Abstract
Advances in basic immunology have led to an improved understanding of the interactions between the immune system and tumours, generating renewed interest in approaches that aim to treat cancer immunologically. As clinical and preclinical studies of tumour immunotherapy illustrate several immunological principles, a review of these data is broadly instructive and is particularly timely now that several agents are beginning to show evidence of efficacy. This is especially relevant in the case of prostate cancer, as recent approval of sipuleucel-T by the US Food and Drug Administration marks the first antigen-specific immunotherapy approved for cancer treatment. Although this Review focuses on immunotherapy for prostate cancer, the principles discussed are applicable to many tumour types, and the approaches discussed are highlighted in that context.
In developed countries, prostate cancer is the most common cancer in men, and it ranks third overall in terms of mortality (behind lung cancer and colon cancer)1. Localized disease is treated surgically or with radiation therapy2 or, alternatively, may be monitored closely if the cancer is thought to be of sufficiently low risk3. If disease returns after initial surgery or radiation therapy, this recurrent disease can be treated with androgen ablation (chemical castration or surgical castration) or observed until metastatic progression. Metastatic prostate cancer is initially treated with androgen ablation, but most patients eventually become refractory to this treatment, developing castration-resistant disease, for which the primary treatment option is chemotherapy4,5. This paucity of therapeutic options, as well as their associated morbidity, has led to a search for new treatments; immunotherapy, in which the patients’ immune system is targeted to induce an antitumour response, is a rapidly evolving treatment option. In many ways, prostate cancer is a typical epithelial adeno carcinoma, so the immunotherapy approaches that are being developed for this disease provide insights that are also applicable to other epithelial cancer types. In this Review, we first briefly discuss the basic biology and natural history of prostate cancer, focusing on issues that relate to immunotherapy. We then outline some of the immunotherapy approaches that have advanced to later stage clinical trials, with an emphasis on the immunological and clinical insights provided by these studies.
Immunological characteristics of prostate cancer
With several notable exceptions, most human cancers develop in immunologically intact hosts. So, the progression of tumours from low-grade, localized disease to metastasis involves an interaction between the tumour cells and the host immune system; here, we focus on what is known regarding that interaction in prostate cancer.
Role of inflammation in the development of prostate cancer
As is the case for most types of cancer, the precise aetiology of prostate cancer is unknown; however, a great deal of literature supports the hypothesis that both genetic6 and environmental7 factors are important. Interestingly, human8 and animal studies indicate that inflammation might have a role in prostate cancer development, as well as in the progression from organ-confined to metastatic disease9,10. Inflammation is also thought to have a role in the development of many other human cancers; well-described examples include gastric, colon and liver cancer11. A causal relationship between ongoing inflammation and prostate cancer has yet to be established, but substantial epidemiological evidence indicates that prostate cancer is more common in demographic groups with a greater degree of baseline inflammation8. Unfortunately, neither the aetiology nor the precise immunological characteristics of intra-prostatic inflammation are well understood. In terms of adaptive immunity, both CD4+ and CD8+ T cells are present in prostate glands, and the CD4+ T cells include both T helper 17 (TH17)12 and regulatory T (TReg)12–15 cell populations. Intraprostatic CD8+ T cells in humans are non-functional and do not upregulate activation markers such as CD69 or CD137 in response to stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin16. These data are consistent with those obtained using antigen-specific CD8+ T cells isolated from melanoma lesions17, as well as with transgenic mouse models of prostate cancer (see below). In terms of immunotherapy, these results indicate that prostate cancer vaccination is targeted at an organ with a pre-existing and complex pattern of inflammation that might be contributing to disease progression.
Early-stage prostate cancer
Like most solid tumours, prostate cancer generally progresses through a series of stages, known as clinical states18 (FIG. 1). In developed countries, many cases of prostate cancer are initially detected by monitoring the levels of prostate-specific antigen (PSA) in the blood (BOX 1). Increased (or changing) levels of PSA prompt a biopsy, and a diagnosis of prostate cancer is based on microscopic evaluation of the biopsy specimen. Diagnosis generally leads to an attempt at local treatment, with either surgery or radiotherapy. For up to 80% of surgically treated men, local treatment is successful in that metastatic disease does not occur within 15 years19. When disease does recur, the initial manifestation is often a rising PSA level without radiologically detectable metastases, a clinical state known as biochemical progression20,21. An analogous state occurs in some gastrointestinal cancers, in which an increasing level of carcinoembryonic antigen (CEA) can be detected before progression as determined by radiographic imaging22. From an immunological perspective, biochemical recurrence provides a unique opportunity for immunological intervention in patients with cancer, as the many immunosuppressive mechanisms (such as TReg cells, myeloid-derived suppressor cells (MDSCs) and transforming growth factor-β (TGFβ))23 associated with an advanced tumour burden are expected to be at a minimum at this stage. However, it is difficult to select appropriate endpoints for clinical trials in patients with biochemically recurrent cancers, as neither PSA nor CEA level is a validated surrogate endpoint acceptable for drug registration (BOX 1). More traditional clinical endpoints, such as the development of overt metastases, might not occur for many years19, leading to unacceptably long follow-up times for trials with such endpoints.
Figure 1. Clinical states of prostate cancer and current therapeutic interventions.
For most patients, prostate cancer is a slowly progressive disease. Most patients are diagnosed with localized disease, and treated with either radiotherapy or surgery. However, a substantial fraction of these men later develop a rising prostate-specific antigen (PSA) level in the absence of radiographically detectable lesions, a state known as biochemical recurrence. Men with biochemically recurrent disease can be either monitored or treated with androgen ablation (surgical or chemical castration). Eventually, a fraction of men develop radiographically detectable metastatic disease; it is in the setting of castration-resistant metastatic disease that most immunotherapy approaches for prostate cancer have been tested.
Box 1. Prostate-specific antigen.
Prostate-specific antigen (PSA) is a glycoprotein with expression mainly confined to the epithelial cells that line prostate glands. Disruption of the normal prostatic architecture owing to inflammation, infection or cancer leads to leakage of PSA into the general circulation, where it can be detected by an enzyme-linked immunosorbent assay (ELISA)-based blood test. In 1986, the US Food and Drug Administration (FDA) approved the use of PSA testing to monitor treatments for prostate cancer and, in 1994, testing of PSA levels was approved by the FDA for disease detection. PSA testing has low sensitivity and specificity for prostate cancer detection, and the value of PSA testing for preventing prostate cancer mortality has been evaluated in two recently published randomized trials, one of which supports testing131, whereas the second trial does not132. Nevertheless, the mortality rate from prostate cancer has declined slowly over the past decade, and there are some data to indicate that the initiation of this decline coincides with the adoption of PSA testing. The American Urological Association (AUA) recommends PSA screening for well-informed men with an estimated ten-year life expectancy133. This qualified screening recommendation is based on the notion that many screened men will be diagnosed with low-risk disease and thus face the therapeutic dilemma of whether to undergo primary therapy. In addition, the five-year survival rate for patients with prostate cancer is nearly 100%, so only men with a predicted lifespan long enough to benefit from intervention should probably undergo a screening PSA test. It is noteworthy that this screening recommendation is not shared by other organizations, most of which recommend against routine screening of asymptomatic men. From an immunological perspective, PSA is a target antigen in several immunotherapeutic approaches for prostate cancer, most notably a poxvirus-based vaccine known as ProstVac VF54.
Late-stage prostate cancer
Prostatic epithelial cells are broadly dependent on androgens for survival; hence men with biochemically recurrent disease can be treated with androgen ablation, through either surgical or chemical castration24. Although an overall survival benefit for androgen ablation in men with biochemically recurrent disease is not well supported by large, randomized clinical trials, it is of interest to note that this commonly used therapy has many immunological effects, several of which would be expected to increase the efficacy of cancer immunotherapy25. Initial observations in this regard came from a study showing that androgen ablation before prostate cancer surgery resulted in a substantial CD4+ T cell infiltration into the gland, and that the infiltrating cells expressed a restricted pattern of use of the T cell receptor β-chain variable region (Vβ) — such an oligoclonal response is consistent with an antigen-specific response26. These findings were well supported by a more recent comprehensive analysis of the post-castration immunological infiltrate in the prostate gland, in which an increased CD8+ T cell infiltrate was noted as well27. Using an autochthonous mouse prostate cancer model, we found that androgen ablation decreases CD4+ T cell tolerance to a prostate cancer-associated antigen: adoptively transferred, clonotypic CD4+ T cells could respond to specific vaccination after androgen ablation but not in intact, tumour-bearing mice28. Perhaps even more intriguing are reports showing that androgen ablation reverses the thymic involution that normally occurs with aging, resulting in increased output of naive T cells29. Taken together, these data strongly support the notion that androgen ablation, through its effects on boosting the prostate-specific immune response, could have an additive effect with immunotherapy, a principle that has been formally evaluated in several clinical trials30. Interestingly, the relative timing of androgen ablation and immunotherapy could be crucial; one study showed that applying immunotherapy before castration was more effective than the converse31.
