Abstract
Organic anion-transporting polypeptides (OATPs) are multispecific transporters that mediate the uptake of numerous drugs and xenobiotics into cells. Here, we examined the effect of green tea (Camellia sinensis) catechins on the function of the four OATPs expressed in human enterocytes and hepatocytes. Uptake of the model substrate estrone-3-sulfate by cells expressing OATP1A2, OATP1B1, OATP1B3, or OATP2B1 was measured in the absence and presence of the four most abundant flavonols found in green tea. Uptake by OATP1A2, OATP1B1, and OATP2B1 was inhibited by epicatechin gallate (ECG) and epigallocatechin gallate (EGCG) in a concentration-dependent way. In contrast, OATP1B3-mediated uptake of estrone-3-sulfate was strongly stimulated by EGCG at low substrate concentrations. The effect of EGCG on OATP1B3 was also studied with additional substrates: uptake of estradiol-17β-glucuronide was unchanged, whereas uptake of Fluo-3 was noncompetitively inhibited. Both ECG and EGCG were found to be substrates of OATP1A2 (Km values of 10.4 and 18.8 μM, respectively) and OATP1B3 (34.1 and 13.2 μM, respectively) but not of OATP1B1 or OATP2B1. These results indicate that two of the major flavonols found in green tea have a substantial effect on the function of OATPs expressed in enterocytes and hepatocytes and can potentially alter the pharmacokinetics of drugs and other OATP substrates. In addition, the diverse effects of EGCG on the transport of other OATP1B3 substrates suggest that different transport/binding sites are involved.
Introduction
Adverse drug-drug interactions are a common result of comorbidity and polypharmacy and pose a significant health threat. Furthermore, dietary supplements are increasingly popular, and some of their ingredients have the potential for additional drug interactions. These adverse drug interactions may be caused by alterations in efflux (Dürr et al., 2000) and uptake transporters (Fattinger et al., 2000), such as the organic anion-transporting polypeptides (OATPs). OATPs are multispecific transporters that mediate the cellular uptake of a wide range of amphipathic compounds, including numerous drugs (Hagenbuch and Gui, 2008). Four well characterized OATPs, OATP1A2, OATP1B1, OATP1B3 and OATP2B1, are expressed in the small intestine and the liver, where the likelihood of drug-drug or drug-food interactions is the greatest.
Both OATP1A2 and OATP2B1 are expressed at the apical membrane of enterocytes (Kobayashi et al., 2003; Glaeser et al., 2007) where they can contribute to the absorption of drugs such as statins, sartans, fexofenadine, talinolol, and methotrexate (Shimizu et al., 2005; Badagnani et al., 2006; Ho et al., 2006; Kitamura et al., 2008; Shirasaka et al., 2010). In the liver, OATP1B1, OATP1B3, and OATP2B1 are expressed at the basolateral membrane of hepatocytes (Abe et al., 1999; König et al., 2000; Kullak-Ublick et al., 2001). Here, these proteins are involved in the removal of drugs from the bloodstream into hepatocytes. With their broader substrate specificity, OATP1B1 and OATP1B3 are thought to play a more important role in hepatocellular drug uptake than OATP2B1 (Smith et al., 2005; Hagenbuch and Gui, 2008; Kindla et al., 2009).
The importance of OATPs to drug disposition has been highlighted by pharmacokinetic studies that correlated changes in the bioavailability of drugs with polymorphisms of OATPs (Kalliokoski and Niemi, 2009). Thus, inhibition or stimulation of OATP function by food or dietary supplements can alter the pharmacokinetics of OATP substrates and potentially lead to adverse effects. Recent studies have indicated that flavonoids found in fruit juices, in green tea, and in many dietary supplements can alter the function of OATP1A2, OATP1B1, and OATP2B1 (Wang et al., 2005; Fuchikami et al., 2006; Bailey et al., 2007). Such interactions can affect the bioavailability of drugs such as fexofenadine and celiprolol, known OATP substrates (Greenblatt, 2009).
Green tea is a commonly consumed beverage and has received much attention for its reputed health benefits. Several epidemiological studies have shown a reduced risk of gastrointestinal cancers among those who regularly consume green tea (for review, see Liu et al., 2008). Compared with other tea preparations, green tea is characterized by very high concentrations of catechins, which make up 30 to 40% of its dry weight. The catechins include epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG), and epigallocatechin gallate (EGCG). EGCG, the most predominant catechin in green tea, has been highly studied for its in vitro effects. Because of the many apparent health benefits of green tea and EGCG, green tea beverages and extract supplements are widely used, creating an increased risk of adverse interactions. EGC and EGCG have been shown to inhibit OATP1B1-mediated uptake of dehydroepiandrosterone sulfate (Wang et al., 2005), whereas all four catechins inhibited estrone-3-sulfate uptake mediated by OATP2B1 (Fuchikami et al., 2006). However, the effect of catechins on the function of OATP1A2 and OATP1B3 has not been reported, and it is not known whether any of these four catechins are transported by any of the OATPs. Therefore, in the present study we asked the question whether all four major catechins inhibit OATP-mediated uptake and whether they are transported by OATPs.
