Abstract
Isoflurane is an inhaled halogenated hydrocarbon anesthetic commonly used for animal research. In our quest to develop a method for measuring bile acid transport in live animals using 19F magnetic resonance (MR) imaging, it occurred to us that isoflurane, which contains five fluorines per molecule and is probably widely distributed, would provide an excellent test drug to evaluate the merits of this approach. Experiments in 20- to 28-g male C57BL/6 mice were performed using a horizontal bore scanner with a 30-mm 19F/1H dual-tuned surface coil used to transmit and receive radiofrequency signals at 300.28 MHz for 1H and 282.55 MHz for 19F nuclei. Proton MR imaging was used to identify the mouse gallbladder in vivo, which was verified by anatomical dissection. Subsequent experiments in mice inhaling 1.5% isoflurane for 1 to 2 h revealed robust 19F signals from the gallbladder, verified by overlying 1H and 19F signals. No 19F signal was detected in mice anesthetized with nonhalogenated anesthetics. The presence of isoflurane in gallbladder bile of isoflurane-treated mice was verified using liquid chromatography-mass spectrometry. Gallbladder bile isoflurane content ranged from 3.2 to 4.7 μg. The data presented here provide proof of concept that this novel approach can be used for in vivo measurement of biliary excretion of both existing and novel 19F-labeled drugs.
Introduction
In the course of studying bile acid transport using an in vitro assay system, we observed that many commonly used drugs inhibit the function of the major intestinal bile acid transporter, the apical sodium-dependent bile acid transporter (ASBT) (gene name SLC10A2) (Zheng et al., 2009). These ASBT inhibitors include HMG-CoA inhibitors (statins) and dihydropyridine calcium-channel blockers; statins and calcium-channel blockers are among the most commonly prescribed drugs in the United States. Inhibition of the ASBT results in reduced intestinal uptake of bile acids and increased fecal bile acid concentrations, risk factors for colon pathology, including cancer (Flynn et al., 2007). Hence, side effects deriving from drug-induced inhibition of the ASBT could pose potential, currently unappreciated, risk.
On the basis of these observations we decided to develop a new in vivo assay to measure the ability of drugs to inhibit bile acid transport. Currently available methods to measure bile acid transport in vivo are limited to 75Se-25-homocholic acid-taurine (Boyd et al., 1981; Fracchia et al., 1998) and luciferase-farnesoid X receptor imaging (Houten et al., 2007). 75Se-25-Homocholic acid-taurine, which was not approved by the U.S. Food and Drug Administration and was available only in Europe, requires administration of a radioactive agent (Pattni and Walters, 2009), and its production was recently discontinued by the supplier (European Amersham catalogs). Luciferase-farnesoid X receptor imaging is applicable only in transgenic mice expressing the reporter gene and provides very limited anatomical detail (Houten et al., 2007). Hence, to measure in vivo bile acid transport we conceived an innovative, nonradioactive approach: 19F-labeled bile acid tracers and in vivo magnetic resonance imaging (MRI) in mice.
19F is particularly well suited for monitoring distribution of administered agents; 19F is second only to 1H in sensitivity for MRI, and background 19F signals in animals and humans are negligible (Jiang et al., 2009). Positron emission tomography was considered but rejected for this purpose because positron emission tomography probes are radioactive (e.g., 18F), are more costly (a cyclotron is needed to create 18F for each use), and have short radiation half-lives (109 min for 18F). In mice, stable nonradioactive 19F-labeled tracers can be imaged for days (Jiang et al., 2009).
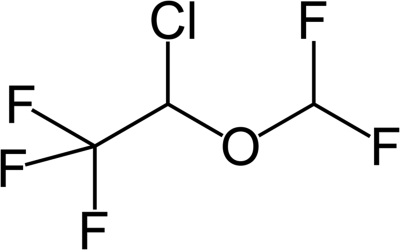
Before we proceeded with the complex and time-consuming development of 19F-labeled bile acid tracers, several key issues had to be addressed. First, for reasons of cost and efficiency, an animal model is needed to measure bile acid uptake by in vivo MRI. After excretion from the liver, bile acids are stored and concentrated in the gallbladder. Hence, gallbladder imaging would be most likely to result in a 19F signal of sufficient strength for detection. Because rats lack a gallbladder, mice would serve this purpose and were previously used as test animals for studies of bile acid transport (Dawson et al., 2003). Second, a fluoridated test agent was required to validate the approach. Fluoridated hydrocarbons, particularly 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane (isoflurane) (Fig. 1), are commonly used to anesthetize mice, and wide tissue distribution, as well as limited hepatic metabolism of this agent, was reported (Kharasch and Thummel, 1993; Wissing et al., 2000; Kharasch, 2008). Hence, isoflurane, which contains five fluorines per molecule (Fig. 1), appeared to be an ideal test agent. Moreover, among the volatile halogenated anesthetics, isoflurane seems to undergo the least amount of defluorination (Kharasch and Thummel, 1993). Almost 30 years ago, it was shown that high-resolution 19F magnetic resonance spectra from isoflurane could be obtained from a surface coil centered over the brain of a living rabbit (Wyrwicz et al., 1983).
