According to a recent report by the National Council on Radiation Protection and Measurements (NCRP 2009), the majority of ionizing radiation exposure of the population of the United States arises from radioactivity in the body. As shown in Figure 1, 12% of the annual exposure can be attributed to diagnostic nuclear medicine, 37% to inhalation of radon and thoron, and 5% to natural internal radioactivity. These internal exposures comprise 54% of the total annual exposure, or an effective dose of about 3.4 mSv per annum. Therefore, they should be addressed when considering responses of complex biological systems to low-level exposure to ionizing radiation (<100 mSv) and their consequences to theoretical assessments and predictions of risk. The present abstract focuses on consideration of risks associated with the nuclear medicine contribution to the US population’s annual exposure to ionizing radiation.
Figure 1.
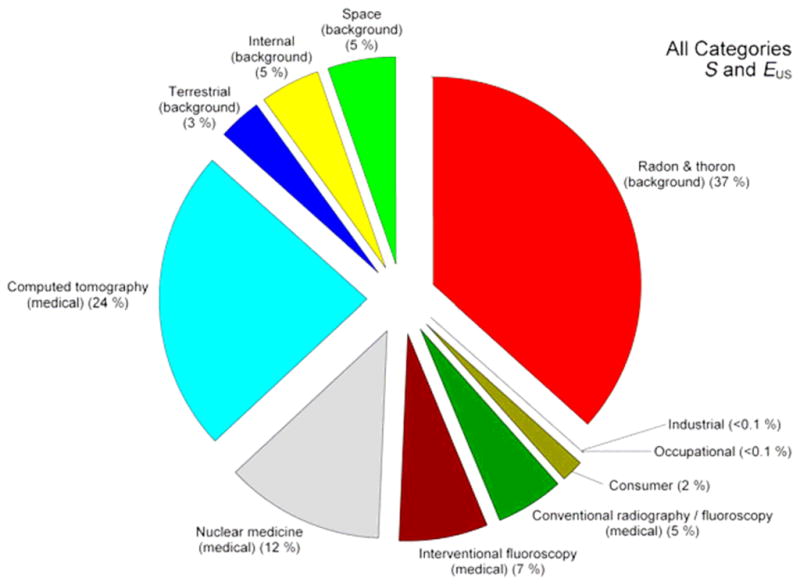
Percent contribution of various sources of exposure to the total collective effective dose (1,870,000 person-Sv) and the total effective dose per individual in the U.S. population (6.2 mSv) for 2006. Percent values have been rounded to the nearest 1%, except for those <1%. Figure is reproduced from NCRP Report 160 (NCRP 2009).
Radionuclides used in nuclear medicine radiopharmaceuticals are drawn from three major categories: beta particle, alpha particle, and Auger electron emitters. The majority of radionuclides used in nuclear medicine are Auger electron emitters, which decay by electron capture (EC) and/or internal conversion (IC). They emit photons for nuclear medicine imaging, and they emit dense showers of very low energy Auger electrons. Examples include 67Ga, 81mKr, 99mTc, 111In, 123I, 125I, and 201Tl. While the majority of nuclear medicine procedures use Auger electron emitters in planar imaging and single photon emission computed tomography (SPECT), positron emitters are also used extensively. Their principal use in diagnostic nuclear medicine is 18F for diagnosis of cancer with positron emission tomography (PET) and 133Xe for lung perfusion imaging. The most common examples of therapeutic beta emitters are 131I for treatment of Grave’s disease and cancer, 90Y for cancer, 89Sr for palliation of bone pain, and occasionally 32P for polycythemia vera. Finally, alpha particle emitters such as 225Ac and 223Ra are currently under experimental evaluation for therapy of a variety of conditions.
Biological damage caused by Auger processes that arise from radioactive decay has been a topic of considerable interest in radiobiology and diagnostic radiology. The keen interest in these radionuclides began when it was observed by Feinendegen (Feinendegen 1968), and later by others (Ertl et al. 1970, Hofer and Hughes 1971, Bradley et al. 1975, Feinendegen 1975), that Auger electron emitters can be highly radiotoxic when they decay within the DNA. Under these conditions, Auger emitters can be as radiotoxic as 210Po which emits 5.3 MeV alpha particles (Howell et al. 1990, Rao et al. 1990). In contrast, cytoplasmic localization and even some intranuclear localizations elicit radiotoxicities analogous to emitters of low linear energy transfer (LET) radiations. Several comprehensive reviews have been published on the biological effects of Auger electron emitters (Sastry and Rao 1984, Sastry 1992, Kassis 2004, Buchegger et al. 2006, Nikjoo et al. 2006, Howell 2008). These are excellent resources for extensive backgrounds and analyses of the field.
