HEY2
- hairy/enhancer-of-split related with YRPW motif 2
- IGFBP
- IGF binding protein
- QPCR
- quantitative PCR
- RQ
- relative quantity
- SCGB1D2
- secretoglobin, family 1D, member 2
- TFAP2C
- transcription factor AP-2γ
- UTR
- untranslated region.
References
- 1. Bulun SE. 2009. Endometriosis. N Engl J Med 360:268–279 [DOI] [PubMed] [Google Scholar]
- 2. Merrill RM, Layman AB, Oderda G, Asche C. 2008. Risk estimates of hysterectomy and selected conditions commonly treated with hysterectomy. Ann Epidemiol 18:253–260 [DOI] [PubMed] [Google Scholar]
- 3. Verkauf BS. 1987. Incidence, symptoms, and signs of endometriosis in fertile and infertile women. J Fla Med Assoc 74:671–675 [PubMed] [Google Scholar]
- 4. Du T, Zamore PD. 2005. microPrimer: the biogenesis and function of microRNA. Development 132:4645–4652 [DOI] [PubMed] [Google Scholar]
- 5. Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. 2009. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol 200:661.e1–661.e7 [DOI] [PubMed] [Google Scholar]
- 6. Chung TK, Cheung TH, Huen NY, Wong KW, Lo KW, Yim SF, Siu NS, Wong YM, Tsang PT, Pang MW, Yu MY, To KF, Mok SC, Wang VW, Li C, Cheung AY, Doran G, Birrer MJ, Smith DI, Wong YF. 2009. Dysregulated microRNAs and their predicted targets associated with endometrioid endometrial adenocarcinoma in Hong Kong women. Int J Cancer 124:1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan Q, Chegini N. 2008. MicroRNA signature and regulatory functions in the endometrium during normal and disease states. Semin Reprod Med 26:479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan Q, Luo X, Chegini N. 2008. Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. J Cell Mol Med 12:227–240 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, Mittal K, Soteropoulos P, Wei JJ. 2007. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer 46:336–347 [DOI] [PubMed] [Google Scholar]
- 10. Marsh EE, Lin Z, Yin P, Milad M, Chakravarti D, Bulun SE. 2008. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil Steril 89:1771–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagaraja AK, Creighton CJ, Yu Z, Zhu H, Gunaratne PH, Reid JG, Olokpa E, Itamochi H, Ueno NT, Hawkins SM, Anderson ML, Matzuk MM. 2010. A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol Endocrinol 24:447–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Creighton CJ, Fountain MD, Yu Z, Nagaraja AK, Zhu H, Khan M, Olokpa E, Zariff A, Gunaratne PH, Matzuk MM, Anderson ML. 2010. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res 70:1906–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, Liu CG, Giannakakis A, Alexiou P, Hasegawa K, Johnstone CN, Megraw MS, Adams S, Lassus H, Huang J, Kaur S, Liang S, Sethupathy P, Leminen A, Simossis VA, Sandaltzopoulos R, Naomoto Y, Katsaros D, Gimotty PA, DeMichele A, Huang Q, Bützow R, Rustgi AK, Weber BL, Birrer MJ, Hatzigeorgiou AG, Croce CM, Coukos G. 2008. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci USA 105:7004–7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teague EM, Print CG, Hull ML. 2010. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update 16:142–165 [DOI] [PubMed] [Google Scholar]
- 15. Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L, Patrignani C, Graziani A, Surico N. 2010. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol 2010:369549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC. 2009. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod 15:625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM. 2009. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol 23:265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan Q, Luo X, Toloubeydokhti T, Chegini N. 2007. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod 13:797–806 [DOI] [PubMed] [Google Scholar]
- 19. Creighton CJ, Benham AL, Zhu H, Khan MF, Reid JG, Nagaraja AK, Fountain MD, Dziadek O, Han D, Ma L, Kim J, Hawkins SM, Anderson ML, Matzuk MM, Gunaratne PH. 2010. Discovery of novel microRNAs in female reproductive tract using next generation sequencing. PloS one 5:e9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. 2010. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod 82:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klemke M, Meyer A, Hashemi Nezhad M, Belge G, Bartnitzke S, Bullerdiek J. 2010. Loss of let-7 binding sites resulting from truncations of the 3′ untranslated region of HMGA2 mRNA in uterine leiomyomas. Cancer Genet Cytogenet 196:119–123 [DOI] [PubMed] [Google Scholar]
