Abstract
Summary
Background and objectives
The early identification of acute heart failure (HF) patients with type 1 cardio-renal syndrome should be the first step for developing prevention and treatment strategies for these patients. This study aimed to assess the performance of neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C in the early detection of type 1 cardio-renal syndrome in patients with acute HF.
Design, setting, participants, & measurements
One-hundred nineteen patients admitted with acute HF were studied. NGAL and creatinine were measured in the first hospitalization morning; creatinine was also measured at least after 48 to 72 hours. Physicians were blinded to NGAL and cystatin C levels. Type 1 cardio-renal syndrome was defined as an increase in the creatinine level of at least 0.3 mg/dl or 50% of basal creatinine.
Results
Type 1 cardio-renal syndrome developed within 48 to 72 hours in 14 patients (11.8%). Admission NGAL levels were higher in these patients: 212 versus 83 ng/dl. At a cutoff value of 170 ng/L, NGAL determined type 1 cardio-renal syndrome with a sensitivity of 100% and a specificity of 86.7%. The area under the receiver-operating characteristic curve of NGAL was 0.93 and that of cystatin C was 0.68.
Conclusions
Above a cutoff value of 170 ng/L, NGAL predicts 48- to 72-hour development of type 1 cardio-renal syndrome with a negative predictive value of 100% and a positive predictive value of 50%. NGAL independently associates with type 1 cardio-renal syndrome and might be a useful biomarker in the early recognition of these patients.
Introduction
The effect of renal dysfunction in heart failure (HF) patients is well known. Renal dysfunction is associated with increased risk of death and hospitalization (1–3). Growing interest in this association has recently led to the description of the cardio-renal syndromes (4,5).
In patients with acute HF, worsening renal function is frequent, occurring in 11% to 40% of the patients (1–4,6). Acute HF patients whose renal function worsens during the acute episode have been classified has having type 1 cardio-renal syndrome. Several retrospective and prospective studies have reported an association of type 1 cardio-renal syndrome occurrence with prolonged length of hospitalization and an ominous prognosis. The early identification of acute HF patients with cardio-renal type 1 syndrome may represent an opportunity to develop strategies aiming for the preservation of kidney function.
Neutrophil gelatinase-associated lipocalin (NGAL) is a glycoprotein belonging to the lipocalin superfamily that is synthesized in the bone marrow during granulocyte maturation. Granulocytes, epithelial cells, renal tubular cells, and hepatocytes release NGAL during injury, and its levels are significantly elevated in epithelial damage (7,8). Several reports have demonstrated that levels of NGAL (urinary and serum) were significantly elevated in patients with acute kidney injury (9–13). This rise in NGAL occurs 24 to 48 hours before plasma creatinine increase.
Cystatin C is a cysteine protease inhibitor synthesized by nucleated cells that is freely filtered in the glomerulus, completely reabsorbed in the convoluted proximal tubule, and is not secreted. Cystatin C levels are not affected by sex, age, race, or muscle mass. A few reports suggested that cystatin C could identify acute kidney injury almost 2 days earlier than creatinine in the intensive care setting (14,15).
We hypothesized that NGAL and cystatin C would be helpful in the early recognition of type 1 cardio-renal syndrome in acute HF patients.
Materials and Methods
We studied all patients admitted to our Internal Medicine Department because of acute HF between May and November 2009. Patients were eligible whether acute HF was de novo or an exacerbation of chronic HF symptoms with an increase in at least one New York Heart Association class. HF diagnosis was based on the European Society of Cardiology criteria. Patients with acute coronary syndromes and patients on chronic renal function replacement therapy were excluded. Patients with type 1 cardio-renal syndrome developing within the first 24 hours were also excluded (four patients).
An echocardiogram was performed on all patients; the ejection fraction was determined using the modified Simpson method. Left ventricular systolic function was considered preserved if the ejection fraction was ≥45%.
