Abstract
Anandamide is an arachidonic acid-derived endogenous cannabinoid that regulates normal physiological functions and pathophysiological responses within the central nervous system and in the periphery. Several cytochrome P450 (P450) isoforms metabolize anandamide to form hydroxylated and epoxygenated products. Human CYP2B6 and CYP2D6, which are expressed heterogeneously throughout the brain, exhibit clinically significant polymorphisms and are regulated by external factors, such as alcohol and smoking. Oxidative metabolism of anandamide by these two P450s may have important functional consequences for endocannabinoid system signaling. In this study, we investigated the metabolism of anandamide by wild-type CYP2B6 (2B6.1) and CYP2D6 (2D6.1) and by their common polymorphic mutants 2B6.4, 2B6.6, 2B6.9, and 2D6.34. Major differences in anandamide metabolism by the two isoforms and their mutants were found in vitro with respect to the formation of 20-hydroxyeicosatetraenoic acid ethanolamide (20-HETE-EA) and 14,15-epoxyeicosatetraenoic acid ethanolamide (14,15-EET-EA). Pharmacological studies showed that both 20-HETE-EA and 14,15-EET-EA bind to the rat brain cannabinoid CB1 receptor with lower affinities relative to that of anandamide. In addition, both products are degraded more rapidly than anandamide in rat brain homogenates. Their degradation occurs via different mechanisms involving either fatty acid amide hydrolase (FAAH), the major anandamide-degrading enzyme, or epoxide hydrolase (EH). Thus, the current findings provide potential new insights into the actions of inhibitors FAAH and EH, which are being developed as novel therapeutic agents, as well as a better understanding of the interactions between the cytochrome P450 monooxygenases and the endocannabinoid system.
Introduction
The endogenous lipid arachidonoyl ethanolamide (anandamide) is a naturally occurring amide of arachidonic acid that binds reversibly and activates the cannabinoid receptors CB1 and CB2, and, therefore, it is referred to as an endocannabinoid. It is estimated that the whole brain basal levels of anandamide are approximately 19 pmol/g (Buczynski and Parsons, 2010). Anandamide binding to its receptors leads to antinociceptive, anti-inflammatory, and neuroprotective effects, which makes the development of pharmacological agents that can selectively elevate the endogenous levels of anandamide, a promising therapeutic approach (Di Marzo, 2008). Such drug candidates include inhibitors of fatty acid amide hydrolase (FAAH), the enzyme that primarily inactivates anandamide, which are being developed for pain, anxiety, and inflammatory disorders (Schlosburg et al., 2009). A better understanding of other metabolic pathways that can exert control over the endogenous levels of anandamide is essential for further progress in this area.
In addition to hydrolysis by FAAH, anandamide is oxygenated by several human cytochrome P450 enzymes, including 3A4, 4F2, 4X1, and the highly polymorphic 2D6, forming a number of metabolites that are likely to have important physiological roles (Snider et al., 2010). For example, the epoxide of anandamide at position C5–C6 formed by hepatic CYP3A4 is a potent agonist at the CB2 receptor (Snider et al., 2009, 2010). Human CYP2D6.1 metabolizes anandamide to produce five monooxygenated metabolites, including a hydroxylated product, the 20-hydroxyeicosatetraenoic acid ethanolamide (HETE-EA), and four epoxides, the 5,6-, 8,9-, 11,12-, and 14,15-epoxyeicosatetraenoic acid ethanolamides (Snider et al., 2008). Because CYP2D6 is expressed and functional in human brain and there are neuropsychiatric differences among individuals with different CYP2D6 genotypes (Funae et al., 2003; Miksys and Tyndale, 2004; Ingelman-Sundberg et al., 2007), it is plausible that CYP2D6-mediated biotransformation of endogenous psychoactive substrates, such as anandamide, results in the formation of metabolites with important activity. The amino acid substitution R296C is present in a large number of CYP2D6 alleles (http://www.cypalleles.ki.se/cyp2d6.htm) either alone (CYP2D6*34) or in conjunction with other mutations that are commonly observed (Marez et al., 1997). Furthermore, CYP2D6 variants containing this mutation have been shown to have an effect on the disposition of neuroactive steroids and tyramine in the brain (Niwa et al., 2004).
As for CYP2D6, CYP2B6 is also expressed in brain and is one of the most polymorphic P450 genes in humans (Miksys et al., 2003; Zanger et al., 2007). Several widely used drugs are metabolized by CYP2B6, including the antidepressant and smoking cessation agent bupropion (Faucette et al., 2000; Hesse et al., 2000), the anticancer agent cyclophosphamide (Chang et al., 1993), and the non-nucleoside reverse transcriptase inhibitor efavirenz (Miksys et al., 2003). Genetic polymorphisms seem to be contributing to changes in the expression and activity of the drug-metabolizing enzymes and thus to drug metabolism reactions. In the brain, CYP2B6 is expressed in neurons and astrocytes in a region-specific manner, and the levels vary depending on an individual's exposure to smoking and alcohol (Miksys et al., 2003). Several common single nucleotide polymorphisms have been identified in the human CYP2B6 gene (Lang et al., 2001; Klein et al., 2005). The frequency of the 2B6.4 allele has been shown to be approximately 50% in Ghanians and close to 30% in African Americans and whites (Klein et al., 2005). The polymorphic 2B6.9 has an allelic frequency of 20% in Japanese populations as reported by Ariyoshi et al. (2001). The frequency of the K262R (*4) and Q172H (*9) CYP2B6 alleles was found to be 0.29 and 0.28, respectively, when screened across a panel of human livers derived from an ethnically diverse population (Hesse et al., 2004), with the CYP2B6*6 haplotype (Q172H/K262R) exhibiting a similar frequency. The objective of the current study was to investigate the major differences in the metabolism of anandamide between wild-type and mutant forms of CYP2D6 and CYP2B6 and to examine some of the pharmacological properties of the differentially generated products.
Materials and Methods
Materials.
