Summary
Background and objectives
Bilirubin is a protective factor with antioxidant and anti-inflammatory properties, but its association with clinical outcomes of hemodialysis patients is unknown. Bilirubin degradation is mainly determined by the activity of hepatic bilirubin uridine diphosphate-glucuronosyltransferase (UGT1A1), which is significantly influenced by a TA-repeat polymorphism in the gene's promoter, an allele designated UGT1A1*28. The study aimed to clarify the association between serum bilirubin and UGT1A1*28 polymorphism and their respective effect on outcomes of chronic hemodialysis patients.
Design, setting, participants, & measurements
The cohort study comprised 661 chronic hemodialysis patients who were prospectively followed for 12 years. The endpoints were cardiovascular events (CVEs) and all-cause mortality.
Results
After adjustment for traditional and dialysis-related risk factors, individuals with bilirubin in the upper tertile had an adjusted hazard ratio of 0.32 for CVEs and 0.48 for all-cause mortality compared with those in the lower tertile. Individuals homozygous for UGT1A1*28 (genotype 7/7) had significantly higher bilirubin levels than those with 6/6 and 7/6 genotypes. In the same multivariable-adjusted model, individuals with 7/7 had approximately one tenth the risk for CVEs and one fourth the risk for all-cause mortality as compared with carriers of the 6 allele.
Conclusions
A graded, reverse association was noted between serum bilirubin and adverse outcomes among chronic hemodialysis patients. Moreover, the UGT1A1*28 polymorphism had strong effects on bilirubin levels and the 7/7 genotype might have an important effect on reducing CVEs and death.
Introduction
The effect of ESRD on public health and health care economics has been a global focus for years. Age-standardized cardiovascular (CV) and non-CV mortality among dialysis patients were, respectively, 8.8- and 8.1-fold higher than in the general population (1). The combined effect of high vascular morbidity and the presence of chronic inflammation and oxidative stress (2,3) may render ESRD patients more prone to develop excessive risks of CV events and death. Although ESRD patients demonstrate a high prevalence of traditional risk factors, the mechanisms underlying the development of complications still remain obscure. Therefore, it is pivotal to recognize a predictive biomarker for CV morbidity and mortality that could be conveniently measured, and allow better identification of high-risk groups.
Bilirubin has both antioxidant (4,5) and anti-inflammatory (6,7) properties. The antioxidant and antiatherogenic effects of bilirubin are thought to result from its ability to inhibit the oxidation of LDL and other lipids (8,9), scavenge oxygen radicals (4), and counteract oxidative stress (10,11). In 1994, a possible role of bilirubin in atherosclerotic vascular disease was first suggested in studies showing an inverse relationship of serum total bilirubin concentrations and risk of coronary artery disease (CAD) (12). Similar inverse associations have now been shown between serum bilirubin concentrations and coronary heart disease, peripheral vascular disease, and stroke (13–15).
Recent independent genome-wide linkage scans have identified a major locus in the chromosomal 2q telomere controlling serum bilirubin concentrations (16,17). The identified chromosomal region harbors the uridine diphosphate-glucuronosyltransferase (UGT1A1) gene. UGT1A1 is the only enzyme that contributes substantially to bilirubin glucuronidation, and consequently, it is the main determinant of bilirubin elimination in humans. A common cause of decreased UGT1A1 activity is the insertion of a TA in the TATAA box in the promoter region of the UGT1A1 gene, designated UGT1A1*28 (18). Individuals homozygous for 7 repeats (7/7) have higher levels of serum bilirubin than heterozygotes (7/6) or those with the wild type of 6 repeats (6/6) (18–20). It is noteworthy that ethnic differences do exist with regard to the UGT1A1*28 polymorphism (21). A few studies have investigated the association of the UGT1A1*28 allele with CV diseases (CVD) (22–26). Only the prospective Framingham Heart Study (22), which followed subjects for 24 years, found an association between the polymorphism and CV endpoints. However, little is known about whether serum bilirubin or UGT1A1*28 is associated with the risk of CV events and death in hemodialysis (HD) patients. In the study presented here, we attempted to clarify the association between serum bilirubin levels and UGT1A1*28 polymorphism, as well as their effect on CV events and all-cause mortality.
