Deletion of Otx2 in GnRH neurons results in fewer GnRH-neurons in the hypothalamus, a delay in pubertal onset, abnormal estrous cyclicity and infertility.
Abstract
GnRH is the central regulator of reproductive function responding to central nervous system cues to control gonadotropin synthesis and secretion. GnRH neurons originate in the olfactory placode and migrate to the forebrain, in which they are found in a scattered distribution. Congenital idiopathic hypogonadotropic hypogonadism (CIHH) has been associated with mutations or deletions in a number of genes that participate in the development of GnRH neurons and expression of GnRH. Despite the critical role of GnRH in mammalian reproduction, a comprehensive understanding of the developmental factors that are responsible for regulating the establishment of mature GnRH neurons and the expression of GnRH is lacking. orthodenticle homeobox 2 (OTX2), a homeodomain protein required for the formation of the forebrain, has been shown to be expressed in GnRH neurons, up-regulated during GnRH neuronal development, and responsible for increased GnRH promoter activity in GnRH neuronal cell lines. Interestingly, mutations in Otx2 have been associated with human hypogonadotropic hypogonadism, but the mechanism by which Otx2 mutations cause CIHH is unknown. Here we show that deletion of Otx2 in GnRH neurons results in a significant decrease in GnRH neurons in the hypothalamus, a delay in pubertal onset, abnormal estrous cyclicity, and infertility. Taken together, these data provide in vivo evidence that Otx2 is critical for GnRH expression and reproductive competence.
GnRH neurons integrate internal homeostatic and external environmental cues and as such are critical regulators of the hypothalamic-pituitary-gonadal axis. Mammalian puberty and subsequent fertility is dependent on the coordinated function of GnRH neurons. Reproductive dysfunction results from a loss of GnRH neurons (1), a disruption of GnRH neuronal migration (2), or a disruption of the GnRH gene or gene expression (3–5). Moreover, congenital idiopathic hypogonadotropic hypogonadism (CIHH) has been shown to result from mutations in GNRH1 and a number of genes that participate in the control of GnRH neurons, such as Kal1, Fgf1, Fgf8, FgfR1, Prok2, ProkR2, Gpr54, Tac3, TacR3, Chd7, and necdin (5–10). However, less than 30% of individuals with CIHH have a known mutation in one of the identified genes (11). Recently mutations in Otx2 have been found in patients with anophthalmia, pituitary hypoplasia, and a combined pituitary hormone deficiency, including disorders of puberty and hypogonadism (12–14). These findings suggest that Otx2 may play a critical role in the development of central components of the hypothalamic-pituitary-gonadal axis.
GnRH neurons arise in the embryonic olfactory placode and undergo a series of developmental events during their migration to their final locations in the ventral hypothalamus (15, 16). GnRH neuronal migration into the basal forebrain, projection of neurosecretory axons to the median eminence, and GnRH hormone release followed by gonadotropin secretion are critical processes required for central regulation of reproduction (2). GnRH neuronal development relies on signals from the surrounding nasal placode and factors that affect neuronal migration as well as an intrinsic developmental program during critical periods (17–24). In mice, characterization of the migratory pathway of GnRH neurons at embryonic day (e) 14.5 reveals that the majority of GnRH neurons have crossed into the rostral forebrain, although some are still present rostral to the cribriform plate (16, 25).
Orthodenticle homeobox 2 (OTX2) is a homeodomain protein necessary for early embryonic development of the head during gastrulation (26–28) and, later, specifies anterior head structures, particularly the forebrain (29–34). Loss-of-function of Otx2 results in malformation of the head and eyes (35–38). Later in development, OTX2 has been localized to migrating neuronal populations in the thalamus (39) and plays a continuing role in eye development and plasticity (40, 41). In addition, Otx2 expression has been documented in adult pinealocyte cells (42). A homozygous null mutation of Otx2 is embryonic lethal due to severe developmental abnormalities of the brain, which has prevented study of the effect of Otx2 on individual neuronal populations (28, 43, 44).
OTX2 is among the regulatory proteins that have been shown to control expression of murine GnRH (45–47) by binding to Otx2 binding sites in the proximal promoter of GnRH both in vitro and in vivo (45, 46). The Otx2 sites in the GnRH promoter are conserved across species (46). In transgenic mice bearing the −356 to +28 bp mouse GnRH promoter fragment fused to the luciferase reporter gene, high levels of luciferase expression were detected; however, when transgenic mice were generated with a −249 to +28 bp promoter fragment, which does not include the Otx2 DNA binding sites, neuronal expression was not detected (45). These data suggest that Otx2 is a critical component in regulating GnRH gene expression.
Given the developmental role of Otx2 in the brain and its role in regulating the GnRH neuron, we generated a conditional knockout (KO) of Otx2 (GnRH-Otx2KO) such that Otx2 expression is lost exclusively in GnRH neurons at the time that they begin to express GnRH. Differences in the number and distribution of GnRH neurons in GnRH-Otx2KO mice were found. These mice had fewer GnRH neurons in the ventral hypothalamus with fewer numbers of cells crossing into the forebrain by e14.5, a time when an increase in cleaved caspase-3 staining was detected. A subsequent decrease in GnRH and LHβ mRNA levels as well as a reduction in sex steroid hormone levels and delayed pubertal onset was observed. Female infertility was associated with persistent estrus, failure to generate a LH surge, and absence of the corpora lutea. Our results demonstrate the critical role that Otx2 plays in GnRH neuronal function and mammalian reproduction.
Results
Construction of GnRH neuron-specific Otx2 KO mice
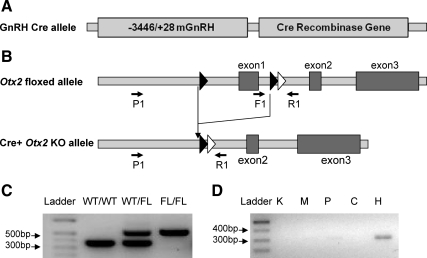
GnRH neuron-specific Otx2 KO (GnRH-Otx2KO) mice were generated using a mouse line in which the first exon of Otx2 was flanked by loxP sites and in which cell-specific expression of cAMP response element (Cre) recombinase causes a loss of Otx2 expression (48). These mice were crossed with GnRH-Cre mice (Fig. 1A) to specifically target the loxP site recombination to the GnRH neurons of the hypothalamus (Fig. 1B) (45, 49).
Fig. 1.
Schematics of constructs used to generate GnRH-Otx2KO mice. Transgenic mice expressing Cre recombinase specifically in GnRH neurons (A) were bred with Otx2 floxed mice (B). A frt recombination site (white triangle) remains from the neomycin excision downstream of the loxP sites (black triangles). Primers used in PCR genotyping are labeled F1 and R1. PCR primers used to detect recombination are labeled P1 and R1. C, Genotyping by PCR analysis of the genomic DNA produced a band migrating at 560 bp in the mice bearing a FL allele and a band at 360 bp in WT mice. D, PCR analysis with primers P1 and R1 yields a band of 300 bp after recombination. This band was seen only in the hypothalamus (H) and not kidney (K), heart (M), pituitary (P), or cerebellum (C) of the GnRH-Otx2KO mice.
Cell-specific KO animals were produced by breeding Cre−/−/Otx2F/F males with Cre+/−/Otx2+/F females. Genotyping was performed by PCR of tail DNA; 360- and 560-bp bands correspond to wild-type (WT) and floxed (FL) alleles, respectively (Fig. 1C). Tissue samples from GnRH-Otx2KO were also analyzed by PCR using primers spanning the targeted region to detect tissue-specific recombination. As expected, only hypothalamic samples from GnRH-Otx2KO animals demonstrated a 300-bp band after recombination (Fig. 1D), confirming that Otx2 recombination occurred only in the hypothalamus (Fig. 1D). Because GnRH neurons are dispersed in the hypothalamus, double-label immunohistochemistry experiments were performed to assess the specificity of the expression of Cre recombinase. Cre expression in control animals (Cre transgenic with Otx2 WT alleles) was restricted to GnRH-positive cells, as has been previously reported (49), and no cells were identified expressing GnRH without Cre recombinase (data not shown).
General physiological parameters such as adult (2 months of age) weight (females: control, 22.8 ± 0.3 g, n = 9; GnRH-Otx2KO, 22.5 ± 0.6 g, n = 6; males: control, 28.5 ± 0.9 g, n = 18; GnRH-Otx2KO, 28.9 ± 1.6 g, n = 3) and gonadal weight (females: control, 8.1 ± 0.4 mg, n = 8; GnRH-Otx2KO, 6.6 ± 0.1 mg, n = 3; males: control, 0.184 ± 0.007 g, n = 18; GnRH-Otx2KO, 0.183 ± 0.006 g, n = 3) were not different between genotypes.