Oligonucleotide primers were obtained from the University of Michigan Core facility. Anandamide and anandamide metabolites used for standards were purchased from Cayman Chemical (Ann Arbor, MI). Catalase, NADPH, and l-α-dilauroyl-phosphatidylcholine were purchased from Sigma-Aldrich (St. Louis, MO). Radiolabeled ligand (−)-cis-3-[2-hydroxy-4(1,1-dimethyl-heptyl)phenyl]-trans-4-(3-hydroxypropyl) (CP-55940) (3H [side chain-2,3,4-3H9(N)]) was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA).
Protein Purification.
P450 reductase was expressed in Topp 3 cells and purified as described previously (Hanna et al., 1998, 2000). The construction of P450s 2B6.4, 2B6.6, 2B6.9, and 2D6.34 was performed using a Stratagene QuikChange Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA). The enzymes were expressed in Escherichia coli C41 DE3 cells and purified according to published protocols (Hanna et al., 2000). The expression plasmid for His-tagged P450s 2B6 and 2D6 was a generous gift from Dr. James R. Halpert (Scott et al., 2001). The enzymes were expressed and purified according to protocols published previously (Hanna et al., 1998, 2000, 2001).
Metabolism of Anandamide by CYP2B6 and CYP2D6 in the Reconstituted System.
CYP2B6 or CYP2D6 enzymes (0.1 nmol) were reconstituted with 0.2 nmol of reductase individually at 4°C for 45 min. The reconstituted enzymes were supplemented with catalase (500 U) and 50 mM potassium phosphate buffer (pH 7.4) and diluted with water to a volume of 500 μl. The substrate anandamide was dissolved in ethanol and added to a final concentration of 2 μM, and the samples were equilibrated at 37°C for 3 min. The reactions were initiated by the addition of 1.2 mM NADPH and allowed to proceed for 45 min in a shaking water bath. The reactions were stopped by the addition of 3 ml of ethyl acetate, vortexed, and centrifuged at 1000 rpm for 5 min. The organic layer (top) was recovered and dried under nitrogen gas. The samples were then suspended in 100 μl of methanol and injected onto a column for ESI-LC-MS for further analysis as described below.
Kinetics of Anandamide Oxidation by CYP2B6, CYP2D6, and Their Mutants.
Incubations of all reconstituted systems containing the recombinant proteins were performed at 37°C in 50 mM potassium phosphate buffer (pH 7.4) in the presence of NADPH (1.2 mM). The linearity of product formation with respect to incubation time and concentration of recombinant protein was established in initial studies. The final incubations to determine the Michaelis-Menten kinetic parameters for the formation of the products were conducted using 100 pmol of P450 enzyme in a final volume of 500 μl with a 45-min incubation time at 37°C. The anandamide concentrations used for these kinetic studies were 0.75, 1.5, 3, 5, and 10 μM and were prepared in ethanol (final concentration of ethanol in the reaction mixture was less than 1%). Standard curves were generated by injecting known molar ratios of anandamide (or a metabolite) to deuterated (d8) anandamide (internal standard). Analyte detection was linear (R2 = 0.99) from at least 0.5 to 100 pmol injected. The amounts of metabolite formed were calculated from their respective curves. Formation of the anandamide metabolites was expressed as picomoles per minute per picomoles of protein using Michaelis-Menten parameters and nonlinear regression using Prism (version 5.01 for Windows; GraphPad Software Inc., San Diego, CA).
LC-MS/MS Analysis of the Incubation Mixtures.
ESI-LS-MS/MS was performed using a ThermoQuest LCQ ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA) interfaced with a Hewlett Packard 1100 series high-performance liquid chromatography system (Hewlett Packard, Palo Alto, CA). Aliquots (10 μl) of the sample extracts were injected onto a Hypersil ODS column (5 μm, 4.6 × 100 mm; Thermo Fisher Scientific). The mobile phase was 0.1% acetic acid in water (A) and 0.1% acetic acid in methanol (B) with a flow rate of 0.3 ml/min. Initial conditions were 75% B for 5 min. The percentage of B was then increased linearly from 75 to 100% over 20 min and 100% B was maintained until 25 min and then decreased to 75% B over 1 minute and maintained at 75% B from 26 to 30 min. The instrument was operated in positive electrospray ionization mode with a capillary temperature of 210°C, capillary voltage of 2 V, sheath gas at 90 arbitrary units, and spray voltage of 4.5 V. The analyses were performed using Xcalibur software in the data-dependent acquisition mode using one full scan from 300 to 500 m/z followed by one data-dependent scan of the most intense ion. Data were acquired in positive ion mode using the Xcalibur software package (Thermo Fisher Scientific).
Competition Binding Assays.
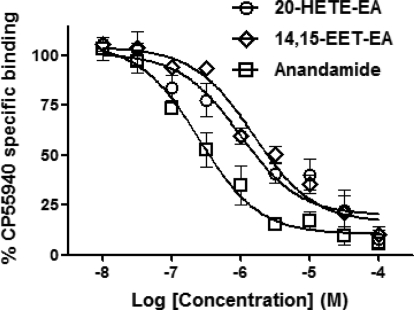
Competition binding assays were performed as described previously (Snider et al., 2009). In brief, the reaction mixtures contained TMEB buffer (50 mM Tris, 3 mM MgCl2, 1 mM EDTA, pH 7.4, and 0.5% bovine serum albumin) with 50 μM phenylmethylsulfonyl fluoride (PMSF), various concentrations of competitor (anandamide, 20-HETE-EA, or 14,15-EET-EA) as described in the legend to Fig. 4, radiolabeled CP-55940 (specific activity = 180 Ci) at its Kd concentration (5 nM), and rat brain membrane proteins. The binding of radiolabeled CP-55940 to the brain membrane proteins in the presence of a 10 μM concentration of the synthetic cannabinoid R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2, 3-de]1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN-55212-2) in the absence of any of the competitors was considered to be nonspecific.
Fig. 4.