Eventually, many men with prostate cancer develop metastatic disease, despite androgen ablation. This disease state is known as metastatic, castration-resistant prostate cancer and is the state in which most immunotherapy approaches have been clinically evaluated. These men have a median survival of ~16 months4,5, allowing the timely completion of trials with a survival endpoint. However, from an immunological perspective, such patients probably have a large number of tumour-induced immunosuppressive mechanisms in place23, including increased levels of TGFβ32, a cytokine that suppresses the activity of CD4+ T cells, CD8+ T cells and natural killer cells. It is noteworthy, however, that tumour-specific tolerance might not be unique to late-stage disease; many mouse cancer models support the notion that the development of tolerance occurs early in tumour progression33,34.
Immunology of prostate cancer in mice
In addition to the human epidemiological and clinical data discussed above, immunological data from mouse models could also potentially inform clinical trial design and interpretation. In this regard, the development of the TRAMP model (transgenic adenocarcinoma of the mouse prostate model)35 has provided a unique opportunity to investigate T cell responses in developing prostate tumours in vivo. This work has included studies of the endogenous T cell repertoire, as well as adoptive T cell transfer experiments. Reassuringly, the results obtained have been generally consistent, with several groups reporting the development of CD8+ T cell tolerance to evolving tumours36–41. These results corroborate earlier studies in other spontaneous mouse cancer models42,43, providing broad support to the notion that evolving tumours are associated with CD8+ T cell tolerance. CD4+ T cell tolerance to prostate tumours has been less well addressed but also seems to be associated with tumour progression34,44. We observed specific CD4+ T cell tolerance to prostate-specific antigens in TRAMP mice as young as 6 weeks of age44 — before overt tumours can be detected pathologically — supporting the notion that the development of specific T cell tolerance is an early event in cancer progression. We also found that naive CD4+ T cells adopted a regulatory phenotype after encounter with tumour antigen44, which is consistent with the hypothesis that tumours can induce TReg cell proliferation and/or development. More recent studies have extended this idea to CD8+ T cells, which might also develop a regulatory phenotype after contact with tumour antigen15,45. Overall, these human and mouse data support a model whereby evolving tumours result in the proliferation of T cells with an anticancer potential but that, in the absence of some intervention, such cells exist in a non-functional or anergic state.
Antigen-specific immunotherapy
The goal of most approaches to cancer immunotherapy is to activate a population of effector T cells, which can then traffic to evolving tumours and mediate the specific lysis of cancer cells. In antigen-specific approaches, a tumour-associated antigen is directly targeted, either by loading that antigen onto antigen-presenting cells (APCs) ex vivo or by incorporating the antigen into a vaccine vector at a protein or DNA level. Below, we summarize the antigen-specific immunotherapy approaches for prostate cancer that have progressed furthest in clinical trials. Although a large number of target antigens could have potentially been selected for prostate cancer, a great deal of clinical work in this area has focused on PSA as a target, most probably because of its long-standing clinical use as a serum marker for the disease. Other prostate-associated antigens include prostatic acid phosphatase (PAP) and prostate-specific membrane antigen (PSMA; also known as GPCII), which is expressed on the vasculature in several types of cancer46. The expression pattern of PSA is nearly ideal for its use as an immunotherapy target; it is expressed fairly exclusively by prostate cancer cells and by non-transformed prostate epithelial cells, making it a specific marker of prostate tissue. Notably, a tumour-specific protein is generally not thought to be necessary because, at the time of vaccine treatment, most men with prostate cancer have undergone primary therapy, and the only remaining PSA-expressing cells would be expected to be tumour cells. A few studies also indicate a role for PSA in the initiation and progression of prostate cancer, making it a potentially functional target as well47. In the absence of immunotherapy, however, T cell responses to PSA are difficult to detect in patients with later-stage prostate cancer, which indicates that, similar to the mouse model, some level of pre-existing tolerance might exist48.
Poxvirus-based vectors
Viral vectors have several inherent advantages for immunotherapy — they are straight-forward to engineer, they can carry large amounts of genetic material and there is a great deal of clinical experience with poxvirus vectors, such as vaccinia virus, as they were used worldwide in the eradication of small-pox49. In vivo, poxvirus vectors most probably infect epithelial cells, a proportion of which undergo cell death. Cellular debris, including encoded antigens, is then taken up by nearby immature APCs, which, when appropriately activated, can present these antigens to CD4+ and CD8+ T cells in a pro-inflammatory context (FIG. 2a). Direct infection of APCs, particularly the langerhans cells in the skin, is another mechanism by which poxvirus vectors can prime an immune response. For prostate cancer, PSA-targeted vaccinia virus-based immunotherapy has proceeded through several steps, including the incorporation of DNA encoding co-stimulatory molecules (lymphocyte function-associated antigen 3 (LFA3), CD80 and intercellular adhesion molecule 1 (ICAM1); known as TRICOM) into the vaccine50, as well as optimization of the MHC class II-binding properties of the vaccine antigen51. The main disadvantage of poxvirus-based vectors results from the immunological properties that render poxviruses efficacious vaccine vectors: their propensity to induce a strong antibody response makes homologous prime–boost regimens ineffective, as the antibody response to viral proteins dominates over the desired response to encoded antigen (or antigens)52. To circumvent this immunological limitation, a semi-heterologous prime–boost strategy involving a vaccinia virus prime followed by an analogous fowlpox virus boost (ProstVac VF–TRICOM; Bavarian Nordic) was optimized53. The clinical development of this agent has been recently reviewed54, and includes several trials in which ProstVac VF was combined with other conventional or experimental agents55. Perhaps most relevant to the present discussion, several recent trials of ProstVac VF provide important immunological and clinical insights into cancer immunotherapy, which are described later.
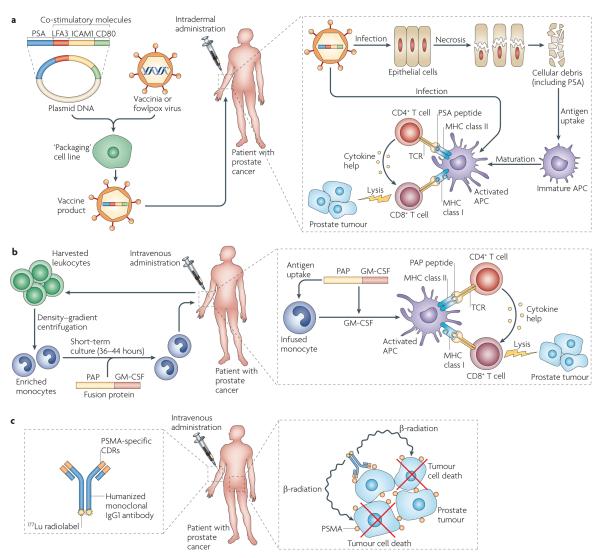
Figure 2. Examples of antigen-specific immunotherapy for prostate cancer.
a ∣ The ProstVac VF ‘vaccine’ consists of a DNA plasmid encoding the target antigen, prostate-specific antigen (PSA), and a series of three co-stimulatory molecules (lymphocyte function-associated antigen 3 (LFA3), CD80 and intercellular adhesion molecule 1 (ICAM1)). The plasmid cassette is incorporated into a poxvirus backbone in a ‘packaging’ cell line, giving a final vaccine product. In this approach, a vaccinia virus-based prime is followed by a fowlpox virus-based boost. The viral vectors are injected intradermally, where they probably infect the patient’s epithelial cells. This in turn leads to epithelial cell death, following which the cellular debris (including the target antigen PSA) is taken up by host antigen-presenting cells (APCs) and presented to host CD4+ and CD8+ T cells. A second potential mechanism for antigen presentation involves direct infection of APCs, including the Langerhans cells in the skin. The incorporation of CD80 into the viral vector facilitates the activation of T cells, through the provision of a co-stimulatory signal for T cell activation. LFA3 and ICAM1 are adhesion molecules; in this context their vector-driven expression on APCs provides additional co-stimulation to facilitate T cell activation. b ∣ Sipuleucel-T immunotherapy is similar to a dendritic cell (DC) vaccine and is based on cells from a patient-derived leukopheresis product. These cells are sent to a central processing facility where monocytes are enriched by density–gradient centrifugation. These monocytes are incubated for 36–44 hours with a specific fusion protein, coupling granulocyte–macrophage colony-stimulating factor (GM-CSF) to the target antigen, in this case prostatic acid phophatase (PAP). In this approach, GM-CSF targets the fusion protein to immature DCs and enhances subsequent DC maturation. Following incubation, the product is sent to the clinic where it is administered intravenously. Once in the patient, the patient’s immature monocytes are thought to mature to fully competent APCs, presenting PAP peptides to the host immune system in a manner that activates CD4+ and CD8+ T cells. c ∣ J591 is an antigen-specific approach using a humanized monoclonal antibody specific for prostate-specific membrane antigen (PSMA). Although early trials used unlabelled antibody, current trials involve 177Lu-labelled J591, a β-ray emitter with a half-life and path-length favourable for radiotherapy and immunotherapy. Here, the antibody specifically targets the radioactive isotope to the target tissue, where tumour cell death is mediated by irradiation. CDR, complementarity-determining region; TCR, T cell receptor.