GnRH-Otx2KO mice have fewer GnRH immunoreactive neurons
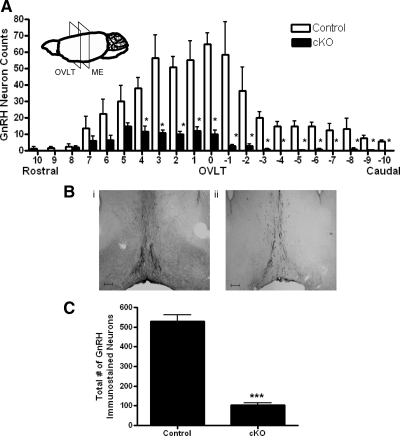
The number and distribution of GnRH immunoreactive neurons in the hypothalamus of control and GnRH-Otx2KO adult (>2 months of age) mice were compared. Immunohistochemistry (IHC) for GnRH was performed on serial coronal sections starting caudal to the ME (Bregma −1.10 mm) through the olfactory bulbs (Bregma 2.10 mm) (50). At the level of the organum vasculosum of the lamina terminalis (OVLT) of control brains, the most dense population of GnRH immunoreactive neurons (65.2 ± 6.8 per 120 μm, n = 5) was observed, whereas equivalent sections in GnRH-Otx2KO mice contained much fewer cells (10.0 ± 2.4 per 120 μm, n = 5, P < 0.05) (Fig. 2A). Control brain sections (40 μm) at the OVLT contained both scattered cell bodies and dense immunoreactive fiber beds, whereas the corresponding GnRH-Otx2KO sections had almost no detectable cell bodies and decreased staining of fibers (Fig. 2B). Similarly, in GnRH-Otx2KO mice, sections rostral and caudal to the OVLT had significantly fewer numbers of cell bodies compared with control mice (data not shown). Thus, whereas control mice have GnRH neurons distributed in a pattern consistent with previous studies (51–53), the cell-specific loss of Otx2 in GnRH-Otx2KO mice resulted in an 80% reduction in GnRH immunoreactive neurons when compared with control mice (105 ± 8, n = 5 vs. 530 ± 33, n = 5, P < 0.0001) (Fig. 2C). Both male (108 ± 12, n = 3) and female (102 ± 15, n = 2) GnRH-Otx2KO mice displayed similar reductions in GnRH neuronal numbers.
Fig. 2.
The total number and distribution of GnRH neurons in the adult brain of GnRH-Otx2KO mice. A, Histogram of the spatial distribution of GnRH immunostaining neurons from control and GnRH-Otx2KO (cKO) mice. The most significant reduction of the GnRH immunostaining neurons in the cKO mice occurs at the OVLT. The inset is a schematic representation of a mouse brain, denoting the relative location of the OVLT and the median eminence. Each bar spans 120 μm, with error bars representing the sem. B, GnRH immunostaining neurons from representative control (i) and cKO (ii) coronal sections at the OVLT. Note the decrease in GnRH neuronal cell bodies and the axonal projections in the cKO mice. Magnification, ×50. Scale bar, 200 μm. C, The total number of GnRH immunostaining neurons are significantly reduced in the cKO mice. *, P < 0.05; ***, P < 0.0001.
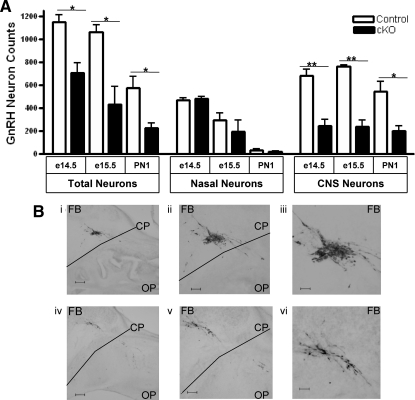
GnRH neurons originate in the olfactory placode and migrate across the cribriform plate during embryonic development (16, 25). Therefore, we quantified the number and location of GnRH neurons along their migratory pathway in control and GnRH-Otx2KO mice. Because GnRH neuronal migration across the cribriform plate is expected to be complete by e14.5, mice were killed at this age and sagittal sections were immunostained for GnRH. The GnRH-Otx2KO animals had fewer GnRH immunoreactive neurons in the forebrain compared with control mice (244 ± 56, n = 4 vs. 684 ± 56, n = 3, P < 0.005) at e14.5 when the average numbers of GnRH immunoreactive neurons in the nasal compartment was nearly equivalent (controls: 468 ± 20; GnRH-Otx2KO: 456 ± 32) (Fig. 3A and Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Figure 3B shows GnRH immunoreactive neurons in control mice (Fig. 3B, i–iii) that have crossed the cribriform plate (CP) from the olfactory placode (OP) to the forebrain (FB), whereas many fewer immunoreactive neurons are seen in the FB of GnRH-Otx2KO mice (Fig. 3B, iv–vi). Mice were also examined at e15.5 and postnatal day (PN) 1, and similar differences were noted in FB regions, control mice having 766 ± 9 (e15.5, n = 3) and 542 ± 91 (PN1, n = 4) GnRH immunoreactive neurons vs. 237 ± 55 (e15.5, n = 3) and 203 ± 41 (PN1, n = 3) in GnRH-Otx2KO mice (Fig. 3A).
Fig. 3.
Decrease of GnRH neurons in forebrain regions at e14.5, e15.5, and PN1 in GnRH-Otx2KO mice. A, Histogram of the total number (±sem) of the GnRH immunostaining neurons, the total number of neurons in the olfactory placode, and the total number of neurons in the CNS for e14.5, e15.5, and PN1 control and GnRH-Otx2KO (cKO) mice. There are significantly fewer total GnRH neurons in GnRH-Otx2KO mice at each time point, and although there are similar numbers of GnRH neurons in the nasal placode, there is a significant reduction in neurons in the CNS of cKO mice at each time point. B, Photomicrographs of 40-μm sagittal sections of e14.5 control (i–iii) and GnRH-Otx2KO (iv–vi) mice immunostained for GnRH at different magnifications. In the cKO mice, fewer GnRH neurons migrate across the CP from the OP into the FB region. Magnifications, ×50 (i and iv), ×100 (ii and v), and ×200 (iii and vi). Scale bars, 200 μm (i and iv), 100 μm (ii and v), 50 μm (iii and vi). *, P < 0.05; **, P < 0.001.
GnRH-Otx2KO mice have fewer neurons marked by enhanced yellow fluorescent protein (EYFP)
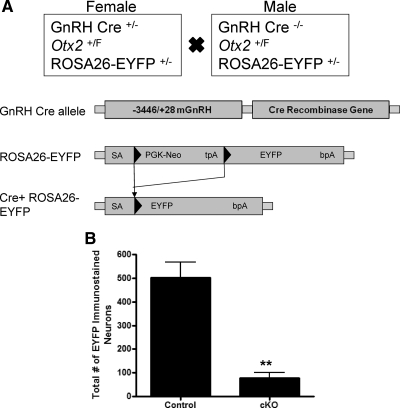
To confirm our observation that GnRH-Otx2KO mice have fewer GnRH neurons within the hypothalamus, we generated a Cre+/−/Otx2F/F/ROSA26-EYFP+/− (GnRH-Otx2KO-EYFP) mouse. GnRH-Cre positive neurons will excise a floxed transcriptional stop sequence inserted between the promoter and the coding sequence of the EYFP gene, permanently labeling all GnRH neurons early in their development (Fig. 4A). IHC for EYFP was then performed on serial coronal sections as before, and the number and distribution of EYFP immunoreactive neurons in the hypothalamus of control and GnRH-Otx2KO-EYFP adult (>2 months of age) mice were compared. Control mice had 502 ± 65 (n = 3) EYFP immunoreactive neurons, whereas GnRH-Otx2KO-EYFP mice had significantly fewer EYFP immunoreactive neurons (78 ± 24, n = 3, P < 0.005) (Fig. 4B). There were no statistically significant differences between the GnRH-staining and the EYFP-staining neurons among control or GnRH-Otx2KO mice.
Fig. 4.
Total number of EYFP immunostaining GnRH neurons is decreased in the adult brain of GnRH-Otx2KO-EYFP mice. A, Schematic of the mating paradigm to generate control and GnRH-Otx2KO mice expressing EYFP. The PGK-neo selectable marker and tpA transcriptional stop sequence are flanked by loxP sites (black triangles). After the GnRH-Cre-mediated excision of the loxP flanked (PGK-neo, tpA) cassette, the EYFP transgene is specifically expressed in GnRH neurons. B, The total number of EYFP immunostaining neurons is significantly reduced in GnRH-Otx2KO-EYFP mice, indicating a defect in migration/survival rather than expression. **, P < 0.005.
