Abstract
Summary
Background and objectives
The efficacy of folic acid therapy to lower homocysteine (Hcy) levels in an effort to reduce cardiovascular disease (CVD) risk in patients with ESRD or advanced chronic kidney disease (ACKD; creatinine clearance, <30 ml/min) remains inconclusive. We conducted a meta-analysis of relevant randomized trials to further examine this issue.
Design, setting, participants, & measurements
This meta-analysis included 3886 patients with ESRD/ACKD from seven qualified randomized trials using folic acid therapy and with CVD reported as one of the end points.
Results
When pooling the seven trials, folic acid therapy reduced the risk of CVD by 15% (RR, 0.85; 95% CI, 0.76 to 0.96; P = 0.009). A greater beneficial effect was observed among those trials with a treatment duration >24 months (RR, 0.84; 95% CI, 0.72 to 0.98; P = 0.02), a decrease in Hcy level >20% (RR, 0.83; 95% CI, 0.73 to 0.95; P = 0.007), and no or partial folic acid fortification (RR, 0.80; 95% CI, 0.65 to 0.99; P = 0.04). The beneficial effect also was seen when Hcy levels decreased >20%, even in the presence of folic acid fortification (RR, 0.85; 95% CI, 0.73 to 0.99; P = 0.04). In the corresponding comparison groups, the estimated RRs were attenuated and insignificant.
Conclusions
Folic acid therapy can reduce CVD risk in patients with ESRD/ACKD by 15%. A greater beneficial effect was observed among those trials with no or partial folic acid fortification or a decrease in Hcy level >20% regardless of folic acid fortification.
Introduction
People with chronic kidney disease (CKD) have a markedly elevated risk for cardiovascular disease (CVD) (1,2) when compared with the general population. For example, approximately 50% of patients with ESRD die from a CVD cause, and CVD mortality for patients with ESRD is 15 to 30 times higher than the age-adjusted CVD mortality in the general population (3). Thus, prevention and treatment of CVD are major considerations in the management of individuals with CKD (1).
Many potential causes may contribute to the elevated risk of CVD in patients with CKD. Traditional cardiovascular risk factors only partially account for this increased CVD morbidity and mortality (3). As such, identification of other significant and treatable risk factors is critical to reduce the excess burden of CVD morbidity and mortality in patients with CKD (4). Homocysteine (Hcy) is of considerable interest because hyperhomocysteinaemia is highly prevalent and significantly related to cardiovascular morbidity and mortality in patients with ESRD (1,5).
However, the question as to whether Hcy-lowering therapy can reduce CVD risk in patients with ESRD or advanced chronic kidney disease (ACKD) remains to be answered. Their characteristically high Hcy levels, extensive vascular disease, and high mortality rates make the ESRD/ACKD population particularly suitable for testing the benefit of Hcy-lowering therapy. In light of the growing number of published trials, each of which by itself is lacking sufficient sample size and power, a comprehensive meta-analysis of all of the available data is warranted to further examine whether folic acid therapy has a beneficial effect on CVD risk in ESRD/ACKD patients.
A meta-analysis by Heinz et al. (5) included three folic acid trials (one not randomized) in patients undergoing maintenance dialysis. This meta-analysis included all of the pertinent published trials up to August 2010 and aimed to assess the relationship between folic acid therapy (with or without vitamin B6 and B12) and the risk of CVD in ESRD/ACKD. We are particularly interested in whether the effect of folic acid therapy on CVD is affected by the factors that could influence the treatment effects and whether there are subgroups that might particularly benefit from folic acid therapy.
Materials and Methods
Search Strategy and Selection Criteria
We attempted to conform to QUOROM (Quality of Reporting of Meta-analyses) guidelines in the report of this meta-analysis (6). To select pertinent studies, we performed a comprehensive and independent literature search of the Medline database from January 1966 to August 2010, with the MESH terms “cardiovascular disease,” “cerebrovascular accident,” “coronary disease,” “coronary thrombosis,” “myocardial ischemia,” “coronary stenosis,” “coronary restenosis,” “cerebrovascular accident,” “cerebrovascular disease,” “stroke” and “folic acid,” “folate,” “multivitamin,” “chronic kidney disease,” “end-stage renal disease,” “advanced chronic kidney disease,” and “dialysis.” Manual searches of the bibliographies of all of the relevant trials and review articles were also conducted. The search was restricted to human studies and clinical trials. There were no language restrictions. A team of experts in the relevant disciplines was assembled.