Sipuleucel-T
In contrast to the ‘off the shelf ’ nature of other immunotherapy agents, sipuleucel-T (Provenge; Dendreon Inc.) is a personalized product that is individually manufactured for each patient with prostate cancer56. It is similar in some ways to dendritic cell (DC) vaccines, which have been extensively studied in many tumour types57. First, leukopheresis is carried out, and monocytes are enriched in the leukopheresis product through density–gradient centrifugation. These cells are then incubated with the targeted immunogen, a fusion protein linking granulocyte–macrophage colony-stimulating factor (GM-CSF) to PAP, before intravenous administration (FIG. 2b). Once infused, these autologous monocytes are thought to mature into functional APCs and to activate PAP-specific CD4+ and CD8+ T cells in treated patients. These activated T cells are then thought to home to tumour lesions, mediating an antitumour response. In this approach, PAP was chosen as the target antigen based on preclinical studies in a rat model that showed that tolerance to PAP in prostate cancer was not mediated by central deletion of PAP-specific T cells, such that PAP-directed vaccination could induce marked T cell infiltration into the prostate gland58. In terms of clinical development of immunotherapies for prostate cancer, this agent has progressed the furthest: three Phase III studies have been completed and US Food and Drug Administration (FDA) approval was granted in April 2010, making sipuleucel-T the first antigen-specific immunotherapy approved for cancer treatment. These Phase III trials provide certain immunological insights, which are discussed further below. It should also be noted that this approach is adaptable to other tumour types by changing the nature of the immunogen — that is, by changing the antigen coupled to GM-CSF in the fusion protein.
Additional approaches
An additional antigen-specific approach to cancer immunotherapy involves DNA-based vaccines; in contrast to the above approaches which use viruses and patient monocytes as vectors, DNA can be rapidly and precisely synthesized, making it straightforward to target nearly any selected antigen59. The main disadvantage of DNA-based vectors is their low level of immunogenicity relative to the highly immunogenic viral vectors described above. To improve the outcome, pro-inflammatory molecules — such as herpes simplex virus type 1 tegument protein VP22 (to enhance spreading from transfected cells to DCs) or Toll-like receptor (TLR) agonists (to activate APCs) — have been incorporated into DNA-based vaccines60, or the vectors have been co-administered with GM-CSF as a nonspecific adjuvant. In this context, GM-CSF is thought to function through the recruitment of APCs, particularly DCs, to the vaccine site61. A recent clinical study62 highlights the potential utility of DNA-based vectors in men with prostate cancer: in a population of men with biochemically recurrent disease given a DNA vaccine encoding PAP, PAP-specific T cell responses were induced, as well as an inhibition of the rate of PSA level increase.
Monoclonal antibodies specific for proteins expressed on the surface of tumour cells are a form of passive immunotherapy, which is in contrast to the above approaches, which are all examples of active immunization. Passive immunotherapy is now commonplace in mainstream clinical oncology, with antibodies specific for CD20 (such as rituximab (Rituxan/Mabthera; Genentech/Roche/Biogen Idec)), human epidermal growth factor receptor 2 (trastuzumab (Herceptin; Genentech/Roche)) and other tumour antigens being widely used. Analogous agents are in earlier stages of development in prostate cancer and focus mainly on PSMA as a target63. Interestingly, PSMA is overexpressed on tumour-associated vasculature, as well as on the cell surface of prostate cancer cells, making this agent potentially applicable to other types of cancer46. Early clinical trials of a humanized, PSMA-specific antibody (J591; Cornell Weill Medical College) showed impressive tumour targeting, but few objective clinical responses were noted in the patients with advanced tumours who were included in these studies64. Similar to monoclonal antibodies developed for the treatment of other types of cancer, the current development of J591 has progressed to a radioisotope-labelled version, with the goal of mediating cancer cell death by localizing a radioactive β-ray emitter close to a patient’s tumour mass65 (FIG. 2c). Several trials involving 177Lu-labelled J591 are currently in progress, including studies combining this agent with conventional cancer therapy.
Polyvalent and non-specific immunotherapy
The antigen-specific immunotherapy approaches discussed above have a distinct advantage in terms of immune monitoring: T cell responses (if they develop) can be assayed using various conventional technologies such as enzyme-linked immunosorbent spot (ELISPOT) assay66. However, skewing of the immune response, as mediated by a potent monovalent antigen-specific immunotherapy, could theoretically lead to tumour antigen loss, which has been documented in melanoma67,68, although not in prostate cancer. Polyvalent immunotherapy vectors might avoid such a situation by simul-taneously inducing an immune response to several tumour-associated antigens.
Cell-based immunotherapy
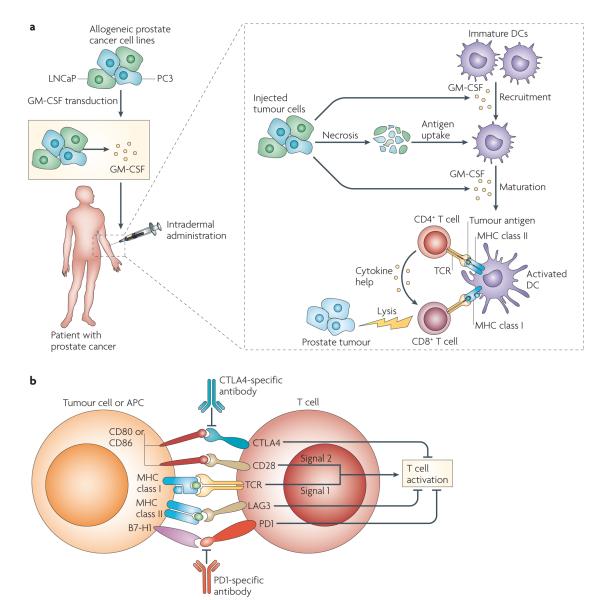
The cell-based immunotherapy known as GVAX (BioSante)69 is one example of a polyvalent approach to tumour immunotherapy, in which GM-CSF-transduced tumour cells are used as a vaccine. Such cells are injected intradermally; the GM-CSF attracts APCs and T cells to the vaccine site, thereby priming an immune response to tumour antigens61,70 (FIG. 3a). Earlier GVAX trials attempted to engineer a vaccine using autologous tumour cells from individual patients71, but it was later appreciated that tumour antigens can be cross-presented on patients’ APCs72, so further clinical development focused on allogeneic tumour cell lines of a particular cancer type transduced to secrete GM-CSF. This approach has been developed for several types of cancer, including pancreatic73, breast74, lung75, haematological76 and prostate70 cancers. Prostate GVAX, for example, includes the androgen-sensitive prostate cancer cell line LNCaP, as well as the castration-resistant prostate cancer cell line PC3 (FIG. 3a), and early phase clinical trials suggested that prostate GVAX could induce new antibodies specific for the cell lines injected77,78. Similar to sipuleucel-T, clinical development of prostate GVAX has advanced to the level of randomized Phase III clinical trials. However, for various reasons, these trials have so far not been successful79, providing important lessons regarding both clinical trial design and tumour immunology in humans (see below).
