The somatotrope lineage restricted expression of hGH-N is mediated by the chromatin conformation of the human growth hormone locus in pituitary.
Abstract
Expression of mammalian GH is normally restricted to somatotropes and somatolactotropes (somatotrope lineages) in the anterior pituitary. The basis for this restriction remains incompletely understood. Recent studies indicate that deoxyribonuclease I hypersensitive site I (HSI) of the hGH locus control region, located at −14.5 kb relative to the hGH-N promoter, acts as a potent long-range enhancer of hGH-N transcription. Here we report that HSI is also critical to somatotrope-restriction of hGH-N expression. Loss of HSI activity, either by direct inactivation of HSI or by interference with HSI-dependent downstream events, results in a relaxation of hGH-N cell-type specification with expansion of hGH-N expression to the full spectrum of Pit-1 positive pituitary cell types. These findings expand the defined roles for HSI of the hGH locus control region to include somatotrope lineage restriction as well as transcriptional enhancement of hGH-N gene expression.
The anterior lobe of the mammalian pituitary is composed of six major cell types: somatotropes, lactotropes, somatolactotropes, thyrotropes, gonadotropes, and corticotropes (1). These cells are marked by the respective expression of GH, prolactin (PRL), GH plus PRL, TSH, FSH/LH, and ACTH, respectively. Differentiation and expansion of these cell lineages is under the control of a complex set of signaling pathways and transcription complexes (1). The POU-homeodomain transcription factor Pit-1, the first and best described of the pituitary-specific transcriptional determinants, is essential for the expansion and differentiation of somatotropes, lactotropes, somatolactotropes, and a subset of thyrotropes lineages and in the expression of their respective hormone products (2). Loss of the Pit-1 gene results in combined deficiencies of GH, Prl, and TSH expression with a corresponding set of phenotypic and clinical disorders (2–4). Despite the common and essential role of Pit-1 in the expression of these three hormones, the expression of each is restricted to its corresponding cell types. The basis for this cell-type restriction remains poorly understood.
The pituitary-expressed human GH gene (hGH-N) is the most 5′ gene within the five-gene hGH cluster (Fig. 1A). Its four paralogs, hCS-L, hCS-A, hGH-V, and hCS-B, are expressed exclusively in the placenta (5). Expression from the hGH cluster in both the pituitary and placenta is dependent on the activities of a set of remote regulatory elements that comprise the hGH locus control region (LCR). These determinants are located from −14.5 to −32 kb 5′ to the hGH-N promoter (6, 7). The components of the hGH LCR colocalize with sites of deoxyribonuclease I hypersensitivity (HS) at the active chromatin locus in the two expressing tissues, pituitary and placenta (6). The closely paired HSI and HSII, located 14.5 kb 5′ to the hGH promoter, constitute the pituitary-specific components of the hGH LCR. These determinants are sufficient to drive high levels of somatotrope-specific expression of hGH-N transgenes in the mouse pituitary (8, 9). HSI contains an array of three Pit-1 binding sites (10–13), and deletion of two of these sites from the hGH locus results in loss of HSI formation and a dramatic decrease of hGH-N expression (10). Of note, the low residual levels of hGH-N expression from this HSI-inactivated hGH transgene remain copy number dependent and site-of-integration independent. The residual low levels of expression most likely reflect basal functions of the hGH-N promoter, which itself contains two Pit-1 binding sites in close proximity to the site of transcription initiation. The consistency of this residual expression among independent transgenic mouse lines (transgene-copy number dependence) most likely reflects the fact that the basal hGH-N promoter is operating in an insulated environment established by retained LCR boundary determinants (6).
Fig. 1.
The hGH-N transgene locus retains consistent expression in the somatotrope lineage in the absence of HSI. A, The structure of the hGH locus and the region encompassed by the hGH/P1 transgene. The genes, their sites of expression, and the direction of their transcription are indicated as are the positions of the HS that constitute the hGH LCR. The hGH/P1(ΔHSI) transgene contains a 99-bp deletion that removes two Pit-1 binding sites that are critical to HSI formation and function. B, Disaggregated pituitary cells from hGH/P1 and hGH/P1(ΔHSI) transgenic mice were immunostained for mGH (red) and hGH (green), and the nuclei were counterstained with DAPI (blue). The individual mGH and hGH signals are displayed in the left and middle panels, respectively. The merged images are shown in the right panels. The top three panels show cells from hGH/P1 pituitaries. The intensities of the hGH and mGH signals are comparable, and 100% of mGH-positive cells are also hGH positive. The bottom three panels show cells from hGH/P1(ΔHSI) pituitaries. The two hGH-positive cells (white arrows) are also mGH positive. The low intensity of the hGH-N signal when compared with that from the hGH/P1 transgene is consistent with the loss of HSI enhancer activity (10). Despite the low level of expression, it was possible with enhanced imaging (not shown) to unambiguously visualize hGH-positive staining in greater than 94% of mGH-positive cells (as summarized in Table 1). Scale bars, 10 μm.
