Abstract
Summary
Background and objectives
Premature cardiovascular (CV) events, especially sudden cardiac death, are common in ESRD patients and associated with uremic cardiomyopathy. Identification of high-risk patients is difficult. Microvolt T-wave alternans (MTWA) is a noninvasive method of detecting variability in electrocardiogram (ECG) T-wave morphology and is a promising technique for identifying patients at high risk of ventricular tachyarrhythmias. MTWA results of ESRD and hypertensive left ventricular hypertrophy (LVH) patients were assessed to determine the prevalence of abnormal results and associations with uremic cardiomyopathy.
Design, setting, participants, & measurements
In this single-center observational study, 200 ESRD and 30 LVH patients underwent assessment including CV history, ECG, cardiac magnetic resonance imaging, and an MTWA exercise test. MTWA results were classified as “negative” or “abnormal” on the basis of previously published reports.
Results
An abnormal MTWA result was more common in ESRD compared with LVH patients (57.5% versus 26.7%, respectively; P = 0.002). In ESRD patients, MTWA was significantly associated with uremic cardiomyopathy, clinical history of atherosclerosis (coronary, cerebral, peripheral) and diabetes mellitus, older age, and hemodialysis therapy. Independent associations with an abnormal MTWA result were older age, macrovascular disease, increased left ventricle (LV) mass, and LV dilation.
Conclusions
Features of uremic cardiomyopathy are associated with an abnormal MTWA result.
Introduction
Patients with end-stage renal disease (ESRD), including those receiving or close to requiring dialysis, have a significantly increased risk of premature cardiovascular (CV) mortality. In contrast to the general population, in which myocardial ischemia and infarction are the leading cause of death, patients with ESRD are more likely to develop cardiac (commonly ventricular) tachyarrhythmias (VTAs) and sudden cardiac death (SCD) (1). Factors associated with SCD in this population include gross myocardial structural abnormalities (left ventricular hypertrophy [LVH], left ventricular systolic dysfunction [LVSD], and left ventricular dilation), collectively called “uremic cardiomyopathy” (2). In addition, accelerated ischemic coronary artery disease (CAD), electrolyte fluctuations, bone mineral disease, uremia, autonomic dysfunction, and inflammation have been implicated in the development of SCD (3–7). Despite identification of potential risk factors, primary prevention of CV death and SCD with conventional strategies has proven ineffective (8–10). Identification of a specific marker for SCD that will provide a therapeutic target is clearly required.
Microvolt T-wave alternans (MWTA; HearTwave II System, Cambridge Heart, Bedford, MA) is a noninvasive technique of assessing abnormal left ventricular repolarization and hence risk of VTA (11). MTWA measures beat-to-beat variations in standard 12-lead electrocardiogram (ECG) T-wave morphology. These data are collected during exercise-induced increase in heart rate and undergo spectral analyses on the basis of established electrophysiological criteria (12–14) to quantify variation at the microvolt level. Several large prospective trials have demonstrated that MTWA analysis is superior or comparable to more invasive electrophysiological methods that predict (negatively and positively) patients at risk of VTA or SCD (15). In the general population, 5.7% of subjects have an abnormal MTWA test (16). In contrast, in patients with ischemic and nonischemic cardiomyopathy and normal renal function, MTWA is not normal in 64% to 72% of patients (14,17), and in diabetic patients without CV or renal disease, MTWA is abnormal in 25% of patients (18).
In order to assess the prevalence and determinants of MTWA in patients with ESRD at risk of SCD, we compared MTWA in patients with ESRD and LVH due to essential hypertension. Using cardiovascular magnetic resonance imaging (CMR), we investigated associations with uremic cardiomyopathy.
Materials and Methods
Patients
The renal transplant unit at the Western Infirmary, Glasgow, provides transplant services to a population of 2.8 million people in the west of Scotland. The transplant waiting list has 300 to 400 patients at any time point; approximately 100 to 120 new patients are waitlisted and approximately 70 adult transplants are performed annually (19). In this study, we evaluated 200 consecutive patients with chronic kidney disease stage 5, including subjects within 6 months of requiring renal replacement therapy (predialysis), who were being assessed for renal transplantation over a 24-month period. A further 30 hypertensive patients with echocardiographic evidence of LVH and normal renal function were recruited from medical outpatient clinics. All patients provided written informed consent and the study was approved by the local ethics committee. All patients underwent CV risk factor assessment including history, clinical examination, ECG, and routine hematologic and biochemical profile. Symptomatic heart failure was defined as presence of New York Heart Failure Association grades 3 or 4. CMR was performed to measure left ventricle (LV) mass and function. All patients underwent MTWA exercise testing within 30 minutes of CMR, and hemodialysis patients were consistently assessed 24 hours after a dialysis session.
CMR Technique and Analysis
Noncontrast CMR was performed using a 1.5-Tesla magnetic resonance imaging scanner (Sonata, Siemens, Erlangan, Germany) as described previously (20). LVH was defined as left ventricular mass index (LVMI; LVMI = LV mass/body surface area [BSA]) >84.1 g/m2 (male) or >76.4 g/m2 (female) and LVSD was defined as left ventricular ejection fraction (LVEF) <55%. LV dilation was defined as end diastolic volume (EDV)/BSA >111.7 ml/m2 (male) or 99.3 ml/m2 (female) or end systolic volume (ESV)/BSA >92.8 ml/m2 (male) or 70.3 ml/m2 (female) (21).
