Abstract
We report a method for formulation of pectin microbeads using microfluidics. The technique uses biocompatible ingredients and allows for controlled external gelation with hydrogen and calcium ions delivered from an organic phase of rapeseed oil. This method allows for encapsulation of nanoparticles into the microparticles of gel and for control of the rate of their release.
INTRODUCTION
In this report we demonstrate the use of a microfluidic system for formation of biocompatible particles of pectin gel, encapsulation of nanoparticles, and the temporal profiles of their release. We discuss the use of various cross-linking agents, including a range of calcium salts and protons. Our results suggest that it is possible to control the profile of release via a judicious choice of concentrations of pectin and cross-linking ions.
The advent and development1 of microfluidic techniques provide the advantages of portability, reduction of the volume of samples, and faster analysis.2 Techniques are being continually developed for automation and high throughput. One of the important developments in microfluidics is the precise control of multiphase flows. Since the first demonstration of formation of monodisperse droplets in microchannels,3 a range of techniques have been proposed for generation of simple3, 4, 5 and multiple6 droplets with an extensive control over the distribution of volumes. The ability to form monodisperse segments of liquids can be successfully utilized to formulate monodisperse polymeric particles7 of various—and easy to control—morphologies8, 9 and capsules.10 Extensions of these techniques are used to encapsulate biomaterials,11 prepare microbubble contrast agents for ultrasound radiography,12 carriers of active substances for targeted drug delivery,13 and for control of the temporal profile of their release.14 Microfluidics offers extensive and unique control over the distribution of volumes of particles and over the process of their gelation. This prompts further development of microfluidic techniques for formulation of biocompatible particles of biopolymers and hydrogels for the applications in pharmacology and medicine.15, 16, 17
The interest in biopolymers is motivated by their low toxicity, good biocompatibility, biodegradability, and ability to easily form gels. For example, microbeads of biopolymers can be used in immunology to encapsulate transplanted cells in order to increase immunosuppression. The separation of cells from the host tissue enhances the efficacy and viability of transplanted cells.18 Likewise, they are used to encapsulate microorganisms (e.g., yeast19 for the fermentation processes) or active substances like drugs,14, 20 enzymes,11 or even blood proteins.21 As opposed to traditional, physicochemical methods of encapsulation, also here microfluidics comes in with a unique advantage of the possibility to encapsulate virtually any material in any matrix via the hydrodynamic control of the process of formation of particles.
The most common method of formulation of microparticles of hydrogels comprises (i) emulsification of solution of biopolymer and (ii) subsequent gelation of the droplets. Emulsification determines the distribution of diameters of the beads while gelation determines their physical and mechanical properties. Microfluidic systems, in contrast to traditional methods of emulsification, can form tightly monodisperse emulsions. Several methods of gelation have been implanted both on-chip and off-chip to gel the droplets. These methods include chemical (commonly radical polymerization22) or physical (thermal setting8, 23 or ionic cross-linking24, 25) processes.
The popular and important hydrogels (alginates and pectins) can be most conveniently cross-linked with ions. Microfluidic techniques offer a range of techniques for introduction of ions at a controlled rate. These include the techniques of external, internal, or coalescence-induced gelation. The external gelation is the most popular form and is conducted by diffusion of cross-linking agent (ions) either from an external bath26 or on-the-chip from the continuous phase.10, 15, 19 In internal gelation,27 the droplets contain not only the biopolymer but also a cross-linking agent in an inactive form (e.g., grains of insoluble calcium carbonate28). Gelation is then triggered by an additional substance (e.g., acetic acid) diffusing into the droplet from the continuous phase.10, 24, 25, 28, 29 Finally, in coalescence-induced gelation two aqueous droplets, one with the biopolymer and one with the ions, are coalesced to induce cross-linking.30, 31, 32 Combined methods are also known.25, 26, 33
These methods have their drawbacks. Internal gelation can lead to nonuniform distribution of ions in the solution of biopolymer and may leave grains of nondissolved cross-linking agent in the beads. Because cross-linking of polymers with ions proceeds rapidly (especially in the case of, e.g., alginates) in the coalescence-induced gelation, it is typically difficult to mix the two solutions thoroughly before gelation deforms the shape of the droplet.
External gelation is most attractive; however, it is often difficult to dissolve appropriate amounts of salt in organic solvents used as the continuous phase in the process of formation of droplets. Here we demonstrate the use of acetic acid as a medium that facilitates incorporation of calcium carbonate into an organic phase of an inexpensive edible rapeseed oil.
Although many hydrogels, including poly(N-isopropylacrylamide),16 carrageenans, celluloses,15 and alginate, have been formulated in microfluidic systems, formation of pectin microbeads has not been extensively studied. The only report34 of gelation of monodisperse droplets of solution of pectin used a microterrace geometry and gelation performed off-chip after an interphase transfer of the droplets into an aqueous solution of bivalent ions. As we discuss below, in this setup diffusion of ions into the beads proceeds while new beads are continuously produced and added to the batch. It is thus impossible to control the amount of the cross-linking agent that is incorporated into the beads. Here we demonstrate a system that allows for precise control of both the rate of delivery of ions to the droplets and the total time during which the transfer proceeds. This, in contrast to traditional methods, allows both for (i) on-line tuning of the time (by adjusting the rates of flow of each of the phases or the length of the fluidic duct in which the transfer occurs) and (ii) perfect reproducibility due to the fact that each of the droplets travels through the system at the same speed.