The robust enhancement of hGH-N by the hGH LCR appears to reflect multiple activities that map to the HSI determinant. These activities include establishment of an extensive (32 kb) domain of histone acetylation encompassing the entire LCR and contiguous hGH-N promoter, establishment of a distinct and more limited domain of histone H3 lysine 4 methylation within the LCR and the establishment of a domain of noncoding Pol II transcription (11). The domain of LCR noncoding transcription encompasses the LCR itself and extends 3′ of HSI to include the adjacent CD79b gene forming a pituitary-specific hGH LCR/CD79b domain of transcription (11). Insertion of an exogenous Pol II termination element between HSI and CD79b interrupts the LCR domain of transcription, interrupts higher-order chromatin looping between HSI and the hGH-N promoter, and markedly decreases hGH expression (11, 12). Thus, the actions of HSI are critical to the establishment of an epigenetically modified and transcriptionally active LCR that is functionally linked to robust enhancement of hGH-N expression.
In the current study, we explore the basis for the cell-type restriction of hGH-N expression. We find that inactivation of HSI either by deletion of its critical Pit-1 binding sites or by interruption of the LCR domain of transcription results in a relaxation of somatotrope lineage specificity with the appearance of hGH-N expression in the two related Pit-1-positive lineages, lactotropes and thyrotropes. These results suggest that the one or more functions of the hGH LCR, and HSI in particular, are involved in the restriction of hGH-N expression to the somatotrope lineage and its exclusion from related Pit-1-positive cell types.
Results
Somatotrope lineage restriction of hGH transgene expression is dependent on HSI activity
hGH LCR activity is essential to robust hGH-N transgene expression in the mouse pituitary (6, 7, 10, 14, 15). Inactivation of HSI by selective deletion of two of its three Pit-1 binding sites results in a 20-fold decrease in hGH-N transgene mRNA (10). Thus, HSI acts in vivo as a somatotrope-specific transcriptional enhancer of hGH expression. To determine whether the loss of HSI function also impacts on somatotrope restriction, we compared the cell-type expression of hGH-N from an intact hGH/P1 transgene with the same transgene lacking a 99-bp segment encompassing two Pit-1 binding sites critical to HSI function (Fig. 1A). Single-cell suspensions of primary pituitary cells from mice carrying the intact hGH/P1 transgene or the hGH/P1(ΔHSI) transgene were stained with antibodies specific to mouse GH (m)GH or to human (h)GH (see Materials and Methods). The analysis revealed that hGH-N was expressed from the intact hGH/P1 transgene in 100% of the mGH-positive cells (n > 500) in two transgenic mouse lines, line 809F and line 811D (7) (see example in Fig. 1B, top). Parallel analysis of pituitary cells from hGH/P1(ΔHSI) mice (lines 960G and 969E) (10) demonstrated hGH-N expression in more than 94% of the somatotropes (n > 400) (see example in Fig. 1B, bottom, and Table 1). The weak hGH staining in these hGH/P1(ΔHSI) samples was consistent with the previously reported 20-fold decrease in overall hGH-N RNA levels in hGH/P1(ΔHSI) transgenic pituitaries. The weakness of the staining is likely to account for the 6% of somatotropes in which hGH could not be visualized with certainty (10). These results indicate that the hGH-N transgene retains consistent expression in the somatotrope lineages (i.e. mGH-positive cells) in the absence of an intact HSI determinant.
Table 1.
Quantification of hGH-positive cells in pituitaries of transgenic mice
| Transgenic line | Somatotropes positive for hGH |
Nonsomatotropes positive for hGH |
Total number of hGH+ cells examined | ||
|---|---|---|---|---|---|
| % | Average of two lines | % | Average of two lines | ||
| hGH/P1 (811D) | 100 | 100 | 0 | 0 | >500 |
| hGH/P1 (809F) | 100 | 0 | >500 | ||
| hGH/P1 (ΔHSI) (960G) | 94 | 94.5c | 12.3a | 14.3b | 457 |
| hGH/P1(ΔHSI) (969E) | 95 | 16.3a | 641 | ||
| hGH/P1(TerF) (1300C) | 95 | 94c | 5.6a | 6.2b | 524 |
| hGH/P1(TerF) (1301G) | 93 | 6.8a | 438 | ||
All hGH-positive cells are also Pit-1 positive.