Materials and Methods
Research Subjects
This prospective cohort study was carried out at six dialysis centers in the Taipei metropolitan area. Study subjects were recruited between March 1997 and February 2003. Initially, all patients (n = 812) undergoing HD were screened in the six centers, and 750 clinically stable patients older than 20 years of age with a HD vintage of more than 6 months before the study were included. Exclusion criteria were weekly dialysis for less than 12 hours; inadequacy of dialysis with urea Kt/V < 1.2; conditions of malignancy, infectious disease, or sepsis; hyperbilirubinemia (total bilirubin > 2.0 mg/dl) caused by drug-induced hepatitis and drugs interfering with bilirubin metabolism; and hepatobiliary disorders including acute or chronic hepatocellular disease, cirrhosis of liver, hepatic tumor, biliary tract stone or tumor, and pancreatic head tumor (Figure 1). Finally, our study population comprised 661 patients (335 men and 326 women; mean age: 58 years). The causes of ESRD were GN (n = 241), interstitial nephritis (n = 65), diabetic nephropathy (n = 195), nephrosclerosis (n = 31), polycystic kidney disease (n = 26), miscellaneous nephropathies (n = 36), and shrunken kidney resulting from unknown causes (n = 67). All of the patients were subjected to a standard bicarbonate dialysis session. HD was performed 3 times weekly using single-use dialyzers with a membrane surface area of 1.6 to 1.7 m2. The median duration of HD before study entry was 50 months (interquartile range [IQR]: 24 to 109). Controls were 152 individuals (82 men and 70 women; mean age: 59 years) with normal renal function, defined on the basis of an estimated GFR value >100 ml/min per 1.73 m2 by a simplified Modifications of Diet in Renal Disease equation, who were enrolled for genotyping of the UGT1A1*28 promoter. These subjects were recruited from volunteers receiving health checkups. The Committee on Human Research of Taipei Veterans General Hospital approved the protocol. Informed consent was obtained from each study subject before study entry.
Figure 1.
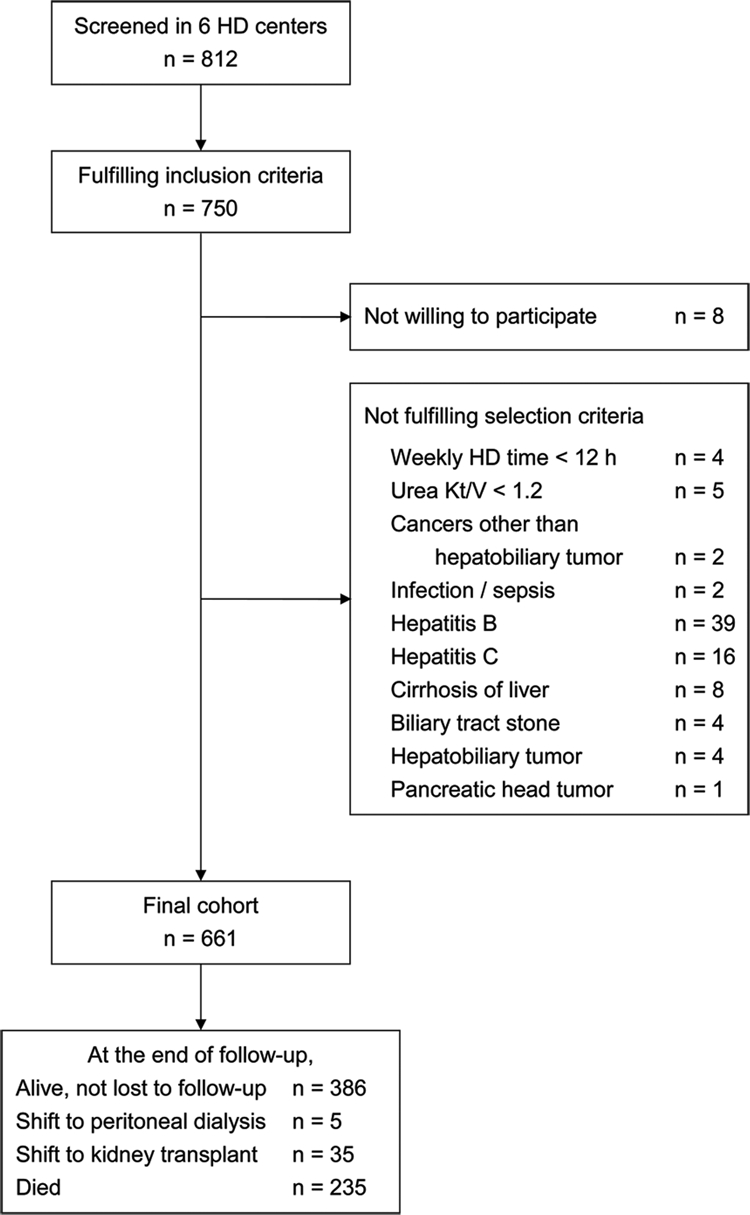
Study flow and design for cohort and follow-up phases.