Figure 3. Immunotherapy for prostate cancer not directed towards a single tumour antigen.
a ∣ In a cell-based immunotherapy approach, allogeneic cancer cell lines specific for a particular cancer type are engineered to secrete granulocyte–macrophage colony-stimulating factor (GM-CSF), which first recruits antigen-presenting cells (APCs), such as dendritic cells (DCs), and T cells (not shown here) to the injection site. The injected vaccine tumour cells undergo necrosis, and cellular debris is taken up by the recruited DCs. Next, the DCs must mature to effectively prime an immune response; GM-CSF secreted by the vaccine cells probably has a role here as well. In the prostate GVAX approach, the injected cancer cells are allogeneic with respect to treated patients, so this immunotherapy relies on cross-presentation to prime a CD8+ T cell antitumour immune response. The prostate cancer cell lines used are LNCaP and PC3, which are androgen-sensitive and castration-resistant prostate cancer cells, respectively. b ∣ The immune checkpoint blockade approach is exemplified by antibodies specific for cytotoxic T lymphocyte antigen 4 (CTLA4) (such as ipilimumab and tremelimumab), which block the immunosuppression mediated by the interaction between CD80 and CD86 (on APCs) and CTLA4 (on CD8+ and CD4+ T cells). A second important immune checkpoint, mediated by the interaction between programmed cell death 1 (PD1) on T cells and its ligand B7-H1 (also known as PDL1) on either APCs or tumour cells, has been the subject of several recent early phase clinical trials. The interaction between lymphocyte activation gene 3 (LAG3) on T cells and MHC class II molecules on APCs is also inhibitory; indeed, CD8+ T cell unresponsiveness may depend on the interaction of several, non-overlapping checkpoints. TCR, T cell receptor.
Immune checkpoint blockade
In addition to the various immunotherapy approaches described above, recent studies in tumour immunology have focused on the concept of immune checkpoints — a series of molecules that function to limit an ongoing immune response80–82 (FIG. 3b). Furthest along in clinical development among the checkpoint inhibitors are antibodies specific for cytotoxic T lymphocyte antigen 4 (CTLA4) (ipilimumab (MDX-010; Bristol-Myers Squibb/Medarex) and tremelimumab (CP-675206; Pfizer)). The importance of CTLA4 in restraining the immune response was apparent from early mouse studies, in which Ctla4-knockout mice died at ~4–6 weeks of age from a lymphoproliferative disorder83,84. CTLA4 blockade has been evaluated in several malignancies, but the most well-developed data come from trials in patients with melanoma, in which the blocking agent is associated with an approximate 10% objective response rate but, also, (as might have been predicted from the knockout mice) a significant rate (25–35%) of clinically important immune-related toxicity85. These data are noteworthy as few significant objective responses have been noted in cancer vaccine trials86; these clinical data indicate that blocking immune checkpoints that restrain existing antitumour immune responses might be more effective than inducing a de novo antitumour response through vaccination. These data also support the notion that certain patients with cancer might have a population of tumour-specific T cells that are poised to mediate an antitumour response but that are effectively restrained by CTLA4 expression. Ipilimumab has been evaluated in several Phase I and Phase II trials in patients with prostate cancer, and objective clinical responses and decreases in PSA levels have been described87. Based on those data, Phase III trial comparing ipilimumab with a placebo is currently underway in men with castration-resistant metastatic disease who have not responded to prior chemotherapy (www.ClinicalTrials.gov, identifier: NCT00861614).
Another immunological checkpoint that has been targeted recently in clinical trials is that mediated by the molecule known as programmed cell death 1 (PD1). PD1 was initially identified in a library-based screen of CD8+ T cells undergoing apoptosis88. Subsequent work identified the ligand for PD1 as B7-H1 (also known as PDl1)89,90 and showed that the interaction between PD1 and B7-H1 leads to an inhibition of T cell function. In animal studies, PD1 blockade potentiates an antitumour immune response91–93, and PD1-deficient animals develop a degree of strain-specific autoimmunity (albeit with a milder phenotype than Ctla4-knockout mice)94,95. Perhaps most importantly, human studies showed that increased expression of B7-H1 was associated with a poor clinical outcome in several tumour types, most notably in renal cell carcinoma96. PD1 has been less well studied in prostate cancer, although we have found that the CD8+ T cells that infiltrate the prostate gland in men with cancer seem to express PD1 (REF. 97). Similar findings were recently reported in patients with melanoma, suggesting that PD1 expression by tumour-infiltrating lymphocytes might be a common occurence98. A Phase I clinical trial of a fully human monoclonal antibody targeting PD1 (MDX-1106; Bristol-Meyers Squibb) has been completed, with interesting results99. First, this agent was remarkably well tolerated, with few serious adverse events noted. Second, several objective clinical responses were noted in patients with various types of cancer, which is unusual for a Phase I trial of immunotherapy in a heavily pre-treated patient population. Taken together, these data reinforce the relative importance of immune checkpoint blockade in tumour immunotherapy. In addition, if confirmed in larger studies, it would seem that the benign toxicity profile of PD1 blockade could render it an ideal candidate for future combinatorial trials.
Clinical trials of prostate cancer immuno therapy
Several of the immunotherapy approaches for prostate cancer discussed above have been tested in large clinical trials (TABLE 1); a targeted overview of these trials can give unique insights into immunotherapy that might apply to other solid tumours. In addition, these trials provide interesting data regarding the translation of immunological concepts from the laboratory to a clinical setting.
Table 1.
Selected clinical trials of immunotherapy for patients with prostate cancer*
| Agent | Sponsoring corporation or institution |
Clinical trial phase |
Design or description of trial |
Number of subjects |
Status | Immune system effects |
Clinical results or comments |
Refs |
|---|---|---|---|---|---|---|---|---|
| Sipuleucel-T | Dendreon Inc. | III | Randomized, placebo-controlled trial of sipuleucel-T versus placebo in men with asymptomatic, metastatic CRPC (the D9901 trial) |
127 | Completed | Increased T cell stimulation index to vector in treated patients |
Primary endpoint (TTP) not met; secondary end point (median survival duration) significantly increased (25.9 versus 21.4 months) |
101 |
| Sipuleucel-T | Dendreon Inc. | III | Randomized, placebo-controlled trial of sipuleucel-T versus placebo in men with asymptomatic, metastatic CRPC (the D9902B trial) |
512 | Completed | Not reported | Primary endpoint (overall survival) met (25.8 versus 21.7 months) |
141 |
| ProstVac | Cancer Therapy Evaluation Program (CTEP) |
II | Randomized trial testing the relative sequencing of recombinant fowlpox virus–PSA and recombinant vaccinia virus–PSA in men with biochemically recurrent prostate cancer (the ECOG 7897 trial) |
64 | Completed | Increased ELISPOT assay response to vaccinia virus–PSA with fowlpox virus boost versus fowlpox virus–PSA with vaccinia virus boost |
Well tolerated | 53 |
| ProstVac VF | Cancer Therapy Evaluation Program (CTEP) |
II | Randomized trial comparing ProstVac VF with hormonal therapy in men with non-metastatic CRPC; crossover allowed |
42 | Completed | Increased ELISPOT assay response to PSA in evaluable patients (n = 8) in ProstVac VF- treated group versus hormonal therapy-treated group |
Increased survival in men treated with ProstVac VF followed by hormonal therapy |
30, 119 |
| ProstVac VF | Therion Biologics (rights now owned by Bavarian Nordic) |
II | Randomized trial comparing ProstVac VF with placebo in men with asymptomatic CRPC (the TBC-PRO-002 trial) |
127 | Completed | No humoral responses to PSA |
Primary endpoint (TTP) not met; overall survival duration (secondary end point) increased (25.1 versus 16.6 months) |
102 |
| Prostate GVAX |
Cell Genesys (rights now owned by BioSante) |
I/II | Dose escalation of prostate GVAX in men with metastatic CRPC (the G0029 trial) |
80 | Completed | Increased antibody response to vaccine cell lines |
Well tolerated; defined dose or schedule for subsequent Phase III trials |
134 |
| Prostate GVAX |
Cell Genesys (rights now owned by BioSante) |
III | Open-label, randomized trial comparing prostate GVAX with docetaxel chemotherapy in men with asymptomatic, metastatic CRPC (the VITAL-1 trial) |
621 | Closed | ND | Closed after an unplanned interim analysis showed futility |
79 |
| Prostate GVAX |
Cell Genesys (rights now owned by BioSante) |
III | Open-label, randomized trial comparing prostate GVAX plus docetaxel chemotherapy with docetaxel chemotherapy in men with symptomatic, metastatic CRPC (the VITAL-2 trial) |
114 (600 planned) |
Halted | ND | Halted after an interim analysis showed imbalance of deaths in combined treatment group (67 versus 47) |
135 |
| Ipilimumab | Bristol-Myers Squibb/Medarex |
III | Randomized, placebo-controlled trial comparing low-dose radiation with or without ipilimumab in men with metastatic CRPC previously treated with docetaxel chemotherapy (the CA184-043 trial) |
800 planned |
Ongoing | ND | ND |
www.ClinicalTrials.gov identifier: NCT00861614 |
|
177Lu–J591 (PSMA- specific antibody) |
Weill Medical College of Cornell University |
I | Single-armed trial of 177Lu-labelled PSMA-specific antibody |
35 | Completed | ND | Dose limiting myelotoxicity; well tolerated; Phase II dose determined |
65 |
|
177Lu–J591 (PSMA- specific antibody) |
Weill Medical College of Cornell University |
II | Randomized trial comparing hormonal therapy (ketoconazole) with or without 177Lu-labelled J591 in men with non-metastatic CRPC |
140 | Ongoing | ND | ND |
www.ClinicalTrials.gov identifier: NCT00859781 |
| DNA vaccine (prostatic acid phosphatase) |
University of Wisconsin Waisman Clinical Biomanufacturing Facility |
I | Testing the safety and tolerability of a new DNA-based immunotherapy platform in men with biochemically recurrent prostate cancer |
22 | Completed | 9 out of 22 patients showed increased T cell proliferation in response to PAP; humoral response to PAP not detected |
No significant treatment- related adverse events; increase in PSA doubling times versus baseline |
62 |
CRPC, castration-resistant prostate cancer; ELISPOT, enzyme-linked immunosorbent spot; ND, not determined; PAP, prostatic acid phosphatase; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; TTP, time to tumour progression.