P < 0.01 in comparison with hGH/P1 lines.
P < 0.05 in comparison with hGH/P1 lines.
We next asked whether the inactivation of HSI resulted in a loss of somatotrope lineage restriction. Analysis of pituitaries of mice carrying the intact hGH/P1 transgene revealed that all hGH expression was restricted to mGH-positive cells (>500 mGH-negative cells studied). In contrast, analysis of two separate hGH/P1(ΔHSI) lines indicated that 14% of hGH-positive cells were mGH negative (see example in Fig. 2 and Table 1). These data lead us to conclude that HSI of the hGH LCR is essential for full restriction of hGH-N transgene expression to the somatotrope lineage.
Fig. 2.
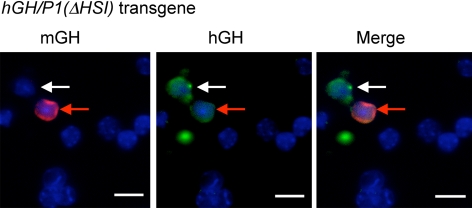
Inactivation of HSI results in a relaxation of hGH-N somatotrope restriction. Cells from an hGH/P1(ΔHSI) pituitary were stained for hGH and mGH, and the nuclei were counterstained with DAPI (blue). The representative field shows an example of a cell that is hGH positive and mGH negative (white arrows) as well as a cell that costains for both mGH and hGH (red arrows). The overall frequency at which hGH is expressed in mGH-negative cells is summarized in Table I. A parallel analysis of hGH/P1 mice failed to reveal evidence for ectopic expression using similar levels of signal enhancement (data not shown). Scale bars, 10 μm.
Ectopic expression of hGH from the hGH/P1(ΔHSI) transgene is restricted to Pit-1-positive cells
The mechanisms by which hGH is restricted to the somatotrope lineages and excluded from the Pit-1-positive lactotropes and thyrotropes remains undefined. Our previous studies revealed that robust expression of the hGH transgene locus in the mouse pituitary reflects complementing actions of Pit-1 bound at HSI and at the hGH promoter (13, 16). Pit-1 binding at the hGH promoter is markedly enhanced by Pit-1 occupancy at HSI (10). To determine whether residual action of Pit-1, most likely at the hGH-N promoter, plays a role in the cell-type determination of hGH-N expression from the hGH/P1(ΔHSI) transgene, we asked whether the ectopically expressed hGH protein remained restricted to Pit-1-positive cells. Immunofluorescent analysis revealed that the nuclei of 61% of the total population of primary pituitary cells in the disaggregated cell preparation were positive for Pit-1 (data not shown). As expected, the majority of the hGH-positive cells in the hGH/P1(ΔHSI) pituitary sample corresponded to the somatotrope lineages and costained for mGH and Pit-1 (example in Fig. 3, yellow arrow). We observed that the cells expressing ectopic hGH, i.e. hGH positive/mGH negative, were consistently Pit-1 positive (see example in Fig. 3, white arrow). The identity of these Pit-1-positive cells supporting ectopic hGH expression from the hGH/P1(ΔHSI) transgene (i.e. hGH-positive/mGH-negative cells) was further defined by staining for markers of the lactotrope and thyrotrope lineages, mPRL or mTSHβ, respectively (1, 16) (Figs. 4 and 5). The hGH/P1(ΔHSI) transgene was expressed in a fraction of lactotropes (mPRL-positive/mGH-negative cells; see example in Fig. 4, yellow arrow) and thyrotropes (mTSHβ positive) (see example in Fig. 5, red arrows). In contrast, expression of the wild-type hGH/P1 transgene was uniformly excluded from these two cell types (n > 500; data not shown). Together, these results indicated that inactivation of HSI resulted in a relaxation of somatotrope lineage restriction and the ectopic expression of hGH-N in the full spectrum of Pit-1-positive cell types.
Fig. 3.
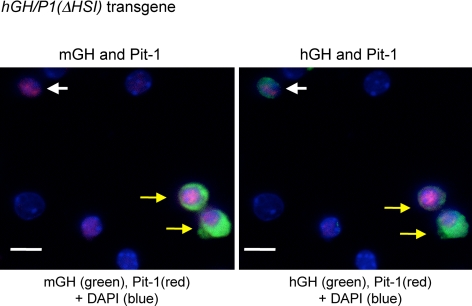
Ectopic detection of hGH from hGH/P1(ΔHSI) transgenic pituitaries is restricted to Pit-1-positive cells. Disassociated pituitary cells were prepared from an hGH/P1(ΔHSI) transgenic mouse. The cells in the left panel were stained with antibodies to mGH (green cytoplasmic staining) and Pit-1 (red nuclear staining). The secondary antibody to detect mGH was conjugated with Cy5 and assigned green as a pseudocolor. The cells in the right panel were stained for hGH and Pit-1. The secondary antibody to detect hGH was conjugated to Cy2. The yellow arrows point to two cells in the somatotrope lineage (mGH positive, Pit-1 positive) that are also hGH positive. In contrast, a cell with ectopic expression of hGH is indicated by the white arrows. This cell is hGH positive and Pit-1 positive but mGH negative. Scale bars, 10 μm.