GnRH neurons in GnRH-Otx2KO mice have an increased rate of programmed cell death
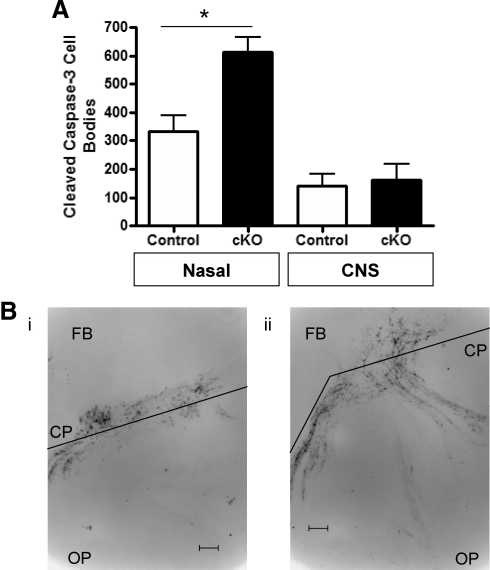
To determine whether GnRH-Otx2KO mice displayed increased rates of programmed cell death, e14.5 brains were assessed for rates of apoptosis using caspase-3 cleavage analysis. Caspase-3 activation, an integral step in programmed cell death pathways, results in protein cleavage, which is detected by specific antibodies. Cleaved caspase immunoreactivity was used to detect dying cells at e14.5 in GnRH-Otx2KO and control mice. This analysis revealed 333 ± 56 (n = 3) and 613 ± 52 (n = 3, P < 0.05) cell bodies immunoreactive for cleaved caspase-3 in the nasal region of control and GnRH-Otx2KO mice, respectively, vs. approximately 150 cell bodies in the central nervous system (CNS) of both control and GnRH-Otx2KO mice (Fig. 5A). The pattern of cleaved caspase-3 immunoreactivity is consistent with the migratory route of GnRH neurons as shown in Fig. 5B.
Fig. 5.
GnRH-Otx2KO mice have an increased rate of programmed cell death along the GnRH neuron migratory route. A, Histogram (±sem) showing significantly more cleaved caspase-3 immunostaining in the olfactory placode of GnRH-Otx2KO (cKO) mice than controls at e14.5, with the majority of apoptotic events occurring at the CP. Cell death was similar in the forebrain of both genotypes. *, P < 0.05. B, Photomicrographs of 40-μm sagittal sections of control (i) and GnRH-Otx2cKO (ii) mice immunostained for cleaved caspase-3 at e14.5. Apoptotic cells can be seen along the migratory route from the OP, at the CP, and in the FB region. Magnification, ×200. Scale bar, 50 μm.
GnRH-Otx2KO mice have decreased hypothalamic GnRH and pituitary LHβ and FSHβ mRNA levels
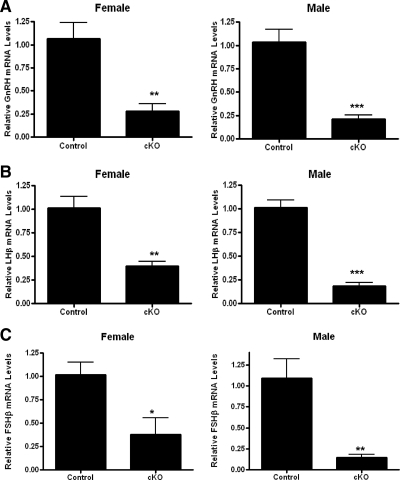
GnRH, LHβ, and FSHβ mRNA levels were measured by quantitative RT-PCR in hypothalamic and pituitary tissue of control and GnRH-Otx2KO mice. Consistent with the IHC results, the absence of Otx2 in GnRH-Otx2KO animals resulted in a significant decrease in hypothalamic GnRH mRNA levels vs. control mice (n = 5) (an approximate 80% decrease); mRNA levels in GnRH-Otx2KO females were 0.28 ± 0.08 (n = 4, P < 0.01) and in males were 0.21 ± 0.05 (n = 5, P < 0.0005) when compared with a normalized value of 1 in control mice (Fig. 6A). There were similar reductions in the pituitary LHβ levels, with GnRH-Otx2KO females having values of 0.40 ± 0.05 (n = 3, P < 0.01) and GnRH-Otx2KO males having values of 0.19 ± 0.03 (n = 3, P < 0.0005) normalized to a value of 1 in control mice (n = 4) (Fig. 6B). Similarly, FSHβ mRNA levels were decreased in female (0.38 ± 0.18, n = 3, P < 0.05) and male (0.15 ± 0.03, n = 3, P < 0.01) GnRH-Otx2KO mice when compared with a normalized value of 1 in control mice (n = 4) (Fig. 6C).
Fig. 6.
Hypothalamic GnRH and pituitary LHβ and FSHβ subunit mRNA levels are reduced in GnRH-Otx2KO mice. Quantitative PCR assessment of hypothalamic GnRH (A), pituitary LHβ subunit (B), and FSHβ subunit (C) mRNA levels demonstrate a significant reduction of GnRH and LHβ and FSHβ subunit expression in GnRH-Otx2KO (cKO) mice in both females and males. *, P < 0.05; **, P < 0.01; ***, P < 0.0005.
GnRH-Otx2KO mice have low basal serum FSH and sex steroid hormone levels but similar serum LH levels
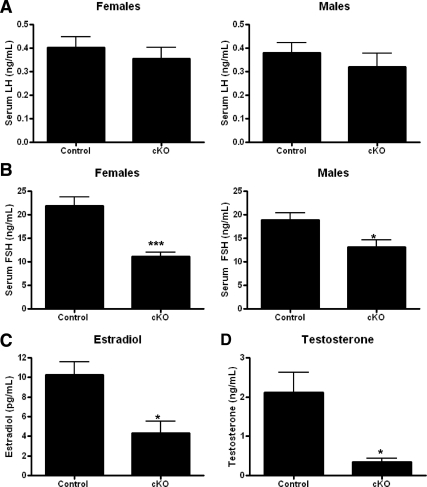
Morning serum samples were obtained from nonbreeding, postpubertal mice before tissue harvest and analyzed for serum LH, FSH, and sex steroid hormone levels. Serum LH values were not significantly different between the GnRH-Otx2KO (female: 0.36±0.05 ng/ml, n = 72; male: 0.32 ± 0.06 ng/ml, n = 13) and control mice (female: 0.40 ± 0.05 ng/ml, n = 74; male: 0.38 ± 0.04 ng/ml, n = 28) (Fig. 7A), whereas the serum FSH levels were lower in both female (11.20 ± 0.80 ng/ml, n = 66, P < 0.001) and male (13.12 ± 1.53 ng/ml, n = 7, P < 0.05) GnRH-Otx2KO mice than controls (female: 21.89 ± 1.91 ng/ml, n = 74; male: 18.88 ± 1.54 ng/ml, n = 16) (Fig. 7B). In GnRH-Otx2KO mice, both estradiol (4.35 ± 1.17 pg/ml, n = 11, P < 0.05) (Fig. 7C) and testosterone (0.34 ± 0.09 ng/ml, n = 10, P < 0.05) (Fig. 7D) were found to be significantly lower compared with controls (female: 10.29 ± 1.32 pg/ml, n = 40; male: 2.12 ± 0.50 ng/ml, n = 30).
Fig. 7.
Serum hormone levels in GnRH-Otx2KO animals are also reduced. Although circulating nonsurge LH levels (A) were not significantly different, FSH (B), estradiol (C), and testosterone (D) levels were significantly decreased in female and male GnRH-Otx2KO (cKO) mice. *, P < 0.05; ***, P < 0.0001.
GnRH-Otx2KO female mice have a delay in puberty
Control and GnRH-Otx2KO mice were examined to determine the age of pubertal onset. In males, the age of preputial separation (PPS) and anogenital distance on the day of full PPS was noted. In females, the age of vaginal opening and the subsequent day of first estrus were recorded. There were no statistically significant differences between male GnRH-Otx2KO and control mice in the age of PPS or anogenital distance (data not shown). In contrast, vaginal opening was delayed by approximately 4 d in GnRH-Otx2KO mice (29.2 ± 1.7 d, n = 5, P < 0.005) compared with control littermates (24.7 ± 0.5 d, n = 15). First estrus occurred approximately 2 d after vaginal opening for both genotypes (control: 27.2 ± 0.5 d; GnRH-Otx2KO: 31.0 ± 1.4 d, P < 0.005).