MTWA Testing Protocol
Acquisition of the MTWA result was performed using a technique that has been used reliably and reproducibly in several patient groups (14–17). MTWA was measured at rest and during treadmill exercise. After skin preparation, high-resolution electrodes (Microvolt Sensors, Cambridge Heart, Inc., Bedford, MA) were placed in the standard 12-lead and Frank orthogonal position, and resting ECG data were acquired initially for 20 seconds. Patients then underwent gentle treadmill exercise to increase heart rate (HR) to between 100 and 110 beats per minute (bpm) for 150 seconds, followed by 90 seconds of walking to achieve a HR of 110 to 120 bpm if tolerated. At the end of the exercise period, patients rested until HR was <90 bpm, after which the test was terminated. ECG data were collected digitally and interpreted according to standard criteria using spectral analysis and reported using standard automated classification (HearTwave II system, Cambridge Heart, Bedford, MA). Briefly, a positive MTWA result was defined as presence of sustained alternans (alternans present for at least 1 minutes) at HR <110 bpm on exercise or at rest even if >110 bpm. A negative MTWA test result was defined as the absence of positive test criteria with a maximum HR ≥105 bpm. A test that did not satisfy positive or negative criteria was classified as indeterminate. On the basis of previously published studies, we further classified results as “abnormal” (for positive and indeterminate test results) or “negative” (negative result) (22). If initially an indeterminate result was obtained, immediate retesting was attempted. The analysis software also provided the reason for an indeterminate test.
Coronary Angiography
Coronary angiography was performed as part of CV assessment on a selected basis. When requested, angiography was performed within 3 months of MTWA assessment. Severity of CAD was recorded according to accepted convention (23): (1) normal epicardial vessels; and (2) nonobstructive coronary disease—CAD but no stenosis >75%—(1 and 2 classified in our study as “normal/nonobstructive”); (3) single-vessel disease (stenosis >75% in one major epicardial vessel); (4) two-vessel disease (stenoses >75% in two major epicardial vessels); and (5) left main or three-vessel disease (3 to 5 classified as “obstructive”).
Exclusion Criteria
Patients with contraindication to CMR (presence of permanent pacemaker or ferromagnetic implants, severe claustrophobia, pregnancy) were not entered into the study. Furthermore, patients with atrial fibrillation were excluded because irregular R-R intervals impair frequency analysis during MTWA testing.
Statistical Analyses
Statistical analyses were performed using SPSS version 15.0 (SPSS, Inc., Chicago, IL). Data are described as mean and SD for parametric data or median and interquartile range for nonparametric data. Comparison was made for patients with ESRD to identify differences between patients with negative and abnormal MTWA by χ2 test for categorical data and t test or Mann–Whitney U test for continuous data as appropriate. Variables identified by univariate logistic regression analysis were entered into a backward stepwise multivariate logistic regression model to identify independent predictors of an abnormal MTWA result in ESRD patients. Survival data including survival time (mean ± SD) are shown as Kaplan–Meier graphs (with statistical comparison using the log rank test).
Sample size for the LVH group was primarily based on comparison of MTWA results because our original aim was to assess the additional effect of uremia and uremic cardiomyopathy on ventricular electrophysiology. Having assessed 200 ESRD patients and assuming a prevalence of an abnormal MTWA result in LVH patients similar to diabetics (approximately 25%), we calculated a power of >90% if 30 controls were assessed (with a 0.05 probability of type 1 error).
Results
Baseline Clinical Parameters
Two-hundred ESRD patients were assessed (71% male; mean age 56.3 ± 12.7 years) and eight patients were excluded (six because of claustrophobia during CMR and two because of presence of atrial fibrillation). The baseline patient demographics are shown in Table 1. The primary renal diseases were diabetic nephropathy in 46 (23%), autosomal dominant polycystic disease in 20 (10%), GN in 42 (21%), renovascular disease in 16 (8%), and chronic pyelonephritis in 20 (10%) of the patients recruited. Other diagnoses accounted for 26 (13%) of the patients and in 30 patients (15%) cause of renal disease was unknown. One-hundred and thirty five (67.5%) patients were established on renal replacement therapy (59% on thrice-weekly maintenance hemodialysis, 8.5% on peritoneal dialysis) and 32.5% of patients were predialysis. There was a high prevalence of traditional CV risk factors in ESRD patients: 24.5% had a past medical history of ischemic heart disease (IHD), 13% had a history of cerebrovascular disease, and 17.5% had a history of peripheral vascular disease. Twenty-seven percent of patients were diabetic, 55.5% were former or current smokers, and 58.5% had hypercholesterolemia. Only 7.5% had a history of symptomatic chronic heart failure. Drug therapy and baseline blood results at the time of assessment are also shown.
Table 1.