Fig. 4.
Ectopic detection of hGH in lactotropes of hGH/P1(ΔHSI) transgenic pituitaries. The hGH/P1(ΔHSI) pituitary cells were stained for mGH (green, pseudocolored as in Fig. 3), mPRL (red), and hGH (green), and the nuclei were counterstained with DAPI (blue). The yellow arrows point to a lactotrope (mPrl positive and mGH negative) with ectopic expression of hGH-N. For comparison, a typical somatotrope with hGH expression (mGH positive/mPrl negative/hGH positive) is identified in the lower left corner of the field (red arrows). The intensities of the hGH signals in this study of the hGH/P1(ΔHSI) cells were enhanced to facilitate accurate identification of hGH. Scale bars, 10 μm.
Fig. 5.
Ectopic detection of hGH in thyrotropes of hGH/P1(ΔHSI) transgenic pituitaries. The cells in this field were stained for mTSHβ (red) and hGH (green). Three mTSHβ-positive cells are seen in this field, two of which also stain for hGH (red arrows), whereas the third cell (white arrows) does not.
Interruption of the LCR domain of noncoding transcription also results in a relaxation of somatotrope specificity of hGH transgene expression
We previously demonstrated that it is possible to interrupt the segment of the HSI-dependent domain of noncoding transcription that encompasses adjacent CD79b gene by inserting a Pol II termination element (TerF) immediately 3′ of HSI (11, 12). This insertion significantly represses looping between HSI and the hGH promoter with a commensurate 5-fold decrease in hGH-N transgene expression. Of note, this insertion, although blocking HSI-dependent activities, has no significant impact on formation of HSI itself, nor does it diminish the level or distribution of HSI-dependent histone acetylation throughout the transgene locus (11). To further test the relationship between LCR function and somatotrope specification, we assessed the impact of this insertion on cell specificity of hGH-N expression. Pituitary cells isolated from two transgenic mouse lines carrying the hGH/P1(TerF) transgene (lines 1301C and 1301G) (11) were stained for mGH and hGH. Although hGH was detected in somatotropes and somatolactotropes (95% of mGH-positive cells were hGH positive) (Figs. 6 and 7A), we observed that 6% of the hGH-positive signals were ectopic, i.e. in mGH-negative cells (Fig. 6, white arrow, and summary in Table 1). As was the case for the hGH/P1(ΔHSI) transgene, the expression of hGH-N from the hGH/P1(TerF) transgene remained limited to Pit-1-positive cells; all of the hGH-positive cells were also Pit-1 positive (n > 400) (Fig. 7A, white arrow).
Fig. 6.
Interruption of the HSI-dependent domain of noncoding transcription across the hGH LCR leads to ectopic expression of hGH-N. Pituitary cells from an hGH/P1(TerF) mouse were analyzed for expression of mGH (red) and hGH (green). The nuclei were counterstained with DAPI (blue). A cell with ectopic expression of hGH (mGH negative/hGH positive) is indicated (white arrows). Six percent of total hGH-positive cells in hGH/P1(TerF) mouse pituitaries were nonsomatotropes (summarized in Table 1). Scale bars, 10 μm.
Fig. 7.
The ectopic expression of hGH after interruption of the HSI-dependent domain of noncoding transcription remains restricted to Pit-1-positive cells. Pituitary cells from an hGH/P1(TerF) mouse were analyzed by immunofluorescent staining for mGH (Cy5 and pseudocolored green), hGH (green), and Pit-1 (red). The nuclei were stained with DAPI (blue). This figure contains an example of an hGH-positive cell that is Pit-1 positive but mGH negative (white arrows). Scale bars, 10 μm.
In summary, the preceding studies, using two distinct models of LCR interruption, lead us to conclude that the intact function of the hGH LCR, and in particular HSI and the domain of noncoding transcription and the native chromatin configuration of the locus, contributes to full retention of somatotrope-specific expression of the hGH-N gene.