GnRH-Otx2KO mice are subfertile
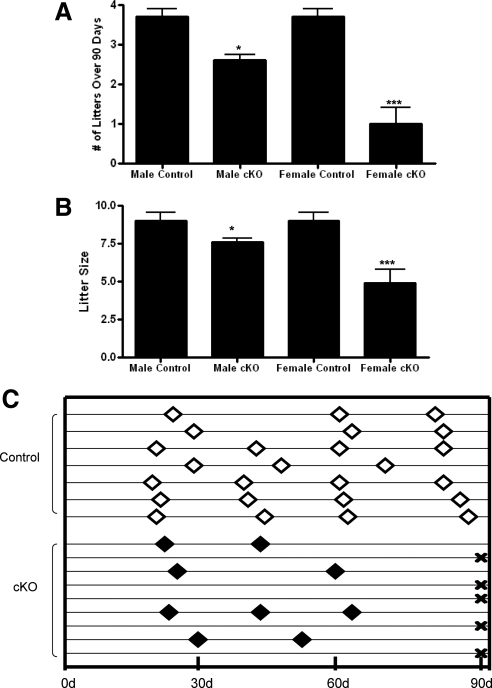
Fertility was determined using a continuous breeding protocol. Measurements included time to first litter, the number and frequency of litters, and size of litters. Each genotype's litter frequency was determined by recording the number of litters produced during a 90-d observation period. Control mice had an average litter frequency of 3.74 ± 0.16 litters per 90 d (n = 7). The litter frequency for male GnRH-Otx2KO mice was 2.62 ± 0.12 litters per 90 d (n = 6, P < 0.05), and female GnRH-Otx2KO mice had an average of only 1.00 ± 0.41 litters per 90 d (n = 9, P < 0.001) (Fig. 8A). The average time to first litter for control mating pairs was 25.6 ± 2.0 d (n = 3), which was not statistically different for female GnRH-Otx2KO mice with at least one litter (25.8 ± 1.4 d, n = 4) (data not shown). GnRH-Otx2KO mice of both sexes had a statistically significant decrease in litter size compared with controls. The control animals produced an average of 9.00 ± 0.54 pups per litter (n = 24 litters), whereas the GnRH-Otx2KO males had an average of 7.65 ± 0.28 pups per litter (n = 96 litters, P < 0.05), and females had an average of just 4.91 ± 0.89 pups per litter (n = 11 litters, P < 0.0005) (Fig. 8B). Five of nine female GnRH-Otx2KO mice were infertile, three animals produced just two litters, and only one female appeared to have relatively normal fertility during the observation period (Fig. 8C). In summary, GnRH-Otx2KO males had a reduced frequency of litters and decreased litter size, whereas female mice were infertile or had smaller litters that occurred more irregularly after the first litter.
Fig. 8.
Reproduction in GnRH-Otx2KO mice is impaired. Both male and female GnRH-Otx2KO (cKO) mice produced fewer litters over 90 d (A) and had fewer pups per litter (B) as compared with controls. C, Schematic representation of GnRH-Otx2KO (cKO) females' reproductive history. Diamonds indicate new litter, whereas X indicates an infertile female. Five of nine female cKO mice failed to produce any litters over 90 d, and only one female produced more than two litters during this period. *, P < 0.05; ***, P < 0.0005.
GnRH-Otx2KO mice have abnormal estrous cycles
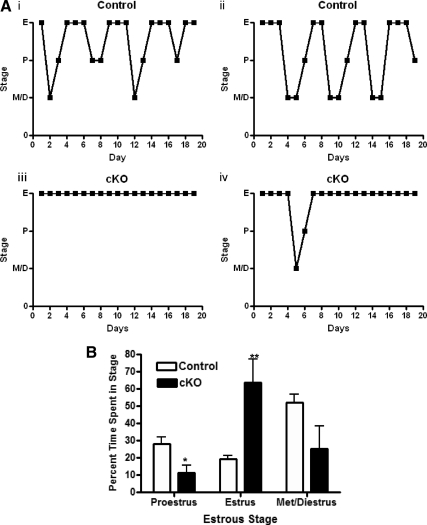
To characterize further the fertility defect exhibited by GnRH-Otx2KO females, we evaluated estrous cyclicity. Vaginal smears were performed daily for 19 d on control and GnRH-Otx2KO mice. Estrous staging was determined by the ratios of cornified epithelia, nucleated epithelia, and polymorphonuclear leukocytes cells found in the smear. Control mice had an average cycle length of 4.95 ± 0.23 d (n = 9) (Fig. 9A). In contrast, GnRH-Otx2KO mice did not exhibit normal estrous cycles, making the calculation of cycle length not possible. GnRH-Otx2KO females were found in estrus 64% of the time, with occasional evidence of proestrus (11%) or metestrus/diestrus (25%), which was a significantly different pattern than that observed in control animals (Fig. 9B).
Fig. 9.
Cycling profiles show a state of persistent estrus in GnRH-Otx2KO females. A, Profile of individual daily vaginal smears for representative control (i and ii) and GnRH-Otx2KO (cKO) (iii and iv) female mice. Control mice progressed normally from estrus (E), to metestrus/diestrus (D/M) to proestrus (P), whereas cKO mice predominantly remained in estrus. B, The increase in time spent in estrus for cKO female mice was offset by a decrease of time spent in proestrus. Each animal was staged for at least 19 consecutive days. *, P < 0.05; **, P < 0.005.
GnRH-Otx2KO mice do not have an LH surge
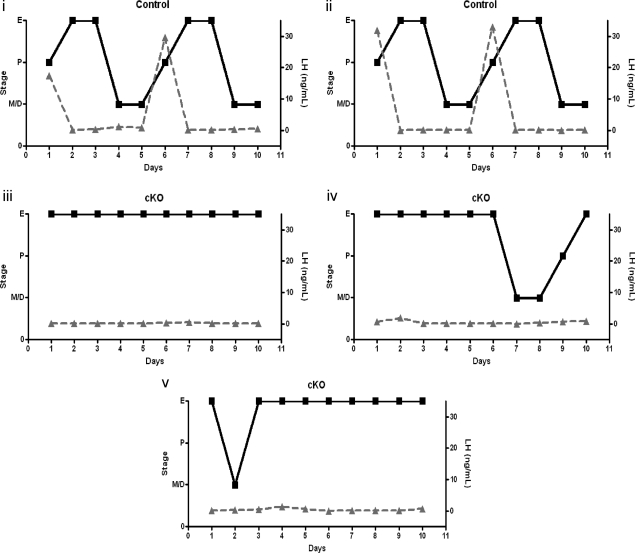
The persistent estrus observed in individually housed female GnRH-Otx2KO mice suggested that central hormonal control of the estrous cycle is perturbed by the lack of GnRH neurons. In vivo, the function of the GnRH neuron can be assessed indirectly by measuring the LH surge during proestrus, which in part results from increased activity of the GnRH pulse generator (54). To determine whether GnRH-Otx2KO females have normal LH surges, estrous cycle staging was evaluated each morning by vaginal cytology and LH levels were measured 1 h before lights out on 10 consecutive evenings in a small volume of blood. Animals were housed individually for at least 1 wk before beginning the protocol. Within the evaluation time period, control animals completed at least one full estrous cycle, whereas GnRH-Otx2KO mice did not cycle as described above. Control animals had a peak LH value of 31.5 ± 1.0 ng/ml (n = 3) on the evening of proestrus as expected and as shown in Fig. 10. In contrast, LH levels in GnRH-Otx2KO mice were always detectable but failed to increase above 1.0 ng/ml, regardless of cycle stage, including one animal found to be in proestrus by cytological analysis.
Fig. 10.
The LH surge is absent in GnRH-Otx2KO females. Profile of LH serum levels (triangles) overlaid on the cycling profile (squares) for representative control (i–ii) and GnRH-Otx2KO (cKO) (iii–v) female mice. The control animals had an LH peak of 31.5 ± 1.0 ng/ml (n = 3) on the evening of proestrus, but cKO animals rarely had a proestrus stage and displayed no change from basal LH levels. The limit of detection in this assay is 48 pg/ml.
GnRH-Otx2KO female mice have few corpora lutea
Control and GnRH-Otx2KO ovaries were hematoxylin and eosin stained and examined for differences in follicular development, luteal tissue, and overall organization. Both control and GnRH-Otx2KO ovaries exhibited follicular development, including primary, secondary, antral, and atretic follicles, but GnRH-Otx2KO ovaries had an almost complete absence of corpora lutea as shown in this representative histological section (Supplemental Fig. 1).
GnRH-Otx2KO testicular histology is unremarkable
Control and GnRH-Otx2KO testes were hematoxylin and eosin stained and examined for differences in testicular structure and spermatozoa differentiation. Both genotypes contained Leydig cells and Sertoli cells and displayed the complete differentiation sequence of spermatozoa from basally situated spermatogonia to primary/secondary spermatocytes to spermatozoa (Supplemental Fig. 2). In addition, the vas deferens of both genotypes were stained and appeared similar (not pictured).
Discussion
Congenital idiopathic hypogonadotropic hypogonadism due to mutations in Otx2 is associated with an absence of puberty and subsequent infertility (12–14). The biochemical profile in patients is characterized by low LH, FSH, and sex steroids. Although patients with Otx2 mutations have pituitary hormone deficiencies, the nature of the molecular abnormality and the level of defect in the reproductive axis remain unknown. Because binding sites for the homeobox Otx2 transcription factor have been identified in the proximal promoter of the mouse and rat GnRH genes (45–47), and Otx2 has been shown to be involved in forebrain patterning in several genetically altered mouse strains (42–44), this study focused on the effect of Otx2 on GnRH neuronal function. We describe the generation and characterization of a mouse model in which Otx2 is selectively deleted from GnRH neurons. The phenotypic characterization of delayed puberty and infertility is consistent with that seen in patients with mutations in Otx2.