Fig. 1.
The chemical structure of 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane (isoflurane) highlighting the five fluorines, including the three equivalent fluorines bonded to the ethane portion.
Therefore, in the present study, we investigated whether isoflurane could be measured in mouse gallbladder bile using in vivo MRI. To verify the presence of isoflurane in gallbladder bile and estimate the limits of detection, we used liquid chromatography-mass spectrometry (LC-MS). The data presented here provide proof of concept that this novel approach can be used for in vivo measurement of biliary excretion of both existing and novel 19F-labeled drugs.
Materials and Methods
Animals.
All animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the U.S. National Academy of Sciences (National Institutes of Health publication 86-23, revised 1985). Mouse studies were approved by both the institutional animal care and use committee at the University of Maryland School of Medicine (Baltimore, MD) and the research and development committee at the VA Maryland Health Care System (Baltimore, MD). C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed under identical conditions in a pathogen-free environment with a 12-h light/dark cycle and free access to standard mouse chow and water for 1 week before treatment. Mice were fasted overnight before imaging studies.
Live Animal MRI.
In vivo experiments were performed on a BioSpec 7.0-T 30-cm horizontal bore scanner using Paravision 5.0 software (Bruker Biospin MRI GmbH, Rheinstetten, Germany). A Bruker 30-mm 19F/1H dual-tuned surface coil was used to transmit and receive radiofrequency signals at 300.28 MHz for 1H and 282.55 MHz for 19F nuclei. Mice (male C57BL/6; 20–28 g b.wt.) were anesthetized in an animal chamber with a gas mixture of O2 (1 l/min) and isoflurane (3%, IsoFlo; Abbott Laboratories, North Chicago, IL). Animals were then placed supine in a Bruker animal bed, and the radiofrequency coil was positioned and fixed with surgical tape in the region of interest on the animal body. The animal bed was moved to the center of the magnet, and the isoflurane level was changed to 1.5% and maintained at this level for the remainder of the experiment. A magnetic resonance-compatible small-animal monitoring and gating system (SA Instruments, Inc., New York, NY) was used to monitor animal respiration rate and body temperature. Mouse body temperature was maintained at 36–37°C using a warm water circulator.
The gallbladder was localized by first obtaining images using 1H MRI. Three-slice (axial, midsagittal, and coronal) scout rapid acquisition with fast low-angle shot MRI was used to first localize the volume of interest. High-resolution proton density-weighted anatomical images were acquired using a rapid acquisition with relaxation enhancement sequence in the axial view with repetition time of 1847 or 2631 ms, echo time of 11 ms, rapid acquisition with relaxation enhancement factor of 8, field of view of 6 × 6 mm2, slice thickness of 1 mm, matrix size of 400 × 400, and in-plane resolution of 0.15 × 0.15 mm2. Number of slices was 12 or 24, and number of averages was 8. Total acquisition time was not more than 18 min.
Low-resolution 19F images were acquired using a fast low-angle shot sequence in the same region of the anatomical 1H MRI with repetition time of 123 or 245 ms, echo time of 6 ms, excitation pulse angle of 30°, field of view of 6 × 6 mm2, matrix size of 32 × 32, in-plane resolution of 1.875 × 1.875 mm2, and slice thickness of 4 mm. Number of slices was 3 or 6, and number of averages was from 600 to 1200. Total acquisition time was less than 2 h and 36 min. Mice inhaled isoflurane from 50 to 94 min before 19F MRI experiments.
Liquid Chromatography-Mass Spectrometry.