Pectins are interesting because they are commonly used in many industries. For example, pectins serve as a thickening and gelling agent in food. Pharmaceutical industry uses pectins in drug delivery systems35 for their perfect biocompatibility, specific pH response that allows for directed (e.g., colon-specific36) delivery. Pectins can be administered to humans without any limits of the daily intake.37 In addition, pectins are believed to decrease the blood cholesterol level,38 slow down the absorption of glucose,39 facilitate excretion of toxic, divalent metals with urine,40 and to have antitumor activity.41
Pectins consist predominantly of methoxylated esters of polygalacturonic acid (G) which are conjugated by α-1-4-glycosidic bonds. Degree of esterification (DE) distinguishes between high-methoxylated pectin (HMP) (above 50% of carboxyl groups are methylated) and low-methoxylated pectin (LMP). DE determines both the chemical and the physical properties of pectin gel. HMP pectins demand the presence of a sugar and a hydrogen ion to gel. In contrast, gelation of LMPs requires cross-linking with divalent ions (via the “egg-box” model42, 43 that is valid also for alginates). For a group of LMPs—amidated low-methoxylated pectins (LMA)—the presence of amino groups43 provides additional hydrogen bonds and changes the macroscopic structure of the network. In this work we used LMA and have cross-linked them with bivalent cations of calcium at low pH.
In the following sections we domonstrate the formation of droplets of aqueous solution of pectin with an extensive control over the volume of these droplets (Sec. 3A), addition of bivalent ions from an organic phase of a rapaseed oil (Sec. 3B), interphase transfer into distilled water, and gelling of the droplets into particles (Sec. 3C). We also demonstrate experimentally the encapsulation of nanoparticles of gold into these pectin particles (Sec. 3D) and the influence of varous parameters on the profile of release of the nanoparticles upon competitive remocval of bivalent ions (Sec. 3E).
MATERIALS AND METHODS
We fabricated microfluidic devices (μFLDs) in polycarbonate by micromilling (MSG 4025 ErgWind, Poland). We bonded the milled plates with the flat ones as described by Ogończyk et al.44 We then modified the surface chemistry of the channels to render it hydrophobic, as described by Jankowski et al.45 A scheme and a photograph of the microdevice are shown in Fig S1 in the supplemental material.46
Digitally controlled syringe pumps (Harvard Apparatus PHD 2000, USA) supplied all liquids to microchannels. To acquire images we used a Nikon SMZ1500 (USA) stereomicroscope and a high-speed Photron FastCam 1024PCI (Japan) camera connected to a PC.
In all experiments we used polyethylene tubing (PE60, Becton, USA) to transfer the liquids between the syringes and the flow-focusing system, and the suspension of droplets between the flow-focusing chip and the collection beaker (either directly or via a second chip used for exchange of the oil, as described in the text).
To the side channels of the flow-focusing μFLDs we administered the rapeseed oil (Kujawski oil, ZT Kruszwica, Poland) with addition of CaCO3 and an acetic acid (both from Chempur, Poland). The discontinuous phase was an aqueous solution of low-methoxylated citrus-apple pectin (NECJ A2 “medium sensitivity to calcium ions,” Pektowin, Poland). A beaker with a distilled water (Millipore, 18 MΩ) served to collect the beads.
To formulate pectin microbeads we used aqueous solutions of pectin of concentration ranging between 0.5% and 1% (w∕w). Gold nanoparticles and silver nanoparticles (AgNPs) nanoparticles were prepared as described in Refs. 47, 48. All NPs had the mean diameter of 5.5 nm and were suspended in distilled water at a concentration of 1.5 mg∕mL. For encapsulation of NPs in pectin, we typically mixed the suspension of NPs with a solution of pectin at a proportion of 2 to 1 by volume. As the source of bivalent cations we used calcium carbonate (CaCO3) dissolved in acetic acid (CH3COOH) and then mixed into the rapeseed oil. We used a range of concentrations of acetic acid and calcium carbonate in oil: between 1% and 10% and between 0.05% and 2% (w∕w with respect to oil), respectively. Typically, the rate of flow of solution of pectin (Qd) was set to 1 mL∕h and of the continuous phase (Qc) to 9 mL∕h. A single batch of particles was typically prepared by producing microbeads for 15 min, collecting them into 1.5 mL of distilled water and allowing them to complete cross-linking in water for 12 h.
In the experiments that tested the profiles of release of NPs from pectin beads, we used a system that contained an additional module for exchange of the oil containing cross-linking agents into a clean rapeseed oil. In this system the outlet of the 100 cm long polyethylene tubing was introduced into a second microfluidic chip, which had an additional inlet for clean oil, and two outlets—one for the oil containing ions and the other for the clean oil. The flow rate of the clean oil (Qc2) was typically set to 30 mL∕h. The outlet of the second chip was connected to the collection beaker via a shorter (∼20 cm) section of polyethylene tubing. The batches were prepared and collected in the same manner as in the case of the first system.