Discussion
The expression of hGH-N is normally restricted to somatotropes and somatolactotropes in adult pituitary. In this study, we investigated the role played by HSI of the hGH LCR in this cell-type specification. The analysis revealed that HSI plays a nonredundant role in this process. Deletion of HSI and/or the interruption of its downstream actions within the LCR resulted in relaxation of somatotrope lineage specification and the appearance of hGH-N expression in the Pit-1-positive lactotrope and thyrotrope lineages.
A central and persistent question in the study of pituitary development is how three Pit-1-dependent hormone genes are differentially expressed in their respective Pit-1-positive cell lineages (1, 17). Previous studies have suggested that the minimal information required for selective expression of mGH in somatotropes but not lactotropes resides in a specific difference in the configuration of the Pit-1 binding sites in the mGH vs. mPrl promoters (18, 19). Subsequent studies revealed that lysine-specific demethylase 1 (LSD1) plays a supportive role in the reciprocal activation and repression of mGH in somatotropes and lactotropes, respectively (19). In contrast to the single GH locus in the mouse genome, the hGH locus contains five highly conserved genes (5) under the control of a complex set of remote regulatory elements that constitute the hGH LCR (6, 7). The robust expression of hGH-N in the pituitary of transgenic mice is dependent on the functions of HSI (10). Deletion of HSI from an extended hGH transgene results in alterations in the chromatin conformation of the hGH locus native to the pituitary somatotrope and 20-fold loss of hGH-N transcription (10, 12). In the present study, we found that loss of HSI or interference with its downstream actions also resulted in a relaxation in somatotrope-lineage restriction. Taken together, these results suggest that the pituitary-specific chromatin configuration of the hGH LCR plays an important and complex role in both the transcriptional enhancement of hGH-N in somatotropes and the restriction of hGH-N expression in lactotropes and thyrotropes.
Why does the loss of HSI activity from the hGH LCR result in ectopic hGH-N expression in lactotropes and thyrotropes? An attractive possibility is that the hGH locus can assume both repressive as well as activating chromatin conformations. It is clear from studies in other model systems that chromatin states can toggle between positive and negative conformations. For example, Kit locus expression is controlled during erythropoiesis by alternative conformations of long-range looping configurations (20). In immature cells, the trans-factor GATA2 is recruited to a remote enhancer and establishes interaction between the enhancer and the Kit promoter. Upon cell differentiation, GATA1 replaces GATA2 and induces an alternative chromatin configuration that represses Kit locus expression (20). Similarly, the repression of the imprinted DLX5 locus is mediated by MeCP2, a methyl-CpG-binding protein, via chromatin looping (21). Mutations in MeCP2 result in loss of the brain-specific silent chromatin looping at the Dlx5-Dlx6 locus and impair the imprinting of Dlx5 in Rett syndrome (22). A recent study demonstrates that the developmental silencing of the human γ-globin gene is mediated by long-range interaction between the LCR and the promoter of the γ-globin gene (22). These studies all point to critical aspects of chromatin domain structure in the silencing as well as the activation of a mammalian locus.
The results of the current study are consistent with a model in which the loss of HSI activity interrupts the ability of the hGH locus to form a repressive chromatin configuration in nonsomatotrope cells. Such an alteration would allow the Pit-1 present in the lactotrope and/or thyrotrope to access a normally inaccessible set of Pit-1 binding sites at the hGH-N promoter, with consequent, ectopic gene activation in a fraction of these two cell types. In support of this model are the previous observations that deletion of HSI in the hGH/P1(ΔHSI) transgene and the insertion of the TerF element 3′ of HSI in the hGH/P1(TerF) transgene both interfere with somatotrope-specific chromatin looping at the hGH locus. This model is also supported by the observation that the ectopic expression of hGH-N from these two transgenes remains restricted to Pit-1-positive cells.
The finding that only a fraction of the lactotropes and thyrotropes are positive for hGH suggests that the altered chromatin configuration of the hGH locus in the HSI-deletion or TerF-insertion transgenes retains a subset of attributes and activities characteristic of the native locus that can be sufficient to repress expression. In the case of the HSI deletion, this includes the retention of HSII, HSIII, and HSV and the looping between HSII and the HSIII/HSV region (10). In the case of the TerF insertion, this includes the retention of the full set of HS, including HSI, and the retention of the 32-kb domain of histone acetylation that encompasses the LCR and hGH promoter (11). Thus, altered chromatin structures due to the inactivation of HSI or interference with its downstream functions might allow Pit-1 to bind to the hGH-N promoter in a fraction of lactotropes and thyrotropes with the consequent activation of hGH-N expression. It is also possible that the hGH-N promoter may itself play a role in the repression of the hGH-N in the absence of the appropriately folded and configured chromatin locus. This attribute would be consistent with the observation that the mGH promoter can play an important role in recruitment of repressive trans-factors in the lactotropes (19).