In parallel studies, to measure the isoflurane content in gallbladder bile, mice were dosed with isoflurane using the same protocol described for live animal MRI. Mice were euthanized immediately after completion of isoflurane inhalation, and the gallbladders were removed and weighed. Tissue samples were transported in tightly sealed containers with minimal head space and stored on ice before sample preparation. The gallbladder was ground using a tissue homogenizer in the presence of 75:25 acetonitrile (ACN)-water solvent. Homogenized tissue was centrifuged (9600g for 1 min at 4°C), and the isoflurane content of the supernatant was measured using LC-MS. LC-MS was performed using a TSQ Vantage (Thermo Fisher Scientific, Waltham, MA) mass spectrometer, with an Accela 1250 pump and a PAL HTC-Accela1-TM autosampler. The column was a Gemini C18 (50 × 4.6 mm, 3 μm, 110 A; Phenomenex, Torrance, CA). The mobile phase (1.2 ml/min, water and ACN) was a gradient as follows: 0 to 0.5 min, 40% ACN; 0.5 to 1.5 min, 40 to 95% ACN; 1.5 to 2.5 min, 95% ACN; 2.5 to 2.6 min, 95 to 40% ACN; and 2.6 to 3.6 min, 40% ACN. MS was performed using an atmospheric pressure chemical ionization probe in negative mode; ion transition m/z 183 → 163 was used to quantify isoflurane. Data collection and processing were performed with Xcalibur 2.3.0 with LCquan 2.6.0 software (see Table 1).
TABLE 1.
Amount and calculated concentration of isoflurane in mouse gallbladder
Isoflurane content of mouse gallbladder bile was measured by LC-MS. See Liquid Chromatography-Mass Spectrometry for details.
| Gallbladder Weight | Gallbladder Isoflurane | Calculated Isoflurane Conc.a | |
|---|---|---|---|
| mg | μg | mM | |
| No isoflurane control | 4.9 | N.D. | N.D. |
| Emptied gallbladder control | 3.3 | N.D. | N.D. |
| Isoflurane-treated mouse 1 | 26.0 | 4.72 | 2.2 |
| Isoflurane-treated mouse 2 | 12.6 | 3.25 | 1.3 |
| Isoflurane-treated mouse 3 | 17.3 | 3.52 | 1.4 |
N.D., not detected.
The calculated isoflurane concentration assumes mouse gallbladder volume of 8 μl/16 g mouse total body weight (Graewin et al., 2004).
Results and Discussion
Exhalation is the major route of elimination for halogenated hydrocarbon anesthetics such as isoflurane (Fig. 1). On the basis of both in vitro and in vivo data, it is estimated that liver metabolism, primarily by CYP2E1, contributes only 0.1 to 2% of isoflurane elimination (Kharasch and Thummel, 1993; Kharasch, 2008). The major metabolite resulting from CYP2E1 metabolism of isoflurane is trifluoroacetyl chloride (Kharasch and Thummel, 1993; Njoku et al., 1997; Kharasch, 2008). Minimal metabolism by the liver is thought to be the major reason that isoflurane poses a lower risk of hepatotoxicity than that of other halogenated anesthetics, such as halothane (Christ et al., 1988; Njoku et al., 1997; Kharasch, 2008).
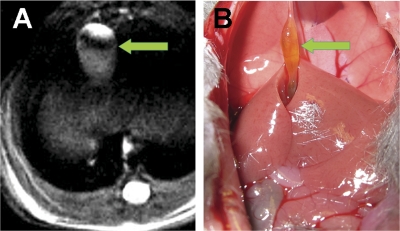
Although isoflurane is widely distributed (Wissing et al., 2000), no report has investigated its distribution into bile. Nevertheless, we focused our studies on imaging isoflurane in the gallbladder. Exobiotics can be excreted by the liver into the biliary tree and both stored and concentrated in the gallbladder; thus, we reasoned that we were most likely to detect the strongest 19F signal in gallbladder bile. The mouse gallbladder is well revealed by proton MRI in the anterior upper abdomen (Fig. 2A). The identity of the gallbladder on MRI was confirmed by immediate animal dissection after imaging (Fig. 2B).
Fig. 2.
Localization of the mouse gallbladder by live animal MRI and verification by anatomical dissection. A, high-resolution proton density-weighted anatomical image of the murine gallbladder (arrowhead) acquired using live animal MRI as described under Materials and Methods. B, gallbladder location (arrow) and appearance were verified by dissection immediately after liver animal MRI. The mouse gallbladder, just to the left of midline, is attached superiorly by the falciform ligament. It is likely that the gallbladder appears less distended than in the magnetic resonance image because of the effects of ketamine/xylazine anesthesia, given after the effects of isoflurane had abated, and as a consequence of surgical manipulation and stretching of the gallbladder to obtain the photograph.