In the experiments that tracked the temporal profile of degradation, we used beads of a volume of 30 pL suspended in solutions of ethylenediaminetetraacetic acid (EDTA) disodium salt (Sigma Aldrich) of concentrations ranging between 10 μM and 10 mM. Typically, we used 10 mL of 1.4 mM solution of EDTA and added it to the suspension of pectin microbeads (1.5 mL). During the experiment, the resulting suspension was gently mixed to prevent sedimentation of gel and to ensure the homogeneity of the sample. The signal was collected with the Shimadzu UV-2401 PC spectrophotometer.
RESULTS
Formation of droplets of an aqueous solution of pectin
We used a standard flow-focusing microfluidic chip to break a continuous stream of solution of pectin into droplets (Fig. 1). All the channels on-the-chip had a uniform square cross-section (400×400 μm). From the side inlets we delivered the continuous, organic phase of a rapeseed oil.
Figure 1.
Micrographs of the flow-focusing junction generating droplets of an aqueous solution of pectin (0.5% w∕w) in rapeseed oil. The rates of flow [Qc,Qd] for the consecutive micrographs are given (in mL∕h) in the figure. All the channels in the device had a uniform, square cross-section of 400×400 μm. The graph illustrates the dependence of the length (diameter for droplets smaller than the cross-section of the channel) of droplets normalized by the width of the channel as a function of the ratio of the rates of flow of the continuous and the droplet phase (Qc∕Qd).
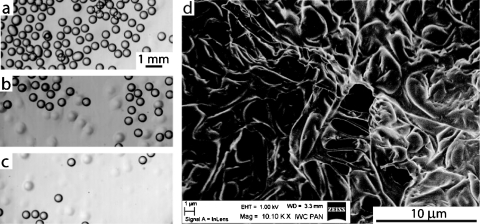
We found that in spite of the non-Newtonian character49 of solutions of pectins, we were able to reproducibly form monodisperse droplets up to a concentration of 1% (w∕w) of pectin in water. Higher concentrations proved impractical, as it was difficult to form monodisperse droplets. Changing the rates of flow of the droplet phase (Qd) and of the continuous phase (Qc) allowed us (Fig. 1) to tune the mean length of the aqueous slugs between approximately half and four times the width of the channel (between ∼200 and 1600 μm) which corresponds to volumes of the droplets ranging between 15 and 150 pL. As shown in the inset of Fig. 1, the volume of the droplets followed the scaling of L∕W∼(Qd∕Qc)−1∕2, which is different from that observed for Newtonian liquids.50
Incorporation of ions into the organic phase
As our goal was to produce biocompatible pectin microbeads for the continuous phase, we chose an edible—and readily available—rapeseed oil. Gelation of pectin requires the presence of both hydrogen ions and bivalent cations of metals. The choice of acid (acetic acid, E260) was dictated by its wide use in industry and solubility both with polar and nonpolar liquids. For the introduction of cations we chose calcium salts for their low toxicity. As salts of calcium are not soluble in rapeseed oil we first prepared solutions of calcium carbonate in an acid. We considered the use of six different salts of calcium (Table 1) and selected calcium carbonate because solutions of only this single salt proved to be miscible with the oil.
Table 1.
Solubility of calcium salts in glacial acetic acid and miscibility of those solutions (acid-salt) with the rapeseed oil. (++) good, (+) poor, (−) insoluble∕immiscible.
| Calcium salt | Solubility in acid | Miscibility with oil | Notes |
|---|---|---|---|
| Chloride | ++ | + | Turbid |
| Lactate | ++ | ⋯ | Stratification and sedimentation |
| Acetate | ++ | ⋯ | Stratification and sedimentation |
| Carbonate | + | ++ | Miscible |
| Oxalate | + | ⋯ | Stratification and sedimentation |
| Citrate | ⋯ | ⋯ | Immiscible |
The final procedure consisted of first dissolving the salt in the acid and then mixing the resulting solution with oil.
Formulation of particles of pectin gel
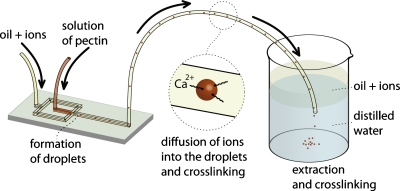
Being able to produce and control the diameter of the droplets and to incorporate the acid and calcium ions into the continuous organic phase, we constructed a system (Fig. 2) for cross-linking of the droplets into particles of gel. To this end, we introduced a long (100 cm) section of polyethylene tubing into the outlet of the flow-focusing chip and inserted the other end of the tubing into a collection beaker filled with distilled water.
Figure 2.
Schematic representation of the experimental system that we used for (i) formation of droplets comprising aqueous solutions of pectin, (ii) incorporation of bivalent and hydrogen ions into the droplets, and (iii) collection, interphase transfer from oil into water, and gelation of droplets.
We observed that the change of a neat oil into the mixture of acetic acid and calcium carbonate in the oil did not significantly affect the process of formation of droplets. Once the droplets were formed in the microfluidic junction, they flew via a 100 cm long section of polyethylene tubing (Fig. 2) where they absorbed the ions from the continuous phase via diffusion and an interphase transfer, and started cross-linking. We inserted the end of the tubing into the aqueous phase in the collection beaker. Because the oil phase is less dense than water, the oil flew to the top while the aqueous droplets of pectin transferred into water and sedimented to the bottom of the flask by gravity.