When an atom undergoing EC or IC is ionized by removing an electron from an inner atomic shell, the residual atom is in an excited state. Relaxation back to the ground state occurs rapidly via radiative and non-radiative processes. Radiative and non-radiative processes involve the emission of characteristic x-rays and Auger electrons, respectively. These are competitive processes, with radiative processes being more probable for K-shell vacancies and non-radiative processes being more probable for vacancies in the L-shell and above. Thus, creation of an initial inner atomic shell vacancy leads to a series of atomic transitions involving the emission of characteristic x-rays and Auger electrons. This phenomenon, historically referred to as the Auger effect, leads to the emission a shower of characteristic x-rays and Auger electrons (Figure 2). Most of these Auger electrons have very low energies (~ 20 eV to 500 eV) with ranges (~ 1–10 nm) in tissue. As a consequence, biomolecules in the vicinity of the decay are subject to the direct effects of electron irradiation and indirect effects caused by radical species that arise from the radiolysis of water (Wright et al. 1990). In addition, the molecule containing the excited atom is subject to damage caused by charge neutralization (Pomplun and Sutmann 2004).
Figure 2.
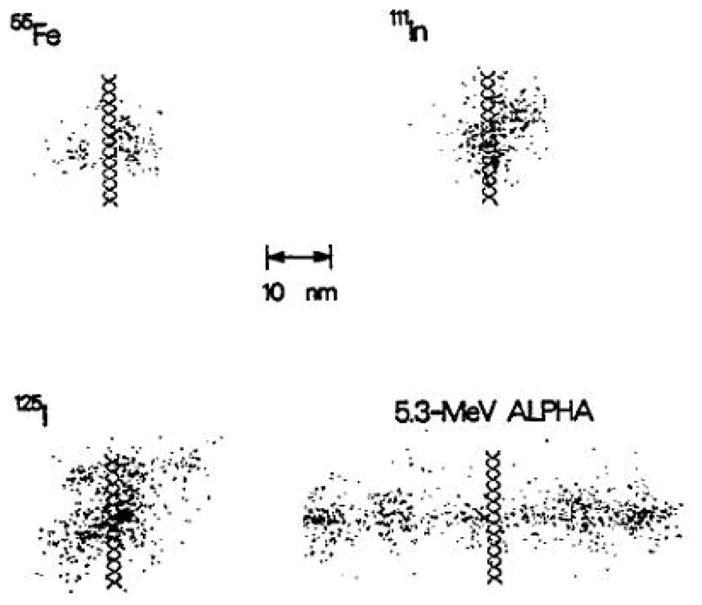
Comparison of the highly localized energy deposition in the immediate vicincity of the decay site of several Auger electron emitters with the energy density along the track of a 5.3 MeV alpha particle. Dots represent the initial positions of the reactive chemical species produced by the radiolysis of liquid water (e.g. OH·, H·, e−aq, etc.). The Auger electron emitters are located at the midpoint on the surface of a hypothetical cylindrical DNA segment. This figure is reproduced from Ref. (Wright et al. 1990).
Many advances have been made in terms of our understanding of the biological effects of Auger processes and their application in medicine. A puzzling dichotomy of opinions exists with respect to the significance of their biological effects. Paradoxically, they are often largely ignored by international organizations that assess radiation risks from low doses of ionizing radiation, while, at the same time, their extreme radiotoxicity is touted as the perfect precision strike against disease (Feinendegen 1975). Thus, there is much to learn about these curious electrons which have seemingly impotent energies when considered alone but, when emitted as a shower of low-energy electrons, deposit an highly localized energy density (HILED) of > 10 MGy in a diameter of about 1 nm around the decay site (Sastry and Rao 1984, Sastry 1992).
The radiotoxicity of β particles emitted by nuclear medicine radionuclides is generally considered low-LET. The common therapeutic β-emitters 131I, 133Xe, 90Sr, 90Y, and 32P emit particles with mean energies of about 0.2, 0.1, 0.2, 0.9, and 0.7 MeV, respectively. While the effects of these emitters are usually commensurate with low-LET X-rays and γ-rays, 131I has sometimes demonstrated itself to be several times more lethal when incorporated into the DNA (Neti and Howell 2004). Whaley and Little have also shown that localized energy deposition by DNA-incorporated 131I and 125I contributes to cytotoxic and mutagenic effects in cells (Whaley and Little 1990). However, the β-emitting radiopharmaceuticals used in diagnostic and therapeutic nuclear medicine do not localize in this fashion.
