Abstract
Imprinting occurs in the endosperm of flowering plants. The endosperm, a product of central cell fertilization, is critical for embryo and seed development. Imprinting in the endosperm is mainly due to the inherited differences in gamete epigenetic composition. Studies have also shown that there are differences in genomic DNA methylation patterns between embryo and endosperm. Examining those differences, along with mutations in the DNA demethylase gene DEMETER, gives insight into the number of imprinted genes and how an antagonistic relationship between TE defense and gene regulation could evolutionarily affect imprinting establishment. Finally, studies demonstrate that DEMETER demethylase activity influences endosperm chromatin composition, and could possibly enhance DNA de novo methylation activity.
Keywords: Embryo, Endosperm, Imprinting, Methylation, Demethylation, Polycomb Group, siRNA, RNA-directed DNA methylation
Introduction
Angiosperm seeds are comprised of the seed coat, the mature embryo, and the endosperm. The endosperm, which serves as a conduit to store and transport nutrients to the embryo during initial phases of seed growth, is a major food source for most of the world. With one exception [1], all known plant gene imprinting occurs in the endosperm. Hence, this review will concentrate on endosperm imprinting.
Gene imprinting is when two alleles are expressed at different levels depending on their parent of origin [2]. This definition includes alleles that are both expressed but at different intensities, and also alleles in which one is expressed and the other is silent. Most of our understanding on the mechanisms of imprinting is from studies of the latter type of imprinting (Table 1) [3,4]. To understand imprinting, it is important to review the different developmental programs of the female and male gametophytes (for a review, see [5,6] and Figure 1). Ultimately, imprinting is often due to the different epigenetic states between the female and male gametes, including differences in DNA methylation.
Table 1.
List of Imprinted Genes in Flowering Plants
| Endosperm Allele Expressed | Potential Function | References | |
|---|---|---|---|
| Arabidopsis thaliana | |||
| MEDEA (MEA) | Maternal | Polycomb Group Complex SET-domain protein | [33,34] |
| FWA | Maternal | Homeodomain transcription factor | [12] |
| FIS2 | Maternal | Zinc-finger transcription factor | |
| MPC | Maternal | C-terminal domain of poly(A) binding proteins (PABPs) | [35] |
| FH5 | Maternal | Actin regulator | [36] |
| MYB3R2 | Maternal | Putative c-myb-like transcription factor | [14] |
| HDG8 | Maternal | Homeobox-leucine zipper transcription factor | [14] |
| HDG9 | Maternal | Homeobox-leucine zipper transcription factor | [14] |
| HDG3 | Paternal | Homeobox-leucine zipper transcription factor | [14] |
| PHE1 | Paternal | MADS-box transcription factor | [26] |
| At5g62110 | Paternal | Putative transcription factor | [14] |
| Zea mays | |||
| R-mottled allelea | Maternal | Transcription factor | [37] |
| dzr1b | Maternal | Regulates zein protein accumulation | [38] |
| fie1 | Maternal | Polycomb group complex | [39], [40], [41] |
| fie2 | Maternal | Polycomb group complex | [39], [40] |
| nrp1 | Maternal | Unknown | [42] |
| mez1 | Maternal | Polycomb group complex | [43] |
| meg1 | Maternal | Cysteine-rich peptide | [44] |
| peg1 | Paternal | Unknown | [45] |
when inherited paternally, kernel has a mottled color pattern. When inherited maternally, kernel is fully colored
allele in the MO17 ecotype is imprinted
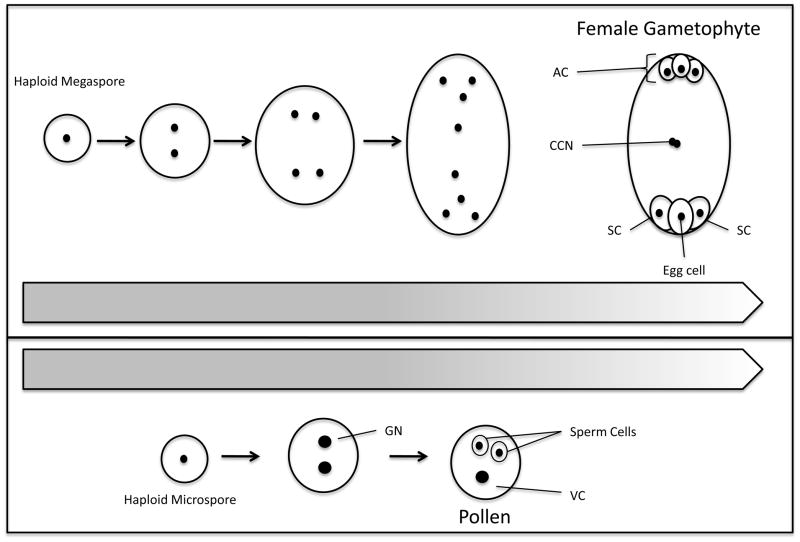
Figure 1. Female and Male Gametogenesis.