These studies show the variety of immunotherapy approaches that have been translated from the laboratory to a clinical setting, as well as the various stages of clinical development. Only selected, illustrative trials are shown here; additional trials are discussed in several recent reviews136–140.
Survival is the most robust clinical trial endpoint
Similar to the readout in a laboratory experiment, a clinical trial must also have a readout, known as a primary endpoint. Clinical trials in patients with cancer often use some measure of tumour progression as the primary endpoint, quantified by a set of formalized criteria known as RECIST (response evaluation criteria in solid tumours) or World Health organization (WHo) criteria. It is important to note that the RECIST system was developed in the era of cytotoxic chemotherapy, and so these criteria are based on two implicit assumptions: effective therapies shrink tumours, and tumour shrinkage translates to patient benefit100. So, a typical endpoint for an oncology clinical trial might be time to tumour progression (TTP), with progression assayed by RECIST. Indeed the first randomized Phase III trial of sipuleucel-T (the D9901 trial)101 was designed with a TTP endpoint, as was a recently published randomized Phase II trial of ProstVac VF102. A statistically significant difference in TTP was not observed between the active immunotherapy and placebo groups in either case. By contrast, both trials showed a clear and statistically significant difference in overall survival between immunotherapy and placebo groups. This observation is consistent with the results of a recently reported randomized Phase III trial of ipilimumab in patients with metastatic melanoma, for which improved survival rates were observed despite a low rate (11%) of objective clinical responses103. The mechanisms behind this discrepancy are not completely understood, but they might involve tumour progression before shrinkage, delayed responses and/or prolonged disease stabilization leading to clinical benefit. Modified versions of RECIST and WHO criteria have been proposed, which use alternative definitions of a response and so might more accurately assess the potential clinical benefits of immunotherapy104. However, reliance on overall survival as a clinical trial endpoint means that trial registration for men with prostate cancer is currently limited to patients with castration-resistant metastatic disease, which, given the belief that cancer immunotherapy is probably most efficacious in a minimal residual disease setting105, means that such trials might not indicate the true potential of a therapy.
Immunotherapy might be more efficacious with a lower disease burden
Although an inverse relationship between tumour burden and immunotherapy response might seem intuitively obvious106, there is some controversy surrounding this idea, as objective clinical responses have been noted in patients with advanced cancers treated with adoptive T cell therapy107, as well as in patients with several tumour types treated with checkpoint blockade108. Nevertheless, a recently published retrospective study of men with prostate cancer enrolled on a Phase II single-armed trial of ProstVac VF provides some clinical evidence for this concept. Here, a well-established predictive algorithm, the Halabi nomogram109, was used to stratify patients into those with predicted survival duration greater than or less than the median at the time of trial enrolment105. Interestingly, patients with less advanced disease (those with a Halabi-predicted survival duration greater than the median) seemed to benefit clinically from immunotherapy with ProstVac VF, in that their observed survival was significantly longer than predicted. Conversely, patients with a predicted survival duration less than the median seemed not to have any survival benefit from the immunotherapy under study.
Phase III trials should be based on data from Phase II studies
In contrast to Phase III trials in other medical disciplines, such trials often end in failure in patients with cancer — the agent under study does not produce the clinical benefit that the trial was intended to assay110. Several hypotheses have been put forward to explain this issue, but perhaps the simplest concerns Phase II trials, which are usually carried out to more accurately quantify the clinical benefit of an agent or approach before moving to a larger, more expensive Phase III study. Although the positive predictive value of Phase II trials for Phase III outcome is not particularly robust, the negative predictive value is strong: a negative Phase II trial in oncology clearly predicts a negative Phase III result111. This issue is exemplified by the development of GVAX immunotherapy for prostate cancer. Early (Phase II) studies established the safety of GVAX71, and immunological correlates (the development of tumour antigen-specific antibodies) were used to select a dosing regimen112. However, in these Phase II studies, GVAX was never compared directly with chemotherapy, and it was not administered in sequence with chemotherapy. Nevertheless, two Phase III trials were launched — both of which used a chemotherapy comparator group. In the first trial (VITAl-1), GVAX immunotherapy was directly compared with chemotherapy in men with asymptomatic, castration-resistant prostate cancer, despite the fact that few radiographically detectable responses were noted in the Phase II GVAX studies. A second Phase III trial (VITAl-2) was subsequently initiated to test the hypothesis that the combination of immunotherapy plus chemotherapy would extend survival in men with more advanced (symptomatic), metastatic disease. Although chemotherapy had been shown to be at least additive with immunotherapy in animal studies113, no Phase II trials were carried out to verify this result in humans or to explore dose and schedule questions. A planned interim analysis of VITAl-2 showed a greater number of deaths in patients treated with the combination of GVAX plus chemotherapy and the trial was closed. Unfortunately, this imbalanced out-come has yet to be explained, by either immunological or clinical mechanisms. These events led to an unplanned interim analysis of the earlier trial (VITAl-1), which showed that the trial was unlikely to meet its primary (survival) endpoint and should be stopped. In hindsight, these data emphasize the importance of Phase II testing, in particular for combination approaches in which dosing and timing might be crucial41,114.
Combination immunotherapy
Most treatment regimens for patients with advanced cancer include a combination of chemotherapy drugs, or a combination of radiation therapy and chemotherapy. So, it is possible that cancer immunotherapy will need to be combined with conventional therapy to achieve maximal patient benefit. Fortunately, many conventional treatments for prostate and other cancers have beneficial immunological effects (see below), making combinatorial trials an attractive proposition. Even chemotherapy, which is broadly viewed as immunosuppressive, might to some extent boost an antitumour response115. However, issues of dose and schedule could be crucial, and Phase II trials are required to explore such issues before the initiation of larger studies.
Androgen ablation
The immunological effects of androgen ablation are surprising because they involve the thymus, which is generally not thought of as an androgen-sensitive organ25 (BOX 2). In aged mice, androgen ablation seems to result in regeneration of the normally involuted thymus and in the output of new T cells, as assayed by increased numbers of T cell receptor excision circles in the peripheral blood29. Similar effects have been observed in humans. As noted above, androgen ablation before prostate cancer surgery results in the infiltration of CD4+ T cells into the prostate gland, and these cells have an activated phenotype26. Also supporting a pro-immunogenic role for androgen ablation are recent data showing the induction of new antibody specificities in treated patients116,117. So, the notion that androgen ablation might enhance an anti-prostate cancer immune response has a strong scientific basis and has been evaluated in several clinical trials. An early study tested one dose of vaccinia virus–PSA vaccine (ProstVac) in combination with androgen ablation, finding the combination to be well tolerated118. In a later randomized study, immune responses to ProstVac were more commonly observed in men who received androgen ablation after active immunotherapy119, as opposed to receiving androgen ablation before immunotherapy. Sipuleucel-T has also been tested in combination with androgen ablation; in this case, immunotherapy was administered after androgen ablation, but data from this study have yet to be published. Taken together, these studies support the notion that combined immune and hormonal therapy might be clinically interesting and worthy of further evaluation. Although this concept could conceivably be applied to other hormone-sensitive cancers, such as breast cancer, we are not aware of any such clinical trials at this time.