Comparisons of clinical, blood, and cardiac data of ESRD patients on the basis of MTWA result
| Variable | Total (n = 200) | MTWA Negative, n = 85 (42.5) | MTWA Abnormal, n = 115 (57.5) | P |
|---|---|---|---|---|
| Age (years) | 56.3 (12.7) | 52.4 (12.8) | 59.1 (12.0) | <0.001 |
| Male (%) | 142 (71) | 59 (69.4) | 83 (72.2) | 0.67 |
| BMI (kg/m2) | 26.5 (4.7) | 26.9 (4.8) | 26.2 (4.6) | 0.29 |
| BSA (m2) | 1.88 (0.2) | 1.89 (0.2) | 1.87 (0.2) | 0.45 |
| Systolic BP (mmHg) | 146 (24.1) | 144 (23.2) | 147 (24.8) | 0.29 |
| Diastolic BP (mmHg) | 82 (14.2) | 83 (12.9) | 82 (15.2) | 0.52 |
| RRT time | 1.1 (0.6, 2.5) | 1.2 (0,6, 2.4) | 1.1 (0.6, 2.6) | 0.67 |
| RRT | ||||
| hemodialysis | 118 (59.0) | 41 (48.2) | 77 (67.0) | 0.008 |
| peritoneal dialysis | 17 (8.5) | 11 (12.9) | 6 (5.2) | 0.09 |
| predialysis | 65 (32.5) | 33 (38.8) | 32 (27.8) | 0.10 |
| Diabetes mellitus | 54 (27.0) | 16 (18.8) | 38 (33.0) | 0.03 |
| IHD | 49 (24.5) | 8 (9.4) | 41 (35.7) | <0.001 |
| Hypertension | 178 (89.0) | 73 (85.9) | 105 (91.3) | 0.23 |
| Heart failure | 15 (7.5) | 4 (4.7) | 11 (9.6) | |
| Cerebrovascular disease | 26 (13) | 3 (3.5) | 23 (20) | 0.001 |
| Peripheral vascular disease | 35 (17.5) | 4 (4.7) | 31 (27) | <0.001 |
| Hypercholesterolemia | 117 (58.5) | 42 (49.4) | 75 (65.2) | 0.03 |
| Smoking | ||||
| never | 89 (44.5) | 45 (52.9) | 44 (38.3) | 0.12 |
| current/former | 111 (55.5) | 40 (47) | 71.34 (61.8) | 0.20 |
| Hemoglobin (g/dl) | 11.4 (1.8) | 11.3 (1.7) | 11.4 (1.9) | 0.96 |
| ESR (mm/h) | 29 (9.5, 49.0) | 28 (8, 37) | 31 (14.5, 44.0) | 0.74 |
| CRP (mg/L) | 4.9 (2.0, 9.9) | 4.7 (1.8, 8.9) | 5.3 (3.0, 44.0) | 0.08 |
| Potassium (mmol/L) | 4.7 (0.7) | 4.7 (0.7) | 4.7 (0.8) | 0.76 |
| Adjusted calcium (mmol/L) | 2.39 (0.3) | 2.39 (0.4) | 2.40 (0.2) | 0.87 |
| Phosphate (mmol/L) | 1.61 (0.5) | 1.69 (0.5) | 1.56 (0.5) | 0.08 |
| Glucose (mmol/L) | 5.5 (4.7, 7.7) | 5.3 (4.6, 6.3) | 5.9 (4.7, 10.6) | 0.02 |
| Creatinine (μmol/L) | 612 (227.5) | 630 (248.4) | 598 (210.3) | 0.33 |
| β-adrenoceptor blocker | 92 (46.0) | 26 (30.6) | 66 (57.4) | <0.001 |
| Aspirin | 102 (51.0) | 41 (48.2) | 61 (53.0) | 0.50 |
| Warfarin | 13 (6.5) | 1 (1.2) | 12 (10.4) | 0.01 |
| Clopidogrel | 17 (8.5) | 3 (3.5) | 14 (12.2) | 0.03 |
| ACEI/ARB | 91 (45.5) | 41 (47.1) | 51 (44.3) | 0.25 |
| Diuretic | 58 (29) | 29 (34.1) | 29 (25.2) | 0.17 |
| Nitrate | 15 (7.5) | 3 (3.5) | 12 (10.4) | 0.07 |
| Calcium channel blocker | 76 (38.0) | 28 (32.9) | 48 (41.7) | 0.21 |
| α-adrenoceptor blocker | 24 (12.0) | 12 (14.1) | 12 (10.4) | 0.43 |
| Statin | 125 (67.5) | 46 (54.1) | 79 (68.7) | 0.04 |
| EF (%) | 61.9 (15.3) | 65.1 (11.4) | 59.5 (17.3) | 0.01 |
| Myocardial mass/BSA (g/m2) | 93.3 (38.2) | 84.9 (33.1) | 99.7 (40.6) | 0.008 |
| EDV/BSA (ml/m2) | 68.9 (35.5) | 60.6 (22.6) | 75.2 (41.8) | 0.004 |
| ESV/BSA (ml/m2) | 28.7 (26.2) | 22.4 (14.2) | 33.5 (31.7) | 0.003 |
| SV/BSA (ml/m2) | 40.1 (18.7) | 38.2 (13.5) | 41.6 (21.9) | 0.21 |
| LVH on MRI | 124 (62.0) | 46 (54.1) | 78 (67.8) | 0.05 |
| LVSD on MRI (EF < 55%) | 45 (22.5) | 14 (16.5) | 31 (27.0) | 0.08 |
| LV dilation | 19 (9.5) | 2 (2.4) | 17 (14.8) | 0.003 |
| CAD severity (n = 60) | ||||
| normal/nonobstructive | 25 (41.7) | 15 (60.0) | 10 (40.0) | 0.23 |
| obstructive | 35 (53.3) | 12 (34.2) | 23 (65.8) |
Data are number with percentage in parentheses and mean ± SD except for RRT time, ESR, CRP, and glucose, where median and interquartile ranges are shown. Tests of significance are t test, Mann–Whitney U test, and χ2 as appropriate. BMI, body mass index; RRT, renal replacement therapy; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor II blocker; EF, ejection fraction; SV/BSA, BSA-corrected stroke volume.