As the process of cross-linking starts already in the microfluidic chip and in the polyethylene tubing, we observed that coalescence of the gelling droplets was only marginal. Once the beads were gelled we were able to collect them from the beaker. Because of vanishingly small contrast of indices of refraction of water and an aqueous pectin gel, we were not able to visualize the particles of gel in water. Micrographs shown in Figs. 3a, 3b, 3c illustrate the process of the transfer of pectin droplets from the oil phase into glycerin. Figure 3d shows a scanning electron microscopy (SEM) image of a dissected microbead which shows clearly that it is gelled.
Figure 3.
A series of micrographs illustrating the transfer of pectin droplets from the organic phase into glycerin. A suspension of microbeads was deposited on a layer of glycerin. Sharply contrasting circles [(a)–(c)] correspond to the beads in organic phase while the faint spots seen in (b) and (c) are particles transferred to glycerin. The experiment was conducted as depicted in Fig. 2 with a 0.5% (w∕w) solution of pectin (Qd=1 mL∕h) and oil with 0.4% CaCO3 and 4% CH3COOH (w∕w) (Qc=6 mL∕h). (d) Scanning electron micrograph of a surface of a pectin microbead. The structure of the obtained gel can be easily observed.
Encapsulation and release of nanoparticles
Microbeads of pectin gel have the potential to serve as drug delivery vesicles. The temporal profile of release of substances encapsulated in the beads can be modulated via the control of the time of degradation of gel. As a model active substance, we used NPs which form stable solutions in water and in aqueous solutions of pectin. We tested the possibility to encapsulate three different types of NPs (Table 2). One type of particles of silver (AgNPs) [diameter of 5.5 nm, coated with SHC11H22N+(CH3)3] that possesses cationic groups at their surface and two types of negatively charged NPs of gold: one draped with (Au–SO3–NPs) and the other with SHC10H20COO− (Au–COO–NPs). We mixed a 0.5% solution of pectin at a ratio of 4:1 with a suspension of NPs (1.5 mg∕mL). After thorough mixing, we pipetted the resulting suspension into an aqueous solution of calcium ions (0.5% w∕w).
Table 2.
Selection of nanoparticles to be encapsulated in pectin gels. During the experiment, we used 1.5 mg∕mL solutions of NPs, 0.5% solution of pectin, and 0.5% solution of CaCl2. Photographs of the beads are presented in Fig. 4.
| Nanoparticles | Charge | Notes |
|---|---|---|
| Ag∕SHC11H22N+(CH3)3 | + | Gelation is extremely slow. |
| Particles coalesce into a cloudy aggregate. | ||
| ⋯ | Gelation is slow. | |
| Particles have liquid cores. | ||
| Au∕SHC10H20COO− | ⋯ | Fast gelation |
| Completely gelled beads |
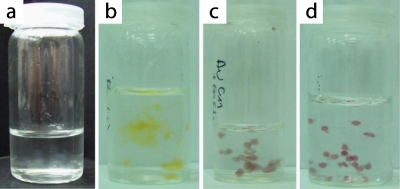
We found that the addition of AgNPs extremely slowed down the process of cross-linking and the resulting gels formed ill-defined, loose “clouds” [Fig. 4b]. Droplets containing Au–SO3–NPs did not assemble into larger aggregates, yet they gelled very slowly leaving a liquid core. In contrast, addition of Au–COO–NPs to the solution of pectin resulted in rapid gelling. The resulting beads were firm and did not coalesce. We thus chose Au–COO–NPs for the further tests of the rate of degradation of pectin microbeads.
Figure 4.
Photographs of beads of pectin gel produced (a) without addition of any NPs or with addition of (b) AgNPs, (c) Au–SO3–NPs, and (d) Au–COO–NPs. The solution of pectin with NPs (mixed 4:1) was pipetted into 0.5% water solution of CaCl2 in order to obtain gel beads.
Profiles of release of nanoparticles
In order to test the profiles of release of the nanoparticles, we immersed cross-linked pectin microbeads in a solution of EDTA that competitively binds cations of calcium and effectively removes them from the gel. This leads to degradation of pectin gel and to release of encapsulated nanoparticles into solution. As we describe it in detail below, the rate of degradation of gel depends on the concentration of pectin, the amount of calcium carbonate added to oil, and the concentration of acetic acid in oil. In addition, this rate depends also on the concentration of EDTA in the test bath and on the time of cross-linking of the particles. The process of gelation of pectin proceeds already in the coflow with the oil and later on in the collection beaker. In the experimental setup depicted schematically in Fig. 2, the aqueous bath into which we collected beads could uptake ions from the organic phase during the time of collection and cross-linking of the pectin beads. We noticed that beads produced in a single run lasting tens of minutes exhibited a wide range of properties and produced nonreproducible profiles of release of NPs. This result suggested that the time of cross-linking and the concentration of ions in the aqueous bath had a large influence on the stability of pectin microbeads.
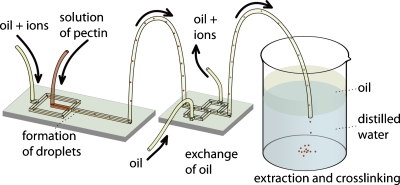
In order to minimize the dispersion of the concentration of calcium ions and of the time of uptake of calcium ions by the droplets∕beads of pectin, we modified the experimental setup, as illustrated in Fig. 5. In this setup, we introduced the outlet of the 100 cm long polyethylene tubing into a second microfluidic chip, which had an additional inlet for clean oil, and two outlets—one for the oil containing ions and the other for the clean oil. This design is based on an earlier report51 on the transfer of beads of alginate with encapsulated cells from oleic acid to mineral oil.