Binding of anandamide, 20-HETE-EA, and 14,15-EET-EA to the rat brain CB1 receptor. Rat brain membranes were incubated with radiolabeled CP-55940 in the presence of vehicle (ethanol) or various concentrations of anandamide, 20-HETE-EA, or 14,15-EET-EA, and the binding reactions were allowed to reach equilibrium over a period of 1 h. The binding of CP-55940 in the presence of a saturating concentration (10 μM) of the cannabinoid agonist WIN-55212-2 was considered to be due to nonspecific binding. After subtraction of the counts due to nonspecific binding, the specific binding in the presence of the various concentrations of the competitors was expressed as a percentage of the specific binding in the presence of vehicle alone. The data were fitted to a one-site competition nonlinear regression model using Prism 5 software, on the basis of which the IC50 values for the three competitors were determined.
Degradation of Anandamide, 20-HETE-EA, and 14,15-EET-EA by Rat Brain Homogenates.
Two-month-old (250 g) male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were used in accordance with the Committee for the Use and Care of Animals at University of Michigan (Jenkins et al., 2006). Immediately after decapitation, rat brain tissues were collected, quick-frozen on dry ice, and stored at −80°C until use. The brain tissues were homogenized in potassium phosphate buffer, pH 7.4, using a Polytron homogenizer. Protein concentrations were measured using the BCA method. Anandamide, 20-HETE-EA, and 14,15-EET-EA (5 μM) were incubated with the rat brain homogenates (0.5 mg protein/reaction) in phosphate buffer at 37°C for 0 to 64 min in the presence or absence of PMSF, as specified in the figure legends. At the designated time points and after the addition of deuterated anandamide as an internal standard, the reaction mixtures were extracted with 3 volumes of ethyl acetate, dried down, and resuspended in 100 μl of methanol followed by ESI-LC-MS analysis as described above. Control experiments contained the same components with the exception that the brain homogenate was first heat-inactivated by boiling for 10 min.
Testosterone Metabolism.
2B6.1 (25 μM) or the mutant enzymes were reconstituted with 0.5 μM reductase in an ice bucket for 45 min. The reconstituted enzymes were then supplemented with catalase (100 units/ml) and diluted with 50 mM potassium phosphate buffer (pH 7.4) to a final volume of 130 μl. The mixture was allowed to equilibrate at 37°C for 10 min, and 100 μl of the volume was transferred into testosterone assay buffer (0.2 mM testosterone and 2 mM NADPH in 50 mM HEPES buffer). The final concentrations of the testosterone used in the buffer were 200, 100, 50, 25, 10, and 0 μM in methanol or methanol in the control sample. Reactions were allowed to proceed at 37°C for 30 min and were terminated with two 2-ml portions of ethyl acetate and extracted. The organic phases were pooled together and dried under nitrogen. The dried extracts were resuspended in 125 μl of 65% methanol, and 100 μl was resolved using high-performance liquid chromatography under isocratic conditions with 65% methanol-35% water and a flow rate of 0.85 ml/min on a Microsorb-MV C18 column (5 μm, 4.6 × 150 mm; Varian, Palo Alto, CA). Metabolites were detected using UV absorption at 254 nm. The area under the peaks for the major metabolites of testosterone, 16α-hydroxytestosterone, and 16β-hydroxytestosterone were integrated to compare the quantities that were formed by each enzyme.
Data Analysis.
Nonlinear regression and statistical analyses of the data were performed using Prism.
Results
Metabolism of Anandamide by Wild-Type CYP2B6 and CYP2D6.
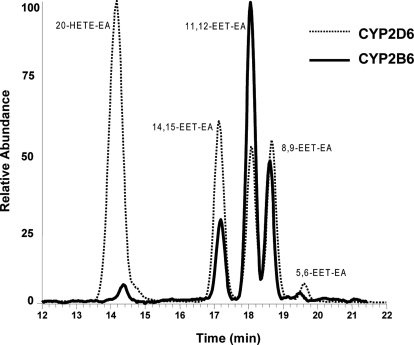
We initially compared the ability of purified human CYP2B6 and CYP2D6 to form 20-HETE-EA and the EET-EAs when incubated with anandamide in the reconstituted system with NADPH. As shown in Fig. 1 and as we have reported previously (Snider et al., 2008), anandamide is metabolized by CYP2D6 to yield five monooxygenated metabolites in the following order of abundance: 20-HETE-EA > 14,15-EET-EA > 8,9-EET-EA ≥ 11,12-EET-EA > 5,6-EET-EA. The incubation of anandamide with CYP2B6 in the presence of NADPH resulted in the same products as CYP2D6, with m/z ratios of 364, 16 mass units higher than that of the parent anandamide (m/z 348). However, the relative ratios of the metabolites formed were very different, as can be seen in Fig. 1. The major difference was the formation of 20-HETE-EA, which was the major metabolite of CYP2D6, but only a minor product of CYP2B6. No changes in the amounts or ratios of product formed were observed when anandamide was metabolized by CYP2B6 in the presence or absence of cytochrome b5 in the reconstituted system (data not shown). Unlike CYP2D6, which is able to further hydroxylate the EET-EAs to yield dioxygenated derivatives (Snider et al., 2008), no such products were formed when anandamide was incubated with CYP2B6 for up to 90 min (data not shown).
Fig. 1.
LC-MS profiles for the metabolism of anandamide by CYP2B6 and CYP2D6. The reconstitution of the enzymes and incubations in the presence of 2 μM anandamide with subsequent analyses of the samples were done as described under Materials and Methods. This figure shows the total ion chromatogram at m/z of 364 for the five hydroxylated and epoxygenated metabolites eluting at 14.5, 17.5, 18.2, 18.7, and 19.5 min.
Differences in the Metabolism of Anandamide between Wild-Type CYP2B6 and Its Three Polymorphic Mutants.