Top Panel: Haploid megaspore, derived from meiosis, proceeds through three rounds of mitosis. After the last mitosis, cell walls form, creating a seven celled structure: the haploid Egg cell, two synergid cells (SC), three antipodal cells (AC), and diploid central cell nuclei (CCN). Bottom Panel: Haploid microspore, derived from meiosis, proceeds through two rounds of mitosis. After the first round, there is a single cell with two haploid nuclei: vegetative nucleus and the generative nucleus (GN). After the second mitotic event, the generative nucleus divides and forms two haploid sperm cells that remain encapsulated in the vegetative cell (VC).
DNA Methylation
Methylation of the 5th carbon of cytosine (5-methylcytosine) is often associated with heterochromatic loci including telomeres and centromeres, and is also coincident with many silent loci, particularly repetitive sequences and transposable elements. In plants, 5-methylcytosine is found in three sequence contexts: CG, CHG, and CHH (H is A, C, or T) [7]. The symmetry of CG and CHG sites allows the template strand to guide methyltransferase to the new unmethylated strand after DNA replication, a process known as maintenance of methylation. METHYLTRANSFERASE 1 (MET1) is required to maintain methylation at CG sites, and CHROMOMETHYLASE 3 (CMT3) is required to maintain methylation of CHG sites. Due to the asymmetry of CHH sites, methylation must always be de novo after each round of DNA replication. DOMAINS REARRANGED METHYLTRANSFERASE 2 (DRM2) is required for de novo methylation of CHH and, to an extent, CHG sites. The de novo methyltransferase is guided to target sites by components of the RNA-directed DNA methylation (RdDM) pathway. For a review of DNA methylation and RdDM, please read Law and Jacobsen [8]. It is important to note that small interfering RNAs (siRNAs), processed from longer double strand RNA (dsRNA), can guide DRM2 to homologous DNA sites for de novo methylation. A pool of siRNAs must be maintained to ensure de novo methylation after each round of DNA replication.
Demethylation and Imprinting
The establishment of imprinting often requires a loss of maternal methylation in the central cell [9–12]. Loss of methylation can occur either passively or actively. Passive demethylation occurs if there is loss of methyltransferase activity, resulting in a failure to maintain DNA methylation after DNA replication [13]. It has been shown that during the late stages of female gametophyte development, MET1 expression is down regulated, which could result in a loss of CG methylation in the mature central cell [11]. Passive demethylation could play an important role in the establishment of imprinting.
Active demethylation requires proteins that can recognize 5-methylcytosine and enzymatically remove it. In plant reproductive organs this is accomplished by DEMETER (DME), a bifunctional DNA glycosylase/lyase that works in conjunction with the base excision repair pathway to remove 5-methylcytsine and replace it with cytosine [9,10]. Expression of DME is detected just before the two haploid polar nuclei fuse, but it quickly disappears after fertilization of the diploid central cell with a haploid sperm cell. DME activity in the central cell is critical for seed development after fertilization because seeds with a maternal dme mutant allele abort development and are inviable. DME expression is not detected in the pollen, and inheriting a mutant paternal dme allele does not effect seed development [9,10]. Because DME is expressed in the central cell, and not the pollen, the endosperm inherits genomes with different epigenetic states, which is critical for imprinting establishment.
Genomic Demethylation and Imprinting Establishment
To identify DME-mediated imprinted genes, Gehring et al. [14] immunoprecipitated methylated DNA, which was sequenced by high throughput methods. Comparing embryos, endosperms, and endosperms that inherited a dme mutant maternal allele (henceforth will be referred to as “dme mutant endosperm”), it was found that the endosperm genome is hypomethylated relative to the embryo, and that methylation increases in dme mutant endosperm. In addition, regions with the greatest difference in methylation between endosperm and embryo were regions enriched for transposable elements (TE) and known siRNA targets. Many of those sites were also located in the proximal regions around genes, including the known imprinted gene MEDEA (MEA).