Materials and Methods
Materials.
[3H]Estrone-3-sulfate (54.26 Ci/mmol) and [3H]estradiol-17β-glucuronide (41.8 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). [3H]Epigallocatechin gallate (10 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis. MO). Unlabeled estrone-3-sulfate, estradiol-17β-glucuronide, (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin gallate, and (−)-epigallocatechin gallate were purchased from Sigma-Aldrich (St. Louis, MO). Fluo-3, pentapotassium salt was purchased from Invitrogen (Carlsbad, CA). Green tea (Camellia sinensis) biomass was provided by the Royal Estates Tea Company, a Division of Thomas J. Lipton, Co. (Englewood Cliffs, NJ). A sample of green tea biomass was extracted exhaustively with 10 ml of H2O (70°C, 10 min). The extract was concentrated in vacuo and dried overnight at 30°C in a vacuum oven. Dulbecco's modified Eagle's medium was purchased from Caisson Laboratories (North Logan, UT), and Eagle's minimum essential medium was purchased from American Type Culture Collection (Manassas, VA). Fetal bovine serum was obtained from HyClone (Logan, Utah). All other materials were purchased from Sigma-Aldrich or Invitrogen.
OATP Expression.
CHO cells stably transfected with human OATP1B1, OATP1B3, and OATP2B1 were generated in our laboratory previously and were cultured as described previously (Gui et al., 2008; Pacyniak et al., 2010). OATP1A2 was transiently expressed in HeLa or HEK-293 cells. HeLa cells were grown in Eagle's minimum essential medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. HEK-293 cells were grown in Dulbecco's modified Eagle's medium containing 4.5 g/l d-glucose, 2 mM l-glutamine, 25 mM Hepes buffer, and 110 mg/l sodium pyruvate, supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cells were maintained in a humidified environment at 37°C and 5% CO2.
Transport Assays.
Cells expressing OATP1B1 or OATP1B3 were seeded on 24-well plates and induced in the absence of geneticin in media containing 5 mM sodium butyrate 24 h before uptake experiments. CHO cells stably expressing OATP2B1 were seeded on 24-well plates 48 h before uptake experiments; OATP2B1 expression in this cell line did not require sodium butyrate induction. OATP1A2 was transiently expressed in HeLa or HEK-293 cells. HeLa cells were seeded on 12-well plates and transfected using the vaccinia virus T7 system, essentially as described previously (Lee et al., 2005). Between 16 and 20 h before uptake experiments, cells were infected with vaccinia virus in serum-free Opti-MEM medium and incubated for 1 h at 37°C. After washing, cells were transfected with pcDNA5/FRT containing the open reading frame of a His-tagged OATP1A2 or with the empty vector using Lipofectamine 2000, as per the manufacturer's instructions. HEK-293 cells were seeded on 24-well plates pretreated with poly-d-lysine and were transfected with pExpress-1 (Express Genomics, Inc., Frederick, MD) containing OATP1A2 or with the empty vector approximately 48 h before uptake experiments.
Uptake experiments were performed essentially as described previously for CHO (Gui et al., 2008), and HEK-293 cells (Weaver and Hagenbuch, 2010). HeLa uptake buffer contained 100 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 10 mM Hepes and was adjusted to pH 7.5 with Trizma base. Catechins were dissolved in DMSO, and stock solutions were stored at −20°C. A final DMSO concentration of 1% was maintained in all experiments. After brief washing, cells were incubated at 37°C with uptake buffer containing substrate and inhibitors. Uptake was terminated by removing the uptake solution and washing four times with ice-cold uptake buffer. To measure uptake of radiolabeled substrates, cells were lysed with 1% Triton X-100 in phosphate-buffered saline, and the radioactivity was quantified with liquid scintillation counting. To measure uptake of Fluo-3, cells were lysed with 1% Triton X-100 in phosphate-buffered saline containing 1 mM CaCl2, and fluorescence was quantified on a Synergy HT microplate reader (BioTek Instruments, Winooski, VT) at an excitation wavelength of 485 nm and an emission wavelength of 528 nm. Unlabeled catechins were detected using a Quattro Premier high-performance liquid chromatography tandem mass spectrometer (Waters, Milford, MA) in electrospray negative ion mode using a C18 column (50 × 2.1 mm, 5 μm; Phenomenex, Torrance, CA) at 40°C. The mobile phase consisted of 60:40 acetonitrile and 1% acetic acid, and was eluted isocratically with a flow rate of 0.3 ml/min. Cells were lysed in mobile phase containing 1 μM ethyl gallate, and lysate was centrifuged at 20,000g for 20 min to remove protein before injection. The transitions monitored were 441.15 > 169.1 for ECG, 457.05 > 169.1 for EGCG, and 197.17 > 124.2 for ethyl gallate (internal standard). QuanLynx software (Waters) was used to quantify mass spectrometry data. Protein concentrations were determined with a BCA assay kit (Thermo Fisher Scientific, Waltham, MA), and uptake was corrected for protein. Net OATP-mediated uptake was defined as the uptake by OATP-expressing cells minus the uptake by the appropriate control cell line (wild-type CHO cells for OATP1B1 and OATP1B3, CHO cells stably expressing empty vector for OATP2B1, and HeLa or HEK-293 cells transiently expressing the empty vector for OATP1A2).