Laboratory Measurements
Venous blood samples were drawn from fasting healthy individuals or from HD patients who had fasted overnight at the start of a midweek dialysis session before administering heparin. A 20-ml fasting venous blood sample was collected at study baseline. Total serum bilirubin was measured using the metavanadate oxidation method (Wako Pure Chemical Industries Ltd., Osaka, Japan). Intra-assay and interassay coefficients of variance for bilirubin measurement were <5%. Albumin was measured using the bromocresol green method, and iron, total cholesterol, triglyceride, HDL-cholesterol, LDL-cholesterol, urea, and creatinine in serum were determined using commercial kits by a Hitachi 7600 autoanalyzer (Roche Modular; Hitachi Ltd., Tokyo, Japan). Total iron binding capacity (TIBC) was measured by using the TIBC Microtest (Daiichi, Tokyo, Japan), and serum ferritin was determined using a RIA (Incstar, Stillwater, MN). Transferrin saturation was calculated as serum iron concentration/TIBC × 100. Serum high-sensitivity C-reactive protein (hsCRP) was measured by an immunoturbidimetric assay using rate nephrelometry (IMMAGE; Beckman Coulter, Galway, Ireland). Adequacy of dialysis was estimated by measuring midweek urea clearance (Kt/V) using the standard method (27).
Genotyping
Genotyping of the UGT1A1 promoter TA-repeat polymorphism in the TATA box at position −53 was performed using the ABI 3130 × l sequencing system as recently described in detail (22). Briefly, PCR was performed with a 5-FAM (carboxyfluorescein)-labeled forward primer (5′-CACGTGACACAGTCAAAC-3′) and an unlabeled reverse primer (5′-CAACAGTATCTTCCCAGC-3′). Amplification was performed for 34 cycles, and each cycle comprised denaturation at 94°C for 45 seconds, annealing at 62°C for 45 seconds, and extension at 72°C for 60 seconds between the initial denaturation at 94°C for 2 minutes and a final extension at 72°C for 1 minute. Finally, the PCR products were sequenced to determine the number of TA repeats over the promoter of the UGT1A1 gene.
Clinical Data Collection
Baseline demographic data were recorded at the time of recruitment. All patients were enrolled by one physician to minimize interobserver variations. Patient history, including smoking status and treatments for hypertension and diabetes mellitus at baseline, were recorded via an interview and confirmed by checking patient records. These data were complemented by clinical assessment of body weight, body mass index (BMI), blood pressure (BP), and fasting blood glucose. Diabetes was diagnosed on the basis of the World Health Organization criteria. Hypertension was defined as BP > 140/90 mmHg and/or the use of antihypertensive medication.
Outcome Data Collection
The cohort was followed up to March 2009. During the follow-up, 119 patients moved away from the dialysis facilities. Among 119 patients, 35 patients received a kidney transplant and 5 were transferred to peritoneal dialysis. The remaining 79 patients transferred to other dialysis units were reviewed using the questionnaire forms filled by the attending physicians at the units. A physician obtained information about the occurrence of interim CV events and cause of death by reviewing hospital record forms, and this information was then analyzed. The composite CV event category comprised fatal and nonfatal myocardial infarction, stroke, congestive heart failure and arrhythmia, as well as CAD, transient ischemic attack, PAD, and sudden death. The overall mortality category comprised death due to CV events, infection, sepsis, malignancy, gastrointestinal bleeding, chronic obstructive lung disease, and cachexia. At the end of the follow-up, 386 patients were confirmed to be alive on HD treatment and 235 patients had died while being treated (Figure 1). The median follow-up period was 54 months (IQR: 27 to 107).