The metabolism of anandamide by wild-type CYP2B6 and its three naturally occurring polymorphic mutants was investigated by comparing the kinetics of product formation. To verify the enzyme activity of the mutant enzymes, testosterone was used as a reference substrate to compare the activity with that of the wild-type enzyme. As shown in Table 1, the Km values for 16α-testosterone formation by the wild-type 2B6 and the variants ranged from 10 to 99 μM. The Vmax values of the mutants shown are normalized to that of the wild-type. The Vmax/Km values ranged over a factor of almost 25 from wild-type 2B6 > 2B6.6 > 2B6.9 > 2B7.4. The Km for 16β-testosterone formation ranged from 18 to 40 μM. The Vmax value for 2B6.6 was comparable to that of the wild type, and the efficiency of catalysis (Vmax/Km) for 16β-testosterone was highest for 2B6.6 with a range of approximately 20.
TABLE 1.
Testosterone metabolism by wild-type 2B6 and its polymorphic mutants
Wild-type and mutant enzymes were reconstituted with reductase, incubated with testosterone in the presence or absence of NADPH, and metabolites were extracted as described under Materials and Methods. The values for the product formation in micromolar concentrations represent the results from two experiments done in duplicate.
| P450 | 16α-Hydroxytestosterone |
16β-Hydroxytestosterone |
||||
|---|---|---|---|---|---|---|
| Km | Vmax | Vmax/Km | Km | Vmax | Vmax/Km | |
| WT | 13.3 ± 3 | 100 ± 2 | 7.52 | 27.7 ± 3 | 100 ± 4 | 3.61 |
| 2B6.4 | 99 ± 17 | 31 ± 4 | 0.31 | 29.8 ± 9 | 8.1 ± 3 | 0.27 |
| 2B6.6 | 20.7 ± 6 | 40 ± 7 | 3.74 | 18.1 ± 2 | 97 ± 19 | 5.36 |
| 2B6.9 | 10 ± 2 | 24 ± 4 | 2.4 | 40 ± 5 | 46 ± 7 | 1.15 |
WT, wild-type.
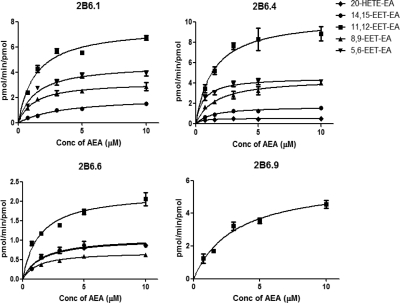
As shown in Fig. 2 and Table 2, CYP2B6.1 metabolized anandamide to give the four EET-EAs with relatively low Km values (1.2–3.6 μM), and the rates of formation (Vmax) ranged from 2.0 to 7.5 pmol of product formed/min/pmol enzyme. The amount of 20-HETE-EA formed by wild-type CYP2B6 was too low to obtain accurate kinetic determinations. The major difference between wild-type CYP2B6 and CYP2B6.4 was an increase in the rate of formation of 20-HETE-EA, although this was still the anandamide product that had the lowest rate of formation. In addition, there was a 3-fold decrease in the Km of CYP2B6.4 for anandamide with respect to the formation of 14,15-EET-EA, an effect that was also seen with the CYP2B6.6 mutant, in which case the Km was decreased by approximately 2-fold. The CYP2B6.6 mutant also exhibited significantly lower rates of formation for 11,12-EET-EA (approximately 3-fold lower) and 8,9-EET-EA (approximately 5-fold lower) relative to those for the wild-type CYP2B6, with no significant changes in the corresponding Km values. The CYP2B6.9 mutant exhibited a 2-fold decrease in the affinity for anandamide and only produced 11,12-EET-EA. However, the rate of formation of that product was comparable to that of the wild-type enzyme.
Fig. 2.
Kinetic profiles for the metabolism of anandamide by CYP2B6 and several of its polymorphic mutants. The expressed enzymes (100 pmol) were reconstituted and incubated with anandamide (0.75–10 μM), and the metabolite formation was determined as described under Materials and Methods. Values are the means of triplicate experiments. AEA, arachidonoyl ethanolamide.
TABLE 2.
Comparison of anandamide metabolism by wild-type and mutant CYP2B6 enzymes
CYP2B6 enzymes were reconstituted with reductase, and the kinetic data were determined as described under Materials and Methods. The concentrations of anandamide ranged from 0.75 to 10 μM. Metabolites were extracted and analyzed by ESI-LC-MS. The data represent the mean of experiments done in triplicate. The data were fitted to the Michaelis-Menton model using Prism software to derive the kinetic constants.
| . | 20-HETE-EA | 14,15-EET-EA | 11,12-EET-EA | 8,9-EET-EA | 5,6-EET-EA |
|---|---|---|---|---|---|
| Km (μM) | |||||
| CYP2B6.1 (WT) | N.D. | 3.6 | 1.320 | 1.210 | 1.320 |
| CYP2B6.4 | 0.362 | 1.237 | 1.380 | 1.510 | 0.591 |
| CYP2B6.6 | N.D. | 1.501 | 1.341 | 1.211 | N.D. |
| CYP2B6.9 | N.D. | N.D. | 3.089 | N.D. | N.D. |
| Vmax (pmol · min−1 · pmol−1) | |||||
| CYP2B6.1 (WT) | N.D. | 2.040 | 7.560 | 3.120 | 4.596 |
| CYP2B6.4 | 0.546 | 1.721 | 10.450 | 4.450 | 4.551 |
| CYP2B6.6 | N.D. | 1.062 | 2.221 | 0.693 | N.D. |
| CYP2B6.9 | N.D. | N.D. | 5.549 | N.D. | N.D. |
WT, wild-type; N.D., not detected.
Comparison of Anandamide Metabolism by Wild-Type CYP2D6 and CYP2D6 R296C (CYP2D6.34).