Calculations and Statistics.
All calculations were performed using Prism 5 (GraphPad Software Inc., San Diego, CA). IC50 values and kinetic parameters were determined within the initial linear period of uptake after correction for protein and subtraction of uptake by the control cell line. Statistical analysis was performed with two-way analysis of variance followed by the Bonferroni post-test.
Results
Characterization of OATP1A2 and OATP2B1 Expression Systems.
OATP2B1 was expressed at high levels on the plasma membrane of the stably transfected OATP2B1-expressing CHO cells as confirmed using an anti-His antibody (data not shown). To minimally characterize OATP2B1 and the transiently expressed OATP1A2 at a functional level, we used the model substrate estrone-3-sulfate. Uptake of 100 μM estrone-3-sulfate was linear for at least 30 s for both OATP1A2 and OATP2B1 (data not shown); therefore, subsequent experiments were performed at 30 s for OATP1A2 and 20 s for OATP2B1. In both systems, transport of estrone-3-sulfate was saturable, with apparent Km and Vmax values of 16.1 ± 0.2 μM and 640 ± 150 pmol/mg · min for OATP1A2 and 14.8 ± 4.0 μM and 2.54 ± 0.57 nmol/mg · min for OATP2B1, respectively.
Effect of Green Tea Extract and Catechins on OATP-Mediated Uptake of Estrone-3-Sulfate.
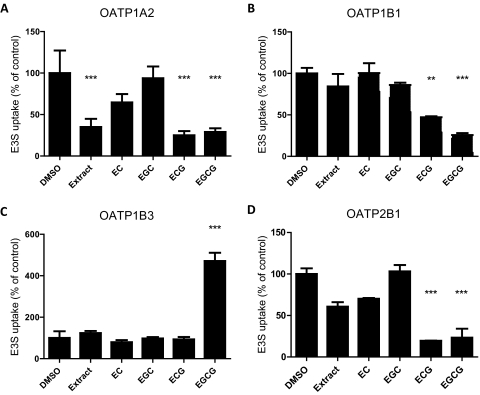
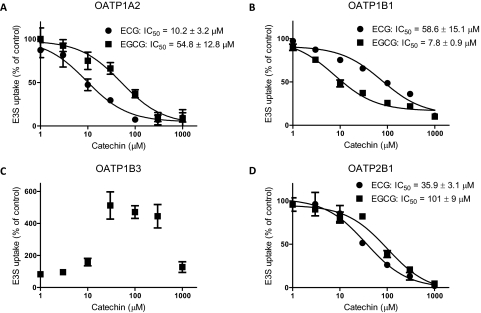
To determine the effects of green tea components on OATP function, we measured OATP-mediated uptake of 0.1 μM estrone-3-sulfate in the presence of 0.03 μg/ml green tea extract or 100 μM green tea catechin under initial linear rate conditions. EC and EGC did not significantly affect estrone-3-sulfate uptake by any of the four cell lines (Fig. 1, A–D). ECG and EGCG inhibited uptake of estrone-3-sulfate by OATP1A2, OATP1B1, and OATP2B1. Of interest, OATP1B3-mediated uptake of estrone-3-sulfate was unaffected by ECG but strongly stimulated by EGCG (Fig. 1C). To further characterize the effect of the gallated catechins on OATP-mediated uptake of estrone-3-sulfate, we determined uptake of 0.1 μM estrone-3-sulfate in the presence of increasing concentrations of ECG or EGCG. As shown in Fig. 2, both ECG and EGCG exhibited a concentration-dependent inhibition of estrone-3-sulfate uptake mediated by OATP1A2, OATP1B1, and OATP2B1 (Fig. 2, A, B, and D). Uptake by OATP1A2 and OATP2B1 was more strongly inhibited by ECG (IC50 values of 10.2 and 35.9 μM, respectively) than by EGCG (54.8 and 101 μM, respectively), whereas uptake by OATP1B1 was more strongly inhibited by EGCG than by ECG (IC50 values of 7.8 and 58.6 μM, respectively). EGCG stimulated estrone-3-sulfate uptake by OATP1B3 5-fold at concentrations of 30 to 300 μM. The stimulatory effect remained at 1 mM EGCG; however, it was greatly reduced (Fig. 2C).
Fig. 1.