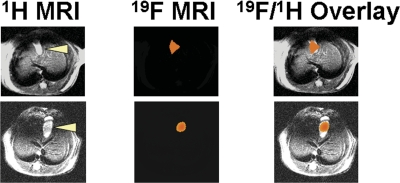
In our initial experiments, we used the same scan resolution for proton and fluorine imaging. However, because of the relatively weaker sensitivity of fluorine, in subsequent studies we modified MRI to obtain a 4-mm slice thickness for 19F images instead of the 1-mm slice thickness used for 1H images. These modifications resulted in stronger 19F signals. As shown in Fig. 3, after isoflurane anesthesia, MRI revealed a robust 19F signal in the mouse gallbladder (two representative examples are shown). No 19F signal was detected in the gallbladder of control mice that were anesthetized with subcutaneous injection of ketamine and xylazine instead of inhaled isoflurane (data not shown). This localization of the 19F signal in the gallbladder suggests that isoflurane is distributed into bile.
Fig. 3.
Comparison of 1H anatomical MRI and 19F isoflurane signal in murine gallbladder. The results of two experiments in live mice are shown. The left panels show 1H anatomical imaging of the murine gallbladder (arrowheads indicate gallbladder). The middle panels show the 19F signal from the corresponding region. The right panels show a reconstruction overlay of the 19F and 1H images revealing that the 19F signal emanates from the gallbladder.
To assess 19F MRI results, parallel studies using LC-MS to measure isoflurane were performed in five mice. Four mice were treated with inhaled isoflurane as described under Materials and Methods, and one control mouse received subcutaneous nonhalogenated anesthetics (no isoflurane). As an unplanned control, the gallbladder of one of the four isoflurane-treated mice was emptied of bile before removal, such that no isoflurane was detected in that mouse.
Table 1 lists the amount of isoflurane in each mouse gallbladder. No isoflurane was found in gallbladders from the two control mice: one that did not receive isoflurane and one whose gallbladder was emptied before the assay. In the remaining three isoflurane-treated mice, 4.72, 3.25, and 3.52 μg of isoflurane was detected in the gallbladder (mean ± S.E., 3.83 ± 0.45 μg) (Table 1). Although the volume of gallbladder contents was not measured, we estimated the isoflurane concentration in gallbladder bile on the basis of published data relating gallbladder volume to mouse weight (Graewin et al., 2004). This analysis yielded gallbladder bile isoflurane concentrations of 2.2, 1.3, and 1.4 mM, respectively (Table 1), suggesting that the limits of detection for isoflurane using 19F MRI are at least 1 to 2 mM.
Regarding the use of this approach for measuring bile acid transport, an advantage of MRI of the gallbladder is that bile acids are stored in this organ. It is estimated that after an overnight fast, at least 80% of the bile acid pool is stored in the gallbladder (Nilsell, 1990). The amount of 19F-labeled bile acid tracer that accumulates in the mouse gallbladder during an overnight fast will depend on the test dose, stability, absorption, mouse body weight, and other factors. Nonetheless, using conservative estimates, we calculate that gavaging mice with 19F-labled chenodeoxycholic acid (15–300 μg/g b.wt.) will result in ∼2 to 15 mM 19F-labeled bile acid in the gallbladder (assuming that ∼50% of 19F-labeled bile acid accumulates in the gallbladder, volume ∼20 μl). These values are above the limits of detection for isoflurane in the present communication (1–2 mM).
In summary, mice inhaling 1.5% isoflurane for 1 to 2 h revealed robust 19F signals in the gallbladder, verified by overlying 1H and 19F signals. No 19F signal was detected in mice anesthetized with nonhalogenated agents. The presence of isoflurane in gallbladder bile of isoflurane-treated mice was confirmed using LC-MS; isoflurane content ranged from 3.2 to 4.7 μg. This finding demonstrates the sensitivity of 19F MRI; the level of detection is at least 1 to 2 mM. The data presented here provide an important proof of concept that this novel approach can be used for in vivo measurement of biliary excretion of both existing and novel 19F-labeled drugs.
Acknowledgments
We acknowledge Steven Roys for helping with overlay of 19F and 1H MRI images.