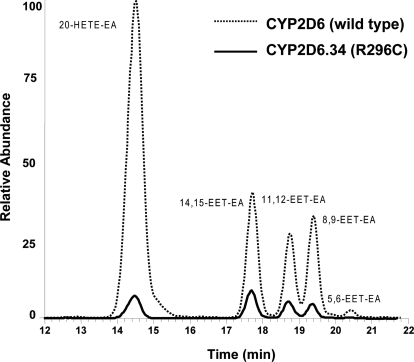
Because there are at least 40 different alleles of the CYP2D6 gene that contain the nucleotide substitution 2850C>T, which results in the substitution of the amino acid cysteine for arginine at position 296 (http://www.cypalleles.ki.se/cyp2d6.htm), we investigated the effect of this single mutation (CYP2D6.34) on the metabolism of anandamide. As shown in Fig. 3, CYP2D6.34, formed all of the same products as the wild-type enzyme, with the exception of 5,6-EET-EA. However, the formation of all four metabolites was significantly lower compared with that for the wild-type enzyme. We were unable to determine the kinetics for the formation of these products because higher concentrations of anandamide resulted in an inhibitory effect on the enzyme activity (data not shown). The formation of 20-HETE decreased the most by far. Another key difference between wild-type CYP2D6 and CYP2D6.34 was that the major product formed by CYP2D6.34 was 14,15-EET-EA, and not 20-HETE-EA, as in wild-type CYP2D6.
Fig. 3.
Metabolism of anandamide by wild-type CYP2D6 and CYP2D6 R296C. Wild-type CYP2D6 or CYP2D6.34 was reconstituted with reductase and incubated with anandamide (2 μM) for 45 min at 37°C, and the samples were analyzed as described under Materials and Methods. The figure shows the total ion chromatogram for the metabolites, demonstrating that the hydroxylated (20-HETE-EA) and epoxygenated (EET-EA) metabolites elute at 14.5, 17.5, 18.6, 19.4, and 20.4 min, respectively.
Binding of 20-HETE-EA and 14,15-EET-EA to the Brain Cannabinoid CB1 Receptor.
We selected 20-HETE-EA and 14,15-EET-EA for further examination of their pharmacological properties because their formation was significantly different between the two wild-type proteins and also between wild-type CYP2B6 and the CYP2B6.4 and CYP2B6.6 variants.
Ligand binding experiments to the CB1 receptor were performed to compare the affinities of 20-HETE-EA and 14,15-EET-EA for the CB1 receptor with that of anandamide. This assay examined the ability of the three compounds to compete with the radiolabeled cannabinoid agonist CP-55940 for binding to the CB1 receptor in rat brain preparations. Rat CB1 shares 97.3% identity with the human receptor (Matsuda et al., 1990). As shown in Fig. 4, anandamide competed with CP-55940 for binding to CB1 with a KI of 275 nM, which is consistent with what has previously been reported in the literature (Pertwee, 2005). Although both 20-HETE-EA and 14,15-EET-EA were also able to compete with CP-55940 for binding to CB1, as evidenced by the dose-dependent decrease in CP-55940 binding, their affinities were significantly lower than that of anandamide. Thus, from the competition curves, the KI values were determined to be 985 nM and 1.56 μM for 20-HETE-EA and 14,15-EET-EA, respectively.
Decreased Biological Stability of 20-HETE-EA and 14,15-EET-EA Relative to Anandamide.
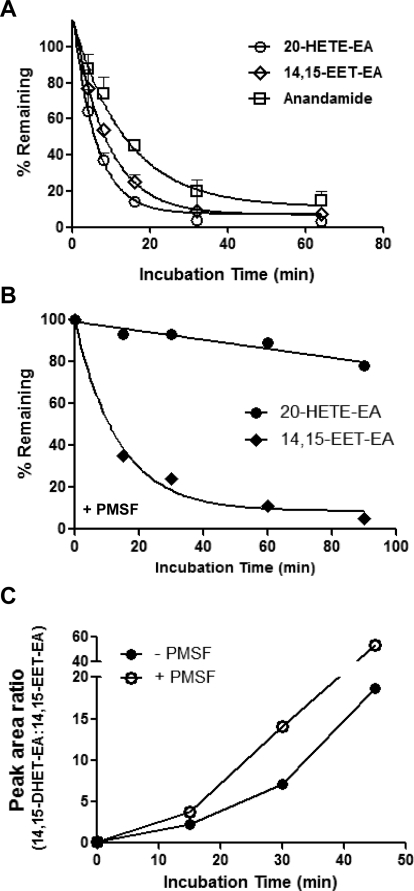
Anandamide is extensively degraded by the enzyme FAAH, which is abundantly expressed in the brain (Giang and Cravatt, 1997). The P450-derived epoxide of anandamide, 5,6-EET-EA, is significantly more stable than anandamide when incubated with mouse brain homogenate (Snider et al., 2009). To compare the relative stabilities of anandamide with those of 20-HETE-EA and 14,15-EET-EA, we incubated each compound in rat brain homogenates for 0 to 64 min and monitored the amounts of the compound remaining as a function of time, as described under Materials and Methods. Anandamide, 20-HETE-EA, and 14,15-EET-EA all disappeared over time with respective half-lives (t1/2) of 9.4, 4.1, and 6.1 min (Fig. 5A). Thus, in addition to having lower affinities for the CB1 receptor, 20-HETE-EA and 14,15-EET-EA are also degraded in rat brain homogenates more rapidly than anandamide.
Fig. 5.
Degradation of anandamide, 20-HETE-EA. and 14,15-EET-EA by rat brain homogenates. In A, each substrate (5 μM) was incubated in the presence of rat brain homogenate (0.5 mg protein/reaction) in phosphate buffer, pH 7.4, at 37°C for 0 to 64 min. At the designated time points, aliquots (500 μl) were removed, and, after the addition of internal standard, the reaction mixtures were extracted with 3 volumes of ethyl acetate and analyzed by ESI-LC-MS as described under Materials and Methods. The amounts of substrate remaining at each time point were plotted as a percentage of the starting amount (at time 0). The data were fitted to a one-phase exponential decay model using Prism 5 software, from which the respective half-lives were obtained. In B, each substrate (5 μM) was incubated in the presence of rat brain homogenate (0.5 mg protein/reaction) in phosphate buffer, pH 7.4, at 37°C for 0 to 90 min in the presence of the FAAH inhibitor, PMSF, at a concentration of 50 μM. At the designated time points aliquots (500 μl) were removed and after the addition of internal standard, the reaction mixtures were extracted with 3 volumes of ethyl acetate and analyzed by ESI-LC-MS as described under Materials and Methods. The amounts of substrate remaining at each time point were plotted as a percentage of the starting amount (at time 0). The data were fitted to a one-phase exponential decay model using Prism 5 software and are representative of three individual experiments. C, determination of the ratios between the epoxide hydrolase-generated product 14,15-DHET-EA and 14,15-EET-EA in the presence and absence of PMSF (50 μM) from the data in B. The data show single measurements at each time point and are representative of the at least three individual experiments.
