Abstract
Work on stem cells is one of the hottest research areas in biology. But are such studies of any therapeutic value? Fortunately, yes, as is evident from successes in treating blindness.
Few people today dispute the enormous potential of stem cells for regenerative medicine. But, despite ever-increasing reports on the Internet of stem cells being used to treat various disorders — from Alzheimer's disease to spinal-cord injuries to severe heart conditions — proven stem-cell therapies remain few and far between. A paper published in the New England Journal of Medicine by Pellegrini, De Luca and colleagues1 stands as a refreshing example of a scientifically documented advance in stem-cell therapies, in this case to treat certain types of blindness.
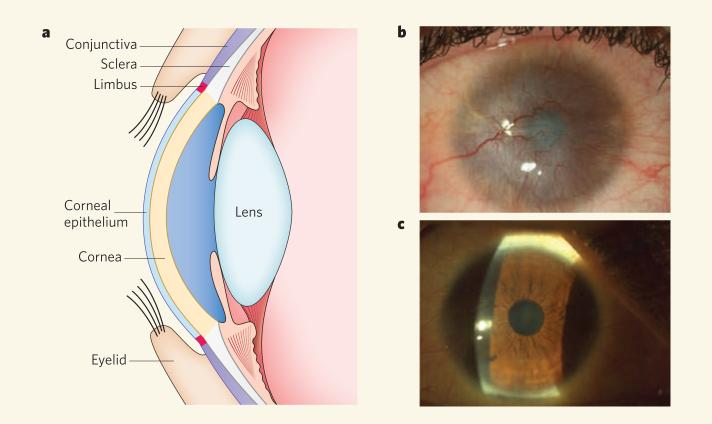
The eyeball is covered by the cornea — the eye's most important light-refracting structure (Fig. 1a). The cornea produces the initial image and casts this onto the lens behind it. The clearness of the cornea is essential to visual acuity and depends on both the integrity of the corneal epithelium covering the eye's surface and a lack of blood vessels in the underlying support tissue (the stroma)2. At its margins, the corneal epithelium is attached to the delicate mucous (conjunctival) epithelium that covers the whites of the eye (sclera) and the internal part of the eyelids. The narrow zone between the cornea and the conjunctiva is known as the limbus. Experimental and clinical evidence indicates that the limbus is the source of corneal epithelial stem cells in humans2.
Figure 1. Help from one eye to its neighbouring eye.
a, The human ocular system. b, When the limbus is permanently damaged, as in the example of a patient shown, conjunctival cells invade the cornea to form a protective epithelial layer. This abnormal ‘rescue’ attempt leads to the formation of new blood vessels, chronic inflammation, stromal scarring and, finally, corneal opacity and loss of vision. c, Pellegrini, De Luca and colleagues1 find that transplantation of corneal stem cells obtained by culturing cells taken from the limbus of the healthy eye regenerates a healthy cornea and permanently restores a patient's normal vision, as shown.
The limbus can be destroyed by ocular burns or infection, causing corneal stem-cell deficiency3. But, in one of nature's remarkable efforts to repair tissues at all costs, abnormal invasion by conjunctival cells provides the damaged cornea with a protective surface layer (Fig. 1b). The consequences are dire, resulting in vascularization of the cornea, chronic inflammation, stromal scarring and, ultimately, corneal opacity and loss of sight2.
Allogeneic corneal transplantations, which involve transplanting cornea from a genetically non-identical donor, have to some extent been successful in restoring patients’ vision. Eventually, however, conjunctival cells invade and replace the transplanted cornea2. What's more, two other factors make treating patients with ocular burns by corneal transplantation problematic: the number of available donors is insufficient to meet demand, and the increasingly popular corrective laser eye surgery often makes the cornea unsuitable for transplantation. Pellegrini, De Luca and colleagues1 now report that limbal stem cells maintained in culture can be a viable alternative source of cells for transplantation to treat burned human corneas.