In summary, the interruption of the pituitary-specific chromatin conformation at the hGH locus results in a partial relaxation of somatotrope-restricted hGH-N expression. These results suggest that the hGH LCR plays both active and repressive roles in hGH-N expression during pituitary development by enhancing its expression in somatotropes while repressing its expression in lactotropes and thyrotropes. Additional studies to investigate the proposed repressive roles of the hGH LCR on hGH-N expression in cell-type restriction may be possible using newly generated mouse pituitary cell lines that contain an intact but repressed hGH-N transgene locus (23). Such studies may provide further insight into the mechanisms of spatial and temporal regulation of gene activation during pituitary cell differentiation.
Materials and Methods
Transgenic mouse lines
The transgenic mouse lines used in this study are as follows: hGH/P1 lines 809F and 811D (7), hGH/P1(ΔHSI) lines 960G and 969E (10), and hGH/P1(TerF) lines 1301C and 1301G (11). These transgenic mouse lines carry similar copy numbers of the respective transgenes (three to five copies). Use of animals was reviewed and approved by the University of Pennsylvania Laboratory Animal Use and Care Committee.
Preparation of the pituitary single-cell suspensions
All preparations were from pituitaries of 8- to 12-wk-old male mice. The intact pituitaries of four mice from the same line were pooled, washed with PBS, and incubated in cell dissociation buffer (Invitrogen, Carlsbad, CA) at room temperature as described (6, 10, 11). The cells were next washed with DMEM containing 10% fetal bovine serum and suspended in DMEM containing 10% fetal bovine serum. The yield averaged 6.6 × 105 cells per pituitary. The prepared cells were directly applied to four to six poly-lysine-coated slides for each preparation and fixed on the slide with 4% formaldehyde in PBS at room temperature for 10 min. The slides were then washed with PBS and incubated in a permeabilization solution of 0.5% saponin and 0.5% Triton X-100 for 10 min at room temperature. The slides underwent a final wash in PBS before the immunofluorescent studies. In each single-cell suspension, more than 90% of the cells had intact nuclei as shown by 4′,6-diamidino-2-phenylindole (DAPI) staining. A manual analysis of each line was repeated a minimum of three times, and two lines were assessed for each transgene. The immunostaining results showed 61% of the cells were Pit-1 positive, 31% of total cells were mGH positive, 15% of the cells were mPrl positive, and 7% of the cells were mTSHβ positive in our single-cell suspensions. The percentages of these hormone-producing cells are similar to previously published results from normal anterior pituitary glands (24–26).
Immunofluorescent staining
The cells were blocked by incubation of the slide in 10% donkey serum, 4× saline-sodium citrate (SSC), 2% BSA, and 0.1% Tween 20 at room temperature for 20 min. mPrl and mTSHβ were detected with corresponding antibodies from the National Hormone and Peptide Program (National Institutes of Health, Torrance, CA). mGH was detected with monkey antirat GH that cross-reacts with mGH but not hGH (16) (National Hormone and Peptide Program, NIH). The monoclonal antibody specific for hGH (mAb9) has been described previously (8, 15). Anti-Pit-1 was purchased (SC-16288; Santa Cruz Biotechnology, Santa Cruz, CA). The anti-rGH antibody was diluted 1:3200; anti-hGH, anti-mPrl, and anti-mTSHβ primary antibodies were diluted 1:2000; and anti-Pit-1 antibody was diluted 1:400 with 4× SSC, 2% BSA, and 0.1% Tween 20 for use in immunostains. The slides were incubated with the diluted primary antibodies for 2 h at room temperature, washed with 4× SSC and 0.1% Triton X-100, and incubated with secondary antibodies for 2 h at room temperature. The secondary antibodies used were as follows: mPrl and mTSHβ were detected with Cy2- or Cy3-conjugated donkey antirabbit IgG, hGH was detected with a Cy2-conjugated donkey antimouse IgG, monkey anti-rGH was detected with Cy3 or Cy5-conjugated donkey antihuman IgG (16), and Pit-1 was detected with a Cy3-conjugated donkey antigoat IgG. Each of the secondary antibodies was purchased from Jackson ImmunoResearch (West Grove, PA). Cell nuclei were counterstained with either DAPI or TO-PRO-3 iodide (Invitrogen). After washing with 4× SSC and 0.1% Triton X-100, the cells were mounted with fluorescent mounting medium (KPL, Gaithersburg, MD).