Long-lived α-emitters were used long ago in diagnostic medicine, sometimes with unfortunate results. For example, thorium dioxide (ThO2), also known as Thorotrast, was used as a contrast agent in X-ray diagnostics from 1930 to 1950. The very long biological clearance time of this agent, coupled with the α-particles emitted by 232Th, resulted high organ equivalent doses and corresponding increases in cancer incidence among this patient population. These and other untoward events slowed the development of α-emitters for therapeutic nuclear medicine. However, over the last two decades, there has been a steady increase in the use α-emitters with relatively short physical half-lives for targeted radioimmunotherapy. These agents are being designed to deliver radiation doses as small as possible to healthy organs, while selectively targeting tumor tissue with high doses.
The maximum equivalent dose to any given organ from diagnostic radiopharmaceuticals is restricted to < 50 mGy, and absorbed doses that approach this magnitude are the exception. While it is generally very difficult to observe statistically significant radiation responses that are caused by acute or chronic irradiation at equivalent doses below 50 mGy, it is possible to measure some biologic responses in highly radiosensitive organs such as the testes and bone marrow. For example, murine spermhead abnormalities can be measured at doses below 50 mGy (Rao et al. 1991). Depending on the subcellular distribution of the radiochemical, the relative biological effectiveness (compared to acute external x-rays) for induction of spermhead abnormalities ranges from 2–60 (Rao et al. 1991). While these RBE values are quite high, it should be noted that no links between induction of spermhead abnormalities and risk to parent or offspring have been demonstrated. Although it has been shown that intravenous injection of the Auger emitter 55Fe can increase the probability of chemically induced cancer in the offspring of irradiated males (Hoyes et al. 2001). In cultured cells, marked induction of cell transformations and hprt mutations at very low doses, with corresponding very high RBE values, have also been demonstrated for DNA-incorporated 125I, a prolific Auger electron emitter (LeMotte et al. 1982, Nagasawa et al. 1992). With this in mind, it is interesting to reflect on Moore and Sastry’s hypothesis that the Auger electrons emitted by 40K may have played a significant role as a mutagen during primordial evolution (Moore and Sastry 1982) These and related findings have been considered by some advisory bodies in making recommendations regarding risk estimates for internal radionuclides (Humm et al. 1994, Goodhead et al. 2004, ICRP 2007). It is generally concluded that there is the possibility of increased risk from Auger emitters depending on the subcellular location and nonuniform distribution between cells within tissues, though the radiation weighting factor for Auger electrons is assigned a value of 1, as it is for β emitters and other low-LET radiations. This implies that their risk for inducing stochastic effects is comparable to x- and γ-rays. However, the ICRP recognizes that Auger electron emitting radiopharmaceuticals represent a special case that require individual attention when they incorporate into DNA (Goodhead et al. 2004, ICRP 2007, Harrison and Day 2008).
In addition to the HILED by Auger electron emitters, a very important aspect of risk assessment for these and other internal radionuclides is the observations of highly nonuniform distribution of radiopharmaceuticals even among cell populations wherein all cells are exposed to the same extracellular radiopharmaceutical concentration. It would appear that log normal distributions of radioactivity are ubiquitous and the standard deviation of this distribution can have a profound impact on the toxicity a given radiopharmaceutical (Neti and Howell 2006). This should be addressed on an individual basis for each radiopharmaceutical (Goodhead et al. 2004, ICRP 2007, Harrison and Day 2008).
Recently, a comprehensive analysis of the cancer risk from internally deposited radionuclides was published (Raabe 2010). This report addressed chronic irradiation by α- and β-emitters localized in lung and in bone. Raabe showed that cancer risk associated with protracted irradiation from internally deposited radionuclides is a non-linear function of lifetime average dose-rate to the affected tissues in beagle dogs. Based on these observations, it was stated that “Cumulative radiation dose is neither an accurate nor an appropriate measure of cancer risk for protracted ionizing radiation exposure.” Observations of other biological endpoints such as killing of murine granulocyte macrophage colony forming cells (GM-CFC) in bone marrow and spermatogonial cells in testes also indicate that cumulative radiation dose is not an appropriate variable to predict response (Howell et al. 1994, Goddu et al. 2000). While a threshold was not observed for murine cell killing in the bone marrow and testes (Howell et al. 1994, Goddu et al. 2000), cancer associated with a protracted low dose-rate exposure of beagle dogs to low- and high-LET ionizing radiation involves a lifespan virtual threshold when the lifetime average dose rate is low (Raabe 2010). The minimum thresholds for induction of lung cancer in young adults were about 0.5 and 5 Gy for α- and β-emitters, respectively, in the lungs. The corresponding thresholds for induction of bone sarcoma were 1 and 20 Gy for α- and β-emitters, respectively, in bone. These absorbed dose thresholds are far beyond the doses delivered by nuclear medicine procedures, although it must be noted that because of the relatively short physical half-lives of radionuclides used in nuclear medicine, exposures in nuclear medicine are protracted over hours, days, or weeks, not over a lifetime.
