Abstract
The influence of drug properties including solubility, lipophilicity, tissue partition coefficients, and in vitro transscleral permeability on ex vivo and in vivo transscleral delivery from corticosteroid suspensions was determined. Solubility, tissue/buffer partition coefficients for bovine sclera and choroid-retinal pigment epithelium (CRPE), and in vitro bovine sclera and sclera-choroid-retinal pigment epithelium (SCRPE) transscleral transport were determined at pH 7.4 for triamcinolone, prednisolone, dexamethasone, fluocinolone acetonide, triamcinolone acetonide, and budesonide in solution. Ex vivo and in vivo transscleral delivery was assessed in Brown Norway rats after posterior subconjunctival injection of a 1 mg/ml suspension of each corticosteroid. Corticosteroid solubility and partition coefficients ranged from ∼17 to 300 μg/ml and 3.0 to 11.4 for sclera and from 7.1 to 35.8 for CRPE, respectively, with the more lipophilic molecules partitioning more into both tissues. Transport across sclera and SCRPE was in the range of 3.9 to 10.7% and 0.3 to 1.8%, respectively, with the transport declining with an increase in lipophilicity. Ex vivo and in vivo transscleral delivery indicated tissue distribution in the order CRPE ≥ sclera > retina > vitreous. Tissue partitioning showed a positive correlation with drug lipophilicity (R2 = 0.66–0.96). Ex vivo and in vivo sclera, CRPE, retina, and vitreous tissue levels of all corticosteroids showed strong positive correlation with drug solubility (R2 = 0.91–1.0) but not lipophilicity (R2 = 0.24–0.41) or tissue partitioning (R2 = 0.24–0.46) when delivered as suspensions. In vivo delivery was lower in all eye tissues assessed than ex vivo delivery, with the in vivo/ex vivo ratios being the lowest in the vitreous (0.085–0.212). Upon exposure to corticosteroid suspensions ex vivo or in vivo, transscleral intraocular tissue distribution was primarily driven by the drug solubility.
Introduction
Whereas corticosteroids in the form of eyedrops, suspensions, and ointments have been available for treating diseases of the ocular surface and anterior segment for several decades, corticosteroid products for treating diseases of the posterior segment of the eye have been developed more recently (Kompella, 2007; Kompella et al., 2010; Edelhauser et al., 2010). Retisert (Bausch and Lomb, Rochester, NY), a surgically placed, sustained-release nondegradable implant of fluocinolone acetonide is in clinical use for the treatment of uveitis. Medidur/Iluvien (pSivida Corporation, Watertown, NY/Alimera Sciences, Alpharetta, GA), an injectable sustained-release nondegradable implant of fluocinolone acetonide, is in phase II clinical trials for the treatment of wet and dry age-related macular degeneration (MAP study), and a new drug application has been filed with the U.S. Food and Drug Administration for the treatment of diabetic macular edema (FAME study). Ozurdex (Allergan, Inc., Irvine, CA), an injectable, sustained release, biodegradable implant of dexamethasone, is in clinical use for the treatment of macular edema after branch retinal vein occlusion or central retinal vein occlusion and noninfectious uveitis affecting the posterior segment of the eye. Furthermore, injectable suspensions of triamcinolone acetonide (Triesence; Alcon Laboratories, Inc., Fort Worth, TX; Trivaris; Allergan, Inc.) were approved for the treatment of uveitis. Thus, corticosteroids are potent anti-inflammatory agents with several applications in treating diseases of extraocular and intraocular tissues including those in the back of the eye.
Although intravitreal injections and sustained release devices deliver adequate drug levels to the retina, repeated intraocular injections as well as surgical placement of intravitreal implants are associated with complications such as cataracts, elevated intraocular pressure, hemorrhage, retinal detachment, and endophthalmitis (Adelman et al., 2010; Sampat and Garg, 2010; Sato et al., 2010). To overcome the risks associated with intravitreal route and to improve on inefficient drug delivery to the back of the eye after topical and oral administration, periocular/transscleral routes such as subconjunctival and subtenon routes may offer more promising alternatives for retinal drug delivery (Cruysberg et al., 2002; Ayalasomayajula and Kompella, 2004; Raghava et al., 2004). Transscleral drug delivery has potential for treating back of the eye disorders (Ayalasomayajula and Kompella, 2005; Amrite et al., 2006). Our prior studies indicated that a periocularly administered microparticle system can sustain the delivery and efficacy of celecoxib for at least 2 months (Amrite et al., 2006). Thus, sustained transscleral retinal drug delivery is feasible.
Anecortave acetate, a steroid drug, was developed for sustained transscleral delivery in treating the wet form of age-related macular degeneration after periocular administration in the posterior juxtascleral space. Anecortave acetate, a drug with very low solubility (0.22 μg/ml in normal saline at 37°C) (Missel et al., 2004), when administered as a suspension allowed sustained delivery/efficacy of the drug (D'Amico et al., 2003; Dahlin and Rahimy, 2007). However, this product did not receive Food and Drug Administration approval in the United States because of its inadequate efficacy, it is likely that inadequate quantities of the drug were delivered to the target tissues due to its poor solubility. Drug delivery by all routes of administration to the eye generally requires the presence of the drug in the soluble form for cell entry and activity, unless the drug particle has special properties to enter the cells, followed by eventual dissolution for drug action. For passive drug diffusion across plasma membranes of cells, concentration gradient and partition coefficient are critical parameters. Depending on the route of administration, the farther the drug is placed from the target tissue, the more critical the concentration gradient becomes for a drug to reach the target tissue at therapeutic levels. For instance, using a 5% eyedrop solution of leukocyte functional antigen-1 antagonist, a drug intended for diabetic retinopathy, we were able to show more significant drug effects in the back of the eye compared with those for a 1% drug solution (Rao et al., 2010). If the drug is placed near the target, drugs with limited solubility may also exert activity, as is the case with intravitreally injected corticosteroids. Another limiting parameter in drug delivery is drug clearance. If the particle or drug is cleared before it can be absorbed (Amrite and Kompella, 2005; Amrite et al., 2008b), drug delivery to the target tissue will suffer. Conjunctival and choroidal circulatory systems contribute the most to drug clearance from periocular space, resulting in limited transscleral retinal drug delivery (Amrite et al., 2008a). Another limitation of subconjunctival and subtenon injections is hyperemia and potential irritation of the conjunctiva.
For drugs in solution at low concentrations, a decrease in transscleral transport has been observed with increasing drug lipophilicity, as a result of drug binding and retention in melanin-rich choroid-retinal pigment epithelium (CRPE) layer or sclera (Cheruvu and Kompella, 2006; Cheruvu et al., 2008). For any drug intended for transscleral delivery to treat chronic diseases, a depot form is preferred. Drug suspensions naturally allow prolonged delivery because of slow drug dissolution (Durairaj et al., 2009). However, it is unclear whether drug solubility and/or lipophilicity influence in vivo transscleral delivery of various corticosteroids from suspensions. Furthermore, it is not clear whether tissue partition coefficients and tissue permeability of corticosteroids can be correlated to in vivo transscleral delivery. Thus, the objective of this study was to determine the influence of drug lipophilicity, tissue partition coefficients, solubility, and permeability on transscleral tissue distribution of six corticosteroids including triamcinolone (T), prednisolone (P), dexamethasone (D), fluocinolone acetonide (FA), triamcinolone acetonide (TA), and budesonide (B). In this study, we assessed sclera and choroid-RPE corticosteroid partition coefficients and sclera and sclera-choroid-RPE transport for solution forms of corticosteroids using pigmented bovine eye tissues. Furthermore, we assessed pigmented rat ex vivo and in vivo transscleral tissue distribution of corticosteroids using suspension forms of the drugs. In vivo rat data were correlated with ex vivo rat data and with drug properties including lipophilicity, solubility, permeability, and partition coefficients.
Materials and Methods
Materials.