A standard protocol for study selection and data abstraction was developed by our multidisciplinary team with expertise in clinical medicine, epidemiology, clinical trials, and biostatistics. Studies were eligible for inclusion if: (1) the study was a randomized controlled trial; (2) the number of cardiovascular events that occurred during the study were reported by intervention and control groups; and (3) the intervention consisted of folic acid therapy (with or without additional B vitamins). To minimize clinical heterogeneity, this meta-analysis was limited to patients with ESRD/ACKD (creatinine clearance, <30 ml/min). Further elaboration on this point can be found in the Discussion.
Data Collection
Of the 49 studies potentially eligible, each of the abstracts was reviewed independently by two investigators to determine whether it met the eligibility criteria for inclusion. All of the data from the eligible trials were independently abstracted in duplicate by two independent investigators using the standard protocol. Discrepancies were resolved by discussion with the third investigator and the multi-disciplinary team who developed the protocol.
Primary and Secondary Outcome
The primary outcome was the occurrence of all of the fatal or nonfatal cardiovascular events (CVD events in each trial are presented in Table 2). The secondary outcome was a composite of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular cause (such data were available from five of the seven trials).
Table 2.
Study design characteristics of individual trials
| Data Source | Blinding | Active Treatment | Control | CVD Events | Fortification | Duration of Intervention (months) | Funding Sources |
|---|---|---|---|---|---|---|---|
| Righetti et al. (12) | Open | Folic acid 5 or 15 mg/d | Usual care | Angina, MI, carotid artery stenoses, thrombotic stroke | No | 12 | Not listed |
| Wrone et al. (18) | Double | Folic acid 5 or 15 mg/d and vitamins B6 and B12 | Folic acid 1 mg/d and vitamins B6 and B12 | MI, revascularization, stroke | Yesa | 24 | Public, corporate |
| Righetti et al. (13) | Open | Folic acid 5 mg/d or 5 mg every other day and vitamins B6 and B12 | Vitamins B6 and B12 | MI, stroke, angina, sudden cardiac arrest, cerebrovascular TIA | No | 29 | Not listed |
| Zoungas et al. (14) | Double | Folic acid 15 mg/d | Placebo | MI, stroke, angina, revascularization, peripheral vascular disease | Partly | 43 | Public, foundation |
| Vianna et al. (15) | Double | Folic acid 10 mg, 3 times/wk | Placebo | Nonfatal/fatal cardiovascular event | No | 24 | Foundation |
| Jamison et al. (16) | Double | Folic acid 40 mg/d and vitamins B6 and B12 | Placebo | MI, stroke | Yes | 38 | Public, corporate |
| Heinz et al. (17) | Double | Folic acid 5 mg, 3 times/wk and vitamins B6 and B12 | Folic acid 0.2 mg/d and vitamins B6 and B12 | MI, stroke, angina, revascularization, sudden cardiac arrest, peripheral vascular disease, pulmonary embolism, thromboses | Yesa | 24 | Public, corporate |
MI, myocardial infarction; TIA, transient ischemic attack.
Folic acid supplement in control group.
Statistical Analyses
Relative risk (RR) with a 95% confidence interval (CI) was used as a measure of the effect of folic acid therapy on CVD risk. Although both fixed-effect and random-effect models yielded similar findings, results from the random-effect models are presented herein because of the different intervention regimens, intervention durations, and dietary intakes of folic acid that were involved in the original trials. Furthermore, many investigators consider the random-effect approach to be a more natural choice than the fixed-effect in contexts of medical decision making (7,8). Heterogeneity between studies was assessed by Cochran's Q with a significance level set at 0.10. We also conducted a sensitivity analysis by removing each individual trial from the meta-analysis. All of the analyses were performed using STATA version 10.0 (StataCorp, College Station, TX).