Box 2. Immunological effects of androgen ablation.
Androgen ablation — which is usually carried out chemically by administration of a leuteinizing-hormone-releasing hormone (LHRH) agonist such as leuprolide acetate or zoledronic acid — is by far the most common treatment for prostate cancer, in patients with both biochemically recurrent and metastatic disease. Recent studies have documented profound immunological effects of this common pharmacological therapy, all of which would be expected to enhance an antitumour immune response:
CD4+ T cell infiltration into the prostate gland26;
Increase in CD8+ T cell and macrophage density in the prostate gland27;
Mitigation of CD4+ T cell tolerance to a prostate- and prostate cancer-restricted antigen28;
Reversal of thymic involution in aged mice29;
Increase in thymic output of T cells29;
Enhancement of efficacy of immunotherapy in animal models31.
Several groups have attempted to make use of these effects in a clinical trial setting25; one notable ongoing trial combines androgen ablation with blockade of the immune checkpoint molecule cytotoxic T lymphocyte antigen 4 (CTLA4) using the monoclonal antibody ipilimumab (MDX-010; Bristol-Myers Squibb/Medarex) in men undergoing surgical resection for prostate cancer (www.ClinicalTrials.gov identifier: NCT00170157).
Radiotherapy
Although the cytotoxic effects of radiation therapy are well appreciated, recent data support the notion that irradiation of cancer cells can prime an antitumour immune response120. On a cellular basis, this process seems to involve the uptake of dying tumour cells by APCs121 and the presentation of tumour antigens to immune cells, as well as the induction of a pro-inflammatory microenvironment by the radiation122. In patients with prostate cancer, evidence for an immunological effect of radiotherapy is provided by data showing the induction of new antibody specificities following radiotherapy treatment116. Although the molecular mechanisms for these immunological effects of radiotherapy are complex115, recent work has shown that high mobility group box 1 (HMGB1) released from dying tumour cells can function as a TLR4 agonist, activating APCs in either the tumour parenchyma or in the draining lymph node (or nodes), and so priming an immune response120. It should be noted that these immunostimulatory effects are not unique to radiation-induced tumour cell death and can also be elicited when tumour cells are killed by certain chemotherapy agents. Several preclinical studies support the notion that combining irradiation with immunotherapy can be either additive or synergistic in terms of the antitumour response123,124. This concept has been evaluated clinically in a small randomized trial of men undergoing primary radiotherapy for prostate cancer125; 13 out of 17 patients in the radiotherapy and immunotherapy combination treatment group had a greater than threefold increase in the number of PSA-specific T cells, whereas no increase in the number of PSA-specific T cells was noted in the group that received radiotherapy alone. However, as is the case for combining chemotherapy with immunotherapy, the relative sequencing of agents might be crucial126. An ongoing, randomized Phase III trial of ipilimumab in men with metastatic castration-resistant prostate cancer, who have failed chemotherapy, includes a low dose of radio therapy in an effort to prime an initial antitumour immune response. As radiotherapy is routinely used in the primary therapy of several tumour types, combination radiotherapy and immunotherapy might be of increasing interest as specific immunotherapy agents are developed for other types of cancer.
Checkpoint blockade
A new approach to immunotherapy involves the combination of several immunological agents to both prime an antitumour response and prevent the suppression of existing and new responses. In prostate cancer, the first published data come from a study combining a CTLA4-specific antibody (ipilimumab) with GM-CSF in an effort to stimulate an endogenous antitumour immune response48. At higher doses of ipilimumab, radiographically detectable antitumour responses were noted; the data strongly suggested a threshold dosing effect, with responses noted at levels of ipilimumab greater than 3 mg per kg. Another relevant concept might be to combine an active, specific immunotherapy with an immune checkpoint-blocking agent. In an early clinical test of this concept, prostate GVAX was combined with ipilimumab in a doseescalation study127. Decreases in PSA levels, as well as radiographically detectable tumour responses, were noted but, as is sometimes the case with ipilimumab, these responses were associated with immune-related adverse events, including hypophisitis128. A similar combination study has been carried out with ProstVac VF, the results of which are currently pending, and a trial combining pancreatic GVAX with ipilimumab is in early stages of accrual. It should be noted that the incidence of high-grade toxicity of ipilimumab may be a limiting factor in these studies. Should PD1-specific antibodies prove to be better tolerated, but still effective as a checkpoint inhibitor, combination trials with PD1-specific antibodies might be more feasible to design and complete.
In summary, there is a strong scientific rationale for combining different types of immunotherapy, as well as for combining immunotherapy with conventional therapy. In some types of cancer, such as breast cancer, these approaches might include tumour-targeted monoclonal antibodies129 or targeted drugs such as imatinib for chronic myelogenous leukaemia130. But such approaches add complexity to clinical trial design, and issues of dosing and sequence are a notable challenge.
Conclusions
The clinical development of immunotherapy for prostate cancer is an instructive example of translational science, in that immunological approaches pioneered in animal studies have eventually proven to have clinical benefit in humans with cancer. The challenges involved in assessing clinical benefit in early disease stages have so far prevented using these therapies in patients with a less prominent tumour burden, but the recent FDA approval of sipuleucel-T could facilitate new opportunities for appropriate earlier stage trials. Achieving long-term remission in most treated patients is an ambitious goal for basic and clinical scientists, and probably requires the careful integration of several treatment modalities in rational combination therapy approaches.
Acknowledgements
C.G.D. is a Damon Runyon-Lilly Clinical Investigator. This work was also supported by US National institutes of Health grants R01 CA127153 and P50 CA058236, the Patrick C. Walsh Fund, the David Koch Foundation and the Prostate Cancer Foundation. The author would like to acknowledge D. Pardoll and E. Antonarakis for review of this manuscript.
Glossary
- Localized disease
In prostate cancer, this usually refers to disease that does not extend beyond the prostate gland itself, which can be treated with radiotherapy, surgery or the removal of androgens.
- Recurrent disease
Cancer that has returned following primary therapy. Recurrent prostate cancer can be detected by a rising level of prostate-specific antigen only (biochemical recurrence) or by computerised tomography or bone scans (metastatic disease).
- Androgen
A type of steroid hormone that controls the male characteristics of vertebrate animals. Testosterone is the best example of an androgen important in prostate cancer, but its metabolite, dihydrotestosterone, is the more potent form in most tissues.
- Chemical castration
A therapy to decrease circulating androgen levels through pharmacological intervention. In patients with prostate cancer, this is carried out using leuteinizing hormone-releasing hormone (LHRH) antagonists, which act on the hypothalamus to centrally mediate a decrease in testosterone secretion.
- Surgical castration
The removal of the testicles to decrease circulating androgen levels. It should be noted that androgens are also secreted by the adrenal cortex, so that surgical castration does not completely eliminate androgens from the blood.
- Castration-resistant disease
Prostate cancer that can be shown to be progressing through a rising prostatespecific antigen level or by imaging studies, despite a low or undetectable level of testosterone in the blood following chemical or surgical castration.
- Surrogate endpoint
A biological marker used in a clinical trial to substitute for a clinically relevant endpoint. Some examples include cholesterol level, which can be a surrogate endpoint for studies aiming to reduce the risk of heart disease, or CD4+ T cell count, which can be a surrogate endpoint for reducing the chance of death from opportunistic infections in patients with HIV.
- Autochthonous
Arising spontaneously over time. Mouse tumour models that are autochronous may more accurately model the natural immune response to cancers, as evolving cancers are recognized by the immune system and induce a tolerogenic state.
- TRAMP model
(transgenic adenocarcinoma of the mouse prostate model). A mouse model of prostate cancer in which prostate cancers arise spontaneously because the SV40 large T antigen is expressed in a prostate-restricted manner, downregulating the tumour suppressor molecules P53 and RB locally.
- Central deletion
Self tolerance that is created at the level of the central lymphoid organs. Developing T cells in the thymus, and B cells in the bone marrow, that strongly recognize self antigen face deletion or marked suppression.
- Passive immunotherapy
The induction of immunity by the transfer of immunoglobulins or T cells.
- Active immunization
The induction of immunity by activation or expansion of the endogenous immune repertoire.
- Primary endpoint
The main result that is measured at the end of a clinical trial to determine whether the hypothesis under study has been fulfilled.
- RECIST
(Response evaluation criteria in solid tumours). A set of formally defined rules used to measure objective clinical responses in cancer patients treated with a particular therapy. These parameters measure a series of index lesions and quantify whether the lesions have decreased in size.