CMR data are also presented in Table 1. LV function was preserved with a mean ejection fraction of 61.9 ± 15.3% and only 22.5% of patients had CMR evidence of LVSD. Sixty-two percent of patients had LVH on CMR (mean LVMI = 93.3 ± 38.2 g/m2) and 9.5% had LV dilation (mean EDV/BSA = 68.9 ± 35.5 ml/m2 and mean ESV/BSA = 28.7 ± 26.2 ml/m2).
Sixty patients underwent coronary angiography as part of their CV assessment. Twelve patients (20.0%) had no evidence of epicardial CAD, 13 (21.7%) had nonobstructive CAD, 19 (31.7%) had single-vessel CAD, 12 (20.0%) had two-vessel disease, and 4 (6.7%) had three-vessel or left main stem disease.
Comparison between ESRD and LVH Patients
There were no differences between age, sex, body mass index, and systolic BP between each group (Table 2). Diastolic BP was higher in the LVH group compared with the ESRD group, probably reflecting increased vascular stiffness in ESRD (24). As one would expect, there was a higher frequency of traditional CV risk factors in ESRD patients compared with patients with LVH. This difference was also reflected in the medications these patients were taking. Furthermore, serum phosphate was higher in ESRD compared with LVH patients.
Table 2.
Comparisons between ESRD and LVH patients
| Variable | ESRD (n = 200) | LVH Only (n = 30) |
|---|---|---|
| MTWA abnormala | 115 (57.5) | 8 (26.7) |
| Age (years) | 56.3 (12.7) | 53.1 (10.7) |
| Male (%) | 142 (71) | 25 (83.3) |
| BMI (kg/m2) | 26.5 (4.7) | 27.1 (4.9) |
| Systolic BP (mmHg) | 146 (24.1) | 152 (22.5) |
| Diastolic BP (mmHg)a | 82 (14.2) | 89 (11.1) |
| Diabetes mellitus | 54 (27.0) | 0 |
| IHD | 49 (24.5) | 2 (9.5) |
| Heart failure | 15 (7.5) | 1 (3.3) |
| Cerebrovascular disease | 26 (13) | 1 (3.3) |
| Peripheral vascular disease | 35 (17.5) | 0 |
| Dyslipidemia | 117 (58.5) | 10 (33.3) |
| Smoking | ||
| never | 89 (44.5) | 19 (61.9) |
| current/former | 111 (55.5) | 11 (38.1) |
| Epo receptor agonist | 162 (81.0) | 0 |
| β-Adrenoceptor blocker | 92 (46.0) | 10 (33.3) |
| Aspirin | 102 (51.0) | 3 (10) |
| Warfarin | 13 (6.5) | 0 |
| Clopidogrel | 17 (8.5) | 0 |
| ACEI/ARB | 91 (45.5) | 12 (40.0) |
| Diuretic | 58 (29) | 6 (20.0) |
| Nitrate | 15 (7.5) | 8 (26.7) |
| Calcium channel blocker | 76 (38.0) | 8 (26.7) |
| α-Adrenoceptor blocker | 24 (12.0) | 1 (3.3) |
| Statin | 125 (62.5) | 9 (30.0) |
| Hemoglobin (g/dl) | 11.4 (1.8) | 11.4 (1.8) |
| ESR (mm/h) | 29 (9.5, 49.0) | 31 (14.5, 44.0) |
| CRP (mg/L) | 4.9 (2.0, 9.9) | 5.0 (3.2, 16.0) |
| Potassium (mmol/L) | 4.7 (0.7) | 4.7 (0.8) |
| Adjusted calcium (mmol/L) | 2.39 (0.3) | 2.41 (0.1) |
| Phosphate (mmol/L)a | 1.61 (0.5) | 1.10 (0.1) |
| Glucose (mmol/L) | 5.5 (4.7, 7.7) | 5.1 (4.6, 6.1) |
| CMR results | ||
| EF (%) | 61.9 (15.3) | 65.8 (11.5) |
| myocardial mass/BSA (g/m2) | 93.3 (38.2) | 82.0 (32.9) |
| EDV/BSA (ml/m2 ) | 68.9 (35.6) | 67.1 (13.7) |
| ESV/BSA (ml/m2 ) | 28.7 (26.2) | 23.5 (12.4) |
| LVH on MRI | 124 (62.0) | 18 (60.0) |
| LVSD on MRI (EF < 55%) | 45 (22.5) | 4 (13.8) |
| LV dilation | 19 (9.5) | 2 (6.9) |
Data are number with percentage in parentheses or mean ± SD except for ESR, CRP, and glucose, where median and interquartile range are shown. Tests of significance are t test, χ2, or Fischer exact test as appropriate.
P < 0.05.
LV systolic function was preserved in both groups. CMR demonstrated a lower ejection fraction, higher LVMI, and higher LV chamber size in the ESRD group compared with the LVH patients. Presence of LVH was high in both groups and despite LVH patients being recruited based on previous echocardiography, only 18 (60%) had LVH on CMR.