Due to the preponderance of differentially methylated regions between embryo and endosperm that also corresponded with TE sites, Gehring et al. suggested that imprinting could be a by-product of TE defense [15]. TEs are potential sites for DNA methylation and silencing [16]. If they insert into, or near, a gene promoter, the methylation could inadvertently silence that gene’s expression. In the central cell, DME removes methylation from the maternal allele and activates expression. Because DME is not expressed in the pollen, DNA methylation remains resulting in endosperms inheriting two parental genomes with differential DNA methylation (maternal hypomethylated, paternal hypermethylated) and allowing for the establishment of imprinting. With this idea, Gehring et al [14] identified regions with the greatest methylation difference between embryo and endosperm, and then analyzed those regions at endosperm expressed genes. The authors discovered 5 new imprinted genes and predicted approximately 50 additional imprinted genes for the Arabidopsis endosperm.
Hsieh et al, [17] concurrently performed shotgun bisulfite sequencing of embryo, endosperm, dme endosperm, and four week old aerial (above-ground) somatic tissue, using a high throughput DNA sequencing platform. Bisulfite converts cytosine to uracil, but 5-methylcytosin is protected from such changes [18]. This allows resolution of each 5-methylcytosine base in the genome. Importantly, this enables the examination of DNA methylation in the CG, CHG, and CHH sequence contexts. As with Gehring et al [14], they also discovered a genome wide loss of methylation in the endosperm compared to embryo, and that DNA methylation was increased in dme mutant endosperm to the level found in aerial tissue [17]. Because DME is a demethylase, it was anticipated that DNA methylation would increase in dme mutant endosperm, which was found at CG sites. Unexpectedly, methylation in dme mutant endosperm at CHG and CHH sites was less than wild type endosperm. Many of those sites that were reduced in methylation were also known target sites for siRNAs/RdDM. The authors hypothesized that the removal of DNA methylation by DME, activates expression of siRNAs, which in turn utilize the RdDM pathway for de novo non-CG methylation (Figure 2). In the dme mutant, there is higher CG methylation, less siRNA activation, which reduces the pool of siRNAs, resulting in a reduction of both the RdDM activity and non-CG methylation. An examination of the siRNA pool in developing seeds supports this hypothesis. A large population of siRNAs in developing seeds is both endosperm specific and imprinted, expressing primarily from the maternal genome [19]. This suggest that not only could protein-coding genes be imprinted, but also any expressing region, including siRNA loci.
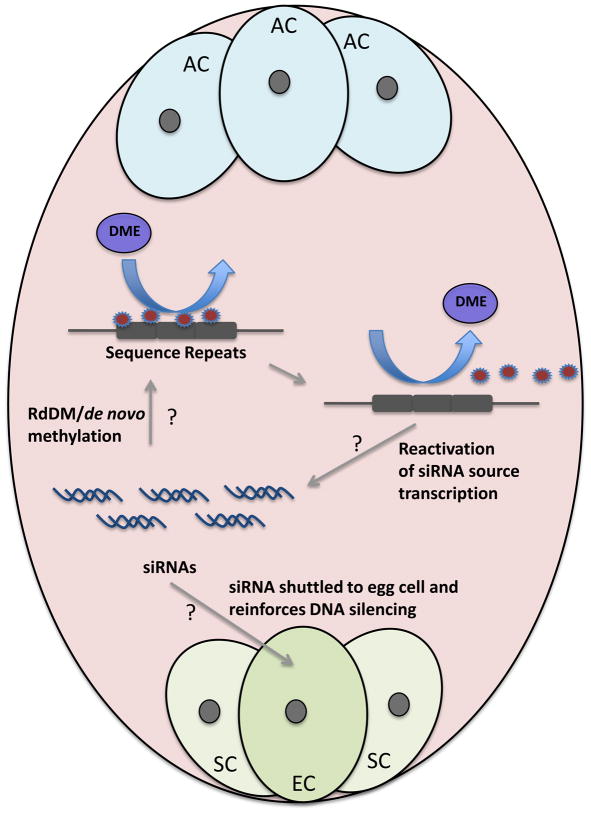
Figure 2. Model of Genome Wide Demethylation.