- Single-armed trial
A clinical trial without a concurrent control group.
- Halabi nomogram
A model that uses historical data to estimate the survival of patients with progressive prostate cancer after castration.
- T cell receptor excision circles
DNA episomes that are normally produced during the thymic maturation of T cells, specifically during recombination of the T cell receptor genes.
- Hypophisitis
Inflammation of the pituitary gland, which can be induced in patients with cancer by CTLA4-blocking antibodies. Clinically, hypophysitis is characterized by decreased levels of thyroid hormone, cortisol and other hormones.
Footnotes
Competing interests statement The author declares competing financial interests: see Web version for details.
DATABASES ClinicalTrials.gov: http://clinicaltrials.gov/
NCT00170157 ∣ NCT00859781 ∣ NCT00861614
UniProtKB: http://www.uniprot.org
B7-H1 ∣ CD80 ∣ CTLA4 ∣ GM-CSF ∣ ICAM1 ∣ LFA3 ∣ PAP ∣ PD1 ∣ PSA ∣ PSMA
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Stat. bite: estimated worldwide cancer mortality among men, 2002. J. Natl Cancer Inst. 2005;97:1402. doi: 10.1093/jnci/dji336. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 2.Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N. Engl. J. Med. 2007;357:2696–2705. doi: 10.1056/NEJMcp0706784. [DOI] [PubMed] [Google Scholar]
- 3.Carter HB, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J. Urol. 2007;178:2359–2364. doi: 10.1016/j.juro.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannock IF, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Petrylak DP, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, et al. Cumulative effect of five genetic variants on prostate cancer risk in multiple study populations. Prostate. 2008;68:1257–1262. doi: 10.1002/pros.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nature Rev. Cancer. 2004;4:519–527. doi: 10.1038/nrc1389. [DOI] [PubMed] [Google Scholar]
- 8.De Marzo AM, et al. Inflammation in prostate carcinogenesis. Nature Rev. Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. A recent study supporting the concept that B cells mediate the progression of prostate cancer from an androgen-dependent to a castration-resistant state in a mouse model.
- 10.Luo JL, et al. Nuclear cytokine-activated IKKα controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 11.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature Rev. Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 12.Sfanos KS, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin. Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller AM, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J. Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 14.Fox SB, et al. The number of regulatory T cells in prostate cancer is associated with the androgen receptor and hypoxia-inducible factor (HIF)-2α but not HIF-1α. Prostate. 2007;67:623–629. doi: 10.1002/pros.20538. [DOI] [PubMed] [Google Scholar]
- 15.Kiniwa Y, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin. Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 16.Bronte V, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J. Exp. Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zippelius A, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 18.Scher HI, Heller G. Clinical states in prostate cancer: toward a dynamic model of disease progression. Urology. 2000;55:323–327. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 19.Pound CR, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 20.Sandler HM, Eisenberger MA. Assessing and treating patients with increasing prostate specific antigen following radical prostatectomy. J. Urol. 2007;178:S20–S24. doi: 10.1016/j.juro.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 21.Freedland SJ, Moul JW. Prostate specific antigen recurrence after definitive therapy. J. Urol. 2007;177:1985–1991. doi: 10.1016/j.juro.2007.01.137. [DOI] [PubMed] [Google Scholar]
- 22.Locker GY, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 23.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv. Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 24.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nature Rev. Cancer. 2002;2:389–396. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front. Biosci. 2007;12:4957–4971. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 26.Mercader M, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc. Natl Acad. Sci. USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. The first clinical demonstration that androgen ablation alters the local immune environment in the prostate gland, resulting in an influx of CD4+ T cells with effector function.
- 27.Gannon PO, et al. Characterization of the intraprostatic immune cell infiltration in androgen-deprived prostate cancer patients. J. Immunol. Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Drake CG, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. A study in mice with autochronous prostate tumours supporting the concept that androgen ablation results in a mitigation of specfic CD4+ T cell tolerance to the prostate gland.
- 29.Sutherland JS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 30.Arlen PM, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J. Urol. 2005;174:539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 31.Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69:571–584. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler HL, et al. Elevated levels of circulating interleukin-6 and transforming growth factor-β1 in patients with metastatic prostatic carcinoma. J. Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- 33.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 34.Mihalyo MA, Hagymasi AT, Slaiby AM, Nevius EE, Adler AJ. Dendritic cells program non-immunogenic prostate-specific T cell responses beginning at early stages of prostate tumorigenesis. Prostate. 2007;67:536–546. doi: 10.1002/pros.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg NM, et al. Prostate cancer in a transgenic mouse. Proc. Natl Acad. Sci. USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Degl’Innocenti E, et al. Peripheral T cell tolerance occurs early during spontaneous prostate cancer development and can be rescued by dendritic cell immunization. Eur. J. Immunol. 2005;35:66–75. doi: 10.1002/eji.200425531. [DOI] [PubMed] [Google Scholar]
- 37.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc. Natl Acad. Sci. USA. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J. Immunol. 2007;178:1268–1276. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- 39.Lees JR, et al. T-cell recognition of a prostate specific antigen is not sufficient to induce prostate tissue destruction. Prostate. 2006;66:578–590. doi: 10.1002/pros.20307. [DOI] [PubMed] [Google Scholar]
- 40.Grosso JF, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. These data show that lymphocyte activation gene 3 (LAG3) is an important immune checkpoint on CD8+ T cells and that blocking LAG3 can enhance T cell effector function in mice with cancer.
- 41.Wada S, et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Speiser DE, et al. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J. Exp. Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyman MA, Aung S, Biggs JA, Sherman LA. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J. Immunol. 2004;172:6558–6567. doi: 10.4049/jimmunol.172.11.6558. [DOI] [PubMed] [Google Scholar]
- 44.Getnet D, et al. Tumor recognition and self-recognition induce distinct transcriptional profiles in antigen-specific CD4 T cells. J. Immunol. 2009;182:4675–4685. doi: 10.4049/jimmunol.0803400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shafer-Weaver KA, et al. Cutting edge: tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J. Immunol. 2009;183:4848–4852. doi: 10.4049/jimmunol.0900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milowsky MI, et al. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J. Clin. Oncol. 2007;25:540–547. doi: 10.1200/JCO.2006.07.8097. [DOI] [PubMed] [Google Scholar]
- 47.Williams SA, Singh P, Isaacs JT, Denmeade SR. Does PSA play a role as a promoting agent during the initiation and/or progression of prostate cancer? Prostate. 2007;67:312–329. doi: 10.1002/pros.20531. [DOI] [PubMed] [Google Scholar]
- 48.Fong L, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PubMed] [Google Scholar]
- 49.Arlen PM, Kaufman HL, DiPaola RS. Pox viral vaccine approaches. Semin. Oncol. 2005;32:549–555. doi: 10.1053/j.seminoncol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Hodge JW, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 51.Terasawa H, Tsang KY, Gulley J, Arlen P, Schlom J. Identification and characterization of a human agonist cytotoxic T-lymphocyte epitope of human prostate-specific antigen. Clin. Cancer Res. 2002;8:41–53. [PubMed] [Google Scholar]
- 52.Harrington LE, Most RR, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaufman HL, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 54.Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin. Investig. Drugs. 2009;18:1001–1011. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gulley JL, Madan RA, Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25:B89–B96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higano CS, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, Phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 57.Gilboa E. DC-based cancer vaccines. J. Clin. Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fong L, Ruegg CL, Brockstedt D, Engleman EG, Laus R. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. J. Immunol. 1997;159:3113–3117. [PubMed] [Google Scholar]
- 59.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nature Rev. Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 60.Tsen SW, Paik AH, Hung CF, Wu TC. Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert Rev. Vaccines. 2007;6:227–239. doi: 10.1586/14760584.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Y, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 62.McNeel DG, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase (PAP) in patients with stage D0 prostate cancer. J. Clin. Oncol. 2009;27:425–430. doi: 10.1200/JCO.2008.19.9968. A recent Phase I trial showing how DNA-based vaccines can be used in prostate cancer. This trial is noteworthy because it targeted a population of patients with early-stage disease and because the DNA vector used is flexible in terms of potential antigens that could be targeted.