- 22. Pan Q, Luo X, Chegini N. 2010. microRNA 21: response to hormonal therapies and regulatory function in leiomyoma, transformed leiomyoma and leiomyosarcoma cells. Mol Hum Reprod 16:215–227 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. 2006. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34:D140–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. 2003. Prediction of mammalian microRNA targets. Cell 115:787–798 [DOI] [PubMed] [Google Scholar]
- 25. Eyster KM, Klinkova O, Kennedy V, Hansen KA. 2007. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril 88:1505–1533 [DOI] [PubMed] [Google Scholar]
- 26. Hever A, Roth RB, Hevezi P, Marin ME, Acosta JA, Acosta H, Rojas J, Herrera R, Grigoriadis D, White E, Conlon PJ, Maki RA, Zlotnik A. 2007. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc Natl Acad Sci USA 104:12451–12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hull ML, Escareno CR, Godsland JM, Doig JR, Johnson CM, Phillips SC, Smith SK, Tavaré S, Print CG, Charnock-Jones DS. 2008. Endometrial-peritoneal interactions during endometriotic lesion establishment. Am J Pathol 173:700–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chand AL, Murray AS, Jones RL, Hannan NJ, Salamonsen LA, Rombauts L. 2007. Laser capture microdissection and cDNA array analysis of endometrium identify CCL16 and CCL21 as epithelial-derived inflammatory mediators associated with endometriosis. Reprod Biol Endocrinol 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lawson C, Bourcier N, Al-Akoum M, Maheux R, Naud F, Akoum A. 2008. Abnormal interleukin 1 receptor types I and II gene expression in eutopic and ectopic endometrial tissues of women with endometriosis. J Reprod Immunol 77:75–84 [DOI] [PubMed] [Google Scholar]
- 30. Zhao H, Wang Q, Bai C, He K, Pan Y. 2009. A cross-study gene set enrichment analysis identifies critical pathways in endometriosis. Reprod Biol Endocrinol 7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scholl B, Bersinger NA, Kuhn A, Mueller MD. 2009. Correlation between symptoms of pain and peritoneal fluid inflammatory cytokine concentrations in endometriosis. Gynecol Endocrinol 25:701–706 [DOI] [PubMed] [Google Scholar]
- 32. Boutten A, Dehoux M, Edelman P, Seta N, Menard A, Madelenat P, Durand G. 1992. IL6 and acute phase plasma proteins in peritoneal fluid of women with endometriosis. Clin Chim Acta 210:187–195 [DOI] [PubMed] [Google Scholar]
- 33. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. 2007. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148:3814–3826 [DOI] [PubMed] [Google Scholar]
- 34. Dizerega GS, Barber DL, Hodgen GD. 1980. Endometriosis: role of ovarian steroids in initiation, maintenance, and suppression. Fertil Steril 33:649–653 [DOI] [PubMed] [Google Scholar]
- 35. Colette S, Defrère S, Lousse JC, Van Langendonckt A, Loumaye E, Donnez J. 2009. Evaluation of estrogen treatment in an immunodeficient mouse endometriosis model. Gynecol Obstet Invest 68:262–268 [DOI] [PubMed] [Google Scholar]
- 36. Alborzi S, Hamedi B, Omidvar A, Dehbashi S, Alborzi S, Alborzi M. 27 July 2010. A comparison of the effect of short-term aromatase inhibitor (letrozole) and GnRH agonist (triptorelin) versus case control on pregnancy rate and symptom and sign recurrence after laparoscopic treatment of endometriosis. Arch Gynecol Obstet 10.1007/s00404-010-1599-6 [DOI] [PubMed] [Google Scholar]
- 37. Alborzi S, Zarei A, Alborzi S, Alborzi M. 2006. Management of ovarian endometrioma. Clin Obstet Gynecol 49:480–491 [DOI] [PubMed] [Google Scholar]
- 38. Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. 2006. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- 39. Hanifi-Moghaddam P, Boers-Sijmons B, Klaassens AH, van Wijk FH, den Bakker MA, Ott MC, Shipley GL, Verheul HA, Kloosterboer HJ, Burger CW, Blok LJ. 2007. Molecular analysis of human endometrium: short-term tibolone signaling differs significantly from estrogen and estrogen + progestagen signaling. J Mol Med 85:471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shiozawa T, Li SF, Nakayama K, Nikaido T, Fujii S. 1996. Relationship between the expression of cyclins/cyclin-dependent kinases and sex-steroid receptors/Ki67 in normal human endometrial glands and stroma during the menstrual cycle. Mol Hum Reprod 2:745–752 [DOI] [PubMed] [Google Scholar]
- 41. Turner KJ, Morley M, MacPherson S, Millar MR, Wilson JA, Sharpe RM, Saunders PT. 2001. Modulation of gene expression by androgen and oestrogens in the testis and prostate of the adult rat following androgen withdrawal. Mol Cell Endocrinol 178:73–87 [DOI] [PubMed] [Google Scholar]