Scientific evidence indicates that the risks associated with exposure to the low dose and low dose rates encountered in diagnostic nuclear medicine are minimal. In contrast, there are enormous medical benefits afforded by the diagnostic capabilities of nuclear medicine in that they often eliminate the need for invasive procedures such as catheterization and interventional radiology and highly invasive exploratory surgery. While the inherent risks associated with therapeutic nuclear procedures are higher, the potential benefits are often lifesaving. Therefore, radiopharmaceuticals will continue to play an indispensable role in medicine, and the continued development of even more effective radiopharmaceuticals will play an essential role in the future of medicine. The continued development of even more effective radiopharmaceuticals, with commensurate consideration of micro- and macroscopic absorbed doses and biologic responses, will play an essential role in the future of medicine.
Acknowledgments
This work was supported in part by NIH Grant Number R01 CA083838-09. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- Bradley EW, Chan PC, Adelstein SJ. The radiotoxicity of iodine-125 in mammalian cells. I. Effects on the survival curve of radioiodine incorporated into DNA. Radiat Res. 1975;64:555–563. [PubMed] [Google Scholar]
- Buchegger F, Perillo-Adamer F, Dupertuis YM, Delaloye AB. Auger radiation targeted into DNA: a therapy perspective. Eur J Nucl Med Mol Imaging. 2006;33:1352–63. doi: 10.1007/s00259-006-0187-2. [DOI] [PubMed] [Google Scholar]
- Ertl HH, Feinendegen LE, Heiniger HJ. Iodine-125, a tracer in cell biology: Physical properties and biological aspects. Phys Med Biol. 1970;15:447–456. doi: 10.1088/0031-9155/15/3/005. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE. Biological Effects of Transmutation and Decay of Incorporated Isotopes. Vienna: International Atomic Energy Agency; 1968. Problems associated with the use of labelled molecules in biology and medicine. General review; pp. 1–15. [Google Scholar]
- Feinendegen LE. Biological damage from the Auger effect, possible benefits. Radiat Environ Biophys. 1975;12:85–99. doi: 10.1007/BF01328970. [DOI] [PubMed] [Google Scholar]
- Goddu SM, Bishayee A, Bouchet LG, Bolch WE, Rao DV, Howell RW. Marrow toxicity of 33P-versus 32P-orthophosphate: implications for therapy of bone pain and bone metastases. Journal of Nuclear Medicine. 2000;41:941–51. [PubMed] [Google Scholar]
- Goodhead DT, Bramhall R, Busby C, Cox R, Darby S, Day P, Harrison J, Muirhead C, Roche P, Simmons J, Wakeford R, Wright EG. Report of the Committee Examining Radiation Risks of Internal Emitters (CERRIE) London: Committee Examining Radiation Risks of Internal Emitters; 2004. [Google Scholar]
- Harrison J, Day P. Radiation doses and risks from internal emitters. J Radiol Prot. 2008;28:137–59. doi: 10.1088/0952-4746/28/2/R01. [DOI] [PubMed] [Google Scholar]
- Hofer KG, Hughes WL. Radiotoxicity of intranuclear tritium, iodine-125 and iodine-131. Radiat Res. 1971;47:94–109. [PubMed] [Google Scholar]
- Howell RW. Auger processes in the 21st century. International journal of radiation biology. 2008;84:959–75. doi: 10.1080/09553000802395527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell RW, Azure MT, Narra VR, Rao DV. Relative biological effectiveness of alpha emitters in vivo at low doses. Radiat Res. 1994;137:352–360. [PMC free article] [PubMed] [Google Scholar]
- Howell RW, Narra VR, Rao DV, Sastry KSR. Radiobiological effects of intracellular polonium-210 alpha emissions: A comparison with Auger-emitters. Radiat Prot Dosim. 1990;31:325–328. [Google Scholar]