Fasting venous blood samples were collected between 8:00 and 9:00 a.m. on the first morning after admission. Creatinine and urea were measured in the emergency department, on the first morning after admission, at 48 to 72 hours, and additionally as requested by the attending physician. All specimens for serum and plasma determinations were centrifuged for 10 minutes at 3000 g within 2 hours after laboratory arrival. All analytical parameters were measured at the Hospital de São João Clinical Pathology Department.
The fasting venous blood sample collected on the first morning after admission was also used to measure NGAL. This measurement was made with the Triage NGAL test system using EDTA-anticoagulated whole blood. All measurements were made within 2 hours after blood collection. This test system is a rapid, point-of-care fluorescence detection immunoassay using the Triage meter (Biosite, Quilaban, Lisboa, Portugal). In brief, several drops of blood are added to the sample port in the device. After addition of the sample, the blood cells are separated from the plasma using a filter contained in the test device. The results are displayed in approximately 15 minutes. The manufacturer provided the calibration curve. For each of the 24 patient samples in which NGAL was determined, one control was performed. The lowest detectable concentration is 60 ng/ml, and the test has been demonstrated to be linear from 60 to 1300 ng/ml NGAL (which is considered the measurable range). We found a within-run precision of 19.3% for a sample with an average of 132 ng/ml. Serum cystatin C was assayed using a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Siemens, Lisboa, Portugal) on a BN II laser nephelometer. The lower limit of detection is 0.05 mg/L, and the within-run and the run-to-run variation was <5%.
Plasma brain natriuretic peptide was measured using an Architect i2000 automated analyzer (Abbott, Lisboa, Portugal). Serum creatinine, urea, and albumin were measured using conventional methods with an Olympus AU5400 automated clinical chemistry analyzer. (Beckman-Coulter, Izasa, Porto, Portugal). Blood counts were obtained using an automated blood counter (Sysmex XE-5000; Emilio de Azevedo Campos, Porto, Portugal).
GFR was estimated according to the Modification of Diet in Renal Disease (MDRD) formula (16).
Patients received standard treatment according to the attending physicians. Physicians were blinded to NGAL and cystatin C levels.
Type 1 cardio-renal syndrome was defined as an increase in the creatinine level of at least 0.3 mg/dl or 50% of basal creatinine during hospitalization. For study purposes, type 1 cardio-renal syndrome was further classified as occurring within the first 48 to 72 hours of admission.
Statistical Analyses
Numerical variables are presented as mean (SD) or median (interquartile range) if non-normally distributed. Categorical variables are presented as count (percent). Patients who developed type 1 cardio-renal syndrome and those who did not were compared: The χ2 test was used to compare categorical variables, a two independent-sample t test was used to compare normally distributed variables, and the Mann–Whitney U test was used for skewed variables. A Spearman correlation was used to correlate NGAL with granulocytes and with GFR as estimated by the MDRD formula. A logistic regression analysis was used to assess determinants of 48- to 72-hour type 1 cardio-renal syndrome. A multivariate model was built taking into consideration the granulocyte count and the GFR because such variables were shown to correlate with NGAL in the study population.
All of the analyses were conducted using SPSS 15.0 (SPSS, Inc., Chicago, IL). P = 0.05 was considered to be statistically significant.
The local ethics committee approved the study. Patients gave informed consent.
Results
During the study period, 119 patients were admitted to our department because of acute HF. Baseline patient characteristics are presented in Table 1. Type 1 cardio-renal syndrome developed in the first 48 to 72 hours in 14 patients (11.8%) and developed anytime during hospitalization in 21 patients (17.6%). Patients with and without type 1 cardio-renal syndrome within 48 to 72 hours upon admission are compared in Table 1.
Table 1.