DME removes DNA methylation in the central cell, causing silent genes to begin expression. Many demethylated sites are also repetitive sequences and transposable elements. Model suggests that demethylation of siRNA source loci, including repetitive DNA and transposable elements, would express. The RdDM pathway would process these RNAs to siRNA, and guide de novo methylation. siRNAs could also be shuttled to the embryo where the RdDM pathway can reinforce silencing of transposable element and other repetitive DNA sequences. AC, antipodal cells; EC, egg cell; SC, synergid cells.
Significance of Endosperm Maternal Genome-Wide Demethylation
Evolutionarily, why would genome-wide demethylation, which includes the demethylation and possible reactivation of TEs, be beneficial? One possible explanation is that regions require demethylation in the central cell for proper endosperm imprinting and development after fertilization. Because the DNA demethylation is not targeted to imprinted genes, but is genome wide, it could result in wide spread TE reactivation. TEs, if reactivated, have the ability to cause unfavorable mutations [20]. However, since the endosperm is a terminal tissue, TEs activated by DME are not inherited by the embryo, and consequently would not be strongly selected against.
An intriguing hypothesis is that genome wide demethylation activates TEs and other siRNA loci in the endosperm, and that the newly created siRNAs can be transported to the egg or embryo to reinforce TE silencing (Figure 2) [17]. These ideas imply a positive selection of a siRNA source during evolution, which would benefit the embryo by reinforcing repetitive DNA silencing, and whose genomic danger imposed by a possible TE reactivation would be confined to the endosperm, a terminal tissue. Although the endosperm collects and distributes nutrients to the embryo [21], and siRNAs can have cell-non-autonomous affects in other parts of the plant [22], it remains to be shown that siRNAs could also be transported between the central cell/endosperm and the egg/embryo. A similar siRNA silencing effect was reported in pollen. Slotkin et al [23] showed that during pollen development (Figure 1), there is a loss of methylation and TE reactivation in the vegetative nucleus (a terminal cell). The authors demonstrated that the TE reactivation allows for additional siRNA production that is shuttled to the two sperm cells to reinforced silencing of siRNA target loci.
Polycomb Group Complex and Imprinting
An additional layer of imprinting regulation involves the Polycomb group complex (PcG). The PcG complex was first indentified in Drosophila and is needed for the suppression of the homeotic, or HOX genes [24]. Two distinct complexes exist in Drosophila, the PcG repressive complex 1 (PRC1), and the PcG repressive complex 2 (PRC2). In plant sexual reproduction, homologous members of the PRC2 play a significant role [25]. The Arabidopsis endosperm PRC2 consists primarily of four proteins: MEA (a SET-domain protein), FERTILIZATION INDEPENDENT ENDOSPERM (FIE, a WD40 protein), FERTILIZATION INDEPENDENT SEED 2 (FIS2, a zinc-finger Su(Z) homolog), and MULTIPLE SUPRESSOR OF IRA1 (MSI1, a p55 homolog). The PRC2 complex methylates lysine 27 on histone H3 (H3K27me), which is associated with compact and silent chromatin. PRC2 is required for the imprinting of PHERES1 (PHE1).
PHE1 is a paternally expressed imprinted gene in the endosperm [26]. PRC2 actively targets the promoter of the maternal PHE1 allele for H3K27me, which is necessary for maternal allele silencing [27,28]. Mutations that disrupt the PRC2 complex result in PHE1 biallelic expression. Interestingly, a differentially methylated region located downstream of PHE1 is thought to be important for imprinting establishment [28,29]. On the paternal allele, this region requires methylation for endosperm expression and imprinting. This region on the maternal PHE1 allele is demethylated, and it is thought that this demethylation allows the PRC2 complex to establish maternal allele silencing. Supporting this idea are comparisons between the endosperm H3K27me profile with both the vegetative and endosperm DNA methylation profile. Weinhofer et al [30], suggest that DNA methylation might prevent the PRC2 complex from binding to target sites, suggesting that DNA demethylation would allow the PRC2 complex to bind and function.
What is the extent of PRC2’s involvement in genomic imprinting? Studies of the cdka;1 mutant suggest that the involvement is quite significant. Pollen carrying a mutation in cdka;1 will produce a single sperm that can fertilize the egg and not the central cell [31]. After the egg is fertilized, the unfertilized central cell begins to develop into an endosperm-like tissue, which ultimately results in seed abortion. Mutations in MEA and FIE, both PRC2 genes, will allow the maternal homodiploid endosperm to develop into a viable seed [32]. One explanation is that loss of the paternal contribution disrupts the ratio of maternal to paternal imprinted loci, resulting is seed abortion. If the paternally-expressed imprinted alleles that are absent in the unfertilized cdka;1 endosperm are expressed in the maternal homodiploid genome due to a loss of PRC2-mediated imprinting, seed development is dramatically rescued.