Figure 5.
Schematic representation of the experimental system that we used in (i) formation of droplets comprising aqueous solutions of pectin, (ii) incorporation of bivalent ions into the droplets, and (iii) collection, interphase transfer from oil into water, and gelation of droplets.
We found that this system is efficient in transferring the microbeads of pectin from the original organic phase used for flow focusing and delivery of ions to the neat oil. The introduction of this chip has significantly improved the reproducibility of the temporal profiles of release that we report below.
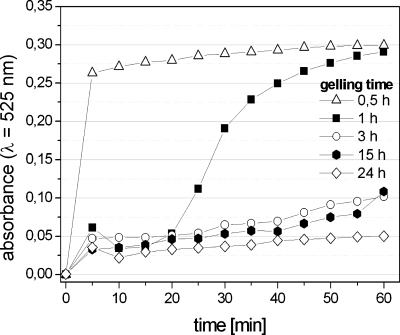
The observation that the time of cross-linking has a significant impact on the profile of release is quantitatively confirmed in an experiment in which we monitored the rate of degradation of the pectin microbeads in a fixed concentration of EDTA (1 mM). We prepared droplets of 0.33% (w∕w) solution of pectin in distilled water with Au–COO–NPs in a continuous phase containing 0.4% (w∕w) of calcium carbonate and 4% of acetic acid. We transferred the droplets to the clean rapeseed oil and then to the distilled water (as shown in Fig. 5). We prepared five batches of beads that were allowed to gel in water for 1∕2, 1, 3, 15, and 24 h. We then filtered out the beads and put them into the solution of EDTA. Figure 6 shows that for short times (tgel<3 h) of cross-linking, the exact interval between the formation of the droplets and introducing of the beads into the solution of EDTA had a pronounced effect on the rate of degradation of the beads. These data suggest that the process of cross-linking involves two steps. The first one involves the absorption of the ions into the droplet and cross-linking of a thin shell at the interface of the droplet. In the second stage, the ions diffuse within the droplet and cross-link its core. We found that our beads cross-linked completely within ∼10 h, and thus for all further experiments we allowed the beads to gel for tgel=12 h.
Figure 6.
Absorbance at λ=525 nm as a function of time measured in suspensions of pectin beads in 1 mM solution of EDTA. The beads were formed from a 0.5% (w∕w) solution of pectin in water mixed at a ratio of 2:1 with a suspension of Au–COO–NPs in water (1.5 mg∕mL), dispersed in rapeseed oil containing 0.4% (w∕w) of CaCO3 and 4% (w∕w) CH3COOH. The five curves correspond to beads that were allowed to cross-link for , 1, 3, 15, and 24 h before they were introduced into the solution of EDTA.
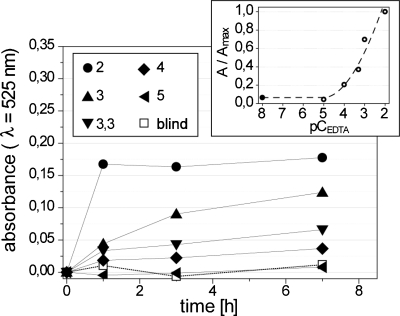
The rate of release of nanoparticles depends also on the concentration of EDTA (CEDTA). We degraded the beads prepared in the same way as in the first set of experiments and cross-linked for 12 h in five solutions of EDTA (10 μM, 100 μM, 500 μM, 1 mM, and 10 mM). These results (Fig. 7 and Fig. S2 in the supplementary information) indicated that indeed CEDTA can be used to control the rate of release of NPs. For all further work, we chose to work with CEDTA=1.4 mM (pCEDTA=2.85) because this value allowed us to observe marked differences in the rate of degradation of pectin beads within 45 min.
Figure 7.
Absorbance at λ=525 nm as a function of time measured in suspensions of pectin beads in five different solutions of EDTA (10 μM, 100 μM, 500 μM, 1 mM, and 10 mM). We express the concentrations of EDTA in the units of pCEDTA=−log10 CEDTA (these values are listed in the legend in the figure). The inset shows the absorbance normalized by the highest value of absorbance (recorded for pCEDTA=2) after 7 h of degradation. The beads were formed from a 0.5% (w∕w) solution of pectin in water mixed with Au–COO–NPs, dispersed in rapeseed oil containing 0.4% (w∕w) of CaCO3 and 4% (w∕w) CH3COOH and cross-linked for 12 h.
In order to check the dependence of the rate of degradation of beads of different composition, we prepared 18 batches of beads in the same conditions: the Qd=1 mL∕h and the Qc=9 mL∕h. In each batch, we formed the beads for 15 min, continuously transferring them into clean oil (Qc2=30 mL∕h) and collecting to 1.5 mL of distilled water. After the completion of formation of beads, we set aside the aqueous dispersion of beads to allow them to cross-link for 12 h. After this time, we removed the layer of oil by suction and added 10 mL of a 1.4 mM solution of EDTA and immediately started recording the absorbance.