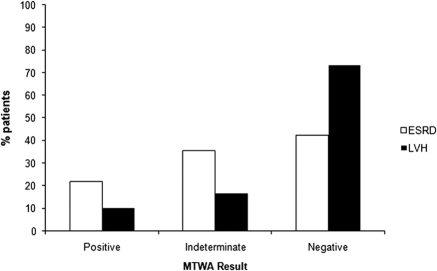
An abnormal MTWA result was significantly more common in ESRD patients compared with LVH patients (57.5% ESRD versus 26.7% LVH; P = 0.002). On comparison of patients with LVH on CMR only (n = 18), an abnormal MTWA result was similarly higher in ESRD compared with LVH patients (27.8% LVH on CMR; P = 0.02). MTWA (Figure 1) was negative in 85 (42.5%), positive in 44 (22%), and indeterminate in 71 (35.5%) of patients with ESRD. In the LVH group, MTWA was negative in 22 (73.3%), positive in 3 (10%), and indeterminate in 5 (16.7%) of the 30 patients assessed (Figure 1).
Figure 1.
Bar chart showing MTWA result for ESRD and LVH patients.
Reasons for indeterminate tests in ESRD patients were failure to achieve HR between 105 and 110 bpm for ≥1 minute (50.7% of all indeterminate results), excessive ventricular ectopy during exercise (23.9%), a noisy recording (21.1%), and rapid rise to a target HR of 105 to 110 bpm or unsustained MTWA (≤1 minute; 4.2%). In LVH patients, indeterminate tests were due to noisy recording (two tests; 40%), rapid rise to a target HR of 105 to 110 bpm or unsustained MTWA (two tests; 40%), and excessive ectopy (one test; 20%).
Comparisons on the Basis of MTWA Result
In the ESRD group, abnormal MTWA was more prevalent in older patients and those with vascular diseases (Table 1). In patients with diabetic nephropathy, 28.7% had abnormal MTWA (versus 15.3% negative MTWA; P = 0.02). In addition, abnormal MTWA was more common in patients receiving warfarin, clopidogrel, or statin therapy.
An abnormal MTWA result was significantly more common in hemodialysis patients (n = 118, abnormal MTWA 67.0% versus negative MTWA 48.2%; P = 0.008). However, there was no significant difference in abnormal MTWA prevalence for peritoneal dialysis (abnormal MTWA 12.9% versus negative MTWA 5.2%; P = 0.09) or predialysis patients (abnormal MTWA 27.8% versus negative MTWA 38.8%; P = 0.10).
To assess the effect of electrolytes on MTWA, venepuncture was performed 10 to 15 minutes before testing (Table 1). There was no significant difference between measured cations (or hemoglobin, phosphate, and inflammatory markers) between patients with or without abnormal MTWA. Random plasma glucose was significantly higher in patients with negative and abnormal MTWA results, reflecting the higher proportion of diabetic patients in this group.
We assessed associations between LV structural abnormalities of uremic cardiomyopathy and MTWA result (Table 1). Abnormal MTWA was significantly associated with abnormalities of uremic cardiomyopathy: higher LV mass, lower ejection fraction, and higher EDV/BSA and ESV/BSA. There were also significantly higher proportions of patients with LVH and LV dilation in the abnormal MTWA group. More patients had LVSD in the abnormal MTWA group, but this did not achieve sufficient statistical significance (P = 0.08). In ESRD patients with no evidence of structural heart disease (n = 68), abnormal MTWA prevalence remained high (32 patients; 47.1%).
Presence of obstructive CAD (n = 35) on coronary angiography was associated with a higher prevalence of an abnormal MTWA result compared with normal/nonobstructive patients (n = 25) (Table 1: obstructive group abnormal MTWA 65.8% versus normal/nonobstructive group abnormal MTWA 40.0%; P = 0.23), although this did not reach statistical significance.
Variables Associated with Abnormal MTWA Result in ESRD Patients
Multivariate logistic regression analyses (backward stepwise) were performed to determine variables independently associated with abnormal MTWA result in ESRD patients only (Table 3). In our model (R2 = 0.40), entering only factors found to be significant after univariate analyses, increasing age and past clinical history of coronary, peripheral vascular, and cerebrovascular diseases were independently associated with abnormal MTWA. In addition, increasing LVMI (or presence of LVH) was independently associated with abnormal MTWA. Although LV dilation was associated with abnormal MTWA, it did not achieve statistical significance in the multivariate model.
Table 3.