Future Directions
To determine the extent imprinting effects seed development, it would be extremely valuable to discover the complete set of imprinted genes, the imprintome. One experimental approach to elucidate the imprintome is to cross different ecotypes, which creates a hybrid endosperm. RNA is isolated, converted to cDNA, and subjected to high-throughput sequencing. In this way, maternal-derived and paternal-derived transcripts can be identified by single-nucleotide polymorphisms (SNPs) that distinguish the two ecotypes. RNA transcripts with a significant parental SNP bias represent potential imprinted genes. A similar approach was recently performed in mice, which revealed over 1,000 candidate-imprinted genes [46,47]. This approach would complement the above genomic studies that have generated DNA methylation profiles in wild type and dme mutant endosperm [14, 17]. Further genomic studies, including mutations in the PRC2 complex, and possibly the components of the RdDM pathway, will aid in our understanding of the various mechanisms that are needed for imprintome establishment.
Acknowledgments
The authors would like to thank Tzung-Fu Hsieh for critical reading of the manuscript. We would also like to acknowledge the support of the National Institutes of Health (R01-GM069415), and the National Science Foundation (MCB 0918821) to Robert L. Fischer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- •1.Jahnke S, Scholten S. Epigenetic resetting of a gene imprinted in plant embryos. Curr Biol. 2009;19:1677–1681. doi: 10.1016/j.cub.2009.08.053. This study describes the first discovery of embryo gene imprinting in plants. [DOI] [PubMed] [Google Scholar]
- 2.Gehring M, Choi Y, Fischer RL. Imprinting and seed development. Plant Cell. 2004;16 (Suppl):S203–213. doi: 10.1105/tpc.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huh JH, Bauer MJ, Hsieh TF, Fischer R. Endosperm gene imprinting and seed development. Curr Opin Genet Dev. 2007;17:480–485. doi: 10.1016/j.gde.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh JH, Bauer MJ, Hsieh TF, Fischer RL. Cellular programming of plant gene imprinting. Cell. 2008;132:735–744. doi: 10.1016/j.cell.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Borg M, Twell D. Life after meiosis: patterning the angiosperm male gametophyte. Biochem Soc Trans. 2010;38:577–582. doi: 10.1042/BST0380577. [DOI] [PubMed] [Google Scholar]
- 6.Yadegari R, Drews GN. Female gametophyte development. Plant Cell. 2004;16 (Suppl):S133–141. doi: 10.1105/tpc.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, Pradhan S, Nelson SF, Pellegrini M, Jacobsen SE. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452:215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi Y, Gehring M, Johnson L, Hannon M, Harada JJ, Goldberg RB, Jacobsen SE, Fischer RL. DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in arabidopsis. Cell. 2002;110:33–42. doi: 10.1016/s0092-8674(02)00807-3. [DOI] [PubMed] [Google Scholar]
- 10.Gehring M, Huh JH, Hsieh TF, Penterman J, Choi Y, Harada JJ, Goldberg RB, Fischer RL. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006;124:495–506. doi: 10.1016/j.cell.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •11.Jullien PE, Mosquna A, Ingouff M, Sakata T, Ohad N, Berger F. Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PLoS Biol. 2008;6:e194. doi: 10.1371/journal.pbio.0060194. Shows that MSI1 and a RBR1 interact to down regulate MET1 expression. This suggests that the down regulation creates hemi-methylated sites, which has a higher binding affinity for the demethylase DME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita T, Miura A, Choi Y, Kinoshita Y, Cao X, Jacobsen SE, Fischer RL, Kakutani T. One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science. 2004;303:521–523. doi: 10.1126/science.1089835. [DOI] [PubMed] [Google Scholar]
- 13.Saze H, Mittelsten Scheid O, Paszkowski J. Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat Genet. 2003;34:65–69. doi: 10.1038/ng1138. [DOI] [PubMed] [Google Scholar]