Microscopic and image analysis
The cells were visualized by confocal microscopy (Leica TCS SP) or on an Olympus IX70 inverted microscopy with a ×60 objective lens. Each fluorescent label was imaged independently. In the case of the confocal studies, we used sequential laser excitation at 488, 561, and 633 nm and emission collection between 495 and 550, 575 and 625, and 655 and 730 nm for Cy2, Cy3, and Cy5 fluorescence, respectively. For Olympus IX70 inverted microscopy, the DAPI, Cy2, Cy3, and Cy5 images were captured with an appropriate set of filters. The field was selected at random by directly observing and selecting cells using the Cy2 (green) channel, which permits detection of the hGH signal only. We then captured images for these cells using blue (DAPI), Cy2, Cy3, and/or Cy5 channels to detect nuclei, hGH, mGH, mPrl, mTSHβ, or Pit-1. For confocal microscopy, images were captured and the cells were counted by using Leica LCS software. For Olympus IX70 microscopy, the images were collected with and processed using the Deltavision Softworx software (Applied Precision, Issaquah, WA). The images were analyzed by using the ImageJ software (National Institutes of Health). When the Cy5-conjugated secondary antibodies were used, the Cy5 channel was pseudocolored to green in the images as indicated in the figure legends. All the cells in the images were included in the analysis. The threshold to score a cell as positive for a particular hormone marker or Pit-1 protein was set using the auto threshold function of the ImageJ software. Only the cells with a clearly intact cytoplasmic hormone-staining pattern over the background level were scored as positives for the hormone markers (hGH, mGH, mPrl, or mTSHβ). More than 400 hGH-positive cells were counted for each transgenic mouse line. Two transgenic lines (unique transgene insertions) were studied for each transgene (Table 1). Four to six slides of each pool of four pituitaries were analyzed for each line. For each multi-immunostaining experiment, at least two slides were analyzed. Each experiment was repeated at least three times.
Statistical analysis
The t test was performed to determine the significance of the ectopic expression of hGH-N in the HSI-deletion and TerF-insertion transgenic mice as compared with the wild-type hGH/P1 transgenic mice.
Acknowledgments
We are grateful to Dr. A. F. Parlow of National Hormone and Pituitary Program of National Institutes of Health for providing the noted antibodies for pituitary hormones. We thank Dr. Andrea Stout of the Microscopy Core in the Department of Cell and Developmental Biology, University of Pennsylvania, for advice concerning the immunostaining.
This work was supported by National Institutes of Health Grants R01 HD25147 and R01 HD046737 (to N.E.C. and S.A.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DAPI
- 4′,6-Diamidino-2-phenylindole
- h
- human
- HS
- hypersensitivity
- LCR
- locus control region
- m
- mouse
- Prl
- prolactin
- SSC
- saline-sodium citrate
- TerF
- termination element.
References
- 1. Zhu X, Gleiberman AS, Rosenfeld MG. 2007. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87:933–963 [DOI] [PubMed] [Google Scholar]
- 2. Li S, Crenshaw EB, 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. 1990. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347:528–533 [DOI] [PubMed] [Google Scholar]
- 3. Camper SA, Saunders TL, Katz RW, Reeves RH. 1990. The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics 8:586–590 [DOI] [PubMed] [Google Scholar]
- 4. Dasen JS, Rosenfeld MG. 1999. Signaling mechanisms in pituitary morphogenesis and cell fate determination. Curr Opin Cell Biol 11:669–677 [DOI] [PubMed] [Google Scholar]
- 5. Chen EY, Liao YC, Smith DH, Barrera-Saldaña HA, Gelinas RE, Seeburg PH. 1989. The human growth hormone locus: nucleotide sequence, biology, and evolution. Genomics 4:479–497 [DOI] [PubMed] [Google Scholar]
- 6. Jones BK, Monks BR, Liebhaber SA, Cooke NE. 1995. The human growth hormone gene is regulated by a multicomponent locus control region. Mol Cell Biol 15:7010–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Su Y, Liebhaber SA, Cooke NE. 2000. The human growth hormone gene cluster locus control region supports position-independent pituitary- and placenta-specific expression in the transgenic mouse. J Biol Chem 275:7902–7909 [DOI] [PubMed] [Google Scholar]