Simple (left) followed by backward stepwise multiple logistic regression analyses (R2 = 0.40) demonstrating independent associations with of abnormal MTWA result
| Variable | Univariate Analyses |
Multivariate Analyses |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (per year) | 1.04 | 1.02, 1.07 | <0.01 | 1.04 | 1.01, 1.07 | 0.01 |
| IHD | 6.19 | 2.74, 13.94 | <0.01 | 3.24 | 1.31, 8.02 | 0.01 |
| Cerebrovascular disease | 6.83 | 1.98, 23.60 | <0.01 | 5.81 | 1.56, 12.87 | <0.01 |
| Peripheral vascular disease | 7.47 | 2.53, 22.11 | <0.01 | 5.34 | 1.68, 16.96 | <0.01 |
| LVMI (per g/m2) | 1.01 | 1.00, 1.02 | 0.01 | 1.02 | 1.01, 1.02 | 0.02 |
| LV dilation | 7.20 | 1.62, 32.07 | 0.01 | 4.34 | 0.98, 21.90 | 0.06 |
| Glucose (per mmol/L) | 1.10 | 1.02, 1.19 | 0.01 | |||
| EF (per %) | 0.98 | 0.95, 0.98 | 0.01 | |||
| Diabetes mellitus | 2.12 | 1.09, 4.15 | 0.03 | |||
| Hypercholesterolemia | 1.92 | 1.08, 3.40 | 0.03 | |||
| RRT | ||||||
| predialysis | 1.00 | |||||
| peritoneal dialysis | 0.57 | 0.19, 1.70 | 0.31 | |||
| hemodialysis | 1.93 | 1.05, 3.59 | 0.04 | |||
| RRT time (per year) | 1.10 | 0.91, 1.33 | 0.30 | |||
| Gender (male versus female) | 1.06 | 0.59, 1.90 | 0.84 | |||
| BMI (per kg/m2) | 0.97 | 0.92, 1.03 | 0.36 | |||
| Systolic BP (per mmHg) | 1.01 | 0.99, 1.02 | 0.07 | |||
| Diastolic BP (mmHg) | 0.97 | 0.95, 1.00 | 0.06 | |||
| Pulse pressure | 1.02 | 0.99, 1.03 | 0.07 | |||
| Chronic heart failure | 2.14 | 0.65, 6.97 | 0.21 | |||
| Smoking | ||||||
| never | 1.00 | |||||
| current/former | 1.73 | 0.88, 3.47 | 0.12 | |||
| Hemoglobin (per g/dl) | 1.02 | 0.86, 1.18 | 0.96 | |||
| Fibrinogen (per g/L) | 0.92 | 0.70, 1.23 | 0.58 | |||
| ESR (per mm/h) | 0.99 | 0.98, 1.01 | 0.83 | |||
| CRP (per mg/L) | 1.01 | 0.99, 1.02 | 0.62 | |||
| Adjusted calcium (per mmol/L) | 1.08 | 0.41, 2.85 | 0.86 | |||
| Phosphate (per mmol/L) | 0.60 | 0.34, 1.07 | 0.08 | |||
| PTH (per pmol/L) | 0.99 | 0.98, 1.01 | 0.42 | |||
| HbA1c (per %) | 0.99 | 0.98, 1.01 | 0.48 | |||
| Potassium (per mmol/L) | 0.94 | 0.63, 1.40 | 0.76 | |||
| Cholesterol (per mmol/L) | 0.60 | 0.32, 1.17 | 0.13 | |||
| Triglyceride (per mmol/L) | 1.12 | 0.69, 1.81 | 0.67 | |||
| HDL-cholesterol (per mmol/L) | 1.34 | 0.70, 2.57 | 0.38 | |||
| LDL-cholesterol (per mmol/L) | 1.17 | 0.63, 2.16 | 0.62 | |||
| Creatinine (per μmol/L) | 0.99 | 0.99 to 1.00 | 0.33 | |||
| EDV/BSA (ml/m2) | 1.00 | 0.98, 1.02 | 0.64 | |||
| ESV/BSA (ml/m2) | 1.02 | 0.98, 1.04 | 0.21 | |||
| LVSD | 1.87 | 0.93, 3.79 | 0.08 | |||
| CAD | ||||||
| obstructive CAD (reference) | 1.00 | |||||
| normal/nonobstructive | 2.08 | 0.53 to 6.60 | 0.23 | |||
Only variables found to be significant on univariate analyses were entered into the multivariate model. OR, odds ratio; CI, confidence interval; PTH, parathyroid hormone; HbA1c, hemoglobin A1c.
Discussion
Sudden cardiac death is the most common cause of premature CV death in ESRD patients. In recent reports from the U.S. Renal Data System, there were 215.3 deaths per 1000 patient-years in all patients on renal replacement therapy. Cardiac diseases accounted for 39.7% of deaths, and of these, 66.1% were caused by cardiac arrhythmia or arrest (1). Patients with ESRD have similar rates (161.0 deaths per 1000 patient-years, 38.0% cardiac deaths, 66.0% due to arrhythmia or cardiac arrest) regardless of mode of therapy. Although very few prospective studies have identified agents that reduce SCD in ESRD patients, those using implantable cardioverter defibrillator (ICD) insertion indicate a possible benefit if patient selection is appropriate. In a study of dialysis patients that survived cardiac arrest, ICD insertion was associated with a 42% reduction in risk (25). Furthermore, patients with ESRD are more likely to experience appropriate ICD shock compared with those with no renal disease (26).
Many prospective studies have evaluated MTWA as a predictive tool for SCD or VTAs (15). These studies, performed in patients with ischemic and nonischemic LV systolic dysfunction, have demonstrated strong negative and positive predictive power (similar to invasive electrophysiological testing) of MTWA results and all-cause mortality. Initially, MTWA was considered an ideal method of noninvasively identifying those patients with reduced LV function (ejection fraction <30% to 35%) who would benefit from primary ICD implantation. However, two more recent prospective studies (MASTER and MTWA substudy SCD-HeFT) concluded that an abnormal MTWA result was not significantly associated with ICD-detected VTAs or able to independently predict VTA/mortality in patients with ischemic cardiomyopathy (27,28). Contrasting results from these heart failure studies suggest that MTWA may be useful for predicting VTAs if appropriate patient selection criteria are implemented.
To this end, we performed a study using MTWA to assess the prevalence and significant associations with an abnormal MTWA result in ESRD patients. This study shows that an abnormal MTWA test result is common (57.5%) in ESRD patients. The prevalence is similar to previous heart failure and cardiomyopathy studies (14,17), although the patients in our cohort had preserved LV function on CMR (ejection fraction 61.9 ± 15.3%). Nonetheless, when compared with studies in patients with diabetes mellitus (approximately 25%) or healthy individuals (approximately 6%), ESRD patients have a higher rate of abnormal results (16,18).