Role of the Funding Source
There was no funding source for this study. The research team had full access to all of the data used for this meta-analysis and had final responsibility for publication. All of the authors have seen and approved the final version of the manuscript.
Results
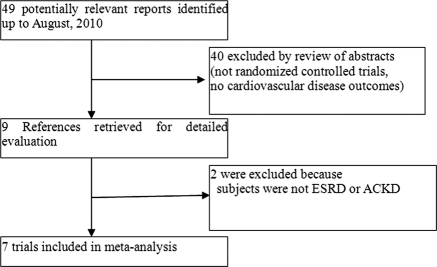
Figure 1 depicts the flow of the study selection process. Of the 49 potentially relevant reports identified and screened, 40 were excluded by review of abstracts because they were not randomized controlled trials (RCTs) or had no cardiovascular disease outcomes. For example, the report by Mann et al. (9) was a post hoc analysis of a subsample of a parent RCT (HOPE-2) and thus was excluded. Of the nine trials retrieved for detailed evaluations, two (10,11) were further excluded because the subjects were not ESRD or ACKD. Our final analysis included seven RCTs (12–18) comprising a total of 3886 subjects with ESRD or ACKD.
Figure 1.
Study selection.
The characteristics of the study participants and design features of each trial are presented in Tables 1 and 2, respectively. Of the seven trials, two (16,18) were conducted in the United States and Canada, three (12,13,17) were in European countries, one (14) was in Australia and New Zealand, and one (15) was in Brazil.
Table 1.
Baseline characteristics and change in Hcy during treatment of individual trials (total n = 3886)
| Data Source | Total Subjects | Age, Mean (SD) (years) | Male | Pre-existing Diseases | History of CVD | Diabetes | Total Cholesterol, Mean (SD) (mg/ml) | Hcy, Mean (SD) (μmol/L) | Change in Hcy during Treatment (%) |
|---|---|---|---|---|---|---|---|---|---|
| Righetti et al. (12) | 81 | 64.0 (14.0) | 55.7 | ESRD | 34.6 | 13.6 | 198.7 (90.0) | 50.3 (6.0) | −52.0 |
| Wrone et al. (18) | 510 | 60.2 (15.1) | 50.0 | ESRD | 34.1 | 45.5 | 183.8 (44.0) | 32.9 (20.0) | −10.9 |
| Righetti et al. (13) | 88 | 64.3 (11.7) | 56.0 | ESRD | 58.0 | 19.3 | 196.0 (78.8) | 35.0 (1.4) | −40.6 |
| Zoungas et al. (14) | 315 | 56.0 (13.5) | 68.0 | ESRD or ACKDa | 35.0 | 23.2 | 200.8 (46.3) | 27 (13.0) | −17.4 |
| Vianna et al. (15) | 186 | 48.5 (12.9) | 59.1 | ESRD | 23.7 | 22.6 | 182.5 (35.9) | Median 25.0 | −55.3 |
| Jamison et al. (16) | 2056 | 65.8 (11.8) | 98.0 | ESRD or ACKDb | NR | 55 | 166.6 (43.9) | 24.1 (8.8) | −25.1 |
| Heinz et al. (17) | 650 | 61.0 (13.0) | 58.0 | ESRD | 48.0 | 40.0 | Median 189.2 | Median 29.0 | −30.0 |
NR, not reported.
ACKD serum creatinine of 0.40 mmol/L or greater (creatinine clearance, <25 ml/min).
ACKD with an estimated creatinine clearance of less than or equal to 30 ml/min.