Effect of green tea extract and catechins on OATP-mediated estrone-3-sulfate uptake. Cells were coincubated with 0.1 μM [3H]estrone-3-sulfate (E3S) and 0.03 μg/ml green tea extract, 100 μM EC, EGC, ECG, EGCG, or the vehicle control (1% DMSO) at 37°C for 20 s [OATP1B1 (B), OATP1B3 (C), and OATP2B1 (D)] or 30 s [OATP1A2 (A)]. After correction for protein, uptake into empty vector (OATP1A2 and OATP2B1) or wild-type control cells (OATP1B1 and OATP1B3) was subtracted to determine OATP-mediated uptake. Values are expressed as a percentage of vehicle control; each value is the mean ± S.E.M. of three independent experiments. Asterisks represent statistically significant differences from the DMSO control (**, p < 0.005; ***, p < 0.001).
Fig. 2.
Concentration-dependent effects of green tea catechins on OATP-mediated estrone-3-sulfate uptake. Cells were coincubated with 0.1 μM [3H]estrone-3-sulfate (E3S) and increasing concentrations of ECG or EGCG at 37°C as described in the legend to Fig. 1. A, OATP1A2. B, OATP1B1. C, OATP1B3. D, OATP2B1. Values are expressed as a percentage of vehicle control; each value represents the mean ± S.E.M. of three independent experiments.
Substrate-Dependent Effect of ECG and EGCG on OATP1B3-Mediated Uptake.
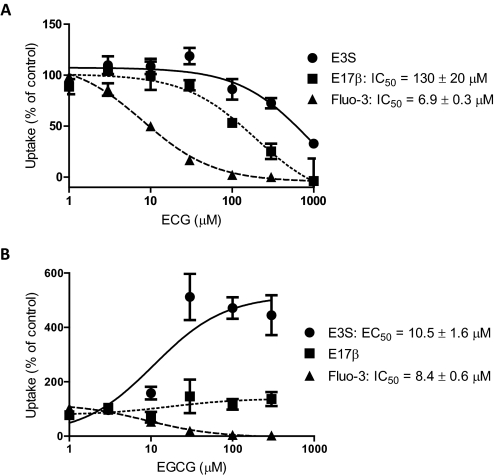
On the basis of previous evidence for substrate-dependent stimulation of OATP1B3 (Gui et al., 2008), we measured the effects of increasing concentrations of ECG and EGCG on the uptake of two additional model substrates of OATP1B3, estradiol-17β-glucuronide (0.1 μM) and Fluo-3 (1 μM), as well as estrone-3-sulfate (0.1 μM). As can be seen in Fig. 3A, ECG inhibited uptake of estradiol-17β-glucuronide with an IC50 value of 130 μM, slightly inhibited estrone-3-sulfate uptake at concentrations higher than 100 μM, and strongly inhibited uptake of Fluo-3 (IC50 = 6.9 μM). EGCG had no effect on the uptake of estradiol-17β-glucuronide; however, it strongly inhibited uptake of Fluo-3 with an IC50 value of 8.4 μM, whereas it stimulated estrone-3-sulfate uptake with an EC50 value of 10.5 μM (Fig. 3B).
Fig. 3.
Substrate-dependent effects of ECG and EGCG on OATP1B3-mediated transport. OATP1B3-expressing and wild-type CHO cells were coincubated with 0.1 μM [3H]estrone-3-sulfate (E3S, ●), 0.1 μM [3H]estradiol-17β-glucuronide (E17β, ■), or 1 μM Fluo-3 (▴) and increasing concentrations of ECG (A) or EGCG (B) at 37°C as described in the legend to Fig. 1. Values are expressed as a percentage of vehicle control; each value represents the mean ± S.E.M. of three independent experiments.
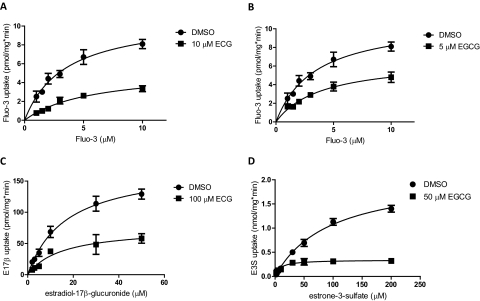
To investigate the mechanism of this substrate dependence, we determined the effect that ECG and EGCG had on the kinetic parameters of each affected OATP1B3 substrate (Fig. 4). Both ECG and EGCG noncompetitively inhibited OATP1B3-mediated uptake of Fluo-3, reducing the Vmax from 9.8 ± 0.6 to 4.3 ± 0.8 and 5.6 ± 0.6 pmol/mg · min, respectively, whereas they had no effect on the Km (2.5 ± 0.8, 2.1 ± 1.8, and 2.7 ± 1.4 μM) (Fig. 4, A and B). ECG also demonstrated noncompetitive inhibition of estradiol-17β-glucuronide uptake, decreasing the Vmax from 240 ± 40 to 110 ± 40 pmol/mg · min, while not affecting Km (19 ± 3 to 17 ± 5 μM) (Fig. 4C). Surprisingly, EGCG also significantly decreased the maximal rate of estrone-3-sulfate transport, reducing the Vmax from 2.1 ± 0.1 to 0.36 ± 0.03 nmol/mg · min (Fig. 4D). However, the Km was also strongly decreased, from 95 ± 9 to 12 ± 5 μM. This 5- to 10-fold increase in affinity results in the stimulation of estrone-3-sulfate transport observed at the low (0.1 μM) concentrations used in the initial inhibition experiments.