Budesonide [16,17-(butylidenebis(oxy))-11,21-dihydroxy-(11-β,16-α)-pregna-1,4-diene-3,20-dione], fluocinolone acetonide [(1S,2S,4R,8S,9S,11S,12R,13S,19S)-12,19-difluoro-11-hydroxy-8-(2-hydroxyacetyl)-6,6,9,13-tetramethyl-5,7-dioxapentacyclo[10.8.0.02,9.04,8.013,18]icosa-14,17-dien-16-one], triamcinolone [(11β,16α)-9-fluoro-11,16,17,21-tetrahydroxypregna-1,4-diene-3,20-dione], and triamcinolone acetonide [(4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS)-4b-fluoro-6b-glycoloyl-5-hydroxy-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2′,1′:4,5]indeno[1,2-d][1,3]dioxol-2-one] were purchased from Spectrum Chemical and Laboratory Products (division of Spectrum Chemical Mfg. Corp.; New Brunswick, NJ). Prednisolone [(11β)-11,17,21-trihydroxypregna-1,4-diene-3,20-dione], dexamethasone [(8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one], and sodium carboxymethylcellulose (low viscosity, 50–200 cps) were purchased from Sigma-Aldrich (St. Louis, MO). High-performance liquid chromatography-grade acetonitrile and methanol were purchased from Thermo Fisher Scientific (Waltham, MA). Freshly excised bovine eyes were purchased from G and C Meat Company (Colorado Springs, CO). Male Brown-Norway (BN) (pigmented) rats weighing 150 to 200 g were purchased from Charles River Laboratories (Wilmington, DE).
Solubility Determination of Corticosteroids in Cocktail Suspension.
Solubility of each corticosteroid was determined at 1 mg/ml in the cocktail suspension in phosphate-buffered saline (PBS) (pH 7.4) containing 0.5 % w/v carboxymethyl cellulose. For comparison purposes, we also determined the solubility of each corticosteroid individually using a 1 mg/ml suspension in PBS (pH 7.4) after incubating for 24 h at 37°C before solubility assessment. The suspension was centrifuged at 25,000 rpm for 45 min using a Beckman Optima LE-80K ultracentrifuge (Beckman Coulter, Fullerton, CA), and the drug concentration in the supernatant was determined using LC-MS/MS analysis.
Bovine Eye Tissue Isolation.
Freshly excised bovine eyes were used in all studies. For isolation of sclera and CRPE (Cheruvu and Kompella, 2006), the anterior segment of the eye was removed with a circumferential cut below the limbus. The eye was cut into two halves along the geometric axis, a line joining the anterior pole (corneal center) and the posterior pole (center of the scleral curve), and the vitreous was removed. Neural retina was removed by exposing the eyecup to isotonic assay buffer at pH 7.4. The equatorial region of the remaining sclera-choroid-RPE (SCRPE) was used as is for SCRPE transport studies and after peeling off the choroid-RPE for transport studies across the sclera. For in vitro partition studies, isolated sclera and choroid-RPE layers were used.
In Vitro Tissue Partition Studies.
These studies were performed to measure the relative affinity of each corticosteroid toward sclera and choroid-RPE compared with PBS. A cocktail of six corticosteroids at three concentrations (0.4, 2, and 10 μg/ml) was used for partition studies. Tissue samples (100 mg; n = 5 for sclera and CRPE) were incubated with 0.5 ml of corticosteroid solution in PBS for 6 h at 37°C. At the end of the incubation period, samples were centrifuged for 15 min at 10,000 rpm (accuSpin Micro 17 centrifuge; Thermo Fisher Scientific). The PBS layer was separated completely, and tissue was rinsed with 0.5 ml of fresh PBS. Corticosterone was added as an internal standard at a fixed concentration (500 ng/ml) to all buffer and tissue samples to account for any loss of drug during the extraction procedure. Tissue samples were homogenized and extracted over 30 min for drug using 2.0 ml of ethyl acetate. Organic layer was removed and dried under a nitrogen atmosphere, and the residue was reconstituted in an acetonitrile-water mixture for LC-MS/MS analysis. Tissue partition coefficients were estimated as the tissue/PBS concentration ratio for each corticosteroid. To determine whether cocktail estimates of partition coefficients differ from individual measures, we selected one corticosteroid (dexamethasone) and measured its tissue partition coefficients (pH 7.4; 0.4, 2 and 10 μg/ml) in a manner similar to what was described above for the cocktail solution.
In Vitro Transscleral Transport of Corticosteroids.
The bovine sclera and sclera-choroid-RPE transport study was conducted as described previously (Cheruvu and Kompella, 2006; Thakur et al., 2010; Kadam et al., 2011). An isotonic assay buffer (pH 7.4) with the following composition was used during the entire tissue isolation procedure and transport study: 122 mM NaCl, 25 mM NaHCO3, 1.2 mM MgSO4, 0.4 mM K2HPO4, 1.4 mM CaCl2, 10 mM HEPES, and 10 mM glucose. After the tissues were mounted in modified Ussing chambers, donor solution (1.5 ml of 10 μg/ml of each corticosteroid in a cocktail) was filled in chambers facing the episcleral side and receiver chambers were filled with the assay buffer (pH 7.4). The transport study was conducted for 6 h at 37°C under 95% air and 5% CO2 aeration. Sample (200 μl) was collected from the receiver side at specific time intervals and replenished with fresh buffer. At the end of the study, tissue regions exposed to the buffer were dissected for drug quantification. The drug content in the receiver and tissue samples was analyzed using an LC-MS/MS method.
Ex Vivo and In Vivo Rat Ocular Distribution of Corticosteroids.
All animals were handled according to The Association for Research in Vision and Ophthalmology statement for the use of Animals in Ophthalmic and Vision Research. A cocktail suspension of six corticosteroids was used for ex vivo and in vivo studies. In the cocktail suspension, each corticosteroid was present at 1 mg/ml, and carboxymethyl cellulose sodium salt (low viscosity, 50–200 cP; Sigma-Aldrich) was present at a concentration of 0.5% w/v as a suspending agent. All corticosteroids were mixed gently with the suspending agent using a pestle and mortar followed by addition of sterilized phosphate buffer saline (pH 7.4). The mixture was incubated for 24 h in a shaker incubator (MAXQ 4000; VWR, West Chester, PA) set at 37°C and 300 rpm. Furthermore, drug suspensions were shaken before each posterior subconjunctival injection. Rats were divided into two groups.
Group 1: in vivo.
Animals were anesthetized with an intraperitoneal injection of 120 μl of 50 mg/ml ketamine and 10 mg/ml xylazine. Then 25 μl of corticosteroid suspension was injected using a 30-gauge needle in the posterior subconjunctival space of one eye of each animal, and the other eye was not treated. At the end of 1 h, animals were sacrificed with an intraperitoneal injection of approximately 350 μl of sodium pentobarbital (250 mg/kg). Eyes were enucleated and immediately frozen in dry ice and an isopentane bath. All samples were stored at −80°C until LC-MS/MS analysis. Different ocular tissues including sclera, choroid-RPE, retina, and vitreous were isolated and analyzed for drug levels.
Group 2: ex vivo.
Animals were euthanized with sodium pentobarbital as mentioned above. Immediately after euthanasia, animals were administered a 25-μl suspension of corticosteroids (1 mg/ml each) in one eye via a posterior subconjunctival injection using a 30-gauge needle, and the other eye was left untreated. At the end of 1 h, the eyes were enucleated and frozen in a manner similar to that above until analysis. Periocular tissue samples were also collected from both groups at the end of the study.
Melanin-Binding Study.
The following procedure was used to determine the extent of binding of corticosteroids in a cocktail with natural melanin. A 1 mg/ml suspension of melanin (Sepia officinalis) in PBS (pH 7.4) was incubated with a series of concentrations of corticosteroids ranging from 0.01 to 30 μM in a cocktail (n = 3 for each concentration). After incubation at 37°C for 6 h, samples were centrifuged at 15,000 rpm (21,130g) for 15 min. The supernatant was withdrawn and analyzed using LC-MS/MS.
LC-MS/MS Analysis of Corticosteroids.