Characteristics of patients with and without type 1 cardio-renal syndrome within 48 to 72 hours of admission
| Characteristic | Total (n = 119) | With Type 1 Cardio-Renal Syndrome (n = 14) | Without Type 1 Cardio-Renal Syndrome (n = 105) | P |
|---|---|---|---|---|
| Age (years), mean (SD) | 75 (12) | 77 (14) | 75 (12) | 0.60 |
| Male gender, n (%) | 57 (47.9) | 4 (28.6) | 53 (50.5) | 0.16 |
| Diabetes, n (%) | 31 (26.1) | 5 (35.7) | 26 (24.8) | 0.52 |
| Ischemic etiology, n (%) | 62 (52.1) | 3 (21.4) | 59 (56.7) | 0.02 |
| Ejection fraction, median (IQR) | 35 (26 to 45) | 40 (24 to 45) | 35 (26 to 45) | 0.81 |
| Preserved left ventricular ejection fraction, n (%) | 38 (32.2) | 4 (28.6) | 34 (32.7) | 1.0 |
| Systolic BP (mmHg), mean (SD) | 124 (28) | 133 (41) | 122 (26) | 0.37 |
| Heart rate (beats/min), mean (SD) | 86 (21) | 88 (19) | 85 (21) | 0.63 |
| Diuretics, n (%) | 106 (89.1) | 14 (100) | 92 (87.6) | 0.35 |
| Loop diuretic dose (first 24 hours) (mg), median (IQR) | 80 (60 to 100) | 80 (60 to 120) | 80 (60 to 100) | 0.24 |
| ACE inhibitors, n (%) | 82 (68.9) | 10 (71.4) | 72 (68.6) | 1.0 |
| Beta-blockers, n (%) | 60 (50.4) | 4 (28.6) | 56 (53.3) | 0.10 |
| Admission creatinine (mg/dl), median (SD) | 1.37 (1.10 to 1.85) | 1.46 (1.27 to 2.48) | 1.33 (1.09 to 1.81) | 0.14 |
| Admission estimated GFR < 60 ml/min per 1.73 m2, n (%) | 88 (73.9) | 12 (85.7) | 76 (72.4) | 0.35 |
| Admission BNP (pg/ml), median, (IQR) | 1510 (718 to 2637) | 1656 (715 to 3506) | 1510 (724 to 2630) | 0.75 |
| Granulocyte count (per mm3), median (IQR) | 5880 (4200 to 7765) | 7260 (5635 to 11872) | 5730 (4108 to 7630) | 0.03 |
| NGAL (ng/ml), median (IQR) | 91 (61 to 166) | 212 (189 to 307) | 83 (60 to 136) | <0.001 |
| Cystatin C (mg/L), median (IQR) | 1.54 (1.11 to 1.93) | 1.78 (1.50 to 2.83) | 1.49 (1.08 to 1.90) | 0.04 |
| Hospital stay, median (IQR) | 9 (8 to 13) | 8 (5 to 13) | 9 (6 to 13) | 0.34 |
| Intrahospital mortality, n (%) | 8 (6.7) | 5 (35.7) | 3 (2.9) | <0.001 |
IQR, interquartile range; ACE, angiotensin converting enzyme; BNP, brain natriuretic peptide.
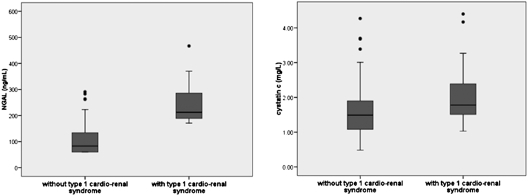
Figure 1 represents NGAL and cystatin C levels in patients who developed type 1 cardio-renal syndrome within the first 48 to 72 hours of admission and in those who did not.
Figure 1.
Depicted are two boxplots. On the left are NGAL value distributions in patients with and without type 1 cardio-renal syndrome within 48 to 72 hours. On the right, the same distribution in shown for cystatin C.
NGAL levels were higher in patients with admission GFR < 60 ml/h per 1.73 m2 than in those with higher GFR: 109.5 ng/ml (78 to 189 ng/ml) versus 64 ng/ml (60 to 86 ng/ml), P < 0.001. Cystatin C levels were also significantly higher in patients with admission GFR < 60 ml/min per 1.73 m2: 1.72 mg/L (1.29 to 2.21 mg/L) versus 1.05 mg/L (0.92 to 1.32 mg/L), P < 0.001. Development of type 1 cardio-renal syndrome was not differently associated with admission GFR.