- 8. Bennani-Baïti IM, Asa SL, Song D, Iratni R, Liebhaber SA, Cooke NE. 1998. DNase I-hypersensitive sites I and II of the human growth hormone locus control region are a major developmental activator of somatotrope gene expression. Proc Natl Acad Sci USA 95:10655–10660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shewchuk BM, Asa SL, Cooke NE, Liebhaber SA. 1999. Pit-1 binding sites at the somatotrope-specific DNase I hypersensitive sites I, II of the human growth hormone locus control region are essential for in vivo hGH-N gene activation. J Biol Chem 274:35725–35733 [DOI] [PubMed] [Google Scholar]
- 10. Ho Y, Elefant F, Cooke N, Liebhaber S. 2002. A defined locus control region determinant links chromatin domain acetylation with long-range gene activation. Mol Cell 9:291–302 [DOI] [PubMed] [Google Scholar]
- 11. Ho Y, Elefant F, Liebhaber SA, Cooke NE. 2006. Locus control region transcription plays an active role in long-range gene activation. Mol Cell 23:365–375 [DOI] [PubMed] [Google Scholar]
- 12. Ho Y, Tadevosyan A, Liebhaber SA, Cooke NE. 2008. The juxtaposition of a promoter with a locus control region transcriptional domain activates gene expression. EMBO Rep 9:891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shewchuk BM, Liebhaber SA, Cooke NE. 2002. Specification of unique Pit-1 activity in the hGH locus control region. Proc Natl Acad Sci USA 99:11784–11789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magoulas C, McGuinness L, Balthasar N, Carmignac DF, Sesay AK, Mathers KE, Christian H, Candeil L, Bonnefont X, Mollard P, Robinson IC. 2000. A secreted fluorescent reporter targeted to pituitary growth hormone cells in transgenic mice. Endocrinology 141:4681–4689 [DOI] [PubMed] [Google Scholar]
- 15. Jin Y, Lu SY, Fresnoza A, Detillieux KA, Duckworth ML, Cattini PA. 2009. Differential placental hormone gene expression during pregnancy in a transgenic mouse containing the human growth hormone/chorionic somatomammotropin locus. Placenta 30:226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shewchuk BM, Ho Y, Liebhaber SA, Cooke NE. 2006. A single base difference between Pit-1 binding sites at the hGH promoter and locus control region specifies distinct Pit-1 conformations and functions. Mol Cell Biol 26:6535–6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asa SL, Ezzat S. 1999. Molecular determinants of pituitary cytodifferentiation. Pituitary 1:159–168 [DOI] [PubMed] [Google Scholar]
- 18. Scully KM, Jacobson EM, Jepsen K, Lunyak V, Viadiu H, Carrière C, Rose DW, Hooshmand F, Aggarwal AK, Rosenfeld MG. 2000. Allosteric effects of Pit-1 DNA sites on long-term repression in cell type specification. Science 290:1127–1131 [DOI] [PubMed] [Google Scholar]
- 19. Wang J, Scully K, Zhu X, Cai L, Zhang J, Prefontaine GG, Krones A, Ohgi KA, Zhu P, Garcia-Bassets I, Liu F, Taylor H, Lozach J, Jayes FL, Korach KS, Glass CK, Fu XD, Rosenfeld MG. 2007. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature 446:882–887 [DOI] [PubMed] [Google Scholar]
- 20. Jing H, Vakoc CR, Ying L, Mandat S, Wang H, Zheng X, Blobel GA. 2008. Exchange of GATA factors mediates transitions in looped chromatin organization at a developmentally regulated gene locus. Mol Cell 29:232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horike S, Cai S, Miyano M, Cheng JF, Kohwi-Shigematsu T. 2005. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat Genet 37:31–40 [DOI] [PubMed] [Google Scholar]
- 22. Xu J, Sankaran VG, Ni M, Menne TF, Puram RV, Kim W, Orkin SH. 2010. Transcriptional silencing of γ-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev 24:783–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sizova D, Ho Y, Cooke NE, Liebhaber SA. 2010. Research resource: T-antigen transformation of pituitary cells captures three novel cell lines in the Pit-1 lineage. Mol Endocrinol 24:2232–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kendall SK, Saunders TL, Jin L, Lloyd RV, Glode LM, Nett TM, Keri RA, Nilson JH, Camper SA. 1991. Targeted ablation of pituitary gonadotropes in transgenic mice. Mol Endocrinol 5:2025–2036 [DOI] [PubMed] [Google Scholar]
- 25. Gage PJ, Roller ML, Saunders TL, Scarlett LM, Camper SA. 1996. Anterior pituitary cells defective in the cell-autonomous factor, df, undergo cell lineage specification but not expansion. Development 122:151–160 [DOI] [PubMed] [Google Scholar]
- 26. Villalobos C, Núñez L, García-Sancho J. 2004. Anterior pituitary thyrotropes are multifunctional cells. Am J Physiol Endocrinol Metab 287:E1166–1170 [DOI] [PubMed] [Google Scholar]