The analytical method for quantification of corticosteroids was described previously (Thakur et al., 2010). A liquid-liquid extraction procedure was used for quantifying corticosteroids in tissues. In brief, weighed amounts of tissues were homogenized in 250 μl of PBS (pH 7.4) containing 500 ng/ml internal standard. Then 1 ml of ethyl acetate was added, and the mixture was vortexed for 20 min using a VX 2500 multitube vortexer (VWR). Organic solvent was separated and evaporated under nitrogen. Samples were reconstituted in 250 μl of acetonitrile-water mixture (75:25) and analyzed using an LC-MS/MS method.
Statistical Analyses.
All data in this study are expressed as the mean ± S.D. Measures for the six corticosteroids were compared using one-way ANOVA followed by a Tukey post hoc analysis. Statistical significance was set at p ≤ 0.05.
Results
Corticosteroid Solubility.
Table 1 depicts the solubility values for six corticosteroids measured individually or in cocktail suspension. A decrease in solubility of fluocinolone acetonide, triamcinolone acetonide, and budesonide was observed when they were present in cocktail suspension compared with their individual suspensions. These three corticosteroids are more lipophilic (logD, Table 1) than the other three, whose solubilities remained unaffected. Solubilities obtained with the cocktail preparation were used for correlations with drug delivery.
TABLE 1.
Molecular weight, PBS solubility, measured lipophilicity, predicted lipophilicity, and particle size of corticosteroids used in the current study
Data are presented as the mean ± S.D. for n = 6 for logD measurements and n = 4 for solubility measurements.
| Steroid (Molecular Weight) | Individual Measures |
Cocktail Measures: PBSSol (n = 4)b | Measured logD (n = 6)c | Predicted logPd | Mean Particle Size in 1 mg/ml Suspensione | |
|---|---|---|---|---|---|---|
| OctSol (n = 4)a | PBSSol (n = 4)b | |||||
| mg/ml | μg/ml | μg/ml | μm | |||
| T (394.43) | 0.29 ± 0.007 | 120.93 ± 19.92 | 129.40 ± 11.84 | 0.71 ± 0.11 | 0.83 | 3.90 ± 0.52 |
| P (360.44) | 7.5 ± 0.11 | 302.95 ± 16.94 | 314.40 ± 31.29 | 1.77 ± 0.11 | 1.49 | 0.84 ± 0.03 |
| D (392.46) | 3.2 ± 0.33 | 86.70 ± 3.81 | 92.32 ± 5.60 | 1.95 ± 0.02 | 1.87 | 2.51 ± 1.15 |
| FA (452.48) | 9.8 ± 0.2 | 17.02 ± 0.64 | 10.17 ± 1.00 | 2.56 ± 0.11 | 2.24 | 4.33 ± 1.50 |
| TA (434.49) | 5.2 ± 0.07 | 26.12 ± 6.02 | 14.81 ± 2.00 | 2.58 ± 0.03 | 2.50 | 1.45 ± 0.21 |
| B (430.50) | 17.2 ± 0.98 | 23.51 ± 1.08 | 5.72 ± 1.20 | 2.97 ± 0.09 | 3.14 | 2.89 ± 0.87 |
Represents solubility of individual corticosteroid in octanol (OctSol). Excess material was added to the octanol at 37°C and the concentration of the dissolved material was determined using a UV spectrophotometer at the end of 24 h. The rank order for octanol solubility measurements on the basis of one-way ANOVA (multiple comparisons) was B > FA > P > TA > D > T (p < 0.05).
Represents solubility of individual corticosteroid in phosphate-buffered saline, pH 7.4 (PBSSol). Excess material was added to PBS at 37°C, and the concentration of the dissolved material was determined using LC-MS/MS at the end of 24 h. The rank order for independent and cocktail solubility measurements on the basis of one-way ANOVA (multiple comparisons) was P > T > D > FA ∼ TA ∼ B (p < 0.05). Individual solubility values of fluocinolone acetonide, triamcinolone acetonide, and budesonide were significantly higher (p < 0.05, Student's t test) than their solubility values in the cocktail.
Distribution coefficient (D) was measured using the USP shake flask method as reported previously (Thakur et al., 2010). The rank order for logD was B > TA ∼ FA > D ∼ P > T (p < 0.05).
Predicted partition coefficient (P) was obtained from SciFinder Scholar 2007 software.
Particle size was determined for 1 mg/ml suspension of each corticosteroid using Zetasizer Nano (Malvern Instruments Ltd., Malvern, Worcestershire, UK). Rank order for the particle size was FA ∼ T ∼ B > TA > P (particle size of D was significantly different from that of P but not other corticosteroids).
Tissue Partition Coefficients.
Percent extraction recovery and percent matrix effect of each corticosteroid were estimated (Table 2) using bovine sclera and CRPE. Ethyl acetate was selected as the organic solvent for extraction. Percent extraction recovery and matrix effect for both tissues ranged between 86.6 and 109.3 and 81.8 and 94.6, respectively.
TABLE 2.
Percentage extraction recovery and percentage matrix effect of corticosteroids in bovine sclera and choroid-RPE
Percentage extraction recovery was calculated as the ratio of analyte peak area obtained with spiking before extraction to the analyte peak area obtained with spiking postextraction multiplied by 100. Percentage matrix effect was calculated as the ratio of analyte peak area of standard with spiking after extraction procedure to analyte peak area of corresponding unextracted standard multiplied by 100. Data are expressed as the mean ± S.D. for n = 5.
| Corticosteroid Used at 400 ng/ml | Sclera |
Choroid-RPE |
||
|---|---|---|---|---|
| % ER | % ME | % ER | % ME | |
| Triamcinolone | 93.22 ± 5.38 | 89.33 ± 5.40 | 90.72 ± 4.40 | 83.04 ± 2.93 |
| Prednisolone | 95.51 ± 6.80 | 94.60 ± 4.37 | 99.12 ± 4.60 | 82.11 ± 2.20 |
| Dexamethasone | 103.44 ± 7.21 | 87.42 ± 6.23 | 86.63 ± 8.75 | 83.72 ± 2.95 |
| Fluocinolone acetonide | 98.09 ± 6.34 | 91.45 ± 8.72 | 93.82 ± 11.81 | 88.20 ± 6.04 |
| Triamcinolone acetonide | 109.32 ± 2.71 | 89.63 ± 3.74 | 90.22 ± 1.61 | 81.83 ± 9.84 |
| Budesonide | 96.56 ± 3.42 | 93.41 ± 5.70 | 86.80 ± 2.73 | 87.24 ± 2.53 |
ER, extraction recovery; ME, matrix effect.
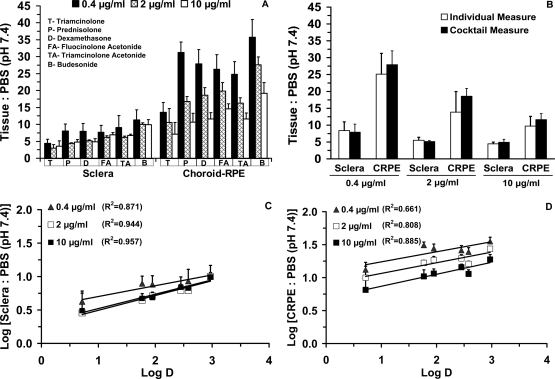
Corticosteroid partition coefficients for sclera and CRPE are shown in Table 3. The ranges of tissue/PBS partition coefficients after drug incubation at 0.4, 2, and 10 μg/ml were 4.3 to 11.4, 3.0 to 9.9, and 3.5 to 9.9 for sclera and 13.5 to 35.8, 10.6 to 27.6, and 7.1 to 19.2 for CRPE, respectively. Corticosteroid partitioning into CRPE was 1.7-to 3.8-fold higher than that in sclera (Fig. 1A). There was no significant difference in the tissue partition coefficients of dexamethasone assessed using individual versus cocktail solution (Fig. 1B). Dexamethasone partition coefficients in the cocktail and as an individual corticosteroid at 0.4, 2, and 10 μg/ml for sclera were 7.9, 5.12, and 4.9 and 8.4, 5.5, and 4.4, respectively. Dexamethasone partition coefficient values in cocktail and individual corticosteroid studies for CRPE were 27.9, 18.57, and 11.62 and 25.1, 13.9, and 9.8, respectively. Partition coefficients generally declined with an increase in drug concentration and showed a positive correlation with the lipophilicity in both tissues (Fig. 1, C and D). Statistically, there was a significant difference between the partition coefficients of triamcinolone (the least lipophilic corticosteroid) and budesonide (the most lipophilic corticosteroid) at all three concentrations in both sclera and CRPE.