- 63.Tagawa ST, et al. Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer. 2010;116:1075–1083. doi: 10.1002/cncr.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nanus DM, et al. Clinical use of monoclonal antibody HuJ591 therapy: targeting prostate specific membrane antigen. J. Urol. 2003;170:S84–S88. doi: 10.1097/01.ju.0000095151.97404.7c. [DOI] [PubMed] [Google Scholar]
- 65.Bander NH, et al. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J. Clin. Oncol. 2005;23:4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 66.Keilholz U, et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the society for biological therapy. J. Immunother. 2002;25:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Jager E, et al. Immunoselection in vivo: independent loss of MHC class I and melanocyte differentiation antigen expression in metastatic melanoma. Int. J. Cancer. 1997;71:142–147. doi: 10.1002/(sici)1097-0215(19970410)71:2<142::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 68.Ohnmacht GA, et al. Short-term kinetics of tumor antigen expression in response to vaccination. J. Immunol. 2001;167:1809–1820. doi: 10.4049/jimmunol.167.3.1809. [DOI] [PubMed] [Google Scholar]
- 69.Dranoff G, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. This study provided the initial immunological and scientific rationale for targeting cancer using tumour cell vaccines engineered to secrete GM-CSF.
- 70.Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol. Oncol. 2006;24:419–424. doi: 10.1016/j.urolonc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 71.Simons JW, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res. 1997;57:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas AM, et al. Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J. Exp. Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaffee EM, et al. Novel allogeneic granulocyte–macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a Phase I trial of safety and immune activation. J. Clin. Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 74.Emens LA, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J. Clin. Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nemunaitis J, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006;13:555–562. doi: 10.1038/sj.cgt.7700922. [DOI] [PubMed] [Google Scholar]
- 76.Borrello IM, et al. Granulocyte–macrophage colony-stimulating factor (GM-CSF)-secreting cellular immunotherapy in combination with autologous stem cell transplantation (ASCT) as postremission therapy for acute myeloid leukemia (AML) Blood. 2009;114:1736–1745. doi: 10.1182/blood-2009-02-205278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Small EJ, et al. Granulocyte macrophage colony-stimulating factor-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin. Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen MC, et al. Antibody responses to galectin-8, TARP and TRAP1 in prostate cancer patients treated with a GM-CSF-secreting cellular immunotherapy. Cancer Immunol. Immunother. 2010;59:1313–1323. doi: 10.1007/s00262-010-0858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Higano C, et al. A Phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC) [online]. American Society of Clinical Oncology 2009 Genitourinary Cancers Symposium.2009. [Google Scholar]
- 80.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J. Clin. Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 81.Chen L. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nature Rev. Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 82.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 84.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 85.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol. Immunother. 2009;58:823–830. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beer TM, et al. Phase I trial of ipilimumab (IPI) alone and in combination with radiotherapy (XRT) in patients with metastatic castration resistant prostate cancer (mCRPC) J. Clin. Oncol. Abstr. 2008;26(Suppl. 15):5004. [Google Scholar]
- 88.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 90.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hirano F, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 93.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int. Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. References 92 and 93 are among the first to highlight a potential role for PD1 blockade in cancer immunotherapy.
- 94.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 95.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 96.Thompson RH, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl Acad. Sci. USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sfanos KS, et al. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1 + Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. References 97 and 98 show that the CD8+ T cells that infiltrate tumours are likely to express PD1, suggesting that PD1 blockade may have therapeutic relevance.
- 99.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. One of the first clinical trials in which the immune checkpoint molecule PD1 was blocked with a monoclonal antibody; these data provided evidence for clinical activity together with a generally benign side-effect profile.
- 100.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 101.Small EJ, et al. Placebo-controlled Phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. The first randomized Phase III trial to show a clinical benefit for antigen-specific immunotherapy in patients with prostate cancer.
- 102.Kantoff PW, et al. Overall survival analysis of a Phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010 Jun 14; doi: 10.1056/NEJMoa1003466. (doi:10.1056/NEJMoa1003466) The first randomized Phase III trial to show a clinical benefit for blockade of the immune checkpoint molecule CTLA4 in patients with cancer.
- 104.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 105.Gulley JL, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol. Immunother. 2009;59:663–674. doi: 10.1007/s00262-009-0782-8. A retrospective analysis showing that virus-based cancer vaccines may be efficacious only in the setting of a low disease burden.
- 106.Finn OJ. Cancer vaccines: between the idea and the reality. Nature Rev. Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 107.Dudley ME, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Halabi S, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J. Clin. Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 110.Hales RK, et al. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann. Oncol. 2010 Mar 17; doi: 10.1093/annonc/mdq048. (doi:10.1093/annonc/mdq048) [DOI] [PubMed] [Google Scholar]
- 111.Ratain MJ. Phase II oncology trials: let’s be positive. Clin. Cancer Res. 2005;11:5661–5662. doi: 10.1158/1078-0432.CCR-05-1046. [DOI] [PubMed] [Google Scholar]
- 112.Higano CS, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 113.Prell RA, Gearin L, Simmons A, Vanroey M, Jooss K. The anti-tumor efficacy of a GM-CSF-secreting tumor cell vaccine is not inhibited by docetaxel administration. Cancer Immunol. Immunother. 2006;55:1285–1293. doi: 10.1007/s00262-005-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Machiels JP, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 115.Zitvogel L, et al. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nesslinger NJ, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin. Cancer Res. 2007;13:1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 117.Morse MD, McNeel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum. Immunol. 2010;71:496–504. doi: 10.1016/j.humimm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanda MG, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 119.Madan RA, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin. Cancer Res. 2008;14:4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. These clinical data support the concept that it is important to consider the relative sequencing of conventional treatment (in this case, androgen ablation) with respect to vaccination.
- 120.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 2007;13:1050–1059. doi: 10.1038/nm1622. An interesting mechansistic study, showing that certain cancer treatments may prime an immune response through TLR4 agonists released by dying tumour cells.
- 121.Lugade AA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 122.Chakraborty M, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J. Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 123.Demaria S, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 124.Chakraborty M, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 125.Gulley JL, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin. Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 126.Harris TJ, et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate. 2008;68:1319–1329. doi: 10.1002/pros.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gerritsen W, et al. A dose-escalation trial of GM-CSF-gene transduced allogeneic prostate cancer cellular immunotherapy in combination with a fully human anti-CTLA4 antibody (MDX-010, ipilimumab) in patients with metastatic hormone-refractory prostate cancer (MHRPC) Ann. Oncol. 2007;18:24. [Google Scholar]
- 128.Blansfield JA, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J. Immunother. 2005;28:593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wolpoe ME, et al. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte macrophage colony-stimulating factor secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in Her-2/neu-transgenic mice. J. Immunol. 2003;171:2161–2169. doi: 10.4049/jimmunol.171.4.2161. [DOI] [PubMed] [Google Scholar]
- 130.Smith BD, et al. K562/GM-CSF immunotherapy reduces tumor burden in chronic myeloid leukemia patients with residual disease on imatinib mesylate. Clin. Cancer Res. 2010;16:338–347. doi: 10.1158/1078-0432.CCR-09-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schroder FH, et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 132.Andriole GL, et al. Mortality results from a randomized prostate-cancer screening trial. N. Engl. J. Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Greene KL, et al. Prostate specific antigen best practice statement: 2009 update. J. Urol. 2009;182:2232–2241. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 134.Higano CS, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 135.Small EJ, et al. A Phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel vs. docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC). [online]. American Society for Clinical Oncology 2009 Genitourinary Cancers Symposium.2009. [Google Scholar]
- 136.Antonarakis ES, Drake CG. Current status of immunological therapies for prostate cancer. Curr. Opin. Urol. 2010;20:241–246. doi: 10.1097/MOU.0b013e3283381793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kipp RT, McNeel DG. Immunotherapy for prostate cancer — recent progress in clinical trials. Clin. Adv. Hematol. Oncol. 2007;5:465–469. [PubMed] [Google Scholar]
- 138.Mohebtash M, Gulley JL, Madan RA, Ferrara T, Arlen PM. Cancer vaccines: current directions and perspectives in prostate cancer. Curr. Opin. Mol. Ther. 2009;11:31–36. [PMC free article] [PubMed] [Google Scholar]
- 139.Fong L, Small EJ. Immunotherapy for prostate cancer. Curr. Oncol. Rep. 2007;9:226–233. doi: 10.1007/s11912-007-0026-z. [DOI] [PubMed] [Google Scholar]
- 140.Slovin SF. Pitfalls or promise in prostate cancer immunotherapy — which is winning? Cancer J. 2008;14:26–34. doi: 10.1097/PPO.0b013e318161bffa. [DOI] [PubMed] [Google Scholar]
- 141.Kantoff P, et al. Updated survival results of the IMPACT trial of sipuleucel-T for metastatic castration-resistant prostate cancer (CRPC) [Online] American Society of Clinical Oncology 2010 Genitourinary Cancers Symposium. 2010 [Google Scholar]