GnRH-Otx2KO mice had a dramatic reduction in the number of GnRH-expressing neurons present in the hypothalamus by immunohistochemistry, fewer than 20% of the normal number of GnRH neurons vs. control mice (Fig. 2C). The reduced GnRH cell number, as determined by IHC could be due to either a loss of GnRH expression, which would prevent detection of the neuron, or a reduction in the overall number of hypothalamic GnRH neurons. In a study of transgenic mice in which GnRH neurons in the hypogonadal (hpg) mouse were labeled with green fluorescent protein (GFP), normal GnRH migration was observed (55), suggesting that expression of GnRH is not required for cell differentiation or migration. We are unable to use this approach in our model because the GnRH promoter is used to drive GFP expression, and therefore GFP expression might also be dependent on Otx2. We chose therefore to determine whether GnRH neuronal migration was affected in the GnRH-Otx2KO mice. Compared with control mice, GnRH-Otx2KO mice had significantly fewer GnRH neurons in the forebrain at e14.5, e15.5, and PN1, as summarized in Fig. 3 and Supplemental Table 1. In contrast, GnRH-Otx2KO mice had relatively equivalent numbers of GnRH neurons in the nasal region at all time points compared with control mice, although in both GnRH-Otx2KO and control mice, most of these neurons were lost during development.
The reduced number of GnRH neurons in their appropriate locations in the hypothalamus of GnRH-Otx2KO, which was confirmed by marking GnRH neurons in the hypothalamus with EYFP (Fig. 4B), suggests a defect in GnRH neuronal migration or an increase in GnRH neuronal cell death. As shown in Fig. 5, A and B, GnRH-Otx2KO mice displayed increased immunostaining for cleaved caspase-3 in the nasal region consistent, with increased programmed cell death being a major cause for the reduction in the number of GnRH neurons in GnRH-Otx2KO mice. This reduction in the GnRH neuronal number correlated with a dramatic decrease in the expression of GnRH and in LHβ and FSHβ subunit mRNA levels by quantitative RT-PCR (Fig. 6, A–C). A reduction in serum FSH levels as well as decreased sex steroid levels was also found (Fig. 7, B–D), whereas the morning basal serum LH levels were unchanged in the GnRH-Otx2KO mice, similar to GPR54−/−, Kiss1−/−, and GNR23−/− mice (56, 57) (Fig. 7A). This is consistent with the finding that basal LH secretion is mediated via a constitutive pathway, whereas pulsatile LH secretion occurs through a GnRH-regulated pathway (58). Furthermore, basal serum LH levels have been shown to be preserved in other models of GnRH deficiency, i.e. the hpg mouse (59). These data may represent an increase in LH serum half-life caused by changes in LH glycosylation seen in GnRH deficiency (60).
Female GnRH-Otx2KO animals lack a normal estrous cycle and exhibit a prolonged estrus stage, with 64% of their time spent in estrus compared with less than 20% for control animals (Fig. 9B). This correlates with a lack of LH surge in the GnRH-Otx2KO animals (Fig. 10). Consequently, the GnRH-Otx2KO females had a reduced fertility, with a dramatic reduction in the number of pups per litter and a reduced frequency of litters (Fig. 8, A and B). Most (five of nine) female mice were infertile over the period of assessment (Fig. 8C). The normal timing to the first litter in a subset of animals could be attributable to a reflex ovulation triggered by the initial introduction of the male and that does not appear to require GnRH in other models (61, 62), but these female GnRH-Otx2KO animals failed to produce any litters after 60 d (Fig. 8C). Other physiological parameters, which can affect fertility such as body weight, were not different between the control and the GnRH-Otx2KO animals at 2 months of age.
Our results are consistent with other models in the literature in which reduced GnRH expression causes infertility. hpg mice, a spontaneously occurring hypogonadal mutation with a defect in GnRH production, do not have detectable GnRH and are infertile (4) whereas the normally fertile heterozygous mice had a normal number of GnRH neurons (55). A second mouse line has been described with a targeted disruption of the GNR23 gene (57). In that line, homozygous transgenic females (GNR23−/−) had about 12% of the normal number of GnRH neurons and were also shown to be in persistent estrus with reduced fertility that was even more profound than in GnRH-Otx2KO mice. Heterozygous GNR23 mice had about 34% of the normal number of GnRH neurons and had normal fertility. Thus, our study together with previous reports suggests that a critical number of GnRH neurons are required for fertility. The minimum number of GnRH neurons required for normal reproductive function in mice is more than the 20% of normal observed in our study and could be as high as 34%, as previously observed (57).
The GnRH-Otx2KO male animals also demonstrated evidence of reduced fertility. The time between litters for GnRH-Otx2KO males was significantly increased compared with controls, and fewer pups per litter were noted (Fig. 8, A and B). Although GnRH-Otx2KO males had decreased serum FSH and testosterone levels (Fig. 7, B and D), testicular histology was not different between GnRH-Otx2KO and control mice (Supplemental Fig. 2). This is the first time a phenotype of reduced fertility has been described in males with reduced numbers of GnRH neurons. Interestingly, GNR23−/− mice, with approximately 70 GnRH neurons, had reduced FSH levels and testicular size and normal reproductive competence (57). As with the female animals, no evidence of a general physiological disturbance with respect to size was found.
Reproduction is a robust process, and our results document that a dramatic decrease in GnRH neuronal cell number is required to completely inhibit reproduction in the mouse. We hypothesize that in patients with CIHH due to a mutation in Otx2, a similar pattern of GnRH neuronal distribution is present and results in the phenotypic presentation associated with hypogonadism.
Interestingly, Otx2 has been shown to have a novel role in critical periods of brain development both prenatally and postnatally. Maturation of the parvalbumin cell network in the visual cortex that controls plasticity is regulated by selective reexpression of Otx2 (41). These authors provided evidence that OTX2 may be secreted, allowing OTX2 protein transfer from cell to cell along the visual pathway (41). Perhaps Otx2 might play a similar role in guiding migration of GnRH neurons along their migratory pathway from the nasal placode to the hypothalamus. Here we provide mechanistic evidence that GnRH neuronal expression of Otx2 is required for normal migration of GnRH neurons into the forebrain and may protect the GnRH neuron from programmed cell death. This work provides new insight into the physiological role of Otx2 in mediating GnRH neuronal function and mammalian reproduction as well as mechanistic understanding of the pathogenesis of CIHH in patients with mutations in Otx2.
Materials and Methods
Animal husbandry
Mice were housed in 14-h light and 10-h dark cycles, with food and water available ad libitum. All experiments were approved and executed in accordance with the Institutional Animal Care and Use Committees at Johns Hopkins University School of Medicine (Baltimore, MD).
GnRH neuron-specific Otx2 KO mouse generation
The floxed Otx2 mouse was kindly provided by Professor Isao Matsuo (48) and contains loxP sites flanking the 5′ untranslated region and the first exon of Otx2, previously shown to generate a complete loss of Otx2 in target cells containing Cre recombinase. Cell-specific KO of Otx2 was accomplished by crossing these floxed animals with a GnRH-Cre mouse previously described (49) in which GnRH neuron-specific Cre expression is driven by 3.4kb of the murine GnRH promoter. Briefly, a first cross between a Cre+/− and a homozygous floxed animal generated heterozygous Cre+/−/Otx2+/F animals. Because of previous reports that Cre can be nonspecifically activated in the testes (63), only female Cre+/− animals were used for these breedings. The F1 Cre+/−/Otx2+/F females were backcrossed with Cre−/−/Otx2F/F males, yielding mixed litters including pups with the desired genotype of Cre+/−/Otx2F/F causing GnRH neuron-specific Otx2 gene deletion (hereafter referred to as GnRH-Otx2KO mice). Cre−/−, Cre+/−/Otx2+/+, and Cre+/−/Otx2+/F littermates were used as controls in all experiments. All mice used in the experiments had a mixed C57BL/6J/CD1 genetic background.
Genotypes were determined by PCR of genomic tail DNA isolated using phenol/chloroform extraction with the following primers: Cre recombinase (sense 5′-CGACCAAGTGACAGCAATGCT-3′ and antisense 5′-GGTGCTAACCAGCGTTTTCGT-3′) and Otx2 (F1 sense 5′-GTATTTTCCTTGCTACCAAACTGCCGAGTG-3′ and R1 antisense 5′-CTGGAGGGAAGCCACACCTCTAAGGATTAA-3′). The Otx2 PCR identified WT and FL alleles with bands of 360 and 560 bp, respectively. Recombination and deletion of the target allele was demonstrated by PCR using the above Otx2 antisense primer (R1) in conjunction with a sense primer upstream of the deletion in the 5′ untranslated region, which would produce a greater than 2 kb product for WT or FL alleles, (P1 5′-TAGAGGGTATTTAAATAAGGACGACTGGG-3′) but yields a 300-bp product after recombination.