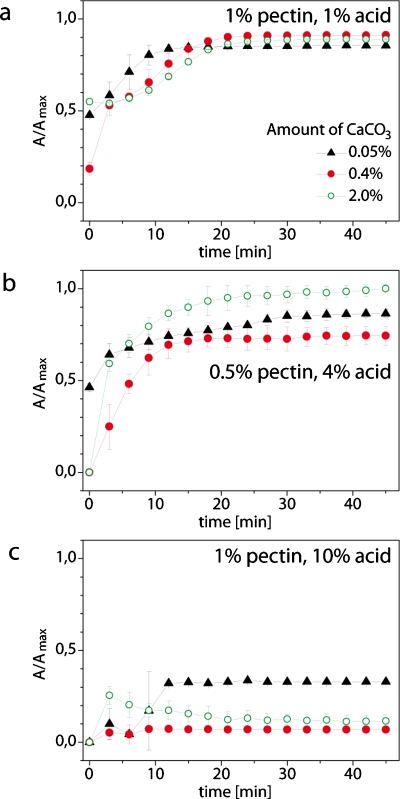
We varied three parameters: (i) the initial concentration of pectin (0.5% and 1% w∕w), (ii) concentration of acetic acid in oil (1%, 4%, and 10% w∕w), and (iii) the amount of calcium carbonate (0.05%, 0.4%, and 2% w∕w in proportion to oil). The most important qualitative observation was that the concentration of acetic acid played the most important role in determining the stability of pectin microbeads. At low concentrations of the acid (1%, all the beads prepared from the 0.5% solution of pectin disintegrated already in the aqueous bath even before addition of EDTA. Beads prepared from the 1% solution of pectin released all NPs within ∼15 min after addition of EDTA without any marked dependence of the rate of release on the amount of calcium carbonate used for cross-linking [Fig. 8a]. In addition, the beads formed with the lowest concentration of acid had a tendency to merge and form large and loose aggregates.
Figure 8.
Profiles of release of nanoparticles encapsulated in pectin microbeads. (a) Beads formed from a 1% (w/w) solution of pectin mixed at a ratio of 2:1 by volume with a suspension of Au–COO–NPs (1.5 mg/mL) dispersed in the oil containing 1% (w/w) acetic acid and different amounts of calcium. (b) Analogous to (a) with 0.5% of pectin and 4% of acetic acid. (c) Same as (a) with 1% of pectin and 10% of acid. See also Figs. S2–S7 in the supplemental material (Ref. 46) which detail the profiles of release under different conditions.
In contrast, at the highest concentration of acid that we tested (10%), the beads never coalesced and were stable even after introduction of the chelate of calcium. Only the beads prepared with the lowest concentration of calcium carbonate have released up to 25% of the encapsulated NPs within the 45 min of the experiment.
We obtained the most interesting results for pectin beads prepared at the intermediate concentration of acid (4%). Beads formed from droplets using 0.5% solution of pectin have all degraded at a similar rate, releasing all of the imbedded NPs within ∼20 min of addition of the solution of EDTA [Fig. 8b]. Interestingly, the beads formed from the 1% solution of pectin and 10% of acid degraded much slower and the rate of release of NPs was faster for beads prepared at higher concentration of calcium ions [Fig. 8c].
DISCUSSION
We presented a microfluidic technique for formation of monodisperse droplets of aqueous solutions of pectin and for cross-linking these droplets into beads of gel. This technique—in contrast to traditional methods of emulsification—produces tightly monodisperse droplets and allows for facile tuning of their diameter.
In order to circumvent the problem associated with poor solubility of salts of metals in oils, we first dissolved calcium carbonate in acetic acid and then mixed this solution with oil. We tested six different salts of calcium and calcium carbonate proved to be best suited for the procedure. Incorporation of both the acid and the calcium carbonate into the organic phase allows for diffusion of both hydrogen and calcium cations into the aqueous droplets. Thus, the microfluidic procedure that we report is not only biocompatible as it uses readily available and nontoxic ingredients, but also allows for tuning the ratio of hydrogen and calcium cations delivered to the pectin droplets.
We were able to successfully transfer the droplets into a neat oil and then into water. This procedure allows for reproducible control of the characteristics of the gel beads. Our results show that the process of cross-linking comprises two stages. The first is fast and cross-links only the external shell of the droplet. The second stage is long—up to several hours for ∼30 pL droplets—as it involves homogenization of the concentration of ions inside the gelling droplet.
We used the microfluidic system that we report here to encapsulate nanoparticles of silver and gold with three different surface chemistries in pectin. We found that only suspensions of Au–COO–NPs gelled rapidly. The tests of the rate of degradation of beads in a solution of EDTA show that this rate depends strongly on the details of formulation of the beads. We found that appropriate concentration of hydrogen ions is critical for obtaining stable beads that do not coalesce and do not disintegrate in water. Beads formed at large concentrations of acid in the oil formed stable gels that are most promising for formulations characterized by prolonged release of encapsulated substances. Interestingly, the ability of tuning of the concentration of protons and cations of calcium—that the microfluidic system offers—can be used to modulate the rate of degradation of pectin microbeads. It is important to note that the exact character of release is likely to depend strongly also on the type—most importantly the degree of methoxylation—of pectin used for preparation of beads.