Statistical Analyses
Descriptive statistics included mean values ± SD for continuous data and percentages for categorical data. The values of serum hsCRP and ferritin were not normally distributed and were reported as median with IQR. Potential differences among the three patient groups of serum bilirubin tertiles at baselines were assessed by ANOVA for normally distributed data, the Kruskal–Wallis test for non-normally distributed data, or the Pearson χ2 test for categorical variables. Comparison of the genotypes and allelic frequencies of the UGT1A1*28 polymorphism in HD patients and healthy individuals were performed using the χ2 test. Linear regression was used to test difference in mean levels of serum bilirubin among three genotypes. The full model included the genotype effect (coded as a recessive model for allele 7) and the covariates of age, sex, HD duration, systolic and diastolic BP, HDL- and LDL-cholesterol, serum albumin, and hsCRP. Differences in serum bilirubin levels between patients with and without CV events and mortality were analyzed using the t test. Cumulative survival curves for first CV events and all-cause mortality were generated using the Kaplan–Meier method. In this analysis, patients who underwent a kidney transplant or transferred to peritoneal dialysis during the 12-year follow-up were censored at the time of the transfer to alternative renal replacement therapy. Between-group survival among the tertiles of serum bilirubin and UGT1A1*28 genotypes was compared using the log-rank test. The multivariate Cox proportional hazards model was used to estimate the hazard ratios of composite CV events and all-cause mortality in relation to serum bilirubin and UGT1A1*28 polymorphism. The analysis was stepwise adjusted for traditional risk factors (age, male gender, smoking history, diabetes, hypertension, prior CVD, LDL-cholesterol, and HDL-cholesterol) and dialysis-related risk factors (HD duration, urea Kt/V, BMI, hemoglobin, serum albumin, hsCRP, ferritin, and transferrin saturation). Because of the significant association between serum bilirubin and the UGT1A1*28 polymorphism, these two variables were not offered simultaneously in a particular model to avoid multicollinearity. An additional model (genotype 7/7 versus 7/6 + 6/6 as the reference group) was used because CV event- and mortality-free survival showed no difference between genotypes 6/6 and 7/6. Statistical analyses were performed using the computer software SPSS version 16.0 (SPSS Inc., Chicago, IL). All P values were two-tailed. P values <0.05 were considered statistically significant.
Results
Serum bilirubin (0.78 ± 0.15 mg/dl) in our cohort was normally distributed. When divided into tertiles, serum bilirubin was distributed as follows: 0.59 ± 0.09 mg/dl (lower tertile), 0.76 ± 0.04 mg/dl (middle tertile), and 0.99 ± 0.25 mg/dl (upper tertile). The baseline demographic characteristics and traditional and dialysis-related risk factors of the study population are displayed in Table 1. There was an even distribution of characteristics among the three bilirubin tertiles including age, gender, smoking history, diabetes, hypertension, prior CVD, LDL- and HDL-cholesterol, urea Kt/V, BMI, dose of epoetin, and duration of HD. Serum bilirubin was negatively correlated with hsCRP and ferritin and positively correlated with serum albumin and hemoglobin.
Table 1.