- ••14.Gehring M, Bubb KL, Henikoff S. Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science. 2009;324:1447–1451. doi: 10.1126/science.1171609. Study shows that the endosperm’s genome is hypomethylated across the genome, and dme mutations increase genome methylation closer to the embryo’s. In addition, the regions of greatest methylation differences corresponds to TE-rich regions, and suggest imprinting might be a by-product of transposon defence and gene regulation by demethylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow DP. Methylation and imprinting: from host defense to gene regulation? Science. 1993;260:309–310. doi: 10.1126/science.8469984. [DOI] [PubMed] [Google Scholar]
- 16.Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007;8:272–285. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- ••17.Hsieh TF, Ibarra CA, Silva P, Zemach A, Eshed-Williams L, Fischer RL, Zilberman D. Genome-wide demethylation of Arabidopsis endosperm. Science. 2009;324:1451–1454. doi: 10.1126/science.1172417. Study shows that DME activity in the central cell causes a genome-wide loss of methylation in the endosperm, as compared to the embryo. The methylation is partially increased to the embryo’s level in dme mutants. Also, the methylation levels, when divided into the three known sequence contexts, are not affected equally by DME, and suggest that DME can affect TE expression. This increased TE expression might activate the RdDM pathway to increase non-CG methylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••19.Mosher RA, Melnyk CW, Kelly KA, Dunn RM, Studholme DJ, Baulcombe DC. Uniparental expression of PolIV-dependent siRNAs in developing endosperm of Arabidopsis. Nature. 2009;460:283–286. doi: 10.1038/nature08084. Study discovered a large population of siRNAs that are endosperm-specific and appear to be imprinted, expressing primarily from a maternal origin. It is the first known imprinting of non-protein coding genes. [DOI] [PubMed] [Google Scholar]
- 20.Lisch D. Epigenetic regulation of transposable elements in plants. Annu Rev Plant Biol. 2009;60:43–66. doi: 10.1146/annurev.arplant.59.032607.092744. [DOI] [PubMed] [Google Scholar]
- 21.Penfield S, Graham S, Graham IA. Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochem Soc Trans. 2005;33:380–383. doi: 10.1042/BST0330380. [DOI] [PubMed] [Google Scholar]
- 22.Chitwood DH, Timmermans MC. Small RNAs are on the move. Nature. 2010;467:415–419. doi: 10.1038/nature09351. [DOI] [PubMed] [Google Scholar]
- •23.Slotkin RK, Vaughn M, Borges F, Tanurdzic M, Becker JD, Feijo JA, Martienssen RA. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell. 2009;136:461–472. doi: 10.1016/j.cell.2008.12.038. Study demonstrates how genome methyaltion is reduced in the vegetative nucleus, causing a reactivation of TE. Study suggests that siRNAs produced from TE reactivation can cross into the sperm cells to possibly reinforce TE silencing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz YB, Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues JC, Luo M, Berger F, Koltunow AM. Polycomb group gene function in sexual and asexual seed development in angiosperms. Sex Plant Reprod. 2010;23:123–133. doi: 10.1007/s00497-009-0131-2. [DOI] [PubMed] [Google Scholar]
- 26.Kohler C, Page DR, Gagliardini V, Grossniklaus U. The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet. 2005;37:28–30. doi: 10.1038/ng1495. [DOI] [PubMed] [Google Scholar]
- 27.Kohler C, Hennig L, Spillane C, Pien S, Gruissem W, Grossniklaus U. The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Genes Dev. 2003;17:1540–1553. doi: 10.1101/gad.257403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villar CB, Erilova A, Makarevich G, Trosch R, Kohler C. Control of PHERES1 imprinting in Arabidopsis by direct tandem repeats. Mol Plant. 2009;2:654–660. doi: 10.1093/mp/ssp014. [DOI] [PubMed] [Google Scholar]
- •29.Makarevich G, Villar CB, Erilova A, Kohler C. Mechanism of PHERES1 imprinting in Arabidopsis. J Cell Sci. 2008;121:906–912. doi: 10.1242/jcs.023077. Shows that MSI1 and a RBR1 interact to down regulate MET1 expression. This suggests that the down regulation creates hemi-methylated sites, which has a higher binding affinity for the demethylase DME. [DOI] [PubMed] [Google Scholar]