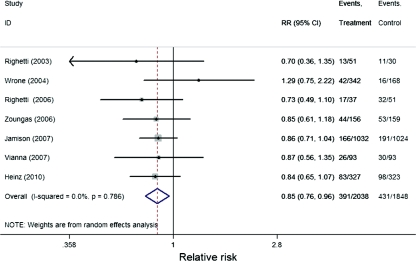
As shown in Figure 2, pooling the seven trials, folic acid therapy (with or without other B vitamins) significantly reduced the risk of primary CVD outcome by 15% (RR, 0.85; 95% CI, 0.76 to 0.96; P = 0.009). Table 3 presents the RRs stratified by duration of folic acid supplementation (≤24 versus >24 months); degree of Hcy lowering (≤20% versus >20%), prior folic acid fortification (yes, no/partly), treatment regimen (folic acid alone versus folic acid plus vitamin B6 and B12), mean daily folic acid dose (<5 mg versus ≥5 mg), and pre-existing renal conditions (ESRD versus ESRD/ACKD). A greater beneficial effect was observed among those trials with a treatment duration of >24 months (RR, 0.84; 95% CI, 0.72 to 0.98; P = 0.02); a decrease in Hcy level >20% (RR, 0.83; 95% CI, 0.73 to 0.95; P = 0.007); and no/partial folic acid fortification (RR, 0.80; 95% CI, 0.65 to 0.99; P = 0.04). The beneficial effect also was seen when Hcy lowering was >20% even in the presence of folic acid fortification/supplement (RR, 0.85; 95% CI, 0.73 to 0.99; P = 0.04). In the corresponding comparison groups, the estimated RRs were attenuated and insignificant. The stratified analysis by treatment regimen, mean daily folic acid dose, and pre-existing renal conditions showed a similar beneficial effect across the strata.
Figure 2.
Forest plot of RR and 95% CI of primary cardiovascular outcome for folic acid treatment versus control in individual trial and pooled data.
Table 3.
Risk estimates of primary cardiovascular outcome for folic acid intervention versus control in pooled and stratified analysis by pertinent factors
| Stratification Variables | Primary CVD Events/Total Subjects |
RR | 95% CI | P | |
|---|---|---|---|---|---|
| Active | Control | ||||
| Overall (12–18) | 391/2038 | 431/1848 | 0.85 | 0.76, 0.96 | 0.009 |
| Intervention duration | |||||
| ≤24 months (12,15,17,18) | 164/813 | 155/614 | 0.88 | 0.72, 1.06 | 0.17 |
| >24 months (13,14,16) | 227/1225 | 276/1234 | 0.84 | 0.72, 0.98 | 0.02 |
| Hcy lowering | |||||
| ≤20% (14,18) | 86/498 | 69/327 | 0.99 | 0.66, 1.48 | 0.95 |
| >20% (12,13,15–17) | 305/1540 | 362/1521 | 0.83 | 0.73, 0.95 | 0.007 |
| Folic acid fortification | |||||
| yes (16–18) | 291/1701 | 305/1515 | 0.88 | 0.76, 1.02 | 0.10 |
| yes and Hcy lowering >20% (16,17) | 249/1359 | 289/1347 | 0.85 | 0.73, 0.99 | 0.04 |
| no or partly (12–15) | 100/337 | 126/333 | 0.80 | 0.65, 0.99 | 0.04 |
| Treatment regimen | |||||
| folic acid only (12,14,15) | 83/300 | 94/282 | 0.83 | 0.65, 1.06 | 0.14 |
| folic acid and vitamins B6 and B12 (13,16–18) | 308/1738 | 337/1566 | 0.86 | 0.75, 0.99 | 0.03 |
| Daily folic acid dose | |||||
| <5 mg (13,15,17) | 126/457 | 160/467 | 0.82 | 0.68, 0.99 | 0.04 |
| ≥5 mg (12,14,16,18) | 265/1581 | 271/1381 | 0.88 | 0.75, 1.02 | 0.09 |
| Pre-existing conditions | |||||
| ESRD (12,13,15,17,18) | 181/850 | 187/665 | 0.85 | 0.71, 1.01 | 0.06 |
| ESRD/ACKD (14,16) | 210/1188 | 244/1183 | 0.86 | 0.73, 1.01 | 0.07 |
We conducted heterogeneity testing for all of the analyses in Table 3. All of the resultant P values were larger than 0.10, meaning that heterogeneity is NS in either the overall analysis or in the stratified analyses. Sensitivity analyses showed that the RRs and 95% CI were not altered substantially by removing any one of the seven trials (data not shown).
As presented in Table 4, we performed an additional analysis of pooling five trials with available data on secondary CVD outcome (a composite of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular cause). Consistently, we found that folic acid therapy (with or without other B vitamins) reduced the risk of secondary CVD end points by 13 to 14%.