Baseline demographic and laboratory characteristics of HD patients stratified by serum bilirubin tertiles
| Parameters | Serum Bilirubin Tertiles |
P | ||
|---|---|---|---|---|
| Lower (n = 221) | Middle (n = 220) | Upper (n = 220) | ||
| Age, years | 58.8 ± 13.9 | 58.8 ± 12.8 | 57.5 ± 13.4 | 0.469a |
| Male gender, n (%) | 114 (51.5) | 107 (48.6) | 114 (51.8) | 0.758b |
| Current smoker, n (%) | 76 (34.3) | 61 (27.7) | 74 (33.6) | 0.260b |
| Hypertension, n (%) | 131 (59.3) | 127 (57.7) | 120 (54.5) | 0.592b |
| Diabetes mellitus, n (%) | 72 (32.6) | 71 (32.3) | 63 (28.6) | 0.632b |
| Previous CV disease, n (%) | 65 (29.4) | 58 (26.3) | 73 (33.1) | 0.277b |
| HD duration, months | 75.3 ± 57.6 | 72.1 ± 60.7 | 73.4 ± 62.8 | 0.254a |
| Kt/V urea | 1.81 ± 0.44 | 1.85 ± 0.66 | 1.83 ± 0.48 | 0.735a |
| Cholesterol, mg/dl | 173 ± 38 | 172 ± 35 | 172 ± 37 | 0.993a |
| Triglyceride, mg/dl | 175 ± 111 | 172 ± 112 | 179 ± 124 | 0.381a |
| HDL-cholesterol, mg/dl | 40 ± 12 | 38 ± 10 | 38 ± 12 | 0.877a |
| LDL-cholesterol, mg/dl | 113 ± 28 | 112 ± 29 | 110 ± 29 | 0.702a |
| Systolic BP, mmHg | 144 ± 22 | 145 ± 29 | 145 ± 22 | 0.851a |
| Diastolic BP, mmHg | 79 ± 12 | 78 ± 10 | 77 ± 11 | 0.727a |
| BMI, kg/m2 | 21.5 ± 2.8 | 22.0 ± 3.6 | 22.2 ± 2.9 | 0.065a |
| hsCRP, mg/L | 3.89 (1.03, 8.02) | 3.46 (0.95, 7.07) | 2.92 (0.73, 6.15) | 0.012c |
| Albumin, g/dl | 3.79 ± 0.36 | 3.95 ± 0.32 | 4.01 ± 0.32 | <0.001a |
| Hemoglobin, g/dl | 9.7 ± 1.0 | 10.3 ± 1.1 | 11.8 ± 1.0 | <0.001a |
| Dose of epoetin, U/kg per week | 69 ± 41 | 68 ± 40 | 70 ± 43 | 0.920a |
| Ferritin, μg/L | 393 (246, 737) | 396 (224, 638) | 307 (148, 478) | <0.001c |
| Transferrin saturation, % | 32 ± 14 | 33 ± 16 | 33 ± 15 | 0.635a |
| Serum bilirubin, mg/dl | 0.59 ± 0.09 | 0.76 ± 0.04 | 0.99 ± 0.25 | <0.001a |
Values in bold are statistically significant. Statistical analysis by aANOVA test,
the Pearson χ2 test, and
the Kruskal–Wallis test.
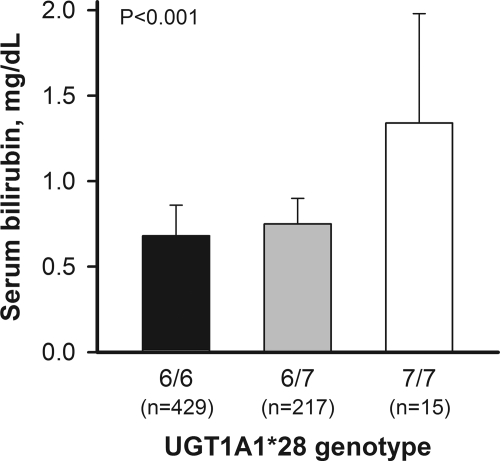
In Table 2, the UGT1A1*28 genotype frequencies were 65.9%, 32.8%, and 2.3% for 6/6, 7/6, and 7/7, respectively, for HD patients. The distribution of 7-allelic frequencies and genotypes was comparable with control subjects. The prevalence of the 7/7 genotype was 2.0% to 2.3%, which is much lower than that reported in Caucasian populations and occurs at a similar frequency in Southeast Asian populations (21). Mean serum bilirubin was highest in 7/7 carriers (1.34 ± 0.64 mg/dl), intermediate in 7/6 carriers (0.75 ± 0.15 mg/dl), and lowest in 6/6 (0.68 ± 0.18 mg/dl) carriers (P < 0.001) (Figure 2). The bilirubin level of 7/7 carriers was 97% higher than that of 6/6 carriers.