GnRH-Otx2KO-EYFP mouse generation
To visualize GnRH neurons in the brains of GnRH-Otx2KO mice devoid of immunoreactive GnRH, we produced GnRH-Cre+/−/Otx2F/F/ROSA26-EYFP+/− mice. ROSA26-EYFP+/− mice contain a floxed transcriptional stop sequence inserted between the promoter and the coding sequences of EYFP (64). GnRH-specific Cre expression in a ROSA26-EYFP+/− background excises the stop codon and results in the expression of EYFP in the GnRH neurons, thus permanently labeling all GnRH neurons. Briefly, a first cross between a Cre+/−/Otx2+/F female and a ROSA26-EYFP+/− male generated heterozygous Cre+/−/Otx2+/F/ROSA26-EYFP+/− mice. The F1 Cre+/−/Otx2+/F/ROSA26-EYFP+/− females were backcrossed with Cre−/−/Otx2F/F/ROSA26-EYFP+/− males, yielding mixed litters including pups with the desired genotype of Cre+/−/Otx2F/F/ROSA26-EYFP+/− causing GnRH neuron-specific Otx2 gene deletion and EYFP expression (hereafter referred to as GnRH-Otx2KO-EYFP mice). Cre+/−/Otx2+/+/ROSA26-EYFP+/− and Cre+/−/Otx2+/F/ROSA26-EYFP+/− littermates were used as controls in all experiments. All mice used in the experiments had a mixed C57BL/6J/CD1 genetic background. ROSA26-EYFP genotypes were determined by PCR of genomic tail DNA isolated using phenol/chloroform extraction with the following three primers: 5′-AAAGTCGCTCTGAGTTGTTAT-3′, 5′-GCGAAGAGTTTGTCCTCAACC-3′ and 5′-GGAGCGGGAGAAATGGATATG-3′ (65). The ROSA26-EYFP PCR identified WT and reporter alleles with bands of an approximate size of 600 and 300 bp, respectively.
Breeding studies
GnRH-Otx2KO mice were mated with control animals in continuous breeding cages to assess fertility of both sexes. Measurements included time to first litter, frequency of litters, and size of litters. Male GnRH-Otx2KO mice were housed with three control female littermates. Female GnRH-Otx2KO mice were housed with a single control male littermate. Cages were checked daily for new litters and number of pups. Frequency of litters was determined by recording each new litter produced over 90 d.
Estrous cycling analysis
Vaginal cytology to determine cycle stage and length was examined by collecting vaginal smears for 19 consecutive mornings. Smears were stained with Diff Quick stain kit (IMEB Inc., San Marcos, CA), and classified as proestrus, estrus, or metestrus/diestrus based on observed ratios of cornified epithelial, nucleated epithelial, and polymorphonuclear leukocytes (66). Cycle length was calculated as the number of days from the beginning of one estrous phase to the beginning of the next.
Histology
Postpubertal male and female mice were anesthetized with an ip injection of 90 mg/kg ketamine and 7.5 mg/kg xylazine, and serum was obtained by a retroorbital or mandibular bleed. Mice were transcardially perfused with ice-cold solutions of 0.9% saline, followed by 100 ml 4% paraformaldehyde [prepared in 0.1 m phosphate buffer (pH 7.4)] and rinsed with 0.9% saline. The brains were removed and postfixed in 4% paraformaldehyde at 4 C for 24 h. Brains were transferred to 30% sucrose at 4 C for another 24 h followed by freezing via immersing in 2-methylbutane at −30 C for 30 min. All samples were stored at −80 C until use. For embryonic time points, timed-pregnant female mice were euthanized at the appropriate day and embryos were removed. The heads were immersed in 4% paraformaldehyde at 4 C for 72 h and then dehydrated and frozen as above.
Frozen adult brains were sectioned using a Microm HM 550 cryostat (Microm International, Walldorf, Germany) in 40 μm coronal sections caudal to the median eminence to the accessory olfactory bulbs. Sequential sections were collected in six-well plates containing 1× PBS so that each well contained every sixth section, and sections were stained while floating. Embryonic brains were sectioned in the sagittal plane and sequential 40-μm sections were collected directly on slides. After quenching endogenous peroxidase for 20 min, slices were washed in 1× PBS and blocked for 1 h with 2% normal goat serum (Vector Laboratories, Burlingame, CA) in d 1 buffer (1× PBS per 1% BSA per 0.3% Triton X-100). Adult sections were then incubated with anti-GnRH antibody (Affinity BioReagents, Golden, CO) at 1:10,000 or anti-GFP antibody (Novus Biologicals, Littleton, CO) at 1:80,000 in d 2 buffer (1× PBS per 0.3% BSA per 0.1% Triton X-100) at 4 C for 48 h. Embryonic sections were incubated with anti-GnRH antibody at 1:1000 or anticleaved caspase-3 antibody (Cell Signaling Technology, Inc., Danvers, MA) at 1:2000 in d 2 buffer at 4 C for 72 h. Sections were then washed twice with d 2 buffer and incubated for at least 1 h at room temperature with biotinylated goat antirabbit IgG antibody (Vector Laboratories) diluted 1:250 with d 2 buffer. After two washes with d 2 buffer, sections were treated with avidin-biotin-horseradish peroxidase (Vector Laboratories) for at least 1 h. Sections were again washed with d 2 buffer followed by 1× PBS and stained using 3, 3′-diaminobenzidine tetrahydrochloride with nickel salts (Vector Laboratories) for 7 min. Sections were serially mounted onto Superfrost/Plus microscope slides (Fisher Scientific, Pittsburgh, PA) in a caudal-to-rostral order and dried overnight. The sections were dehydrated through a series of ethanol and xylene washes and then sealed with Permount (Fisher Scientific). Only the cell bodies of GnRH and EYFP immunoreactive neurons were counted, by a single individual, on a Primo Star microscope (Carl Zeiss Microimaging, Thornwood, NY).
Ovaries from perfused females were removed and postfixed in 10% formalin. The next day ovaries were washed twice in 1× PBS and transferred to 70% ethanol overnight at 4 C. After dehydration, the ovaries were processed for paraffin embedding, and 5-μm sections were cut by the Johns Hopkins Medical Institutions Reference Histology Laboratory. Sections were hematoxylin and eosin stained and corpora lutea were quantified by counting from 20 sections per ovary from four different anatomical locations on a Primo Star microscope (Carl Zeiss Microimaging).
Male mice were perfused with a saline heparin flush and Bouin's fixative. After perfusion, the entire reproductive pluck (testes, epididymis, and seminal vesicles) was submerged in Bouin's fixative overnight and then washed and submerged in 70% ethanol for 2 h. The testes, epididymis, and seminal vesicles were longitudinally sectioned and hematoxylin and eosin stained. Sections were viewed using a Primo Star microscope (Carl Zeiss Microimaging). Perfusion, sectioning, and staining were performed by the Johns Hopkins Department of Molecular and Comparative Pathobiology Phenotyping Core.
Hormone analysis
Blood samples were collected as above for terminal bleeds, whereas the small volumes obtained daily for cycling studies used either femoral or mandibular bleeding techniques. All samples were centrifuged at 3500 rpm at 4 C for 15 min to separate the serum, which was stored at −20 C until use. Assays were performed at the RIA Core of the Baltimore-Chicago Specialized Cooperative Reproductive Center for LH, FSH, estradiol, and testosterone as appropriate.
For the cycling study, single measurements were performed with 10 μl of serum. LH and FSH were analyzed using the xMap technology (Millipore, Billerica, MA) with a rat pituitary panel and a standard curve generated by xPonent software (Luminex, Austin, TX). Standards and samples were incubated with antibody-coated beads on a microplate shaker overnight at 4 C followed by three washes using a vacuum manifold apparatus. Detection antibody was then added to the wells and incubated on a microplate shaker at room temperature for 30 min. Streptavidin-phycoerythrin solution was added for a 30-min incubation at room temperature using the microplate shaker. Plates were then washed three times, and beads were resuspended in sheath fluid on the microplate shaker for 5 min before being read on the Luminex 200IS system with xPonent software. Data were analyzed with five-parametelogistic curve fitting. The limit of detection for the assay for LH was 48 pg/ml, the intraassay coefficient of variance was 2.3–9.5%, and the interassay coefficient of variance was 12.5–14.5% for low- and high-quality controls. The limit of detection for the assay for FSH was 32 pg/ml, the intraassay coefficient of variance was 1.2–8.7%, and the interassay coefficient of variance was 17.2–18.3% for low- and high-quality controls.
Quantitative real-time PCR
Total RNA was extracted from pituitary and hypothalamic tissue using Trizol (Invitrogen, Carlsbad, CA) as previously described (67). Two micrograms of RNA were reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) to produce cDNA. The cDNA obtained from 50 ng of total RNA was used in each subsequent 25 μl quantitative PCR. PCR reactions were performed using the MyiQ quantitative real-time PCR machine (Bio-Rad). Primer sets included: mouse GnRH (sense 5′-CCCTTTGACTTTCACATCC-3′ and antisense 5′-GGGTCTGCCATTTGATCCAC-3′), LHβ subunit (sense 5′-CAGTCTGCATCACCTTCACCA-3′ and antisense 5′-GGTAGGTGCACACTGGCTGA-3′), FSHβ subunit (sense 5′-GCCGTTTCTGCATAAGC-3′ and antisense 5′-CAATCTTACGGTCTCGTATACC-3′), and a ribosomal 18S control (sense 5′-TGGTTGATCCTGCCAGTAG-3′ and antisense 5′-CGACCAAAGGAACCATAACT-3′).