Table 4.
Risk estimates of secondary cardiovascular outcome for folic acid intervention versus control in individual trial and pooled data
| Data Source | Secondary CVD Events/Total Subjects |
RR | 95% CI | P | |
|---|---|---|---|---|---|
| Active | Control | ||||
| Wrone et al. (18) | 28/342 | 12/168 | 1.15 | 0.60, 2.20 | 0.68 |
| Righetti et al. (13) | 6/37 | 13/51 | 0.64 | 0.27, 1.52 | 0.31 |
| Zoungas et al. (14) | 46/156 | 55/159 | 0.85 | 0.62, 1.18 | 0.33 |
| Heinz et al. (17) | 53/327 | 58/323 | 0.90 | 0.64, 1.27 | 0.55 |
| Jamison et al. (16) | 166/1032 | 191/1024 | 0.86 | 0.71, 1.04 | 0.12 |
| Overall (13,14,16–18) | 299/1894 | 329/1725 | 0.87 | 0.75, 1.00 | 0.06 |
| Overall (13,14,16,17)a | 271/1552 | 317/1557 | 0.86 | 0.74, 0.99 | 0.04 |
Wrone et al. was excluded because the control group was given folic acid 1 mg/d.
Discussion
A recent meta-analysis concluded that the total Hcy level may be a risk factor for CVD events and total mortality in patients with ESRD not receiving vitamin supplementation or folic acid fortification (5). However, randomized clinical trials have not been able to demonstrate a convincing beneficial effect of folic acid therapy on CVD (12–18) in ESRD/ACKD. Our power analysis demonstrated that each of the individual trials did not have sufficient power to discern a 15% reduction in CVD risk (data not shown). This meta-analysis, by pooling seven randomized controlled trials (12–18) comprising a total of 3886 subjects with ESRD or ACKD, achieved greater statistical power to reach a valid conclusion. More importantly, our meta-analysis examined factors that could have contributed to the inconsistent or null findings from previously published trials and provided new insight on the efficacy and causality of folic acid therapy on CVD risk in patients with ESRD/ACKD.
Of note, in discussing our findings, one must keep in mind that meta-analysis has inherent limitations, including its retrospective and aggregate nature and its inability to adjust for individual variables. The sample size of the trials included in this analysis varied, and the results were more likely influenced by the trials with larger sample sizes. However, we performed sensitivity testing and found that removing any single trial did not significantly alter our results. Furthermore, our testing of heterogeneity between studies did not demonstrate a significant difference. Publication bias is an important issue for meta-analysis, in which positive results are more likely to be published, and as such, meta-analysis may overestimate the true effect or association. However, because of the highly controversial nature of this topic, the published trials so far have ranged from positive findings to no effect and/or negative findings. These trials varied in quality according to treatment assignment procedure, adherence and follow-up, and statistical analysis (data not shown), but sensitivity analyses showed that the RRs and 95% CI were not altered substantially by removing any of the trials. Finally, our meta-analysis included trials in patients with ESRD and ESRD/ACDK. These two groups of patients appear to have comparable effects of folic acid treatment on CVD outcome. Our finding is consistent with other published reports (HOST study [16] and Jungers et al. [19]). The generalizability of our findings to other chronic renal conditions remains to be determined.
Who Can Particularly Benefit from Folic Acid Therapy?
The question remains whether there is a subgroup of patients with ESRD/ACKD who can particularly benefit from folic acid therapy. Our meta-analysis showed that, on average, folic acid therapy reduced the risk of CVD by 15% (RR, 0.85; 95% CI, 0.76 to 0.96; P = 0.009) in patients with ESRD/ACKD. In the stratified analyses, a greater beneficial effect was observed among those trials with a treatment duration >24 months (RR, 0.84; 95% CI, 0.72 to 0.98; P = 0.02); a decrease in Hcy level >20% (RR, 0.83; 95% CI, 0.73 to 0.95; P = 0.007); and no/partial grain fortification (RR, 0.80; 95% CI, 0.65 to 0.99; P = 0.04). The beneficial effect also was seen when the decrease in Hcy levels was >20% in the presence of folic acid fortification (RR, 0.85; 95% CI, 0.73 to 0.99; P = 0.04).