Table 2.
UGT1A1*28 genotype and allelic frequency in HD patients and healthy subjects
| Genotype | HD Patients (n = 661) | Healthy Controls (n = 152) |
|---|---|---|
| 6/6, n (%) | 429 (65.9) | 103 (67.7) |
| 6/7, n (%) | 217 (32.8) | 46 (30.3) |
| 7/7, n (%) | 15 (2.3) | 3 (2.0) |
| 7-allelic frequency | 0.187 | 0.172 |
Figure 2.
Serum bilirubin at baseline in 661 HD patients stratified by the UGT1A1*28 genotype.
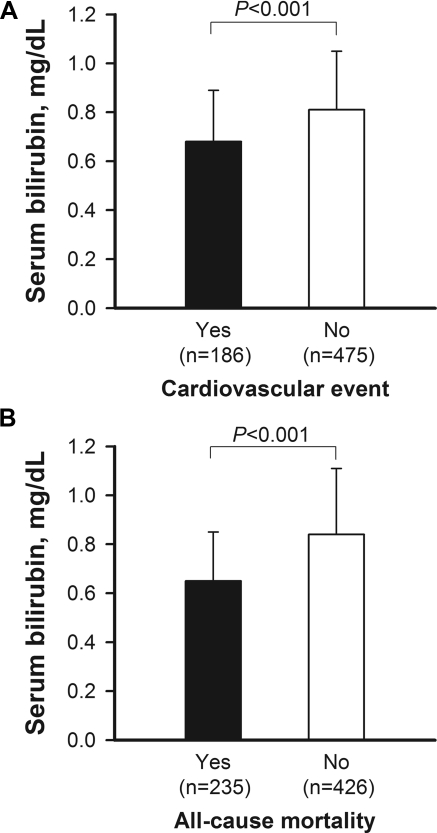
Table 3 displays the CV events and causes of mortality in HD patients at the end of the study. The all-cause mortality rate was 35.6% and 108 deaths (46%) were CV-related, whereas 127 deaths (56%) were non-CV-related. The proportions of CV and non-CV mortality are in accordance with the reports of United States (28) and Europe (1). Infections and malignancies were the most important causes of non-CV mortality. In subjects having the CV events and all-cause mortality (Figure 3), serum bilirubin levels were significantly lower than in individuals not reaching the endpoints (P < 0.001), respectively.
Table 3.
CV disease events and cause of death during follow-up
| CV Events | All-Cause Mortality | |
|---|---|---|
| CV origin | ||
| myocardial infarction, n (%) | 38 (12.7a) | 23 (9.8b) |
| heart failure, n (%) | 64 (21.4a) | 10 (4.3b) |
| arrhythmia, n (%) | 38 (12.7a) | 11 (4.7b) |
| stroke, n (%) | 47 (15.7a) | 24 (10.2b) |
| mesenteric infarction, n (%) | 4 (1.3a) | 3 (1.3b) |
| sudden death, n (%) | 37 (12.4a) | 37 (15.7b) |
| CAD, n (%) | 17 (5.7a) | – |
| transient ischemic attack, n (%) | 5 (1.7a) | – |
| peripheral arterial disease, n (%) | 35 (11.7a) | – |
| hypotension, n (%) | 14 (4.7a) | – |
| subtotal, n (%) | 299 (100a) | 108 (46.0b) |
| Non-CV origin | ||
| sepsis/infection | – | 70 (29.8b) |
| cancer | – | 29 (12.3b) |
| gastrointestinal bleeding | – | 16 (6.8b) |
| chronic obstructive lung disease | – | 8 (3.4b) |
| cachexia | – | 4 (1.7b) |
| subtotal, n (%) | 0 (0) | 127 (54.0b) |
| Total, n | 299 (100a) | 235 (100b) |
Percent of all CV events.
Percent of all mortality cases.
Figure 3.
Serum bilirubin at baseline in 661 patients with or without reaching endpoints of (A) the CV events and (B) all-cause mortality.