PCR conditions were optimized to generate greater than 95% PCR efficiency, and only those reactions between 95 and 105% efficiency were included in subsequent analysis. Cycle threshold (Ct) was obtained for each sample. A corrected Ct (ΔCt) was calculated by subtracting the 18S Ct from the unknown sample Ct for each sample. Relative differences from the control sample were then calculated by using the formula: fold change = 2^ (control ΔCt − sample ΔCt). PCR products were also analyzed by gel electrophoresis.
Statistical analysis
All results are expressed as the sem (±sem) Statistical significance was assessed by unpaired t test using GraphPad Prism 4 (GraphPad, San Diego, CA), with P < 0.05 considered significant.
Acknowledgments
Special thanks to Jennifer Mammen, Helen Kim, Yewade Ng, Tameka Williams, Horacio Novario, Sheng Wu, Melissa Yates, George Park, and David Cooke for their expert contributions as well as Brigette Mann at the at the RIA core for performing the hormone analysis.
This work was supported by research grants from the National Institutes of Health [R01HD044608 (to A.W.) and R01HD034551 (to S.R.)]; National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement [U54 HD041859 (The Baltimore-Chicago Center for Reproductive Research)] as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research; and the Baltimore Diabetes Research and Training Center Grant [P60DK079637].
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CIHH
- Congenital idiopathic hypogonadotropic hypogonadism
- CNS
- central nervous system
- CP
- cribriform plate
- Cre
- cAMP response element
- Ct
- cycle threshold
- ΔCt
- corrected Ct
- e
- embryonic day
- EYFP
- enhanced yellow fluorescent protein
- FB
- forebrain
- FL
- floxed
- hpg
- hypogonadal
- GFP
- green fluorescent protein
- IHC
- immunohistochemistry
- KO
- knockout
- OP
- olfactory placode
- OTX2
- orthodenticle homeobox 2
- OVLT
- organum vasculosum of the lamina terminalis
- PN
- postnatal day
- PPS
- preputial separation
- WT
- wild type.
References
- 1. Gamble JA, Karunadasa DK, Pape JR, Skynner MJ, Todman MG, Bicknell RJ, Allen JP, Herbison AE. 2005. Disruption of ephrin signaling associates with disordered axophilic migration of the gonadotropin-releasing hormone neurons. J Neurosci 25:3142–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwanzel-Fukuda M, Bick D, Pfaff DW. 1989. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res 6:311–326 [DOI] [PubMed] [Google Scholar]
- 3. Cattanach BM, Iddon CA, Charlton HM, Chippa SA, Fink G. 1977. Gonadotropin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269:338–340 [DOI] [PubMed] [Google Scholar]
- 4. Mason AJ, Hayflick JS, Zoeller RT, Young WS, 3rd, Phillips HS, Nikolics K, Seeburg PH. 1986. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science 234:1366–1371 [DOI] [PubMed] [Google Scholar]
- 5. Chan YM, de Guillebon A, Lang-Muritano M, Plummer L, Cerrato F, Tsiaras S, Gaspert A, Lavoie HB, Wu CH, Crowley WF, Jr, Amory JK, Pitteloud N, Seminara SB. 2009. GNRH1 mutations in patients with idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 106:11703–11708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trarbach EB, Silveira LG, Latronico AC. 2007. Genetic insights into human isolated gonadotropin deficiency. Pituitary 10:381–391 [DOI] [PubMed] [Google Scholar]
- 7. Seminara SB, Hayes FJ, Crowley WF., Jr 1998. Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev 19:521–539 [DOI] [PubMed] [Google Scholar]
- 8. Bhangoo A, Jacobson-Dickman E. 2009. The genetics of idiopathic hypogonadotropic hypogonadism: unraveling the biology of human sexual development. Pediatr Endocrinol Rev 6:395–404 [PubMed] [Google Scholar]
- 9. Bouligand J, Ghervan C, Tello JA, Brailly-Tabard S, Salenave S, Chanson P, Lombès M, Millar RP, Guiochon-Mantel A, Young J. 2009. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med 360:2742–2748 [DOI] [PubMed] [Google Scholar]
- 10. Miller NL, Wevrick R, Mellon PL. 2009. Necdin, a Prader-Willi syndrome candidate gene, regulates goandotropin-releasing hormone neurons during development. Hum Mol Genet 18:248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bianco S, Kaiser U. 2009. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol 5:569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diaczok D, Romero C, Zunich J, Marshall I, Radovick S. 2008. A novel dominant negative mutation of OTX2 associated with combined pituitary hormone deficiency. J Clin Endocrinol Metab 93:4351–4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dateki S, Fukami M, Sato N, Muroya K, Adachi M, Ogata T. 2008. OTX2 mutation in a patient with anophthalmia, short stature, and partial growth hormone deficiency: functional studies using the IRBP, HESX1, and POU1F1 promoters. J Clin Endocrinol Metab 93:3697–3702 [DOI] [PubMed] [Google Scholar]
- 14. Dateki S, Kosaka K, Hasegawa K, Tanaka H, Azuma N, Yokoya S, Muroya K, Adachi M, Tajima T, Motomura K, Kinoshita E, Moriuchi H, Sato N, Fukami M, Ogata T. 2010. Heterozygous orthodenticle homeobox 2 mutations are associated with variable pituitary phenotype. J Clin Endocrinol Metab 95:756–764 [DOI] [PubMed] [Google Scholar]
- 15. Schwanzel-Fukuda M, Pfaff DW. 1989. Origin of luteinizing hormone-releasing hormone neurons. Nature 338:161–164 [DOI] [PubMed] [Google Scholar]
- 16. Wray S, Grant P, Gainer H. 1989. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86:8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kramer PR, Guerrero G, Krishnamurthy R, Mitchell PJ, Wray S. 2000. Ectopic expression of luteinizing hormone-releasing hormone and peripherin in the respiratory epithelium of mice lacking transcription factor AP-2α. Mech Dev 94:79–94 [DOI] [PubMed] [Google Scholar]
- 18. Gill JC, Moenter SM, Tsai PS. 2004. Developmental regulation of gonadotropin-releasing hormone neurons by fibroblast growth factor signaling. Endocrinology 145:3830–3839 [DOI] [PubMed] [Google Scholar]
- 19. Kramer PR, Wray S. 2000. Novel gene expressed in nasal region influences outgrowth of olfactory axons and migration of luteinizing hormone-releasing hormone (LHRH) neurons. Genes Dev 14:1824–1834 [PMC free article] [PubMed] [Google Scholar]
- 20. Tobet SA, Chickering TW, King JC, Stopa EG, Kim K, Kuo-Leblank V, Schwarting GA. 1996. Expression of gamma-aminobutyric acid and gonadotropin-releasing hormone during neuronal migration through the olfactory system. Endocrinology 137:5415–5420 [DOI] [PubMed] [Google Scholar]
- 21. Fueshko SM, Key S, Wray S. 1998. GABA inhibits migration of luteinizing hormone-releasing hormone neurons in embryonic olfactory explants. J Neurosci 18:2560–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwarting GA, Kostek C, Bless EP, Ahmad N, Tobet SA. 2001. Deleted in colorectal cancer (DCC) regulates the migration of luteinizing hormone-releasing hormone neurons to the basal forebrain. J Neurosci 21:911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tobet SA, Schwarting GA. 2006. Minireview: recent progress in gonadotroprin-releasing hormone neuronal migration. Endocrinology 147:1159–1165 [DOI] [PubMed] [Google Scholar]
- 24. Pierce A, Bliesner B, Xu M, Nielsen-Preiss S, Lemke G, Tobet S, Wierman ME. 2008. Axl and Tyro3 modulate female reproduction by influencing gonadotropin-releasing hormone neuron survival and migration. Mol Endocrinol 22:2481–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu TJ, Gibson MJ, Rogers MC, Silverman AJ. 1997. New observations on the development of the gonadotropin-releasing hormone system in the mouse. J Neurobiol 33:938–998 [DOI] [PubMed] [Google Scholar]
- 26. Kinder SJ, Tsang TE, Ang SL, Behringer RR, Tam PP. 2001. Defects of the body plan of mutant embryos lacking Lim1, Otx2, or Hnf3β activity. Int J Dev Biol 45:347–355 [PubMed] [Google Scholar]
- 27. Rhinn M, Dierich A, Shawlot W, Behringer RR, Le Meur M, Ang SL. 1998. Sequential roles for Otx2 in visceral endoderm and neuroectoderm for forebrain and midbrain induction and specification. Development 125:845–856 [DOI] [PubMed] [Google Scholar]
- 28. Ang SL, Jin O, Rhinn M, Daigle N, Stevenson L, Rossant J. 1996. A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development 122:243–252 [DOI] [PubMed] [Google Scholar]