Fig. 4.
Effect of ECG and EGCG on the kinetics of OATP1B3-mediated transport. OATP1B3-expressing and wild-type CHO cells were coincubated with the stated concentrations of ECG or EGCG (■) or the vehicle control (1% DMSO, ●) and increasing concentrations of Fluo-3 (A and B), estradiol-17β-glucuronide (E17β) (C), or estrone-3-sulfate (E3S) (D). A–C are representative graphs from at least three independent experiments. Each value shown in D represents the mean ± S.E.M. of at least three independent experiments.
OATP-Mediated Uptake of ECG and EGCG.
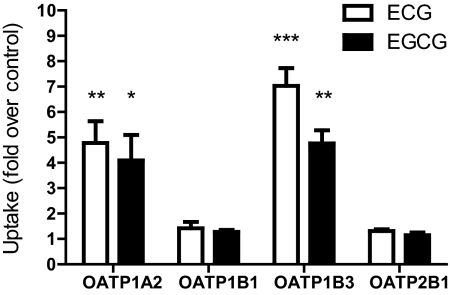
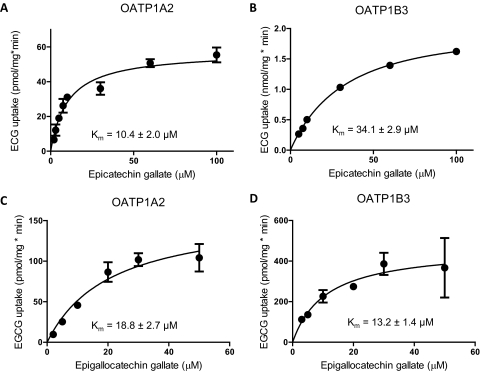
Given that inhibitors of transport are sometimes also substrates, we tested whether either ECG or EGCG was transported by these four OATPs. We measured accumulation of 100 μM ECG or EGCG in the OATP-expressing or control cells after a 10-min incubation. As summarized in Fig. 5, both OATP1A2 and OATP1B3 transported ECG and EGCG. Although ECG and EGCG were clear inhibitors of OATP1B1 and OATP2B1, we did not detect significant uptake by either OATP. Uptake of both catechins by OATP1A2 and OATP1B3 increased with time and was linear for at least 2 to 5 min (data not shown). Uptake was saturable, with apparent Km values between 10 and 34 μM (Fig. 6). OATP1A2 transports EGCG with a maximal rate of transport (Vmax) almost twice that of ECG (100 and 60 pmol/mg · min, respectively), whereas OATP1B3 has a Vmax approximately 6 times higher for ECG than for EGCG (2.2 and 0.340 nmol/mg · min, respectively).
Fig. 5.
Uptake of green tea catechins by OATPs. Cells were incubated with 100 μM ECG or EGCG at 37°C for 10 min. Uptake by each OATP-expressing cell line was divided by the uptake by its appropriate control cell and is expressed as fold uptake over control. Each value represents the mean ± S.D. of at least two experiments performed in triplicate. Asterisks represent statistically significant uptake compared with control cell lines (*, p < 0.05; **, p < 0.005; ***, p < 0.001).
Fig. 6.
Kinetics of epicatechin gallate and epigallocatechin gallate uptake mediated by OATP1A2 or OATP1B3. Uptake of increasing concentrations of ECG (A and B) or [3H]EGCG (C and D) was measured at 37°C under the initial linear rate conditions. After subtraction of the values obtained with control cells, net OATP1A2-mediated (A and C) or OATP1B3-mediated (B and D) uptake was fitted to the Michaelis-Menten equation to determine Km and Vmax values. A and B, plot of mean data points from at least three independent experiments. C and D, representative graphs with mean ± S.E.M. of three independently determined Km values.
Discussion
The present study addressed the question of whether the four major green tea catechins affect the activity of all OATPs expressed in the small intestine and liver. In addition, we investigated whether the catechins that do alter OATP activity are transported by OATPs. Our results demonstrate that although EC and EGC have minimal effect on OATPs, ECG and EGCG significantly alter the function of all four OATPs investigated. We found that the effects of ECG and EGCG on OATP1B3-mediated transport were substrate-dependent and could cause noncompetitive inhibition or stimulation of activity. In addition, we showed that both ECG and EGCG are substrates of OATP1A2 and OATP1B3 but are not transported by OATP1B1 or OATP2B1, despite the strong inhibition of estrone-3-sulfate transport by these two proteins.