- Hoyes KP, Lord BI, McCann C, Hendry JH, Morris ID. Transgenerational effects of preconception paternal contamination with 55Fe. Radiat Res. 2001;156:488–94. doi: 10.1667/0033-7587(2001)156[0488:teoppc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Humm JL, Howell RW, Rao DV. Dosimetry of Auger electron emitting radionuclides: Report No. 3 of the AAPM Nuclear Medicine Task Group No. 6. Med Phys. 1994;21:1901–1915. doi: 10.1118/1.597227. [DOI] [PubMed] [Google Scholar]
- ICRP. Publication 103: The 2007 Recommendations of the International Commission on Radiological Protection. Annals of the ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Kassis AI. The amazing world of Auger electrons. Int J Radiat Biol. 2004;80:789–803. doi: 10.1080/09553000400017663. [DOI] [PubMed] [Google Scholar]
- LeMotte PK, Adelstein SJ, Little JB. Malignant transformation induced by incorporated radionuclides in BALB/3T3 mouse embryo fibroblasts. Proc Natl Acad Sci. 1982;79:7763–7767. doi: 10.1073/pnas.79.24.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FD, Sastry KSR. Intracellular potassium: K-40 as a primordial gene irradiator. Proc Natl Acad Sci USA. 1982;79:3556–3559. doi: 10.1073/pnas.79.11.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H, Kassis AI, Berman RM, Sahu SK, Nickoloff JA, Okinaka RT, Adelstein SJ, Little JB. Biophysical Aspects of Auger Processes. Woodbury, NY: American Institute of Physics; 1992. Comparison of mutation induction by external and internal radiation sources in synchronized Chinese hamster ovary (CHO) cells; pp. 194–209. http://www.aapm.org/pubs/books/PROC_8.pdf. [Google Scholar]
- NCRP. Ionizing Radiation Exposure of the Population of the United States. Vol. 160. Bethesda: National Council on Radiation Protection and Measurements; 2009. p. 160. [Google Scholar]
- Neti PV, Howell RW. Isolating effects of microscopic nonuniform distributions of 131I on labeled and unlabeled cells. Journal of Nuclear Medicine. 2004;45:1050–8. [PMC free article] [PubMed] [Google Scholar]
- Neti PVSV, Howell RW. Log normally distributed cellular uptake of radioactivity: Implications for biological responses to radiopharmaceuticals. J Nucl Med. 2006;47:1049–1058. [PMC free article] [PubMed] [Google Scholar]
- Nikjoo H, Girard P, Charlton DE, Hofer KG, Laughton CA. Auger electrons--a nanoprobe for structural, molecular and cellular processes. Radiation Protection Dosimetry. 2006;122:72–79. doi: 10.1093/rpd/ncl441. [DOI] [PubMed] [Google Scholar]
- Pomplun E, Sutmann G. Is coulomb explosion a damaging mechanism for125IUdR? Int J Radiat Biol. 2004;80:855–60. doi: 10.1080/09553000400017614. [DOI] [PubMed] [Google Scholar]
- Raabe OG. Concerning the health effects of internally deposited radionuclides. Health Phys. 2010;98:515–36. doi: 10.1097/HP.0b013e3181c20e25. [DOI] [PubMed] [Google Scholar]
- Rao DV, Narra VR, Govelitz GF, Lanka VK, Howell RW, Sastry KSR. In vivo effects of 5.3 MeV alpha particles from Po-210 in mouse testes: Comparison with internal Auger emitters. Radiat Prot Dosim. 1990;31:329–332. [Google Scholar]
- Rao DV, Narra VR, Howell RW, Lanka VK, Sastry KSR. Induction of spermhead abnormalities by incorporated radionuclides: dependence on subcellular distribution, type of radiation, dose rate, and presence of radioprotectors. Radiat Res. 1991;125:89–97. [PMC free article] [PubMed] [Google Scholar]
- Sastry KSR. Biological effects of the Auger emitter 125I: A review. Report No. 1 of AAPM Nuclear Medicine Task Group No. 6. Med Phys. 1992;19:1361–1370. doi: 10.1118/1.596926. [DOI] [PubMed] [Google Scholar]
- Sastry KSR, Rao DV. Physics of Nuclear Medicine: Recent Advances. New York: American Institute of Physics; 1984. Dosimetry of low energy electrons; pp. 169–208. [Google Scholar]
- Whaley JM, Little JB. Efficient mutation induction by I-125 and I-131 decays in DNA of human cells. Radiat Res. 1990;123:68–74. [PubMed] [Google Scholar]
- Wright HA, Hamm RN, Turner JE, Howell RW, Rao DV, Sastry KSR. Calculations of physical and chemical reactions with DNA in aqueous solution from Auger cascades. Radiat Prot Dosim. 1990;31:59–62. [Google Scholar]