A 1998 meta-analysis of 12 RCTs evaluating the effects of folic acid and B vitamins on Hcy showed that reductions in Hcy are significantly greater when the pretreatment Hcy level is high (20). Of note, folic acid fortification of North Americans (140 μg/100 g of flour) lowered the population mean of Hcy to about 8 to 10 μmol/L (21). As a consequence, the ability of folic acid to reduce Hcy among North Americans was reduced from 25 to 15% (22). This attenuated Hcy lowering effect was not considered in the design of some RCTs, and it is likely that folic acid fortification might have contributed to the no-effect findings among these trials. As expected, our analysis showed that folic acid fortification was an important determinant for the treatment effect of the trials.
Is There a Ceiling Effect of Folic Acid Therapy in CVD Prevention?
Among the published trials of folic acid therapy on CVD outcomes, there were considerable variations in the dosage of folic acid. Uncertainty remains regarding an optimum dose of folic acid supplementation in CVD prevention. Like any essential nutrient, one would expect that in populations with adequate folic acid intake, further reductions in Hcy level and CVD risk by folic acid supplementation would be limited. Likewise, excessive doses of folic acid therapy will not lead to an additional beneficial effect. A meta-analysis of 25 randomized controlled trials (22) showed that daily doses of 0.8 mg of folic acid had achieved the maximum reduction in plasma Hcy concentrations produced by folic acid supplementation.
Of the seven trials included in this meta-analysis, three trials used <5 mg of folic acid daily and four used ≥5 mg of folic acid daily. As shown in Table 3, there was no evidence of an added benefit from larger doses of folic acid in comparison with lower doses on CVD outcome in patients with ESRD/ACKD. Of note, the placebo group in some trials also received folic acid treatment, which might have contributed to the no-effect findings. For example, the placebo group in the trial by Wrone et al. (18) received 1 mg/d of folic acid, which exceeded the ceiling of 0.8 mg daily. It is not surprising then that this trial yielded no-effect findings with regard to folic acid therapy.
Is There an Adverse Effect of Folic Acid Therapy in ESRD/ACKD?
A trial recently reported by House et al. (11) suggested that among patients with diabetic nephropathy, high doses of B vitamins compared with a placebo resulted in a greater decrease in GFR and an increase in vascular events. However, there were a number of factors that could have affected the results and interpretation of the trial, such as small sample size, the between-group imbalance in important baseline characteristics, low adherence with the intervention, and the difference in cumulative proportion of composite outcome between the placebo and intervention groups within the first 8 months. When the events that occurred in the first eight months were removed, there was no group difference from 8 to 36 months of the trial.
The finding by House et al. was not seen in any previous B-vitamin trials, including trials in patients with ESRD/ACKD. Even in trials with high-dose folic acid (40 mg/d) (16), there was no significant difference in the number and types of adverse events (including serious adverse events) between the treatment and control groups. Our meta-analysis provided no evidence that folic acid therapy (with or without B vitamins) increased the risk of primary and secondary CVD outcomes.
Clinical and Research Implications
Our findings remain to be confirmed by data from large, ongoing and future trials. As with any meta-analysis, our findings should be interpreted in the context of available evidence in the fields. Given the ongoing controversy over homocysteine-lowering therapy to reduce CVD risk in the general population and in patients with ESRD/ACKD, clarifying whether there are targeted groups of individuals who may benefit from this simple intervention is very important from a research and population health perspective. To efficiently assess the efficacy and causality of folic acid therapy on CVD in the general population or in patients with ESRD/ACKD, future clinical trials should be conducted in regions without folic acid fortification and in populations with low intake of folic acid, high Hcy concentrations, and high prevalence of CVD. For example, in many developing countries such as China, Hcy plasma concentrations (median, 13 to 15 μmol/L) and prevalence of CVD are high (23). We speculate that in populations with these characteristics, folic acid supplementation could be a safe and inexpensive strategy to reduce CVD risk. The issue of folic acid therapy alone or in combination with other B vitamins, as well as optimal dosage, should also be carefully considered in future trials.