TABLE 3.
Tissue/PBS (pH 7.4) partition coefficients of corticosteroids in bovine sclera and CRPE
The partition coefficient was calculated as a ratio of drug concentration in tissue to that in PBS (pH 7.4) after incubation for 6 h at 37°C. Three different concentrations (0.4, 2, and 10 μg/ml) were used for estimating the partition coefficient. Data are expressed as the mean ± S.D. for n = 5.
| Steroid | Concentration | Sclera/PBS | CRPE/PBS |
|---|---|---|---|
| μg/ml | |||
| Triamcinolone | 0.4 | 4.36 ± 1.30 | 13.53 ± 2.97 |
| 2 | 3.00 ± 0.97 | 10.59 ± 4.07 | |
| 10 | 3.55 ± 1.60 | 7.12 ± 3.42 | |
| Prednisolone | 0.4 | 8.06 ± 2.00 | 31.27 ± 3.08 |
| 2 | 4.42 ± 0.12 | 16.76 ± 1.49 | |
| 10 | 4.75 ± 0.77 | 10.67 ± 2.61 | |
| Dexamethasone | 0.4 | 7.90 ± 2.38 | 27.90 ± 4.14 |
| 2 | 5.12 ± 0.29 | 18.57 ± 2.32 | |
| 10 | 4.90 ± 0.87 | 11.62 ± 1.83 | |
| Triamcinolone acetonide | 0.4 | 7.64 ± 2.09 | 26.30 ± 4.33 |
| 2 | 6.22 ± 0.43 | 19.80 ± 2.53 | |
| 10 | 6.93 ± 0.66 | 14.60 ± 1.57 | |
| Fluocinolone acetonide | 0.4 | 9.12 ± 3.55 | 24.83 ± 3.68 |
| 2 | 6.20 ± 0.36 | 16.30 ± 1.52 | |
| 10 | 6.72 ± 0.42 | 11.59 ± 1.77 | |
| Budesonide | 0.4 | 11.34 ± 2.98 | 35.75 ± 5.22 |
| 2 | 9.99 ± 0.43 | 27.60 ± 2.35 | |
| 10 | 9.96 ± 1.41 | 19.20 ± 3.20 |
Fig. 1.
Tissue partition coefficients measured using the cocktail approach were not significantly different from those measured using an individual corticosteroid solution and correlated positively with lipophilicity. A, sclera and choroid-RPE tissue/PBS (pH 7.4) partition coefficients of six corticosteroids at 0.4, 2, and 10 μg/ml in a cocktail solution. B, sclera and choroid-RPE tissue/PBS (pH 7.4) partition coefficients of dexamethasone alone at 0.4, 2, and 10 μg/ml. Correlation of lipophilicity (logD; pH 7.4) with sclera (C) and CRPE tissue/PBS (pH 7.4) (D) partition coefficients in cocktail mixture at 0.4, 2, 10 μg/ml. Data are expressed as the mean ± S.D. for n = 5.
Transport of Corticosteroids across Bovine Sclera and SCRPE.
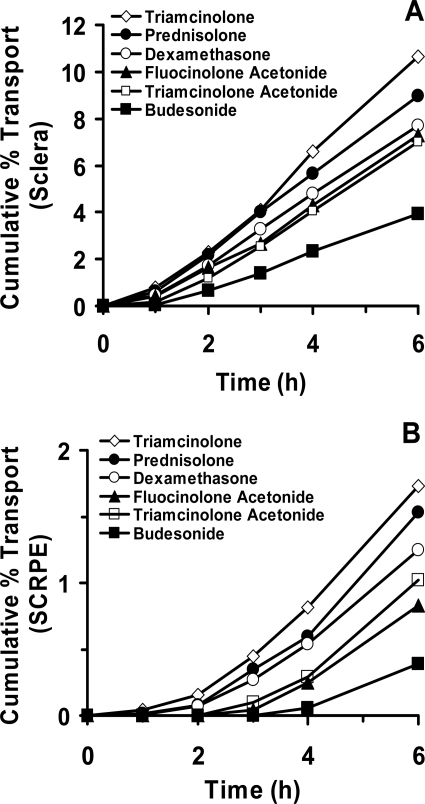
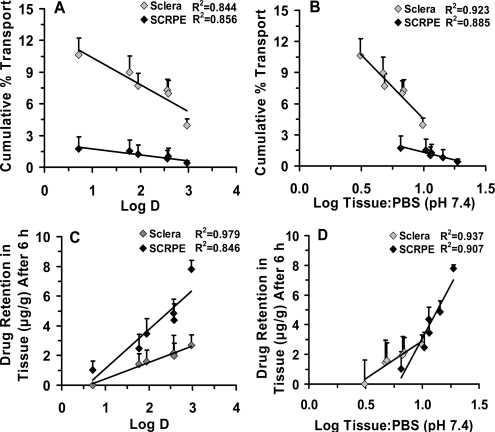
The cumulative transport of corticosteroids across sclera and SCRPE at the end of 6 h ranged from 3.9 to 10.7 and 0.3 to 1.8%, respectively (Fig. 2, A and B). Rank order for cumulative percent transport across sclera and SCRPE based on the ANOVA was triamcinolone ∼ prednisolone ∼ dexamethasone ∼ fluocinolone acetonide ∼ triamcinolone acetonide > budesonide. Budesonide, the most lipophilic corticosteroid, exhibited the highest levels in tissues (sclera and SCRPE) at the end (6 h) of the transport study. These levels were significantly (p < 0.05) different from those of other corticosteroids. The cumulative percent transport across the tissues showed a strong inverse correlation (R2 ≥ 0.8) with the drug lipophilicity for both sclera and SCRPE. It showed inverse correlation with tissue partitioning of corticosteroids for sclera (R2 ≥ 0.9) and CRPE (R2 ≥ 0.8) (Fig. 3, A and B). Tissue retention of corticosteroids at the end of the transport study in sclera and SCRPE showed a positive correlation with lipophilicity and with tissue partition coefficients (R2 ≥ 0.8) (Fig. 3, C and D).
Fig. 2.
Transscleral transport corticosteroids. In vitro bovine sclera (A) and SCRPE (B) transport of corticosteroids was performed at 10 μg/ml and at 37°C. Data are expressed as the mean for n = 4 for sclera and n = 6 for SCRPE. Error bars are not shown for clarity.
Fig. 3.
Cumulative percent transport of corticosteroids across bovine sclera and SCRPE correlates inversely with lipophilicity (logD; pH 7.4) (A) and solute tissue partition coefficients measured at 10 μg/ml (B). Linear correlation of amount of corticosteroids retained in the tissue at the end (6 h) of transport study with drug lipophilicity (C) and tissue partition coefficients measured at 10 μg/ml (D). Data are expressed as the mean ± S.D. (n = 4 for sclera and n = 6 for SCRPE).
Ocular Distribution of Corticosteroids in BN Rats.