ECG and EGCG significantly inhibited the uptake of estrone-3-sulfate by all four OATPs at a concentration of 100 μM (Fig. 2). The U.S. Department of Agriculture Database for the Flavonoid Content of Selected Foods reported average concentrations in brewed green tea to be 19.73 mg/100 ml (450 μM) ECG and 77.81 mg/100 ml (430 μM) EGCG, with the maximal concentrations of each catechin in the low millimolar range (U.S. Department of Agriculture, www.nal.usda.gov/fnic/foodcomp/Data/Flav/Flav02-1.pdf). These compounds inhibited estrone-3-sulfate uptake by OATP1A2 and OATP2B1, expressed at the lumen of enterocytes, with IC50 values ranging from 10 to 100 μM (Fig. 2). Assuming a gastric fluid volume of 100 to 500 ml, drinking a cup or two of tea on an empty stomach would result in intestinal concentrations of ECG and EGCG within the range that alters OATP transport. The physiological relevance of altered OATP1B1 and OATP1B3 transport is more ambiguous, because the bioavailability of catechins is low. A single-dose study in healthy volunteers showed that consumption of 1600 mg of EGCG resulted in mean peak plasma concentrations (Cmax) of 7.4 μM, with values ranging from 5.8 to 11.3 μM (Ullmann et al., 2003). The IC50 and EC50 values of EGCG on OATP1B3-mediated uptake of Fluo-3 and estrone-3-sulfate (8.4 and 10.5 μM, respectively) and on OATP1B1-mediated transport of estrone-3-sulfate (IC50 = 7.8 μM), are well within this range, indicating the physiological relevance of these interactions for those who take high-dose supplements. The same authors found that daily consumption of 800 mg of EGCG resulted in an average Cmax of 5.3 μM after 10 days (Ullmann et al., 2004). In addition, the bioavailability of EGCG was shown to increase with increasing doses, indicating that a saturable presystemic elimination process is involved in the low systemic bioavailability (Chow et al., 2001). If this presystemic elimination occurs via the liver, the OATP1B1- and OATP1B3-expressing hepatocytes may be exposed to these EGCG concentrations at more moderate doses as well.
We identified both ECG and EGCG as novel substrates for OATP1A2 and OATP1B3 (Figs. 5 and 6). It is important to note that although these two catechins were inhibitors of OATP1B1 and OATP2B1 (Figs. 1 and 2), we did not see any uptake by either of these OATPs (Fig. 5). This result corroborates the finding that many inhibitors of transporters are not substrates of those transporters. It has been shown that ECG and EGCG are taken up into Caco-2 cells and that uptake of ECG was saturable and stimulated by low pH (Vaidyanathan and Walle, 2003). The authors suggested that this transport was mediated by the monocarboxylate transporter MCT1 (Vaidyanathan and Walle, 2003). However, direct transport of ECG or EGCG by MCT1 to our knowledge has not been reported, and so far no uptake transporter has been identified for green tea catechins. Given that OATP1A2 is expressed in enterocytes, our results suggest that OATP1A2 could be involved in the absorption of ECG and EGCG from the gut. Furthermore, given that OATP1A2 can transport numerous drugs including fexofenadine (Cvetkovic et al., 1999), several antibiotics such as levofloxacin (Maeda et al., 2007), methotrexate (Badagnani et al., 2006), statins (Fujino et al., 2005; Ho et al., 2006), and talinolol (Shirasaka et al., 2010), there is the potential for food-drug interactions such as the ones described for fruit juices (Dresser et al., 2002; Lilja et al., 2004; Greenblatt, 2009) in patients that complement their prescription drugs with over-the-counter green tea supplements. A similar danger may exist for OATP1B3. It is not known whether the low systemic bioavailability of ECG and EGCG is due to efflux from enterocytes or to a high first-pass effect. However, efficient uptake via OATP1B3 into hepatocytes could contribute to the low bioavailability of these compounds.
This study clearly demonstrates that OATP-mediated transport may be affected in different ways by the same compound, depending on the substrate being transported. We found that ECG inhibited OATP1B3-mediated uptake of estrone-3-sulfate, estradiol-17β-glucuronide, and Fluo-3, but to very different extents (Fig. 3A). Inhibition of estrone-3-sulfate transport was too weak to further characterize; however, estradiol-17β-glucuronide and Fluo-3 were both inhibited in a noncompetitive manner. EGCG, which differs from ECG by a single hydroxyl group, stimulated OATP1B3 activity with respect to estrone-3-sulfate transport, inhibited transport of Fluo-3, and had no effect on the transport of estradiol-17β-glucuronide (Fig. 3B). Uptake of Fluo-3 was noncompetitively inhibited, as was uptake of estrone-3-sulfate at high substrate concentrations. The stimulation of estrone-3-sulfate at low substrate concentrations was found to be caused by increased substrate affinity. In addition, although 100 μM ECG did not inhibit OATP1B3-mediated estrone-3-sulfate uptake and although EGCG did not affect OATP1B3-mediated estradiol-17β-glucuronide uptake, both catechins are substrates of OATP1B3 (Figs. 1, 3, and 5). Together, these results suggest the presence of multiple substrate binding sites or translocation pathways on OATP1B3.