Conclusions
Our meta-analysis provided coherent evidence that, on average, folic acid therapy can reduce CVD risk in patients with ESRD/ACKD by approximately 15%, and greater beneficial effect may be expected in patients with adequate duration of treatment, in patients with greater reduction in Hcy during treatment, and in populations without folic acid fortification/supplementation.
Disclosures
None.
Acknowledgments
We thank Ms. Tami Bartell for intensive English editing.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Wright J, Hutchison A: Cardiovascular disease in patients with chronic kidney disease. Vasc Health Risk Manag 5: 713–722, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Heinz J, Kropf S, Luley C, Dierkes J: Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: A meta-analysis. Am J Kidney Dis 54: 478–489, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF: Improving the quality of reports of Meta-analyses of randomized controlled trials: The QUOROM statement. Lancet 354: 1896–1900, 1999 [DOI] [PubMed] [Google Scholar]
- 7. DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7: 177–188, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Ades AE, Lu G, Higgins JP: The interpretation of random-effects meta-analysis in decision models. Med Decis Making 25: 646–654, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Mann JF, Sheridan P, McQueen MJ, Held C, Arnold MO, Fodor G, Yusuf S, Lonn EM: Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease: Results of the renal Hope-2 study. Nephrol Dial Transplant 23: 645–653, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS, McKenney JL, Mercier RY, Pfeffer MA, Selhub J: Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis 53: 121–128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, Dresser GK, Spence JD: Effect of B-vitamin therapy on progression of diabetic nephropathy: A randomized controlled trial. JAMA 303: 1603–1609, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Righetti M, Ferrario GM, Milani S, Serbelloni P, Rosa LL, Uccellini M, Sessa A: Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit 9: PI19–PI24, 2003 [PubMed] [Google Scholar]
- 13. Righetti M, Serbelloni P, Milani S, Ferrario G: Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif 24: 379–386, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, Atkins RC, Nicholls K, Fraenkel M, Hutchison BG, Walker R, McNeil JJ: Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: A multicenter, randomized, controlled trial. J Am Coll Cardiol 47: 1108–1116, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Vianna AC, Mocelin AJ, Matsuo T, Morais-Filho D, Largura A, Delfino VA, Soares AE, Matni AM: Uremic hyperhomocysteinemia: a randomized trial of folate treatment for the prevention of cardiovascular events. Hemodial Int 11: 210–216, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM: Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end stage renal disease: A randomized controlled trial. JAMA 298: 1163–1170, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Heinz J, Kropf S, Domröse U, Westphal S, Borucki K, Luley C, Neumann KH, Dierkes J: B vitamins and the risk of total mortality and cardiovascular disease in end-stage renal disease: Results of a randomized controlled trial. Circulation 121: 1432–1438, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Wrone EM, Hornberger JM, Zehnder JL, Mccann LM, Coplon NS, Fortmann SP: Randomized trial of folic acid for prevention of cardiovascular events in end stage renal disease. J Am Soc Nephrol 15: 420–426, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Jungers P, Joly D, Massy Z, Chauveau P, Nguyen AT, Aupetit J, Chadefaux B: Sustained reduction of hyperhomocysteinaemia with folic acid supplementation in predialysis patients. Nephrol Dial Transplant 14: 2903–2906, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Homocysteine Lowering Trialists' Collaboration: Lowering blood homocysteine with folic acid based supplements: Meta-analysis of randomized trials. BMJ 316: 894–898, 1998 [PMC free article] [PubMed] [Google Scholar]
- 21. Jacques PF, Selhub J, Bostom AG, Wilson PWF, Rosenberg IH: The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med 340: 1449–1454, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Homocysteine Lowering Trialists'Collaboration: Dose-dependent effects of folic acid on blood concentrations of homocysteine: A meta-analysis of the randomized trials. Am J Clin Nutr 82: 806–812, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Li Z, Zhang H, Liao Y, Wang D, Zhao B, Zhu Z, Zhao J, Ma A, Han Y, Wang Y, Shi Y, Ye J, Hui R: Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke: A multicenter case-control study in China. Stroke 34: 2085–2090, 2003 [DOI] [PubMed] [Google Scholar]