Next, we investigated the primary routes for the degradative metabolism for the two P450-derived products of anandamide by incubating them in rat brain homogenates in the presence of 50 μM PMSF, a serine protease inhibitor that inhibits FAAH activity (Deutsch and Chin, 1993). As shown in Fig. 5B, there was minimal loss of 20-HETE-EA under these conditions (less than 20% after 90 min of incubation). In contrast, 14,15-EET-EA disappeared fairly rapidly over time in the presence of PMSF with a half-life of 9.4 min, which was slightly higher than the t1/2 of 6.1 min that was observed in the absence of PMSF (Fig. 5A). The EET-EAs can be further metabolized by epoxide hydrolase to their corresponding dihydroxyeicosatrienoic acid ethanolamides (DHET-EAs) (Snider et al., 2007, 2010). To determine the relative contribution of brain epoxide hydrolase activity to the disappearance of 14,15-EET-EA, we measured the amount of 14,15-DHET-EA formed in the presence and absence of PMSF. As shown in Fig. 5C, the ratios of 14,15-DHET-EA to 14,15-EET-EA increased significantly over time and were determined to be approximately 18 and 53 in the absence and presence of PMSF, respectively, after 45 min of incubation time, indicating that metabolism of the 14,15-EET-EA by epoxide hydrolase is the predominant metabolic pathway when FAAH is not inhibited and is significantly augmented upon FAAH inhibition.
Discussion
Understanding the outcomes of the metabolism of various endogenous substrates by brain P450s has physiological and pharmacological significance. For example, it was recently demonstrated that brain P450 epoxygenase activity is essential for achieving pain-relieving effects upon administration of μ opioids (Conroy et al., 2010), raising the possibility that this could be the case for cannabinoids as well. The unique regulation of brain CYP2B6 and CYP2D6 by drugs and genetics (Miksys and Tyndale, 2004) requires a much better understanding of their contribution to the metabolism of endogenous psychoactive substrates. The endocannabinoid anandamide has been shown to exert neuroprotective effects that may be mediated either by cannabinoid receptors or independently of them. The latter include activation of nuclear peroxisome proliferator-activated receptors or the modulation of ion channels, including the transient receptor potential vanilloid type 1 receptor (Rockwell and Kaminski, 2004; van der Stelt and Di Marzo, 2005; Hegde et al., 2008). The CB1 receptor, like CYP2B6 and CYP2D6, is expressed heterogeneously within the brain, where its activation leads to the impairment of cognition and memory, alterations in motor function, and antinociception, among other effects (Mackie, 2008).
P450 enzymes are known to oxidize arachidonic acid to form the physiologically active 5,6-, 8,9-, 11,12-, and 14,15-EETs and several hydroxyeicosatetraenoic acids including 20-HETE (Capdevila et al., 1992). Purified rabbit CYP2B4 and rat CYP2B1 have previously been shown to metabolize arachidonic acid, producing primarily EETs (Zeldin et al., 1995). In the study reported here, anandamide, a derivative of arachidonic acid, is shown to be oxidized by both CYP2B6 and CYP2D6 to yield different EET-EAs and HETEs. We have also investigated the major differences in the metabolism of anandamide by the human wild-type and some of the major polymorphically expressed mutants of CYP2B6 and CYP2D6 in vitro in the reconstituted system and have examined some of the pharmacological properties of the products resulting from anandamide metabolism by these P340s. We found that the main difference between wild-type CYP2B6 and CYP2D6 is the formation of the hydroxylated anandamide product 20-HETE-EA, which is the major metabolite formed by CYP2D6, and it is not formed in significant amounts by CYP2B6. In addition, there was increased formation of 20-HETE-EA by 2B6.4 compared with that by the wild type. It has previously been shown that the 2B6.4 allelic protein has higher 7-ethoxy-4-trifluromethyl coumarin O-dethylase activity and a higher bupropion clearance rate (Kirchheiner et al., 2003), whereas the 2B6.6 allele has decreased protein expression (Lang et al., 2001) and decreased efavirenz clearance. The mutant CYP2B6 G516T (2B6.9) metabolized anandamide to form only 11,12-EET-EA. This mutation has previously been shown to be associated with a reduction in the catalytic function of this enzyme (Rotger et al., 2005). Thus, the effects of CYP2B6 polymorphisms on the metabolism of anandamide appear to be similar to the influence of the polymorphic alleles on substrates such as bupropion, efavirenz, and cyclophosphamide.
Although CYP2D6 accounts for only 2 to 9% of the total hepatic P450 content, it is involved in the metabolism of many clinically used drugs, including ones that affect the central nervous system. Many variants of CYP2D6 carry the mutation R296C, and individuals with this variant have been characterized as poor metabolizers.
Intrinsic clearance (Vmax/Km) values that can be used to compare the abilities of CYP2B6 and CYP2D6 to metabolize anandamide and the various metabolites are shown in Table 3. The intrinsic clearance (Clint) based on 11,12-EET-EA formation was approximately 13-fold greater for CYP2B6 compared with that for CYP2D6. In comparison, only a slight increase was observed for the Clint to form 14,15-EET-EA and a 3-fold increase for 8,9-EET-EA. Overall, lower intrinsic clearance values were found for all the EET-EA metabolites formed by CYP2D6, whereas Clint for the formation of 20-HETE-EA was much greater for CYP2D6 than for CYP2B6. Major differences were also seen between the wild-type enzymes and their mutants that involved some significant changes in the product formation ratios and Km values. For example, both 2B6.4 and 2B6.6 exhibit decreased Km values for anandamide when formation of 14,15-EET-EA was catalyzed. In addition, 14,15-EET-EA is the major product formed by 2D6.34.