- 29. Vernay B, Koch M, Vaccarino F, Briscoe J, Simeone A, Kageyama R, Ang SL. 2005. Otx2 regulates subtype specification and neurogenesis in the midbrain. J Neurosci 25:4856–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brodski C, Weisenhorn DM, Signore M, Sillaber I, Oesterheld M, Broccoli V, Acampora D, Simeone A, Wurst W. 2003. Location and size of dopaminergic and serotonergic cell populations are controlled by the position of the midbrain-hindbrain organizer. J Neurosci 23:4199–4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fossat N, Chatelain G, Brun G, Lamonerie T. 2006. Temporal and spatial delineation of mouse Otx2 functions by conditional self-knockout. EMBO Rep 7:824–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Omodei D, Acampora D, Mancuso P, Prakash N, Di Giovannantonio LG, Wurst W, Simeone A. 2008. Anterior-posterior graded response to Otx2 controls proliferation and differentiation of dopaminergic progenitros in the ventral mesencephalon. Development 135:3459–3470 [DOI] [PubMed] [Google Scholar]
- 33. Puelles E, Acampora D, Lacroix E, Signore M, Annino A, Tuorto F, Filosa S, Corte G, Wurst W, Ang SL, Simeone A. 2003. Otx dose-dependent integrated control of antero-posterior and dorso-ventral patterning of midbrain. Nat Neurosci 6:453–460 [DOI] [PubMed] [Google Scholar]
- 34. Puelles E, Annino A, Tuorto F, Usiello A, Acampora D, Czerny T, Brodski C, Ang SL, Wurst W, Simeone A. 2004. Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development 131:2037–2048 [DOI] [PubMed] [Google Scholar]
- 35. Hide T, Hatakeyama J, Kimura-Yoshida C, Tian E, Takeda N, Ushio Y, Shiroishi T, Aizawa S, Matsuo I. 2002. Genetic modifiers of otocephalic phenotypes in Otx2 heterozygous mutant mice. Development 129:4347–4357 [DOI] [PubMed] [Google Scholar]
- 36. Wyatt A, Bakrania P, Bunyan DJ, Osborne RJ, Crolla JA, Salt A, Ayuso C, Newbury-Ecob R, Abou-Rayyah Y, Collin JR, Robinson D, Ragge N. 2008. Novel heterozygous OTX2 mutations and whole gene deletions in anophthalmia, microphthalmia and coloboma. Hum Mutat 29:E278–E283 [DOI] [PubMed] [Google Scholar]
- 37. Tajima T, Ohtake A, Hoshino M, Amemiya S, Sasaki N, Ishizu K, Fujieda K. 2009. OTX2 loss of function mutation causes anophthalmia and combined pituitary hormone deficiency with a small anterior and ectopic posterior pituitary. J Clin Endocrinol Metab 94:314–319 [DOI] [PubMed] [Google Scholar]
- 38. Simeone A, Acampora D, Mallamaci A, Stornaiuolo A, D'Apice MR, Nigro V, Boncinelli E. 1993. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J 12:2735–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inverardi F, Beolchi MS, Ortino B, Moroni RF, Regondi MC, Amadeo A, Frassoni C. 2007. GABA immunoreactivity in the developing rat thalamus and Otx2 homeoprotein expression in migrating neurons. Brain Res Bull 73:64–74 [DOI] [PubMed] [Google Scholar]
- 40. Rebsam A, Mason CA. 2008. Otx2's incredible journey. Cell 134:386–387 [DOI] [PubMed] [Google Scholar]
- 41. Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. 2008. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134:508–520 [DOI] [PubMed] [Google Scholar]
- 42. Rath MF, Muñoz E, Ganguly S, Morin F, Shi Q, Klein DC, Moller M. 2006. Expression of the Otx2 homeobox gene in the developing mammalian brain; embryonic and adult expression in the pineal gland. J Neurochem 97:556–566 [DOI] [PubMed] [Google Scholar]
- 43. Acampora D, Mazan S, Lallemand Y, Avantaggiato V, Maury M, Simeone A, Brûlet P. 1995. Forebrain and midbrain regions are deleted in Otx2−/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development 121:3279–3290 [DOI] [PubMed] [Google Scholar]
- 44. Matsuo I, Kuratani S, Kimura C, Takeda N, Aizawa S. 1995. Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev 9:2646–2658 [DOI] [PubMed] [Google Scholar]
- 45. Kim HH, Wolfe A, Cohen RN, Eames SC, Johnson AL, Wieland CN, Radovick S. 2007. In vivo identification of a 107-base pair promoter element mediating neuron-specific expression of mouse gonadotropin-releasing hormone. Mol Endocrinol 21:457–471 [DOI] [PubMed] [Google Scholar]
- 46. Kelley CG, Lavorgna G, Clark ME, Boncinelli E, Mellon PL. 2000. The Otx2 homeoprotein regulates expression from the gonadotropin-releasing hormone proximal promoter. Mol Endocrinol 14:1246–1256 [DOI] [PubMed] [Google Scholar]
- 47. Larder R, Mellon PL. 2009. Otx2 induction of the gonadotropin-releasing hormone promoter is modulated by direct interaction with Grg co-repressors. J Biol Chem 284:16966–16978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tian E, Kimura C, Takeda N, Aizawa S, Matsuo I. 2002. Otx2 is required to respond to signals from anterior neural ridge for forebrain specification. Dev Biol 242:204–223 [DOI] [PubMed] [Google Scholar]
- 49. Wolfe A, Divall S, Singh SP, Nikrodhanond AA, Baria AT, Le WW, Hoffman GE, Radovick S. 2008. Temporal and spatial regulation of CRE recombinase expression in GnRH neurons in the mouse. J Neuroendocrinol 20:909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Franklin KBJ, Paxinos G. 1997. The mouse brain in stereotaxic coordinates. San Diego: Academic Press [Google Scholar]
- 51. Silverman AJ, Livne I, Witkin JW. 1994. The gonadotropin-releasing hormone (GnRH), neuronal systems: immunocytochemistry and in situ hybridization. In: Knobil E, Neill JD. eds. The physiology of reproduction. New York: Raven Press; 1683–1709 [Google Scholar]
- 52. King JC, Tobet SA, Snavely FL, Arimura AA. 1982. LHRH immunopositive cells and their projections to the median eminence and organum vasculosum of the lamina terminalis. J Comp Neurol 209:287–300 [DOI] [PubMed] [Google Scholar]
- 53. Witkin JW, Paden CM, Silverman AJ. 1982. The luteinizing hormone-releasing hormone (LHRH) systems in the rat brain. Neuroendocrinology 35:429–438 [DOI] [PubMed] [Google Scholar]
- 54. Gallo RV. 1981. Pulsatile LH release during the ovulatory LH surge on proestrus in the rat. Biol Reprod 24:100–104 [DOI] [PubMed] [Google Scholar]
- 55. Gill JC, Wadas B, Chen P, Portillo W, Reyna A, Jorgensen E, Mani S, Schwarting GA, Moenter SM, Tobet S, Kaiser UB. 2008. The gonadotropin-releasing hormone (GnRH) neuronal population is normal in size and distribution in GnRH-deficient and GnRH receptor-mutant hypogonadal mice. Endocrinology 149:4596–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. 2007. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 57. Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. 2008. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR. 2003. The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reprod Suppl 61:463–476 [PubMed] [Google Scholar]
- 59. Gibson MJ, Kasowski H, Dobrjansky A. 1994. Continuous gonadotropin-releasing hormone infusion stimulates dramatic gonadal development in hypogonadal female mice. Biol Reprod 50:680–685 [DOI] [PubMed] [Google Scholar]
- 60. Wide L, Eriksson K, Sluss PM, Hall JE. 2009. Serum half-life of pituitary gonadotropins is decreased by sulfonation and increased by sialylation in women. J Clin Endocrinol Metab 94:958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brown-Grant K, Davidson JM, Greig F. 1973. Induced ovulation in albino rats exposed to constant light. J Endocrinol 57:7–22 [DOI] [PubMed] [Google Scholar]
- 62. Gibson MJ, Krieger DT, Charlton HM, Zimmerman EA, Silverman AJ, Perlow MJ. 1984. Mating and pregnancy can occur in genetically hypogonadal mice with preoptic area brain grafts. Science 225:949–951 [DOI] [PubMed] [Google Scholar]
- 63. Schmidt EE, Taylor DS, Prigge JR, Barnett S, Capecchi MR. 2000. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc Natl Acad Sci USA 97:13702–13707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- 66. Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. 1982. A longitudinal study of extrous cyclicity in aging C57BL/6L mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod 27:327–339 [DOI] [PubMed] [Google Scholar]
- 67. DiVall SA, Radovick S, Wolfe A. 2007. Egr-1 binds the GnRH promoter to mediate the increase in gene expression by insulin. Mol Cell Endocrinol 270:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]