- 30.Weinhofer I, Hehenberger E, Roszak P, Hennig L, Kohler C. H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation. PLoS Genet. 2010:6. doi: 10.1371/journal.pgen.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A. A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet. 2006;38:63–67. doi: 10.1038/ng1694. [DOI] [PubMed] [Google Scholar]
- 32.Nowack MK, Shirzadi R, Dissmeyer N, Dolf A, Endl E, Grini PE, Schnittger A. Bypassing genomic imprinting allows seed development. Nature. 2007;447:312–315. doi: 10.1038/nature05770. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita T, Yadegari R, Harada JJ, Goldberg RB, Fischer RL. Imprinting of the MEDEA polycomb gene in the Arabidopsis endosperm. Plant Cell. 1999;11:1945–1952. doi: 10.1105/tpc.11.10.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vielle-Calzada JP, Thomas J, Spillane C, Coluccio A, Hoeppner MA, Grossniklaus U. Maintenance of genomic imprinting at the Arabidopsis medea locus requires zygotic DDM1 activity. Genes Dev. 1999;13:2971–2982. doi: 10.1101/gad.13.22.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiwari S, Schulz R, Ikeda Y, Dytham L, Bravo J, Mathers L, Spielman M, Guzman P, Oakey RJ, Kinoshita T, et al. MATERNALLY EXPRESSED PAB C-TERMINAL, a novel imprinted gene in Arabidopsis, encodes the conserved C-terminal domain of polyadenylate binding proteins. Plant Cell. 2008;20:2387–2398. doi: 10.1105/tpc.108.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitz Gerald JN, Hui PS, Berger F. Polycomb group-dependent imprinting of the actin regulator AtFH5 regulates morphogenesis in Arabidopsis thaliana. Development. 2009;136:3399–3404. doi: 10.1242/dev.036921. [DOI] [PubMed] [Google Scholar]
- 37.Kermicle JL. Dependence of the R-mottled aleurone phenotype in maize on mode of sexual transmission. Genetics. 1970;66:69–85. doi: 10.1093/genetics/66.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaudhuri S, Messing J. Allele-specific parental imprinting of dzr1, a posttranscriptional regulator of zein accumulation. Proc Natl Acad Sci U S A. 1994;91:4867–4871. doi: 10.1073/pnas.91.11.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danilevskaya ON, Hermon P, Hantke S, Muszynski MG, Kollipara K, Ananiev EV. Duplicated fie genes in maize: expression pattern and imprinting suggest distinct functions. Plant Cell. 2003;15:425–438. doi: 10.1105/tpc.006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gutierrez-Marcos JF, Costa LM, Dal Pra M, Scholten S, Kranz E, Perez P, Dickinson HG. Epigenetic asymmetry of imprinted genes in plant gametes. Nat Genet. 2006;38:876–878. doi: 10.1038/ng1828. [DOI] [PubMed] [Google Scholar]
- 41.Hermon P, Srilunchang KO, Zou J, Dresselhaus T, Danilevskaya ON. Activation of the imprinted Polycomb Group Fie1 gene in maize endosperm requires demethylation of the maternal allele. Plant Mol Biol. 2007;64:387–395. doi: 10.1007/s11103-007-9160-0. [DOI] [PubMed] [Google Scholar]
- 42.Guo M, Rupe MA, Danilevskaya ON, Yang X, Hu Z. Genome-wide mRNA profiling reveals heterochronic allelic variation and a new imprinted gene in hybrid maize endosperm. Plant J. 2003;36:30–44. doi: 10.1046/j.1365-313x.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- 43.Haun WJ, Laoueille-Duprat S, O’Connell MJ, Spillane C, Grossniklaus U, Phillips AR, Kaeppler SM, Springer NM. Genomic imprinting, methylation and molecular evolution of maize Enhancer of zeste (Mez) homologs. Plant J. 2007;49:325–337. doi: 10.1111/j.1365-313X.2006.02965.x. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez-Marcos JF, Costa LM, Biderre-Petit C, Khbaya B, O’Sullivan DM, Wormald M, Perez P, Dickinson HG. maternally expressed gene1 Is a novel maize endosperm transfer cell-specific gene with a maternal parent-of-origin pattern of expression. Plant Cell. 2004;16:1288–1301. doi: 10.1105/tpc.019778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutierrez-Marcos JF, Pennington PD, Costa LM, Dickinson HG. Imprinting in the endosperm: a possible role in preventing wide hybridization. Philos Trans R Soc Lond B Biol Sci. 2003;358:1105–1111. doi: 10.1098/rstb.2003.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]