Plasma NGAL showed a positive and mild correlation with granulocyte count (r = 0.20, P = 0.03) and a moderately strong negative correlation with renal function as estimated by the MDRD (r = −0.49, P < 0.001).
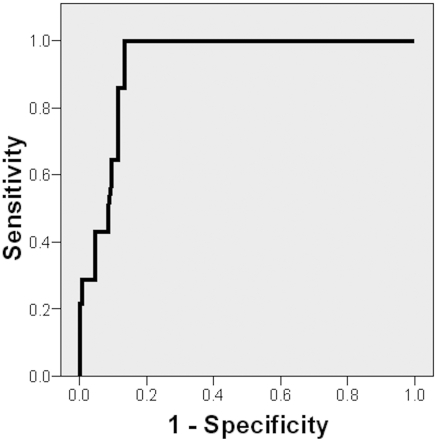
The area under the receiver-operating characteristic (ROC) curve of NGAL for type 1 cardio-renal syndrome was 0.93 (88 to 98) (P < 0.001; Figure 2). The best cutoff value (170 ng/ml) had a sensitivity of 100% and a specificity of 86.7% (positive predictive value of 50.1% and negative predictive value of 100%). The area under the ROC curve of cystatin C for type 1 cardio-renal syndrome was 0.68 (0.54 to 0.82) (P = 0.04).
Figure 2.
ROC curve of NGAL for type 1 cardio-renal syndrome prediction. AUC = 0.93 (88 to 98), P < 0.001.
The odds ratio (OR) of cardio-renal syndrome for each 10-ng/ml increase in NGAL was 1.26 (1.13 to 1.41) (P < 0.001). After adjustment for granulocyte count and renal dysfunction at admission, NGAL was independently associated with type 1 cardio-renal syndrome development within 48 to 72 hours: OR = 1.47 (1.20 to 1.80), P < 0.001.
Discussion
In our study, we evaluated NGAL and cystatin C for the early diagnosis of type 1 cardio-renal syndrome in prospectively recruited acute HF patients. Our results strongly suggest that NGAL can be an ancillary tool in the early recognition of acute HF patients that will develop type 1 cardio-renal syndrome. Our results in acute HF patients show that plasma NGAL has a role in the detection of patients with type 1 cardio-renal syndrome. The area under the curve (AUC) of NGAL was 0.91, and a single NGAL measurement (first day onward) could identify all patients developing type 1 cardio-renal syndrome within 48 to 72 hours with a 50% false positive rate. No patient with <170-ng/ml first-morning NGAL developed type 1 cardio-renal syndrome, and half of the patients with a first-morning value >170 ng/ml develop type 1 cardio-renal syndrome.
The association of higher serum NGAL levels with worsening renal function was already demonstrated by Aghel et al. in a population of 91 acute HF patients (17). Different from the latter study in which worsening renal function was considered from the 5th day onward, we restricted type 1 cardio-renal syndrome to the first 48 to 72 hours of admission. We did so because we considered that renal deterioration beyond such period could be associated with factors not anticipated by admission NGAL.
NGAL is one of the earliest markers of kidney ischemia and nephrotoxic injury in animal models, and it is detected in human blood and urine soon after acute kidney injury (7–9). Urinary and plasma NGAL levels predict acute kidney dysfunction in patients undergoing cardiopulmonary bypass (11,12). The performance of NGAL in acute kidney dysfunction prediction has varied widely across studies, probably because different definitions of acute kidney injury have been used (10,18,19). Concern that NGAL performance in acute kidney injury prediction can possibly depend on baseline renal function has been raised. McIlroy and colleagues reported that, in patients undergoing cardiopulmonary surgery, NGAL only performed well in those with an estimated GFR >60 ml/min (20). Our sample size precluded one such stratification.