This work was supported by the National Institutes of Health National Cancer Institute [Grant CA120407]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK67530, DK067872, DK081479]; and the National Institutes of Health National Center for Research Resources [Grant 1S10-RR019935].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.037358.
- ASBT
- apical sodium-dependent bile acid transporter
- MRI
- magnetic resonance imaging
- LC
- liquid chromatography
- MS
- mass spectrometry
- ACN
- acetonitrile.
Authorship Contributions
Participated in research design: Raufman, Xu, Cheng, Johnson, Gullapalli, and Polli.
Conducted experiments: Raufman, Xu, Cheng, Khurana, Johnson, Shao, Kane, Shi, Gullapalli, and Polli.
Contributed new reagents or analytic tools: Xu, Johnson, Shao, Kane, Shi, Gullapalli, and Polli.
Performed data analysis: Raufman, Xu, Cheng, Johnson, Shao, Kane, and Polli.
Wrote or contributed to the writing of the manuscript: Raufman, Xu, Cheng, Khurana, Johnson, Gullapalli, and Polli.
References
- Boyd GS, Merrick MV, Monks R, Thomas IL. (1981) Se-75-labeled bile acid analogs, new radiopharmaceuticals for investigating the enterohepatic circulation. J Nucl Med 22:720–725 [PubMed] [Google Scholar]
- Christ DD, Satoh H, Kenna JG, Pohl LR. (1988) Potential metabolic basis for enflurane hepatitis and the apparent cross-sensitization between enflurane and halothane. Drug Metab Dispos 16:135–140 [PubMed] [Google Scholar]
- Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. (2003) Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem 278:33920–33927 [DOI] [PubMed] [Google Scholar]
- Flynn C, Montrose DC, Swank DL, Nakanishi M, Ilsley JN, Rosenberg DW. (2007) Deoxycholic acid promotes the growth of colonic aberrant crypt foci. Mol Carcinog 46:60–70 [DOI] [PubMed] [Google Scholar]
- Fracchia M, Pellegrino S, Secreto P, Pera A, Galatola G. (1998) Biliary lipid composition in idiopathic bile acid malabsorption. Gut 43:812–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graewin SJ, Lee KH, Kiely JM, Svatek CL, Nakeeb A, Pitt HA. (2004) Gallbladder myocytes are short and cholecystokinin-resistant in obese diabetic mice. Surgery 136:431–436 [DOI] [PubMed] [Google Scholar]
- Houten SM, Volle DH, Cummins CL, Mangelsdorf DJ, Auwerx J. (2007) In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol Endocrinol 21:1312–1323 [DOI] [PubMed] [Google Scholar]
- Jiang ZX, Liu X, Jeong EK, Yu YB. (2009) Symmetry-guided design and fluorous synthesis of a stable and rapidly excreted imaging tracer for 19F MRI. Angew Chem Int Ed Engl 48:4755–4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharasch ED. (2008) Adverse drug reactions with halogenated anesthetics. Clin Pharmacol Ther 84:158–162 [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Thummel KE. (1993) Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology 79:795–807 [DOI] [PubMed] [Google Scholar]
- Nilsell K. (1990) Bile acid pool size and gallbladder storage capacity in gallstone disease. Scand J Gastroenterol 25:389–394 [DOI] [PubMed] [Google Scholar]
- Njoku D, Laster MJ, Gong DH, Eger EI, 2nd, Reed GF, Martin JL. (1997) Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury. Anesth Analg 84:173–178 [DOI] [PubMed] [Google Scholar]
- Pattni S, Walters JR. (2009) Recent advances in the understanding of bile acid malabsorption. Br Med Bull 92:79–93 [DOI] [PubMed] [Google Scholar]
- Wissing H, Kuhn I, Rietbrock S, Fuhr U. (2000) Pharmacokinetics of inhaled anaesthetics in a clinical setting: comparison of desflurane, isoflurane and sevoflurane. Br J Anaesth 84:443–449 [DOI] [PubMed] [Google Scholar]
- Wyrwicz AM, Pszenny MH, Schofield JC, Tillman PC, Gordon RE, Martin PA. (1983) Noninvasive observations of fluorinated anesthetics in rabbit brain by fluorine-19 nuclear magnetic resonance. Science 222:428–430 [DOI] [PubMed] [Google Scholar]
- Zheng X, Ekins S, Raufman JP, Polli JE. (2009) Computational models for drug inhibition of the human apical sodium-dependent bile acid transporter. Mol Pharm 6:1591–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]