These results emphasize that, at least in the case of OATP1B1- and OATP1B3-mediated transport, it is crucial to test more than one substrate when screening for potential inhibitors. The International Transporter Consortium recently suggested that possible OATP inhibition by new molecular entities should be tested using a prototypical substrate such as estradiol-17β-glucuronide (Giacomini et al., 2010). However, we showed that OATP1B3-mediated uptake of estradiol-17β-glucuronide was not affected by EGCG, whereas uptake of Fluo-3 was inhibited and uptake of estrone-3-sulfate was stimulated (Fig. 3). Likewise, although a previous study found that EGC inhibited OATP1B1-mediated uptake of dehydroepiandrosterone sulfate (Wang et al., 2005), we did not see significant inhibition of OATP1B1-mediated uptake of estrone-3-sulfate in the presence of EGC. Thus, we propose that for OATP1B1 and OATP1B3, the effect of potential inhibitors should always be tested by using several substrates instead of a single prototypical substrate. Similar substrate-dependent effects have previously been observed for rat Oatp1a4 (Sugiyama et al., 2002), for human OATP1B1 (Noé et al., 2007), and for human OATP1B3 (Gui et al., 2008). In our previous study (Gui et al., 2008), the non-OATP substrate clotrimazole stimulated OATP1B3-mediated estradiol-17β-glucuronide uptake, did not affect uptake of estrone-3-sulfate, and inhibited uptake of Fluo-3. Together, these data clearly demonstrate that stimulation as well as inhibition are substrate-dependent and indicate the presence of multiple substrate binding sites.
A previous study showed inhibition of OATP2B1-mediated uptake of estrone-3-sulfate by all four catechins (Fuchikami et al., 2006). However, we found that only ECG and EGCG significantly inhibit OATP2B1-mediated estrone-3-sulfate transport. These differences could be explained by the 10-fold higher substrate concentration used in the current study and highlights the difficulty in predicting in vivo effects based on in vitro data. However, in both studies the effects of EC and EGC on OATP2B1-mediated transport were much weaker than the effects of ECG and EGCG, suggesting that ECG and EGCG are the green tea catechins most likely to alter OATP-mediated drug uptake.
In conclusion, we have demonstrated that the green tea compounds ECG and EGCG are substrates for OATP1A2 and OATP1B3 suggesting that these two transporters could be involved in the disposition of these two catechins. We also demonstrated that compounds such as ECG and EGCG can affect OATPs in a substrate-dependent manner. This finding highlights the importance of using multiple and clinically relevant substrates when screening for potential drug-drug interactions with this family of transporters. Because of increasing use of green tea catechins, particularly EGCG, in dietary supplements and because ECG and EGCG can significantly alter the function of OATPs involved in drug disposition, the results of this study suggest that there is a significant possibility of adverse drug-catechin interactions.
Acknowledgments
We thank Gemma O'Donnell for performing the green tea extraction. We also thank Colleen Flynn for technical assistance.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM077336]; and the National Institutes of Health National Center for Research Resources [Grant P20-RR021940].
Parts of this work were previously presented in poster form at the following conference: Roth M, Timmermann B, and Hagenbuch B (2009) Interaction of green tea catechins with organic anion transporting polypeptides. Experimental Biology. 2009 Apr 18–22; New Orleans, LA; The American Society for Pharmacology and Experimental Therapeutics, Bethesda, MD. FASEB J 23:748.4.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.036640.
- OATP
- organic anion-transporting polypeptide
- EC
- (−)-epicatechin
- EGC
- (−)-epigallocatechin gallate
- ECG
- (−)-epicatechin gallate
- EGCG
- (−)-epigallocatechin gallate
- CHO
- Chinese hamster ovary
- HEK
- human embryonic kidney
- DMSO
- dimethyl sulfoxide.
Authorship Contributions
Participated in research design: Roth, Timmermann, and Hagenbuch.
Conducted experiments: Roth.
Performed data analysis: Roth and Hagenbuch.
Wrote or contributed to the writing of the manuscript: Roth, Timmermann, and Hagenbuch.