TABLE 3.
Intrinsic clearance (Vmax/Km) values for CYP2B6 and CYP2D6 for the formation of the different oxidative metabolites of anandamide
The kinetic data used to calculate the intrinsic clearance values for CYP2B6 were obtained from Table 2 and for CYP2D6 from Snider et al. (2008).
| Vmax/Km | 20-HETE-EA | 14,15-EET-EA | 11,12-EET-EA | 8,9-EET-EA | 5,6-EET-EA |
|---|---|---|---|---|---|
| CYP2B6 | N.D. | 0.57 | 5.73 | 2.58 | 3.38 |
| CYP2D6 | 2.85 | 0.46 | 0.42 | 0.76 | N.D. |
N.D., not detected.
We also found that 20-HETE-EA binds to the CB1 receptor with a 3.6-fold lower affinity relative to anandamide and is more rapidly degraded in the brain than anandamide in a PMSF-sensitive manner, most likely by FAAH. Like 20-HETE-EA, 14,15-EET-EA also binds to the CB1 receptor, but with a 5.7-fold lower affinity relative that of to anandamide. In addition, 14,15-EET-EA is degraded more rapidly than anandamide, in an epoxide hydrolase-dependent manner, based on the appearance of the 14,15-DHET-EA product (Snider et al. 2007). The current findings may contribute to a better understanding of the potential pharmacological effects of inhibitors of both FAAH and epoxide hydrolase in the brain. A study reported that administration of the soluble epoxide hydrolase inhibitor 12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl ester either before or after experimental ischemic stroke reduced the infarct size by 40 to 50% (Zhang et al., 2007). This protective effect was almost completely reversed by coadministration of the P450 epoxygenase inhibitor 6-(2 propargyloxyphenyl) hexanoic acid, and this occurred apparently independent of P450-derived arachidonic acid metabolites. During ischemic stroke in laboratory animals and in human patients, there is an increased synthesis of anandamide (Muthian et al., 2004), which might be expected to lead to an increase in the generation of P450-derived anandamide products. It remains to be investigated whether some of the metabolites generated by CYP2B6 and CYP2D6 are involved in the neuroprotective effects of epoxide hydrolase inhibitors. Although these metabolites bind more weakly to the CB1 receptor, it is possible that they have other molecular targets.
In summary, the metabolism of anandamide by CYP2B6 and CYP2D6 and their polymorphically expressed mutants leads to the generation of different profiles of metabolites, with the major changes being primarily the formation of 20-HETE-EA and 14,15-EET-EA. Although these two products are CB1 receptor ligands, they exhibit decreased stability in the brain. Future functional characterization of the P450-derived metabolites of anandamide and their secondary products will yield additional information regarding the biological significance of these metabolic pathways in the brain.
Supplementary Material
Acknowledgments
We thank Hsia-lien Lin for providing purified reductase.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant CA16954] (to P.F.H.); the National Institutes of Health National Institute of General Medical Sciences [Grant T32-GM007767]; Merck and Co., Inc. (predoctoral fellowship support); and the Michigan Institute for Clinical and Health Research Postdoctoral Translational Scholars Program [Award UL1-RR024986] (to N.T.S.).
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.036707.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
ABBREVIATIONS:
- FAAH
- fatty acid amide hydrolase
- HETE
- hydroxyeicosatetraenoic acid
- HETE-EA
- hydroxyeicosatetraenoic acid ethanolamide
- P450
- cytochrome P450
- ESI
- electrospray ionization
- LC
- liquid chromatography
- MS
- mass spectrometry
- MS/MS
- tandem mass spectrometry
- PMSF
- phenylmethylsulfonyl fluoride
- EET
- epoxyeicosatrienoic acid
- EET-EA
- epoxyeicosatrienoic acid ethanolamide
- CP-55940
- (−)-cis-3-[2-hydroxy-4(1,1-dimethyl-heptyl)phenyl]-trans-4-(3-hydroxypropyl)
- WIN-55212-2
- R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2, 3-de]1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate
- DHET-EA
- dihydroxyeicosatrienoic acid ethanolamide.
Authorship Contributions
Participated in research design: Sridar, Snider, and Hollenberg.
Conducted experiments: Sridar and Snider.
Performed data analysis: Sridar and Snider.
Wrote or contributed to the writing of the manuscript: Sridar, Snider, and Hollenberg.