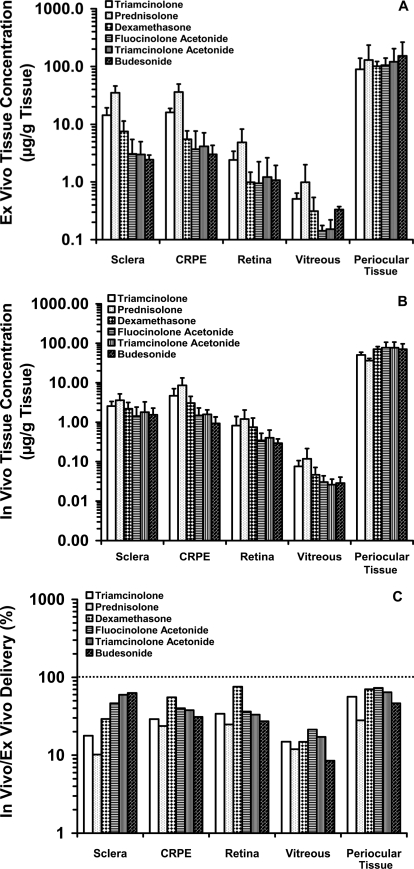
The ex vivo drug levels in sclera, CRPE, retina, and vitreous for the corticosteroids were in the range of 2.43 to 35.03, 3.00 to 35.98, 0.95 to 4.85, and 0.15 to 0.99 μg/g tissue, respectively. The in vivo drug levels in sclera, CRPE, retina, and vitreous were in the range of 1.42 to 3.56, 0.93 to 8.52, 0.29 to 1.20, and 0.02 to 0.11 μg/g tissue, respectively (Fig. 4, A and B). Ex vivo levels of prednisolone were significantly different from those of all other corticosteroids in sclera and in CRPE. In retina and vitreous, ex vivo levels of prednisolone were significantly different from those of all corticosteroids except triamcinolone, which is immediately next in solubility ranking after prednisolone.
Fig. 4.
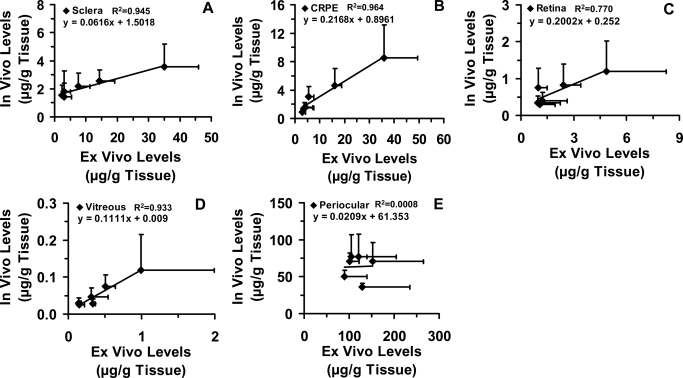
Transscleral retinal delivery of corticosteroids. Ex vivo (A) and in vivo (B) ocular tissue distribution, and in vivo/ex vivo percent delivery (C) of six corticosteroids at the end of 1 h in BN rats. Twenty-five microliters of 1 mg/ml suspension was injected into the posterior subconjunctival space of euthanized (ex vivo study) or live (in vivo) rats. Data are expressed as the mean ± S.D. for n = 4.
With respect to in vivo distribution, there was no significant difference among the six corticosteroids in sclera. Levels of prednisolone in CRPE were different from those of all other corticosteroids except triamcinolone. In vivo retinal and vitreal levels of prednisolone were significantly different from those of fluocinolone acetonide, triamcinolone acetonide, and budesonide but not triamcinolone and dexamethasone.
Ocular tissue levels for the in vivo study were lower in general compared with those for the ex vivo study (Fig. 4C). The corticosteroid dose remaining at the end of 1 h in the periocular tissue in the ex vivo study was approximately 1.36- to 3.56-fold higher than that remaining in the in vivo study. Corticosteroid levels in sclera, choroid-RPE, retina, and vitreous were approximately 1.59- to 9.81-, 1.80- to 4.22-, 1.31 to 4.04-, and 4.7- to 11.7-fold higher, respectively, in the ex vivo study than in the in vivo study. Prednisolone with the highest solubility (∼300 μg/ml) among all corticosteroids (15–100 μg/ml for other corticosteroids) used in this study, diffused more into all tissues.
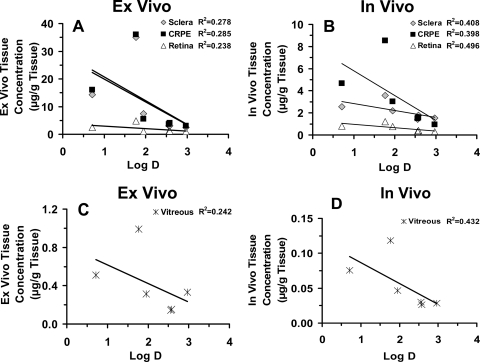
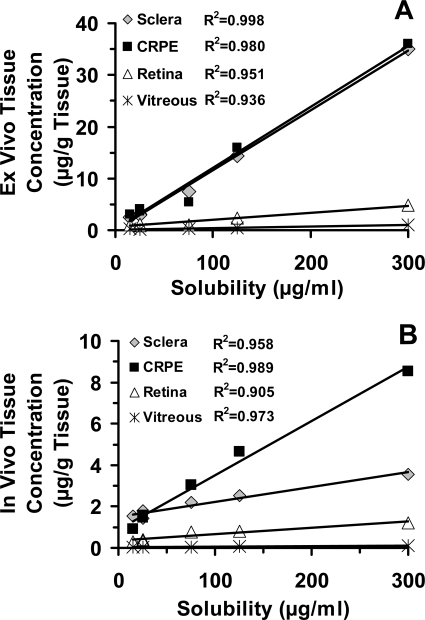
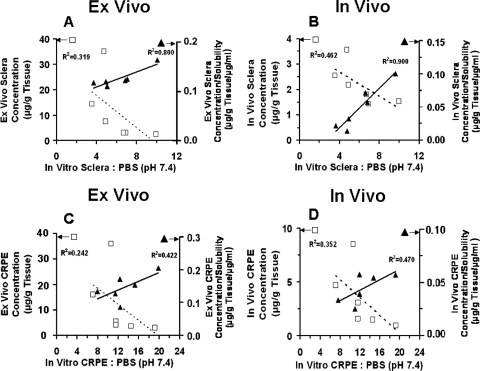
We observed a positive relationship between ex vivo and in vivo tissue levels (R2 ≥ 0.7 for sclera, CRPE, retina, and vitreous) (Fig. 5, A–D). However, we did not observe any relationship between ex vivo periocular tissue levels and in vivo periocular tissue levels (Fig. 5E). Furthermore, we did not observe a positive relationship between tissue levels and lipophilicities of corticosteroids (Fig. 6). On the other hand, the correlation between ex vivo/in vivo tissue concentrations (Fig. 7, A and B) and solubilities of corticosteroids in the suspension exhibited strong positive relationships, with the correlation coefficients for all the tissues being ≥0.9. The relationship between rat ex vivo/in vivo tissue levels and bovine in vitro tissue partitioning studies was poor (R2 < 0.5 for sclera and R2 < 0.4 for CRPE). However, upon normalizing the observed tissue concentrations to the solubility of corticosteroids present in the cocktail suspension, we could see an improved positive relationship with in vitro tissue partition coefficients (R2 ≥ 0.8 for sclera and R2 ≥ 0.4 for CRPE) in bovine tissues measured using the solution form of corticosteroids (Fig. 8). A modest yet positive correlation (R2 ≥ 0.3) was observed between rat ex vivo/in vivo retinal/vitreal concentrations obtained with drug suspensions and bovine in vitro cumulative percent transport of corticosteroids across sclera and SCRPE measured using drug solutions (Fig. 9). One reason for the poor correlation between in vitro partition/transport studies and the in vivo/ex vivo delivery studies is the fact that the former studies were done using solution forms of the drug, whereas the latter studies were done using suspension forms of the drug.
Fig. 5.
Good correlation of in vivo tissue concentrations with ex vivo tissue concentrations for sclera (A), CRPE (B), retina (C), and vitreous (D) after transscleral delivery from corticosteroid suspensions in Brown Norway rats. Data obtained at 1 h postdosing was correlated. E, no correlation was observed for periocular tissue. Data are expressed as the mean ± S.D. for n = 4.
Fig. 6.
Poor correlation of tissue concentrations with corticosteroid lipophilicity after suspension administration for transscleral delivery. A and C, correlations of ex vivo transscleral delivery in euthanized Brown Norway rats with lipophilicity. B and D, correlations of in vivo transscleral delivery in Brown Norway rats with lipophilicity. Data are expressed as the mean ± S.D. for n = 4.
Fig. 7.