In summary, the microfluidic system presented in this report is simple and operates on inexpensive compounds to formulate monodisperse microbeads of pectin gel. The use of microfluidics to control the delivery of ions allows for precise tailoring of the process of encapsulation of nanoparticles and for tuning the rate of their subsequent release from pectin beads.
ACKNOWLEDGMENTS
The authors thank Maciej Paszewski for his cooperation. Project operated within the Foundation for Polish Science Team Programme cofinanced by the EU European Regional Development Fund.
References
- Whitesides G. M., Nature (London) 442, 368 (2006). 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- Arora A., Simone G., Salieb-Beugelaar G., Kim J., and Manz A., Anal. Chem. 82, 4830 (2010). 10.1021/ac100969k [DOI] [PubMed] [Google Scholar]
- Thorsen T., Roberts R. W., Arnold F. H., and Quake S. R., Phys. Rev. Lett. 86, 4163 (2001). 10.1103/PhysRevLett.86.4163 [DOI] [PubMed] [Google Scholar]
- Gañán-Calvo A. M., Phys. Rev. Lett. 80, 285 (1998). 10.1103/PhysRevLett.80.285 [DOI] [PubMed] [Google Scholar]
- Anna S. L., Bontoux N., and Stone H. A., Appl. Phys. Lett. 82, 364 (2003). 10.1063/1.1537519 [DOI] [Google Scholar]
- Shah R. K., Shum H. C., Rowat A. C., Lee D., Agresti J. J., Utada A. S., Chu L.-Y., Kim J.-W., Fernandez-Nieves A., Martinez C. J., and Weitz D. A., Mater. Today 11, 18 (2008). 10.1016/S1369-7021(08)70053-1 [DOI] [Google Scholar]
- Serra C. A. and Chang Z., Chem. Eng. Technol. 31, 1099 (2008). 10.1002/ceat.200800219 [DOI] [Google Scholar]
- Xu S., Nie Z., Seo M., Lewis P., Kumacheva E., Stone H. A., Garstecki P., Weibel D. B., Gitlin I., and Whitesides G. M., Angew. Chem., Int. Ed. 44, 724 (2005). 10.1002/anie.200462226 [DOI] [PubMed] [Google Scholar]
- Yuet K. P., Hwang D. K., Haghgooie R., and Doyle P. S., Langmuir 26, 4281 (2010). 10.1021/la903348s [DOI] [PubMed] [Google Scholar]
- Zhang H., Tumarkin E., Sullan R. M. A., Walker G. C., and Kumacheva E., Macromol. Rapid Commun. 28, 527 (2007). 10.1002/marc.200600776 [DOI] [Google Scholar]
- Um E., Lee D.-S., Pyo H.-B., and Park J.-K., Microfluid. Nanofluid. 5, 541 (2008). 10.1007/s10404-008-0268-6 [DOI] [Google Scholar]
- Stride E. and Edirisinghe M., Soft Matter 4, 2350 (2008). 10.1039/b809517p [DOI] [Google Scholar]
- Sirsi S. R. and Borden M. A., Bubble Science, Engineering & Technology 1, 3 (2009). 10.1179/175889709X446507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Hashimoto M., Dang T. T., Hoare T., Kohane D. S., Whitesides G. M., Langer R., and Anderson D. G., Small 5, 1575 (2009). 10.1002/smll.200801855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Tumarkin E., Peerani R., Nie Z., Sullan R. M. A., Walker G. C., and Kumacheva E., J. Am. Chem. Soc. 128, 12205 (2006). 10.1021/ja0635682 [DOI] [PubMed] [Google Scholar]
- Shah R. K., Kim J.-W., Agresti J. J., Weitz D. A., and Chu L. -Y., Soft Matter 4, 2303 (2008). 10.1039/b808653m [DOI] [Google Scholar]
- Morimoto Y., Tan W.-h., Tsuda Y., and Takeuchi S., Lab Chip 9, 2217 (2009). 10.1039/b900035f [DOI] [PubMed] [Google Scholar]
- Schmidt J. J., Rowley J., and Kong H. J., J. Biomed. Mater. Res. 87A, 1113 (2008). 10.1002/jbm.a.32287 [DOI] [PubMed] [Google Scholar]
- Choi C.-H., Jung J.-H., Rhee Y., Kim D.-P., Shim S.-E., and Lee C.-S., Biomed. Microdevices 9, 855 (2007). 10.1007/s10544-007-9098-7 [DOI] [PubMed] [Google Scholar]
- Yu C.-Y., Cao H., Zhang X.-C., Zhou F.-Z., Cheng S.-X., Zhang X.-Z., and Zhuo R.-X., Langmuir 25, 11720 (2009). 10.1021/la901389v [DOI] [PubMed] [Google Scholar]
- Liu Z., Jiao Y., Wang Y., Zhou C., and Zhang Z., Adv. Drug Delivery Rev. 60, 1650 (2008). 10.1016/j.addr.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Seiffert S. and Weitz D. A., Soft Matter 6, 3184 (2010). 10.1039/c0sm00071j [DOI] [Google Scholar]
- Capretto L., Mazzitelli S., Tosi A., and Nastruzzi C., J. Controlled Release 132, e55 (2008). 10.1016/j.jconrel.2008.09.054 [DOI] [Google Scholar]