References
- Ariyoshi N, Miyazaki M, Toide K, Sawamura Yi, Kamataki T. (2001) A single nucleotide polymorphism of CYP2b6 found in Japanese enhances catalytic activity by autoactivation. Biochem Biophys Res Commun 281:1256–1260 [DOI] [PubMed] [Google Scholar]
- Buczynski MW, Parsons LH. (2010) Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Br J Pharmacol 160:423–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR, Estabrook RW. (1992) Cytochrome P450 and the arachidonate cascade. FASEB J 6:731–736 [DOI] [PubMed] [Google Scholar]
- Chang TK, Weber GF, Crespi CL, Waxman DJ. (1993) Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 53:5629–5637 [PubMed] [Google Scholar]
- Conroy JL, Fang C, Gu J, Zeitlin SO, Yang W, Yang J, VanAlstine MA, Nalwalk JW, Albrecht PJ, Mazurkiewicz JE, et al. (2010) Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nat Neurosci 13:284–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. (1993) Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol 46:791–796 [DOI] [PubMed] [Google Scholar]
- Di Marzo V. (2008) Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov 7:438–455 [DOI] [PubMed] [Google Scholar]
- Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM. (2000) Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos 28:1222–1230 [PubMed] [Google Scholar]
- Funae Y, Kishimoto W, Cho T, Niwa T, Hiroi T. (2003) CYP2D in the brain. Drug Metab Pharmacokinet 18:337–349 [DOI] [PubMed] [Google Scholar]
- Giang DK, Cravatt BF. (1997) Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci USA 94:2238–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna IH, Kim MS, Guengerich FP. (2001) Heterologous expression of cytochrome P450 2D6 mutants, electron transfer, and catalysis of bufuralol hydroxylation: the role of aspartate 301 in structural integrity. Arch Biochem Biophys 393:255–261 [DOI] [PubMed] [Google Scholar]
- Hanna IH, Reed JR, Guengerich FP, Hollenberg PF. (2000) Expression of human cytochrome P450 2B6 in Escherichia coli: characterization of catalytic activity and expression levels in human liver. Arch Biochem Biophys 376:206–216 [DOI] [PubMed] [Google Scholar]
- Hanna IH, Teiber JF, Kokones KL, Hollenberg PF. (1998) Role of the alanine at position 363 of cytochrome P450 2B2 in influencing the NADPH- and hydroperoxide-supported activities. Arch Biochem Biophys 350:324–332 [DOI] [PubMed] [Google Scholar]
- Hegde VL, Hegde S, Cravatt BF, Hofseth LJ, Nagarkatti M, Nagarkatti PS. (2008) Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Mol Pharmacol 74:20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, Court MH. (2004) Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics 14:225–238 [DOI] [PubMed] [Google Scholar]
- Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. (2000) CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos 28:1176–1183 [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. (2007) Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther 116:496–526 [DOI] [PubMed] [Google Scholar]
- Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR. (2006) Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol 16:1211–1216 [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Klein C, Meineke I, Sasse J, Zanger UM, Mürdter TE, Roots I, Brockmöller J. (2003) Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics 13:619–626 [DOI] [PubMed] [Google Scholar]
- Klein K, Lang T, Saussele T, Barbosa-Sicard E, Schunck WH, Eichelbaum M, Schwab M, Zanger UM. (2005) Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet Genomics 15:861–873 [DOI] [PubMed] [Google Scholar]
- Lang T, Klein K, Fischer J, Nüssler AK, Neuhaus P, Hofmann U, Eichelbaum M, Schwab M, Zanger UM. (2001) Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics 11:399–415 [DOI] [PubMed] [Google Scholar]
- Mackie K. (2008) Cannabinoid receptors: where they are and what they do. J Neuroendocrinol 20 (Suppl 1):10–14 [DOI] [PubMed] [Google Scholar]
- Marez D, Legrand M, Sabbagh N, Lo Guidice JM, Spire C, Lafitte JJ, Meyer UA, Broly F. (1997) Polymorphism of the cytochrome P450 CYP2D6 gene in a European population: characterization of 48 mutations and 53 alleles, their frequencies and evolution. Pharmacogenetics 7:193–202 [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564 [DOI] [PubMed] [Google Scholar]
- Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. (2003) Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology 45:122–132 [DOI] [PubMed] [Google Scholar]
- Miksys S, Tyndale RF. (2004) The unique regulation of brain cytochrome P450 2 (CYP2) family enzymes by drugs and genetics. Drug Metab Rev 36:313–333 [DOI] [PubMed] [Google Scholar]
- Muthian S, Rademacher DJ, Roelke CT, Gross GJ, Hillard CJ. (2004) Anandamide content is increased and CB1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience 129:743–750 [DOI] [PubMed] [Google Scholar]
- Niwa T, Hiroi T, Tsuzuki D, Yamamoto S, Narimatsu S, Fukuda T, Azuma J, Funae Y. (2004) Effect of genetic polymorphism on the metabolism of endogenous neuroactive substances, progesterone and p-tyramine, catalyzed by CYP2D6. Brain Res Mol Brain Res 129:117–123 [DOI] [PubMed] [Google Scholar]
- Pertwee RG. (2005) Pharmacological actions of cannabinoids. Handb Exp Pharmacol 168:1–51 [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Kaminski NE. (2004) A cyclooxygenase metabolite of anandamide causes inhibition of interleukin-2 secretion in murine splenocytes. J Pharmacol Exp Ther 311:683–690 [DOI] [PubMed] [Google Scholar]
- Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL, Keiser O, Biollaz J, Décosterd L, Telenti A, et al. (2005) Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics 15:1–5 [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Lichtman AH. (2009) Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J 11:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EE, Spatzenegger M, Halpert JR. (2001) A truncation of 2B subfamily cytochromes P450 yields increased expression levels, increased solubility, and decreased aggregation while retaining function. Arch Biochem Biophys 395:57–68 [DOI] [PubMed] [Google Scholar]
- Snider NT, Kornilov AM, Kent UM, Hollenberg PF. (2007) Anandamide metabolism by human liver and kidney microsomal cytochrome P450 enzymes to form hydroxyeicosatetraenoic and epoxyeicosatrienoic acid ethanolamides. J Pharmacol Exp Ther 321:590–597 [DOI] [PubMed] [Google Scholar]
- Snider NT, Nast JA, Tesmer LA, Hollenberg PF. (2009) A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist. Mol Pharmacol 75:965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider NT, Sikora MJ, Sridar C, Feuerstein TJ, Rae JM, Hollenberg PF. (2008) The endocannabinoid anandamide is a substrate for the human polymorphic cytochrome P450 2D6. J Pharmacol Exp Ther 327:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider NT, Walker VJ, Hollenberg PF. (2010) Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: physiological and pharmacological implications. Pharmacol Rev 62:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. (2005) Anandamide as an intracellular messenger regulating ion channel activity. Prostaglandins Other Lipid Mediat 77:111–122 [DOI] [PubMed] [Google Scholar]
- Zanger UM, Klein K, Saussele T, Blievernicht J, Hofmann MH, Schwab M. (2007) Polymorphic CYP2B6: molecular mechanisms and emerging clinical significance. Pharmacogenomics 8:743–759 [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Plitman JD, Kobayashi J, Miller RF, Snapper JR, Falck JR, Szarek JL, Philpot RM, Capdevila JH. (1995) The rabbit pulmonary cytochrome P450 arachidonic acid metabolic pathway: characterization and significance. J Clin Invest 95:2150–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. (2007) Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab 27:1931–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.