Stem-cell transplantation is not a new concept. More than half a century ago, E. Donnall Thomas showed that intravenous infusion of donor bone-marrow cells can repopulate the bone marrow and produce new blood cells4; he later won a Nobel prize for this first demonstration of the use of stem cells for regeneration of damaged or diseased tissues and organs. By the 1970s, physicians were successfully performing bone-marrow transplants, which are now used to treat blood disorders ranging from severe combined immunodeficiency to sickle-cell anaemia to leukaemias, as well as other cancers of the human immune system. By the early 1980s, human skin stem cells were being cultured to make epidermal sheets to repair the skin of badly burned patients.
Pellegrini, De Luca and colleagues have been culturing corneal stem cells from small biopsies of human limbal tissue for the past decade. The appreciable similarities between limbal and epidermal cells allowed the researchers to adapt methods5,6 developed for human epidermal stem-cell cultures. Cultured epidermal-cell colonies can be classified according to cell number and capacity for growth. The smaller-sized colonies generate epidermal cells that stop growing over time. By contrast, the larger-sized colonies — referred to as holoclones — display quintessential features of stem cells, namely long-term self-renewal and the ability to regenerate tissue. This makes them suitable for burn therapy.
Pellegrini, De Luca and co-workers7 discovered that human limbal cells cultured using a similar protocol also form small and large colonies. Interestingly, only the limbal holoclones and not the smaller colonies expressed p63, a transcription factor that is essential for the proliferative potential of epidermal stem cells8. In the impressive accompanying clinical studies9, the researchers obtained limbal stem cells from the healthy eye of 112 patients with ocular burns, cultivated them and then transplanted the cultured cells onto the patients’ damaged eye. After an extensive 10-year monitoring period, the authors now report1 permanent restoration of a transparent, self-renewing corneal epithelium in three-quarters of the study patients (Fig. 1c). Notably, 78% of the successful transplantations involved cultures in which p63-expressing cells constituted more than 3% of the cells capable of forming colonies. These observations unveil a direct correlation between the percentage of p63-positive corneal stem cells in a culture and their transplantability. The correlation presents a powerful diagnostic tool for predicting whether any given limbal culture is likely to be suitable for long-term transplantation.
This work1 also offers hope for exploring alternative sources of limbal stem cells to treat patients who have suffered severe injuries to both eyes, and who therefore lack limbal stem cells. Indeed, in the future it might be possible to create corneal stem cells by culturing other cells from the patient — for instance, skin stem cells — and then either directly inducing their transdifferentiation to limbal cells, or transforming them first to an embryonic-stem-cell-like state (induced pluripotent stem cells, or iPS cells) before inducing their differentiation along the limbal lineage. To achieve such stem-cell therapies and improve on the existing ones, researchers will need to learn more about how corneal stem cells differ from their skin counterparts. Better protocols must be devised for purifying stem cells and for preserving and enhancing their self-renewal in vitro. And protocols for transdifferentiation and/or iPS-lineage differentiation will need to be established for the generation of limbal stem cells.
Pellegrini, De Luca and colleagues’ work1 elegantly demonstrates how the knowledge of one type of stem cell — in this case, the human epidermal stem cell — can be used to advance a clinical treatment for another, the limbal stem cell. Their paper sets the gold standard for the level of scientific proof that is needed for each new stem-cell therapy, and provides a blueprint that can be applied to the development of other adult stem cells for clinical therapies. Stem-cell therapy still has a long journey ahead, but the light is beginning to shine brightly on its path.
References
- 1.Rama P, et al. N. Engl. J. Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 2.Pellegrini G, Rama P, Mavilio F, De Luca M. J. Pathol. 2009;217:217–228. doi: 10.1002/path.2441. [DOI] [PubMed] [Google Scholar]
- 3.Dua HS, Azuara-Blanco A. Surv. Ophthalmol. 2000;44:415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 4.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. N. Engl. J. Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 5.Barrandon Y, Green H. Proc. Natl Acad. Sci. USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green H. Sci. Am. 1991;265:96–102. doi: 10.1038/scientificamerican1191-96. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrini G, et al. J. Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senoo M, Pinto F, Crum CP, McKeon F. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini G, et al. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]