Good correlation of tissue concentrations with corticosteroid solubility after suspension administration for transscleral delivery. Correlation of ex vivo transscleral delivery in euthanized Brown Norway rats (A) and in vivo transscleral delivery in live Brown Norway rats (B) with drug solubility. Data are expressed as the mean ± S.D. for n = 4.
Fig. 8.
Correlation between ex vivo or in vivo tissue concentrations obtained after transscleral delivery from corticosteroid suspensions in Brown Norway rats with in vitro bovine tissue/PBS partition coefficients. A and C, correlations between ex vivo tissue concentrations and tissue partition coefficients for sclera and choroid-RPE, respectively. B and D, correlations between in vivo tissue concentrations and tissue partition coefficients for sclera and choroid-RPE, respectively. Data are expressed as the mean ± S.D. (n = 4 for ex vivo and in vivo studies and n = 5 for tissue partition studies). □, ex vivo or in vivo tissue levels (micrograms per gram of tissue) without normalization to corticosteroid solubility in the cocktail suspension; ▴, ex vivo or in vivo tissue levels (micrograms per gram of tissue per microgram per milliliter) normalized to corticosteroid solubility in the cocktail suspension.
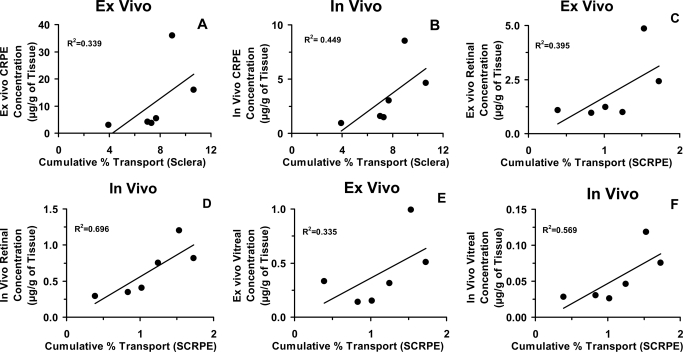
Fig. 9.
Correlation between in vitro sclera (A and B) and sclera-choroid-RPE (C–F) transport of corticosteroid solutions and ex vivo or in vivo tissue concentrations obtained after transscleral delivery from corticosteroid suspensions. Data are expressed as the mean ± S.D. for n = 4 for in vitro transport across sclera and ex vivo or in vivo tissue concentrations and n = 6 for in vitro transport across SCRPE.
Melanin-Binding Study.
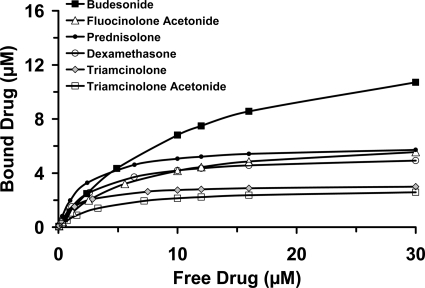
After incubation with melanin, free drug concentrations in the supernatant were quantified using LC-MS/MS. The amount of bound drug was estimated after subtraction of the free drug amount from the initial amount used. Means of free and bound drug concentrations are plotted in Fig. 10.
Fig. 10.
Concentration-dependent binding of corticosteroids to natural melanin. Various concentrations of corticosteroids in solution (0.01–30 μM; n = 3 for each concentration) were incubated as a cocktail with natural melanin (from S. officinalis) dispersed in PBS (pH 7.4), for 6 h at 37°C. At the end of the study, mean bound and free drug concentrations were estimated and plotted.
Discussion
The influence of physicochemical properties of corticosteroids on ocular tissue distribution via the transscleral route has not been explained previously. For the first time in this study, we report that 1) in vivo as well as ex vivo transscleral retinal and vitreal corticosteroid delivery from suspension depots correlates well and positively with drug solubility but does not correlate well with lipophilicity, in vitro transport, or in vitro tissue partitioning; 2) in vitro sclera-choroid-RPE permeability for corticosteroid solutions decreases with an increase in drug lipophilicity; 3) sclera and choroid-RPE partition coefficients for corticosteroids correlate positively with drug lipophilicity and decrease with an increase in dose; and 4) in vivo transscleral ocular delivery is less than ex vivo delivery in all eye tissues assessed.
All corticosteroids used in this study are neutral lipophilic drug molecules (pKa >7; SciFinder Scholar 2007 software; American Chemical Society, Washington, DC) with very low aqueous solubilities. Dexamethasone (Ozurdex), fluocinolone acetonide (Retisert), and triamcinolone acetonide (Trivaris) are in clinical use for treating disorders of the back of the eye. The remaining corticosteroids were chosen to have a selection of corticosteroids with a broad span of lipophilicity and solubility. Because therapy of back of the eye diseases requires chronic dosing, corticosteroids are typically administered as suspensions (Tsujikawa et al., 2005; Lin et al., 2007; Toda et al., 2007; Shima et al., 2008). Furthermore, injection of a small volume at the soluble concentrations of corticosteroids was inadequate in rat in vivo studies to detect drug levels in the back of the eye tissues. Therefore, we injected a 1 mg/ml suspension (25-μl volume) of corticosteroids for ex vivo/in vivo studies in the rat model. The ex vivo rat study is akin to a permeability study for drug suspension because drug clearance mechanisms via the circulation are impaired. In addition to tissue distribution studies with drug suspension, we assessed various drug properties including drug solubility, in vitro tissue permeability, and tissue partition coefficients using corticosteroid solutions.
Our biodistribution studies demonstrated that the higher the amount of soluble corticosteroid in the suspension (Table 1), the higher was its concentration in intraocular tissues (Fig. 4), consistent with Fick's first law of diffusion. Retinal and vitreal levels of prednisolone, the most soluble corticosteroid assessed, were the highest. Correlation plots (Figs. 6 and 7) between ex vivo/in vivo delivery and lipophilicity/solubility indicated that corticosteroid solubility instead of lipophilicity plays a dominant role in corticosteroid distribution to the back of the eye tissues from suspensions, within the range of drug lipophilicities (logD range, 0.71–2.97) assessed.
Corticosteroid solubility was in the order: P > T > D > FA ∼ TA ∼ B (Table 1). Apart from the inherent solubility imparted by the chemical structure, dissolution of particles in suspension at the site of injection is a key parameter controlling the driving force or concentrations. According to the modified Noyes-Whitney equation, under sink as well as nonsink conditions, total surface area of the dissolving particle is directly proportional to the rate of dissolution (Kumar and Tewari, 2005). Therefore, the smaller the particle size is, the greater the total particle surface area and hence the dissolution rate are. The mean particle diameter for prednisolone in the suspension was the lowest at 0.84 μm, which may be contributing to its higher dissolution rate compared with that of the other corticosteroids, whose diameters ranged from 1.45 to 4.33 μm. Possibly because of its greater solubility and smaller particle size, transscleral delivery was the highest for prednisolone. A decrease in the solubility of FA, TA, and budesonide in the cocktail mixture is consistent with the limited availability of water for dissolution and competition among various corticosteroids for the same. Low solubility of these lipophilic corticosteroids may have contributed to their lower transscleral delivery. Because all experiments were performed with a mixture of steroids, we used solubility values obtained with the cocktail for various correlations and observed that in vivo and ex vivo tissue distribution generally increases with an increase in corticosteroid solubility.
Tissue distribution in the ex vivo/in vivo rat study followed the general trend: CRPE ≥ sclera > retina > vitreous (Fig. 4). Because choroid-RPE is rich in melanin content (Cheruvu et al., 2008) and because melanin has high affinity for basic and neutral molecules with pKa values greater than 7 (Leblanc et al., 1998), high levels of corticosteroids in CRPE might be due to melanin binding. Excluding CRPE with binding elements, tissue levels for each corticosteroid decreased with an increase in inward distance from the site of administration, indicating that periocular injections deliver the drug via local diffusion as opposed to systemic recirculation of the drug (Ayalasomayajula and Kompella, 2004). Upon normalizing the ex vivo/in vivo tissue levels of corticosteroids to their observed solubility in the suspension used for injection, we observed positive, improved correlations with in vitro partition coefficients (Fig. 8) (R2 values before and after normalization: ex vivo sclera, 0.32 versus 0.80; in vivo sclera, 0.46 versus 0.90; ex vivo CRPE, 0.24 versus 0.42; in vivo CRPE, 0.35 versus 0.47).