These data show higher prevalence of abnormal MTWA in ESRD patients compared with hypertensive LVH patients. We chose to evaluate patients with hypertensive LVH and normal renal function as controls to determine any additional effect of uremia in addition to cardiac hypertrophy. An earlier study comparing MTWA results between patients with hypertrophic cardiomyopathy and hypertensive LVH patients found a much higher prevalence of a positive test result (31%) in LVH patients compared with the data presented here (29). However, this study used different MTWA exercise protocols (only HR up to 110 bpm were performed) and older classification criteria (indeterminate tests were not reported).
In ESRD patients, the abnormalities of uremic cardiomyopathy are common and independently predict CV morbidity and mortality (2,30). This study demonstrates independent associations between features of uremic cardiomyopathy (elevated LVMI, LV dilation) and abnormal MTWA result. As stated earlier, patients with other cardiomyopathies have an abnormal MTWA result prevalence between 65% and 78%, and it follows that nonuniform repolarization is more likely to occur in the presence of atypical myocardial cytoarchitecture (e.g., LVH, myocardial scarring) (31). Cardiac action potential (AP) propagation is also interrupted by areas of myocardial ischemia (32) caused by accelerated macro- and microvascular coronary disease, which are common in ESRD patients. Thus, the MTWA result may provide a useful indicator of patients with uremic cardiomyopathy at risk of SCD.
Furthermore, in ESRD patients with normal myocardial structure, the abnormal MTWA result was high (47.1%), suggesting that abnormal ventricular repolarization may occur independently of development of cardiac structural changes. Presumably this is due to abnormalities of intracellular electrolyte control or the effect of uremia on cardiomyocyte membrane recovery.
Older age was independently associated with an abnormal MTWA test as has been demonstrated in previous studies of patients with ischemic and nonischemic cardiomyopathy (15). Presence of an abnormal MTWA result was also independently associated with established macrovascular atheromatous disease (i.e., coronary, peripheral, cerebrovascular disease), presumably reflecting these patients' higher risk of CV morbidity and mortality. These findings are supported by our coronary angiography results, which demonstrate an association between abnormal MTWA result and higher prevalence of obstructive CAD, although this did not reach statistical significance. Diabetic patients with ESRD had a significantly higher rate of abnormal tests, and this most likely represents their additional (and usually silent) CV morbidity. Interestingly, patients receiving maintenance hemodialysis had a significantly higher rate of an abnormal test. All of these patients were established on renal replacement therapy for 6 months, removing early elevated SCD risk associated with hemodialysis commencement that has previously been demonstrated (33). In addition, testing was performed 24 hours after the end of the patients' last hemodialysis dose, thereby removing the effect of rapid postdialysis cation diffusion.
Our rate of indeterminate tests was high (35.5%), and a large proportion of these patients were unable to achieve adequate HR elevation, reflecting poor exercise tolerance of ESRD patients which has previously been demonstrated (34). Some prospective trials (35,36) have demonstrated an indeterminate result alone as a significant independent predictor of death (arrhythmic and nonarrhythmic), but the relevance in ESRD patients remains to be assessed.
In conclusion, this study demonstrates a significant association between an abnormal MTWA result and uremic cardiomyopathy. Furthermore, underlying atherosclerosis is also associated with an abnormal MWTA result. MTWA may identify ESRD patients at high risk of cardiac arrhythmia and SCD and allow identification of subjects amenable to future interventional studies.
Disclosures
None.
Acknowledgments
The study was supported by the Darlinda's Charity for Renal Research. The British Heart Foundation provides funding for Rajan Patel (FS/08/030/24993) and Patrick Mark.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2009 [Google Scholar]
- 2. Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE: Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11: 1277–1285, 1996 [PubMed] [Google Scholar]
- 3. Herzog CA, Strief JW, Collins AJ, Gilbertson DT: Cause-specific mortality of dialysis patients after coronary revascularization: Why don't dialysis patients have better survival after coronary intervention? Nephrol Dial Transplant 23: 2629–2633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bleyer AJ, Russell GB, Satko SG: Sudden and cardiac death rates in hemodialysis patients. Kidney Int 55: 1553–1559, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Zoccali C, Mallamaci F, Tripepi G, Parlongo S, Cutrupi S, Benedetto FA, Cataliotti A, Malatino LS: Norepinephrine and concentric hypertrophy in patients with end-stage renal disease. Hypertension 40: 41–46, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez-Benot A, Martin-Malo A, Alvarez-Lara MA, Rodriguez M, Aljama P: Mild hyperphosphatemia and mortality in hemodialysis patients. Am J Kidney Dis 46: 68–77, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Stenvinkel P: Inflammation in end-stage renal disease: The hidden enemy. Nephrology (Carlton) 11: 36–41, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F: Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Cice G, Ferrara L, D'Andrea A, D'Isa S, Di Benedetto A, Cittadini A, Russo PE, Golino P, Calabro R: Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: A prospective, placebo-controlled trial. J Am Coll Cardiol 41: 1438–1444, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Zannad F, Kessler M, Lehert P, Grunfeld JP, Thuilliez C, Leizorovicz A, Lechat P: Prevention of cardiovascular events in end-stage renal disease: Results of a randomized trial of fosinopril and implications for future studies. Kidney Int 70: 1318–1324, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Estes