References
- Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T, et al. (1999) Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem 274:17159–17163 [DOI] [PubMed] [Google Scholar]
- Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, Kawamoto M, Johns SJ, Ferrin TE, Carlson EJ, et al. (2006) Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther 318:521–529 [DOI] [PubMed] [Google Scholar]
- Bailey DG, Dresser GK, Leake BF, Kim RB. (2007) Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther 81:495–502 [DOI] [PubMed] [Google Scholar]
- Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, Crowell JA, Yang CS, Hara Y. (2001) Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev 10:53–58 [PubMed] [Google Scholar]
- Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. (1999) OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos 27:866–871 [PubMed] [Google Scholar]
- Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. (2002) Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther 71:11–20 [DOI] [PubMed] [Google Scholar]
- Dürr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ, Fattinger K. (2000) St John's wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther 68:598–604 [DOI] [PubMed] [Google Scholar]
- Fattinger K, Cattori V, Hagenbuch B, Meier PJ, Stieger B. (2000) Rifamycin SV and rifampicin exhibit differential inhibition of the hepatic rat organic anion transporting polypeptides, Oatp1 and Oatp2. Hepatology 32:82–86 [DOI] [PubMed] [Google Scholar]
- Fuchikami H, Satoh H, Tsujimoto M, Ohdo S, Ohtani H, Sawada Y. (2006) Effects of herbal extracts on the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos 34:577–582 [DOI] [PubMed] [Google Scholar]
- Fujino H, Saito T, Ogawa S, Kojima J. (2005) Transporter-mediated influx and efflux mechanisms of pitavastatin, a new inhibitor of HMG-CoA reductase. J Pharm Pharmacol 57:1305–1311 [DOI] [PubMed] [Google Scholar]
- International Transporter Consortium, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, et al. (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, et al. (2007) Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther 81:362–370 [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ. (2009) Analysis of drug interactions involving fruit beverages and organic anion-transporting polypeptides. J Clin Pharmacol 49:1403–1407 [DOI] [PubMed] [Google Scholar]
- Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, Hagenbuch B. (2008) Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol 584:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Gui C. (2008) Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 38:778–801 [DOI] [PubMed] [Google Scholar]
- Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB. (2006) Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology 130:1793–1806 [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Niemi M. (2009) Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 158:693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindla J, Fromm MF, König J. (2009) In vitro evidence for the role of OATP and OCT uptake transporters in drug-drug interactions. Expert Opin Drug Metab Toxicol 5:489–500 [DOI] [PubMed] [Google Scholar]
- Kitamura S, Maeda K, Wang Y, Sugiyama Y. (2008) Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos 36:2014–2023 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2003) Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther 306:703–708 [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. (2000) A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol 278:G156–G164 [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. (2001) Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 120:525–533 [DOI] [PubMed] [Google Scholar]
- Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, Leake BF, Kim RB. (2005) Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem 280:9610–9617 [DOI] [PubMed] [Google Scholar]
- Lilja JJ, Juntti-Patinen L, Neuvonen PJ. (2004) Orange juice substantially reduces the bioavailability of the β-adrenergic-blocking agent celiprolol. Clin Pharmacol Ther 75:184–190 [DOI] [PubMed] [Google Scholar]
- Liu J, Xing J, Fei Y. (2008) Green tea (Camellia sinensis) and cancer prevention: a systematic review of randomized trials and epidemiological studies. Chin Med 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Takahashi K, Ohtsu N, Oguma T, Ohnishi T, Atsumi R, Tamai I. (2007) Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm 4:85–94 [DOI] [PubMed] [Google Scholar]
- Noé J, Portmann R, Brun ME, Funk C. (2007) Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos 35:1308–1314 [DOI] [PubMed] [Google Scholar]
- Pacyniak E, Roth M, Hagenbuch B, Guo GL. (2010) Mechanism of polybrominated diphenyl ether uptake into the liver: PBDE congeners are substrates of human hepatic OATP transporters. Toxicol Sci 115:344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, Sugiyama Y. (2005) Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos 33:1477–1481 [DOI] [PubMed] [Google Scholar]
- Shirasaka Y, Kuraoka E, Spahn-Langguth H, Nakanishi T, Langguth P, Tamai I. (2010) Species difference in the effect of grapefruit juice on intestinal absorption of talinolol between human and rat. J Pharmacol Exp Ther 332:181–189 [DOI] [PubMed] [Google Scholar]
- Smith NF, Figg WD, Sparreboom A. (2005) Role of the liver-specific transporters OATP1B1 and OATP1B3 in governing drug elimination. Expert Opin Drug Metab Toxicol 1:429–445 [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Shitara Y, Abe T, Sugiyama Y. (2002) Effect of 17β-estradiol-d-17β-glucuronide on the rat organic anion transporting polypeptide 2-mediated transport differs depending on substrates. Drug Metab Dispos 30:220–223 [DOI] [PubMed] [Google Scholar]
- Ullmann U, Haller J, Decourt JD, Girault J, Spitzer V, Weber P. (2004) Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. Int J Vitam Nutr Res 74:269–278 [DOI] [PubMed] [Google Scholar]
- Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, Pineau B, Weber P. (2003) A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res 31:88–101 [DOI] [PubMed] [Google Scholar]
- Vaidyanathan JB, Walle T. (2003) Cellular uptake and efflux of the tea flavonoid (−)epicatechin-3-gallate in the human intestinal cell line Caco-2. J Pharmacol Exp Ther 307:745–752 [DOI] [PubMed] [Google Scholar]
- Wang X, Wolkoff AW, Morris ME. (2005) Flavonoids as a novel class of human organic anion-transporting polypeptide OATP1B1 (OATP-C) modulators. Drug Metab Dispos 33:1666–1672 [DOI] [PubMed] [Google Scholar]
- Weaver YM, Hagenbuch B. (2010) Several conserved positively charged amino acids in OATP1B1 are involved in binding or translocation of different substrates. J Membr Biol 236:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]