Strong positive correlations between ex vivo and in vivo tissue distributions were observed in all the tissues including sclera, CRPE, vitreous, and retina (Fig. 5). Kim et al. (2004) reported that transscleral delivery of gadolinium-diethylenetriaminepentaacetic acid to the vitreous in rabbits was approximately 30-fold higher after euthanasia because of the impairment of dynamic barriers, which include blood and lymphatic clearance pathways. These authors also showed that under in vivo conditions, the elimination rate constant from the subconjunctival space into episcleral veins and conjunctival lymphatics was 3 log units higher than the transport rate constant into the vitreous. In our ex vivo rat study, corticosteroid levels in sclera, choroid-RPE, and retina were approximately 1.59- to 9.81-, 1.80- to 4.22-, and 1.31- to 4.04-fold higher than those in the in vivo study (Fig. 4).
Partitioning of corticosteroids from solution into sclera and CRPE generally increased with an increase in drug lipophilicity (Table 3; Fig. 1). Furthermore, partition coefficients were the highest at the lowest concentration (0.4 μg/ml) assessed and decreased steeply with an increase in drug concentration for all corticosteroids in CRPE. A similar but less prominent decline in partition coefficients with an increase in dose was evident for sclera (Fig. 1). For some β-blockers, we previously observed a modest decline in partition coefficients with an increase in drug concentration in sclera and choroid-RPE (Kadam and Kompella, 2010). These results suggest that corticosteroid binding is more avid but limited in its capacity in the CRPE compared with that in sclera, possibly due to the presence of high melanin content in bovine CRPE, unlike the sclera (Cheruvu et al., 2008).
Correlation coefficients for the relationship between partition coefficients and logD (Fig. 1) increased with an increase in concentration in sclera (R2 of 0.87, 0.94, and 0.96 at 0.4, 2, and 10 mg/ml, respectively) as well as CRPE (R2 of 0.66, 0.81, and 0.89 at 0.4, 2, and 10 μg/ml, respectively). Furthermore, it is evident that the correlations were superior in sclera compared with those in CRPE. A potential explanation for these differences is the nature of the tissues. Although sclera has a low quantity of melanin pigment, CRPE is rich in melanin pigment. Saturability of melanin binding and tissue differences in melanin content/binding might account for the observed differences with drug concentrations and tissues, respectively. With an increase in drug concentration, melanin binding may be saturated, resulting in predominance of true tissue partitioning that correlates well with logD. However, in a previous study (Kadam and Kompella, 2010), we did not observe concentration-dependent increases or tissue-dependent differences in the partition coefficient versus logD correlation coefficients of β-blockers. Because sustained drug delivery at low concentrations is the norm for corticosteroid delivery to the back of the eye, nonsaturating concentrations are likely to be present in vivo.
Ideally, the volume of aqueous and organic layers in a partition study should be equal. In this study, we used a higher volume of aqueous medium (500 μl) and a volume equivalent to approximately 100 μl (100 mg of tissue) for the organic/tissue phase. The U.S. Environmental Protection Agency (1982) stated that the octanol/water volume ratios in partition coefficient estimations can be adjusted on the basis of the relative solubility of the chemical in octanol and water to minimize errors originating from dividing large numbers by small numbers. For the corticosteroids used in this study, although the octanol solubility ranged from 0.3 to 18 mg/ml, the aqueous solubility ranged from 17 to 303 μg/ml. On the basis of this low relative solubility of corticosteroids in the aqueous phase, we used a larger volume for the aqueous phase during partition studies. Another important factor is that tissue partition coefficient estimates might differ between homogenized and intact tissues. Because drug delivery is our primary goal and because the drug is exposed to intact tissue in vivo, we used intact tissue for partition studies. We previously compared various techniques including the tissue homogenization technique for partition coefficients and demonstrated that the partition coefficients are higher in intact tissues compared with homogenized tissues for β-blockers (Kadam and Kompella, 2010). Because of the dose dependence and nonhomogenous nature of the tissue compartment, the measures in this study should be considered to be apparent partition coefficients.
Results from our in vitro studies using corticosteroid solution demonstrated that transscleral transport of these molecules across sclera and SCRPE is reduced with an increase in lipophilicity, with the decline being greater for SCRPE transport (Fig. 2). It has been shown previously by our group (Cheruvu and Kompella, 2006) that bovine transscleral transport of celecoxib (aqueous solubility, ∼0.002 mg/ml; logD, 3.8) was less than that of fluorescein (aqueous solubility, ∼600 mg/ml; logD, −0.99), despite the fact that both solutes have similar molecular radii of 0.53 nm and the donor celecoxib concentration was 2.5- to 3.0-fold higher than that of fluorescein. Likewise, Cruysberg et al. (2002) have shown that the transscleral permeability of corticosteroids is related to molecular weight and lipid solubility with tissue transport of the most lipophilic molecule being the least and that of the least lipophilic molecule being the most. Consistent with our β-blockers transport studies across sclera-choroid-RPE in various species (Kadam et al., 2011), we observed that hydrophilic corticosteroids were transported better than lipophilic molecules during the course of the study (Figs. 2 and 3). Sclera and SCRPE transport of corticosteroids related inversely with solute lipophilicity (R2 ≥ 0.8). As tissue partitioning and/or binding increases, corticosteroids are likely to be preferentially retained in the CRPE, resulting in reduced free drug levels and SCRPE transport. A modest (R2 = 0.3–0.7) yet positive correlation was observed between in vitro transport across sclera/SCRPE and ex vivo/in vivo tissue levels (CRPE/retina/vitreous) (Fig. 9), indicating that more soluble corticosteroids are delivered better in vitro as well as in vivo. The modest correlation between ex vivo/in vivo tissue levels and in vitro transport data is possibly due to the differences in the drug form among these studies.
All studies including partition, transport, and ex vivo/in vivo delivery were performed using pigmented tissues or animals. Corticosteroids used in this study exhibit different affinities and/or binding capacities for melanin pigment (Fig. 10). These differences may account in part for some of the observed drug delivery differences among various corticosteroids. Our studies indicate that the solubility is the major parameter responsible for ocular biodistribution of corticosteroids from a suspension dosage form. Lipophilicity plays a major role in tissue partitioning and transscleral transport from the solution forms. Highly lipophilic corticosteroids when presented at low, soluble concentrations, partition/bind more in the tissue, resulting in reduced transscleral transport to the vitreous.
In conclusion, after periocular administration of corticosteroid suspensions, transscleral delivery to the back of the eye tissues including sclera, CRPE, retina, and vitreous is primarily governed by the drug solubility. In addition, at low concentrations, transscleral transport of hydrophilic corticosteroids was greater than that of lipophilic corticosteroids. Therefore, more hydrophilic corticosteroids may be of potential value in treating back of the eye disorders by the transscleral route. Partitioning as well as in vivo accumulation of corticosteroids was the highest in CRPE, most likely because of the high melanin content in this tissue.
This work was supported by the National Institutes of Health National Eye Institute [Grants R01-EY018940, R01-EY017533].
Parts of this work were previously presented at the following conferences: Thakur A, Kadam RS, and Kompella UB (2009) Affinity of various corticosteroids to bovine lens, trabecular meshwork, choroid-RPE, and sclera. ARVO 2009 Annual Meeting; 2009 May 3–7; Fort Lauderdale, FL. Association for Research in Vision and Ophthalmology, Rockville, MD; and Thakur A, Kadam RS, and Kompella UB (2010) Influence of drug lipophilicity on bovine sclera and sclera-choroid-RPE permeability of corticosteroids. ARVO 2010 Annual Meeting; 2010 May 2–6; Fort Lauderdale, FL. Association for Research in Vision and Ophthalmology, Rockville, MD.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.037408.