- Tan W.-H. and Takeuchi S., Adv. Mater. (Weinheim, Ger.) 19, 2696 (2007). 10.1002/adma.200700433 [DOI] [Google Scholar]
- Capretto L., Mazzitelli S., Balestra C., Tosi A., and Nastruzzi C., Lab Chip 8, 617 (2008). 10.1039/b714876c [DOI] [PubMed] [Google Scholar]
- Rondeau E. and Cooper-White J. J., Langmuir 24, 6937 (2008). 10.1021/la703339u [DOI] [PubMed] [Google Scholar]
- Reis C. P., Neufeld R. J., Vilela S., Ribeiro A. J., and Veiga F., J. Microencapsul. 23, 245 (2006). 10.1080/02652040500286086 [DOI] [PubMed] [Google Scholar]
- Workman V. L., Dunnett S. B., Kille P., and Palmer D. D., Biomicrofluidics 1, 014105 (2007). 10.1063/1.2431860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici E., Tetradis-Meris G., de Torres C. P., and Jousse F., Food Hydrocolloids 22, 97 (2008). 10.1016/j.foodhyd.2007.01.022 [DOI] [Google Scholar]
- Liu K., Ding H.-J., Liu J., Chen Y., and Zhao X.-Z., Langmuir 22, 9453 (2006). 10.1021/la061729+ [DOI] [PubMed] [Google Scholar]
- Zhao L. B., Pan L., Zhang K., Guo S. S., Liu W., Wang Y., Chen Y., Zhao X. Z., and Chan H. L. W., Lab Chip 9, 2981 (2009). 10.1039/b907478c [DOI] [PubMed] [Google Scholar]
- Saeki D., Sugiura S., Kanamori T., Sato S., and Ichikawa S., J. Biosci. Bioeng. 108, S162 (2009). 10.1016/j.jbiosc.2009.08.437 [DOI] [PubMed] [Google Scholar]
- Shin S.-J., Park J.-Y., Lee J.-Y., Park H., Park Y.-D., Lee K.-B., Whang C.-M., and Lee S.-H., Langmuir 23, 9104 (2007). 10.1021/la700818q [DOI] [PubMed] [Google Scholar]
- Kawakatsu T., Trägårdh G., and Trägårdh C., Colloids Surf., A 189, 257 (2001). 10.1016/S0927-7757(01)00508-8 [DOI] [Google Scholar]
- Liu L., Fishman M., and Hicks K., Cellulose 14, 15 (2007). 10.1007/s10570-006-9095-7 [DOI] [Google Scholar]
- Sriamornsak P., Silpakorn University International Journal 3, 206 (2003). [Google Scholar]
- Compendium of Food Additive Specifications (FAO, United Nations Information Centre Library, Rome, 2009).
- Sriamornsak P., Silpakorn University International Journal 21–22, 206 (2001). [Google Scholar]
- Voragen A., Coenen G.-J., Verhoef R., and Schols H., Struct. Chem. 20, 263 (2009). 10.1007/s11224-009-9442-z [DOI] [Google Scholar]
- Kohn R., Carbohydr. Polym. 2, 273 (1982). 10.1016/0144-8617(82)90030-3 [DOI] [Google Scholar]
- Ovodov Y., Russ. J. Bioorg. Chem. 35, 269 (2009). 10.1134/S1068162009030017 [DOI] [Google Scholar]
- Sikorski P., Mo F., Skjåk-Bræk G., and Stokke B. T., Biomacromolecules 8, 2098 (2007). 10.1021/bm0701503 [DOI] [PubMed] [Google Scholar]
- Fang Y., Al-Assaf S., Phillips G. O., Nishinari K., Funami T., and Williams P. A., Carbohydr. Polym. 72, 334 (2008). 10.1016/j.carbpol.2007.08.021 [DOI] [Google Scholar]
- Ogończyk D., Węgrzyn J., Jankowski P., Dąbrowski B., and Garstecki P., Lab Chip 10, 1324 (2010). 10.1039/b924439e [DOI] [PubMed] [Google Scholar]
- Jankowski P., Ogończyk D., Kosiński A., Lisowski W., and Garstecki P., Lab Chip 11, 748 (2011). 10.1039/c0lc00360c [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.3569944 for a schematic diagram and a photograph of the microfluidic system and for graphs that detail the profiles of release of nanoparticles encapsulated in pectin microbeads.
- Kalsin A. M., Fialkowski M., Paszewski M., Smoukov S. K., Bishop K. J. M., and Grzybowski B. A., Science 312, 420 (2006). 10.1126/science.1125124 [DOI] [PubMed] [Google Scholar]
- Jana N. R. and Peng X., J. Am. Chem. Soc. 125, 14280 (2003). 10.1021/ja038219b [DOI] [PubMed] [Google Scholar]
- Kjøniksen A.-L., Hiorth M., and Nyström B., Eur. Polym. J. 41, 761 (2005). 10.1016/j.eurpolymj.2004.11.006 [DOI] [Google Scholar]
- Christopher G. F. and Anna S. L., J. Phys. D 40, R319 (2007). 10.1088/0022-3727/40/19/R01 [DOI] [Google Scholar]
- Kim C., Lee K. S., Kim Y. E., Lee K.-J., Lee S. H., Kim T. S., and Kang J. Y., Lab Chip 9, 1294 (2009). 10.1039/b819044e [DOI] [PubMed] [Google Scholar]