In patients undergoing coronary angiography, NGAL has been shown as an early marker of contrast nephrotoxicity (21–23). Recent studies in critical ill patients suggested that NGAL is a powerful predictor of acute kidney dysfunction. In a pediatric population with systemic inflammatory response or septic shock, serum NGAL has been found to be highly sensitive (89%) but nonspecific (39%) for the early detection of acute kidney dysfunction (24). This observation can be related to granulocyte degranulation, a known source of NGAL. Urinary NGAL has also been reported to be highly sensitive (91%) and specific (96%) for acute kidney dysfunction in adult intensive care polytrauma patients (25). Additionally, a very relevant clinical investigation in a broad sample of heterogeneous emergency department patients has shown that urinary NGAL is an early marker of acute kidney dysfunction: The AUC was 0.9, and 90% sensitivity and 99% specificity were observed for the best NGAL cutoff (130 ng/ml creatinine) (26).
In our population, half of the patients with NGAL levels >170 ng/ml did not develop type 1 cardio-renal syndrome as detected by plasma creatinine. These patients might have had mild kidney stress and been unable to increase plasma creatinine levels. Such NGAL increases may represent systemic inflammation and not be related to kidney dysfunction itself. In fact, in our population, serum NGAL correlated with granulocytes, supporting a contribution of neutrophils to circulating NGAL levels (7,27). Additionally, as suggested by Yndestad et al., NGAL can also express myocardial remodeling because its gene expression is significantly increased by innate immune responses such as Toll-like receptors (2 and 4) and IL-1β agonists in an experimental HF model leading to matrix degradation (27).
Elevated NGAL levels in patients not developing clinically identifiable kidney injury have been associated with poor outcome in chronic HF patients; the meaning of this is still unknown in the acute setting (28).
Cystatin C is a renal function biomarker not affected by gender, age, race, or muscle mass. Previous studies suggested that serum and urinary cystatin C could be early markers of acute kidney dysfunction (14,15).
Studies including NGAL and cystatin C have suggested that NGAL had a better performance in identifying acute kidney dysfunction than cystatin C (29,30). In our patients, although those developing type 1 cardio-renal syndrome had higher levels of NGAL and cystatin C than the others, the performance of cystatin C was nonsatisfactory as expressed by an AUC of 0.68. Whereas cystatin C is a functional marker, NGAL is an injury marker and is therefore ideal for detecting acute kidney dysfunction.
Risk factors for worsening renal function have been extensively evaluated but are still largely unknown. Also not completely understood are the mechanisms leading to type 1 cardio-renal syndrome. A previous observation found that venous congestion is the most important hemodynamic factor leading to renal function deterioration (31); in such investigation, BP was not different among patients who did and did not develop renal function deterioration. As in this study, our groups of patients developing and not developing type 1 cardio-renal syndrome had similar BP. Our results suggest that in patients with high NGAL levels a more intensive approach toward lowering venous pressure could be one strategy to prevent type 1 cardio-renal syndrome. A possible clinical implication of our observation is that the use of higher diuretic doses in these high NGAL patients could improve renal perfusion by venous pressure reduction and consequent increase in transrenal pressure. Ultimately renal protection could be achieved, at least in part, by increased, although still careful, diuretic therapy in this group of patients. Clinical investigation exploring this issue is still needed.
Limitations
Our study is a single-center study and results cannot be extrapolated. Its reproduction in other centers or by multicenter studies would argue for its validity. More studies are needed to identify the best NGAL cutoff for recognizing type 1 cardio-renal syndrome. However, our sample included old and very old patients and HF patients with preserved systolic function representing real-world decompensated HF patients in medical wards. Only patients first admitted to medical wards were included; therefore, the most severe patients admitted to intensive care units were missed. The low-risk profile of the patients enrolled in the study presented here does not limit the clinical relevance of the study because the value of NGAL in early acute kidney dysfunction detection has already been reported in intensive care populations.
In conclusion, in patients with acute HF, serum NGAL can help clinicians in the early identification of patients developing very short-term renal deterioration. NGAL is an independent predictor of type 1 cardio-renal syndrome development. Per each 10-mg/L increase in NGAL levels, there was a 31% higher risk of renal dysfunction. The early recognition of such patients is the first step to the development of preventive and treatment strategies for these very high-risk patients.
Disclosures
None.