- T
- triamcinolone
- P
- prednisolone
- D
- dexamethasone
- FA
- fluocinolone acetonide
- TA
- triamcinolone acetonide
- B
- budesonide
- RPE
- retinal pigment epithelium
- CRPE
- choroid-retinal pigment epithelium
- BN
- Brown Norway
- LC
- liquid chromatography
- MS/MS
- tandem mass spectrometry
- SCRPE
- sclera-choroid-retinal pigment epithelium
- PBS
- phosphate-buffered saline
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Thakur, Kadam, and Kompella.
Conducted experiments: Thakur.
Contributed new reagents or analytic tools: Thakur, Kadam, and Kompella.
Performed data analysis: Thakur and Kompella.
Wrote or contributed to the writing of the manuscript: Thakur, Kadam, and Kompella.
References
- Adelman RA, Zheng Q, Mayer HR. (2010) Persistent ocular hypertension following intravitreal bevacizumab and ranibizumab injections. J Ocul Pharmacol Ther 26:105–110 [DOI] [PubMed] [Google Scholar]
- Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. (2006) Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci 47:1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrite AC, Edelhauser HF, Kompella UB. (2008a) Modeling of corneal and retinal pharmacokinetics after periocular drug administration. Invest Ophthalmol Vis Sci 49:320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrite AC, Edelhauser HF, Singh SR, Kompella UB. (2008b) Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis 14:150–160 [PMC free article] [PubMed] [Google Scholar]
- Amrite AC, Kompella UB. (2005) Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol 57:1555–1563 [DOI] [PubMed] [Google Scholar]
- Ayalasomayajula SP, Kompella UB. (2004) Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm Res 21:1797–1804 [DOI] [PubMed] [Google Scholar]
- Ayalasomayajula SP, Kompella UB. (2005) Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur J Pharmacol 511:191–198 [DOI] [PubMed] [Google Scholar]
- Cheruvu NP, Amrite AC, Kompella UB. (2008) Effect of eye pigmentation on transscleral drug delivery. Invest Ophthalmol Vis Sci 49:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheruvu NP, Kompella UB. (2006) Bovine and porcine transscleral solute transport: influence of lipophilicity and the Choroid-Bruch's layer. Invest Ophthalmol Vis Sci 47:4513–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruysberg LP, Nuijts RM, Geroski DH, Koole LH, Hendrikse F, Edelhauser HF. (2002) In vitro human scleral permeability of fluorescein, dexamethasone-fluorescein, methotrexate-fluorescein and rhodamine 6G and the use of a coated coil as a new drug delivery system. J Ocul Pharmacol Ther 18:559–569 [DOI] [PubMed] [Google Scholar]
- D'Amico DJ, Goldberg MF, Hudson H, Jerdan JA, Krueger DS, Luna SP, Robertson SM, Russell S, Singerman L, Slakter JS, et al. (2003) Anecortave acetate as monotherapy for treatment of subfoveal neovascularization in age-related macular degeneration: twelve-month clinical outcomes. Ophthalmology 110:2372–2383; discussion 2484–2485 [DOI] [PubMed] [Google Scholar]
- Dahlin DC, Rahimy MH. (2007) Pharmacokinetics and metabolism of anecortave acetate in animals and humans. Surv Ophthalmol 52 (Suppl 1):S49–S61 [DOI] [PubMed] [Google Scholar]
- Durairaj C, Kim SJ, Edelhauser HF, Shah JC, Kompella UB. (2009) Influence of dosage form on the intravitreal pharmacokinetics of diclofenac. Invest Ophthalmol Vis Sci 50:4887–4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhauser HF, Rowe-Rendleman CL, Robinson MR, Dawson DG, Chader GJ, Grossniklaus HE, Rittenhouse KD, Wilson CG, Weber DA, Kuppermann BD, et al. (2010) Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci 51:5403–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam RS, Cheruvu NP, Edelhauser HF, Kompella UB. (2011) Sclera-choroid-RPE transport of eight β-blockers in human, bovine, porcine, rabbit, and rat models. Invest Ophthalmol Vis Sci doi: 10.1167/iovs.10-6233 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kadam RS, Kompella UB. (2010) Influence of lipophilicity on drug partitioning into sclera, choroid-retinal pigment epithelium, retina, trabecular meshwork, and optic nerve. J Pharmacol Exp Ther 332:1107–1120 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim H, Robinson MR, Lizak MJ, Tansey G, Lutz RJ, Yuan P, Wang NS, Csaky KG. (2004) Controlled drug release from an ocular implant: an evaluation using dynamic three-dimensional magnetic resonance imaging. Invest Ophthalmol Vis Sci 45:2722–2731 [DOI] [PubMed] [Google Scholar]
- Kompella UB. (2007) Drug delivery to the back of the eye. Arch Soc Esp Oftalmol 82:667–668; 669–670 [PubMed] [Google Scholar]
- Kompella UB, Kadam RS, Lee VH. (2010) Recent advances in ophthalmic drug delivery. Ther Deliv 1:435–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Tewari D. (2005) Dissolution, in Remington: The Science and Practice of Pharmacy, 21st ed (Troy DB. ed) pp 495–672, Lippincott Williams & Wilkins, Baltimore [Google Scholar]
- Leblanc B, Jezequel S, Davies T, Hanton G, Taradach C. (1998) Binding of drugs to eye melanin is not predictive of ocular toxicity. Regul Toxicol Pharmacol 28:124–132 [DOI] [PubMed] [Google Scholar]
- Lin JM, Chiu YT, Hung PT, Tsai YY. (2007) Early treatment of severe cystoid macular edema in central retinal vein occlusion with posterior sub-tenon triamcinolone acetonide. Retina 27:180–189 [DOI] [PubMed] [Google Scholar]
- Missel PJ, Stevens LE, Mauger JW. (2004) Dissolution of anecortave acetate in a cylindrical flow cell: re-evaluation of convective diffusion/drug dissolution for sparingly soluble drugs. Pharm Dev Technol 9:453–459 [DOI] [PubMed] [Google Scholar]
- Raghava S, Hammond M, Kompella UB. (2004) Periocular routes for retinal drug delivery. Expert Opin Drug Deliv 1:99–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Prescott E, Shelke NB, Trivedi R, Thomas P, Struble C, Gadek T, O'Neill CA, Kompella UB. (2010) Delivery of SAR 1118 to the retina via ophthalmic drops and its effectiveness in a rat streptozotocin (STZ) model of diabetic retinopathy (DR). Invest Ophthalmol Vis Sci 51:5198–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampat KM, Garg SJ. (2010) Complications of intravitreal injections. Curr Opin Ophthalmol 21:178–183 [DOI] [PubMed] [Google Scholar]
- Sato T, Emi K, Ikeda T, Bando H, Sato S, Morita S, Oyagi T, Sawada K. (2010) Severe intraocular inflammation after intravitreal injection of bevacizumab. Ophthalmology 117:512–516, 516.e1–516.e2 [DOI] [PubMed] [Google Scholar]
- Shima C, Ogata N, Minamino K, Yoshikawa T, Yoshikawa T, Matsuyama K, Matsumura M. (2008) Posterior sub-Tenon injection of triamcinolone acetonide as pretreatment for focal laser photocoagulation in diabetic macular edema patients. Jpn J Ophthalmol 52:265–268 [DOI] [PubMed] [Google Scholar]
- Thakur A, Kadam RS, Kompella UB. (2010) Trabecular meshwork and lens partitioning of corticosteroids: Implications for elevated intraocular pressure and cataracts. Arch Ophthalmol doi:10.1001/archophthalmol.2011.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda J, Fukushima H, Kato S. (2007) Injection of triamcinolone acetonide into the posterior sub-tenon capsule for treatment of diabetic macular edema. Retina 27:764–769 [DOI] [PubMed] [Google Scholar]
- Tsujikawa A, Fujihara M, Iwawaki T, Yamamoto K, Kurimoto Y. (2005) Triamcinolone acetonide with vitrectomy for treatment of macular edema associated with branch retinal vein occlusion. Retina 25:861–867 [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (1982) Partition coefficient CG 1400, in Chemical fate testing guidelines, EPA 560/6-82-003, National Technical Information Services, Springfield, VA [Google Scholar]