NA, III, Michaud G, Zipes DP, El Sherif N, Venditti FJ, Rosenbaum DS, Albrecht P, Wang PJ, Cohen RJ: Electrical alternans during rest and exercise as predictors of vulnerability to ventricular arrhythmias. Am J Cardiol 80: 1314–1318, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Richter S, Duray G, Hohnloser SH: How to analyze T-wave alternans. Heart Rhythm 2: 1268–1271, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Bloomfield DM, Hohnloser SH, Cohen RJ: Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol 13: 502–512, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, Kaufman ES, Davidenko JM, Shinn TS, Fontaine JM: Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 47: 456–463, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Gehi AK, Stein RH, Metz LD, Gomes JA: Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: A meta-analysis. J Am Coll Cardiol 46: 75–82, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Furlanello F, Galanti G, Manetti P, Capalbo A, Pucci N, Michelucci A, Marangoni D, Terrasi F, Pettinati G, Cappato R: Microvolt T-wave alternans as predictor of electrophysiological testing results in professional competitive athletes. Ann Noninvasive Electrocardiol 9: 201–206, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow T, Kereiakes DJ, Bartone C, Booth T, Schloss EJ, Waller T, Chung ES, Menon S, Nallamothu BK, Chan PS: Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll Cardiol 47: 1820–1827, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Molon G, Costa A, Bertolini L, Zenari L, Arcaro G, Barbieri E, Targher G: Relationship between abnormal microvolt T-wave alternans and poor glycemic control in type 2 diabetic patients. Pacing Clin Electrophysiol 30: 1267–1272, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Scottish Renal Registry. Available at: www.srr.scot.nhs.uk Accessed February 12, 2010
- 20. Stewart GA, Mark PB, Johnston N, Foster JE, Cowan M, Rodger RS, Dargie HJ, Jardine AG: Determinants of hypertension and left ventricular function in end stage renal failure: A pilot study using cardiovascular magnetic resonance imaging. Clin Physiol Funct Imaging 24: 387–393, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU: Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging 17: 323–329, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Salerno-Uriarte JA, De Ferrari GM, Klersy C, Pedretti RF, Tritto M, Sallusti L, Libero L, Pettinati G, Molon G, Curnis A, Occhetta E, Morandi F, Ferrero P, Accardi F: Prognostic value of T-wave alternans in patients with heart failure due to nonischemic cardiomyopathy: Results of the ALPHA Study. J Am Coll Cardiol 50: 1896–1904, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, Pijls NH, Siebes M, Spaan JA: Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: A scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 114: 1321–1341, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Yildiz A, Memisoglu E, Oflaz H, Yazici H, Pusuroglu H, Akkaya V, Erzengin F, Tepe S: Atherosclerosis and vascular calcification are independent predictors of left ventricular hypertrophy in chronic haemodialysis patients. Nephrol Dial Transplant 20: 760–767, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Herzog CA, Li S, Weinhandl ED, Strief JW, Collins AJ, Gilbertson DT: Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int 68: 818–825, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Robin J, Weinberg K, Tiongson J, Carnethon M, Reddy M, Ciaccio C, Quadrini M, Hsu J, Fan J, Choi P, Kadish A, Goldberger J, Passman R: Renal dialysis as a risk factor for appropriate therapies and mortality in implantable cardioverter-defibrillator recipients. Heart Rhythm 3: 1196–1201, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Chow T, Kereiakes DJ, Onufer J, Woelfel A, Gursoy S, Peterson BJ, Brown ML, Pu W, Benditt DG: Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. J Am Coll Cardiol 52: 1607–1615, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Gold MR, Ip JH, Costantini O, Poole JE, McNulty S, Mark DB, Lee KL, Bardy GH: Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: Primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation 118: 2022–2028, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Francis D, Lane R, Mayet J, Foale RA, Thom S, Peters NS: Microvolt T wave alternans in patients with hypertension and left ventricular hypertrophy. J Hum Hypertens 15[Suppl 1]: S95–S96, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Krane V, Heinrich F, Meesmann M, Olschewski M, Lilienthal J, Angermann C, Stork S, Bauersachs J, Wanner C, Frantz S: Electrocardiography and outcome in patients with diabetes mellitus on maintenance hemodialysis. Clin J Am Soc Nephrol 4: 394–400, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS: Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 99: 1385–1394, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Wyse DG, Friedman PL, Brodsky MA, Beckman KJ, Carlson MD, Curtis AB, Hallstrom AP, Raitt MH, Wilkoff BL, Greene HL: Life-threatening ventricular arrhythmias due to transient or correctable causes: High risk for death in follow-up. J Am Coll Cardiol 38: 1718–1724, 2001 [DOI] [PubMed] [Google Scholar]
- 33. U.S. Renal Data System: USRDS 2002 Annual Data Report. Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2002 [Google Scholar]
- 34. Sharma R, Pellerin D, Gaze DC, Gregson H, Streather CP, Collinson PO, Brecker SJ: Dobutamine stress echocardiography and the resting but not exercise electrocardiograph predict severe coronary artery disease in renal transplant candidates. Nephrol Dial Transplant 20: 2207–2214, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Kaufman ES, Bloomfield DM, Steinman RC, Namerow PB, Costantini O, Cohen RJ, Bigger JT, Jr: “Indeterminate” microvolt T-wave alternans tests predict high risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 48: 1399–1404, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Chan PS, Bartone C, Booth T, Kereiakes D, Chow T: Prognostic implication of redefining indeterminate microvolt T-wave alternans studies as abnormal or normal. Am Heart J 153: 523–529, 2007 [DOI] [PubMed] [Google Scholar]