- 42. Pavlova A, Boutin E, Cunha G, Sassoon D. 1994. Msx1 (Hox-7.1) in the adult mouse uterus: cellular interactions underlying regulation of expression. Development 120:335–345 [DOI] [PubMed] [Google Scholar]
- 43. Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ. 2005. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 146:3490–3505 [DOI] [PubMed] [Google Scholar]
- 44. Creighton CJ, Nagaraja AK, Hanash SM, Matzuk MM, Gunaratne PH. 2008. A bioinformatics tool for linking gene expression profiling results with public databases of microRNA target predictions. RNA 14:2290–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luo X, Ding L, Chegini N. 2004. Gonadotropin-releasing hormone and TGF-β activate MAP kinase and differentially regulate fibronectin expression in endometrial epithelial and stromal cells. Am J Physiol Endocrinol Metab 287:E991–E1001 [DOI] [PubMed] [Google Scholar]
- 46. Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. 2003. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4:P3. [PubMed] [Google Scholar]
- 47. Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protoc 4:44–57 [DOI] [PubMed] [Google Scholar]
- 48. Pellicano M, Bramante S, Guida M, Bifulco G, Di Spiezio Sardo A, Cirillo D, Nappi C. 2008. Ovarian endometrioma: postoperative adhesions following bipolar coagulation and suture. Fertil Steril 89:796–799 [DOI] [PubMed] [Google Scholar]
- 49. Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. 2009. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod 80:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sengupta S, den Boon JA, Chen IH, Newton MA, Stanhope SA, Cheng YJ, Chen CJ, Hildesheim A, Sugden B, Ahlquist P. 2008. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA 105:5874–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. 2006. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril 85:564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. 2007. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282:31725–31732 [DOI] [PubMed] [Google Scholar]
- 53. Guo H, Ingolia NT, Weissman JS, Bartel DP. 2010. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466:835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aghajanova L, Tatsumi K, Horcajadas JA, Zamah AM, Esteban FJ, Herndon CN, Conti M, Giudice LC. 22 September 2010. Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol Reprod 10.1095/biolreprod.110.086181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. 2002. Global gene profiling in human endometrium during the window of implantation. Endocrinology 143:2119–2138 [DOI] [PubMed] [Google Scholar]
- 56. Toloubeydokhti T, Pan Q, Luo X, Bukulmez O, Chegini N. 2008. The expression and ovarian steroid regulation of endometrial micro-RNAs. Reprod Sci 15:993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57. Qian K, Hu L, Chen H, Li H, Liu N, Li Y, Ai J, Zhu G, Tang Z, Zhang H. 2009. Hsa-miR-222 is involved in differentiation of endometrial stromal cells in vitro. Endocrinology 150:4734–4743 [DOI] [PubMed] [Google Scholar]
- 58. Donnez J, Nisolle M, Smoes P, Gillet N, Beguin S, Casanas-Roux F. 1996. Peritoneal endometriosis and “endometriotic” nodules of the rectovaginal septum are two different entities. Fertil Steril 66:362–368 [PubMed] [Google Scholar]
- 59. Nisolle M, Donnez J. 1997. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril 68:585–596 [DOI] [PubMed] [Google Scholar]
- 60. Heilier JF, Donnez O, Van Kerckhove V, Lison D, Donnez J. 2006. Expression of aromatase (P450 aromatase/CYP19) in peritoneal and ovarian endometriotic tissues and deep endometriotic (adenomyotic) nodules of the rectovaginal septum. Fertil Steril 85:1516–1518 [DOI] [PubMed] [Google Scholar]
- 61. Irwin JC, Kirk D, King RJ, Quigley MM, Gwatkin RB. 1989. Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril 52:761–768 [DOI] [PubMed] [Google Scholar]
- 62. Saldanha AJ. 2004. Java Treeview—extensible visualization of microarray data. Bioinformatics 20:3246–3248 [DOI] [PubMed] [Google Scholar]
- 63. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-ΔΔC(T)] method. Methods (San Diego, Calif) 25:402–408 [DOI] [PubMed] [Google Scholar]
- 64. Friedman RC, Farh KK, Burge CB, Bartel DP. 2009. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. 2005. Combinatorial microRNA target predictions. Nat Genet 37:495–500 [DOI] [PubMed] [Google Scholar]
- 66. Betel D, Wilson M, Gabow A, Marks DS, Sander C. 2008. The microRNA.org resource: targets and expression. Nucleic Acids Res 36:D149–D153 [DOI] [PMC free article] [PubMed] [Google Scholar]