Acknowledgments
This investigation received a grant from “Fundação Ciênica e Tecnologia,” project PIC/IC/82773/2007.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Froman DE, Butler J, Wang Y, Adraham WT, O'Conner CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevensen LW, Young JB, Krumholz HM: Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B: Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: Results of the prospective outcomes study in heart failure (POSH). Eur Heart J 17: 1216–1222, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L: Worsening renal function in patients hospitalized for acute heart failure: Clinical implications and prognostic significance. Eur J Heart Fail 10: 188–195, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R: Cardiorenal syndrome. J Am Coll Cardiol 52: 527–539, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P; for the Acute Dialysis Quality Initiative (ADQI) consensus group Cardio-renal syndromes: Report from the consensus conference of the Acute Dialysis Quality Initiative. Eur Heart J Dec 31: 703–711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarraf M, Masoumi A, Schrier RW: Cardiorenal syndrome in acute decompensated heart failure. Clin J Am Soc Nephrol 12: 2013–2026, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Xu S, Venge P: Lipocalins as biochemical markers of disease. Biochim Biophys Acta 1482: 298–307, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J: Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. Clin Invest 3: 610–621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mori K, Nakao K: Neutrophil gelatinase associated lipocalin as the real time indicator of kidney damage. Kidney Int 71: 967–970, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Haase-Fielitz A, Bellomo R, Devarajan P, Bennett M, Story D, Matalanis G, Frei U, Dragun D, Haase M: The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant 11: 3349–3354, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT: Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis 52: 425–433, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fieltz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 54: 1012–1024, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Herget-Rsenthal S, Marggrat G, Husing J, Goring F, Pietruck F, Janssen O, Phillip T, Kribben A: Early detection of acute renal failure by serum cystatin C. Kidney Int 66: 1115–1122, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Villa P, Jimenez M, Soriano MC, Manzanares J, Casasnovas P. Serum cystatin C concentrations as a marker of acute renal dysfunction in critically ill patients. Crit Care 9: R139–R143, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Meara E, Chong KS, Gardner RS, Jardine AG, Neilly JB, McDonagh TA: The Modification of Diet in Renal Disease (MDRD) equations provide valid estimations of glomerular filtration rates in patients with advanced heart failure. Eur J Heart Fail 1: 63–67, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Aghel A, Shrestha K, Mullens W, Borowsky A, Tang W: Serum neutrophil gelatinase associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail 16: 49–54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P: Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: A prospective uncontrolled cohort study. Crit Care 11: R127, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P: Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin J Am Soc Nephrol 3: 665–673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McIlroy DR, Wagener G, Lee HT: Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: The effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol 5: 211–219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Poniatowski B, Pawlak K, Dobrzycki S: NGAL (neutrophil gelatinase-associated lipocalin) and cystatin C: Are they good predictors of contrast nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine? Int J Cardiol 127: 290–291, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q: Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract 108: c176–c181, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, III, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Devarajan P: NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 22: 2089–2095, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR: Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med 36: 1297–1303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Makris K, Markou N, Evodia E, Dimopoulou E, Drakopoulos I, Ntetsika K, Rizos D, Baltopoulos G, Haliassos A: Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med 47: 79–82, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J: Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 148: 810–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yndestad A, Landrø L, Ueland T, Dahl CP, Flo TH, Vinge LE, Espevik T, Frøland SS, Husberg C, Christensen G, Dickstein K, Kjekshus J, Øie E, Gullestad L, Aukrust P: Increase systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J 30: 1229–1236, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Bolignano D, Basile G, Parisi P, Coppolino G, Nicocia G, Buemi M. Increased plasma neutrophil gelatinase-associated lipocalin levels predict mortality in elderly patients with chronic heart failure. Rejuvenation Res 1: 7–14, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, Malyszko JS, Dobrzycki S: Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren Fail 31: 910–919, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M: Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery—A prospective cohort study. Crit Care Med 37: 553–560, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WT: Importance of venous congestion for worsening of renal function in advanced heart failure. J Am Coll Cardiol 53: 589–596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]