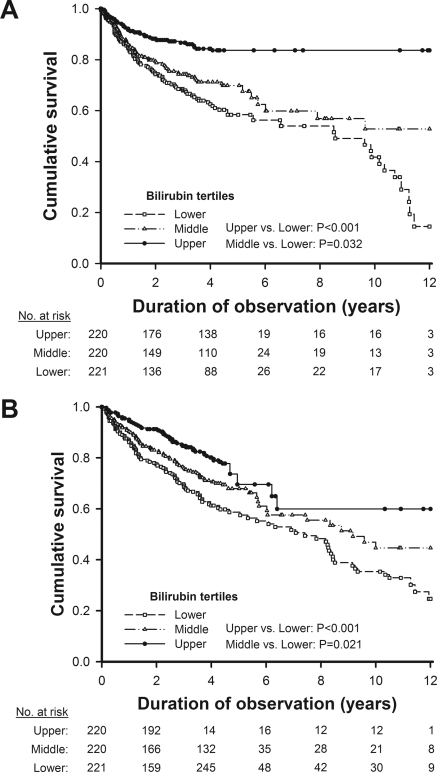
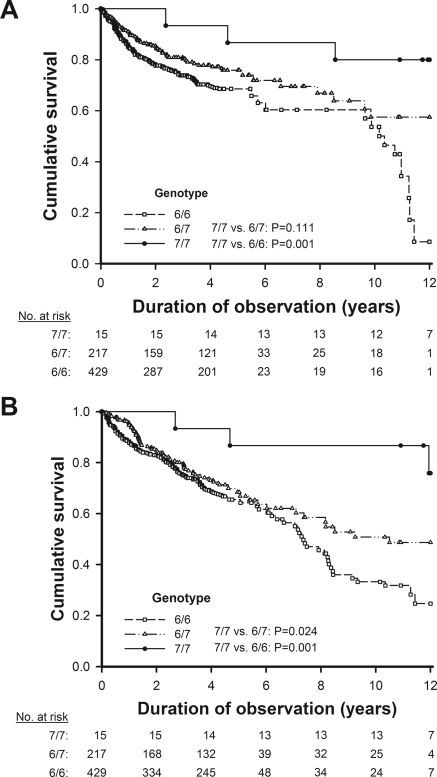
In Kaplan–Meier analysis curves for endpoints of the first CV event and all-cause mortality among 661 HD patients, the risk of CV events and death in the lower tertile of serum bilirubin was significantly higher than those in the middle and upper tertiles (Figure 4). The 7/7 homozygotes of UGT1A1*28 had a significantly lower risk of CV events than the 6/6 homozygotes and a lower risk of all-cause mortality than the carriers of the 6 allele (Figure 5).
Figure 4.
Kaplan–Meier analysis curves for endpoints of (A) the first cardiovascular event and (B) all-cause mortality among 661 HD patients in relation to the tertiles of serum bilirubin.
Figure 5.
Kaplan–Meier analysis curves for endpoints of (A) the first cardiovascular event and (B) all-cause mortality among 661 HD patients in relation to the UGT1A1*28 genotype.
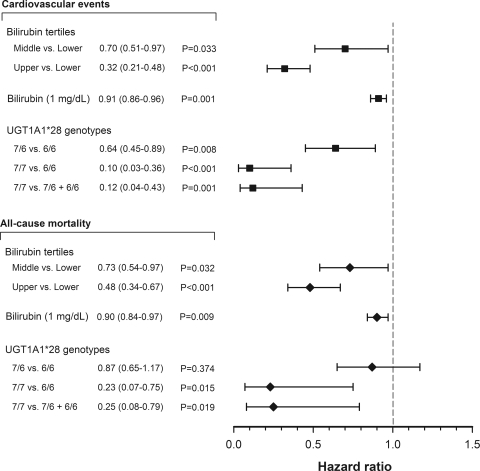
In multivariate Cox regression analysis (Figure 6), compared with the lower bilirubin tertile, the fully adjusted hazard ratios in the middle and upper tertiles for CV events were 0.70 (95% confidence interval [CI], 0.51 to 0.97) and 0.32 (95% CI, 0.21 to 0.48), respectively. An association of bilirubin serum levels with each outcome further showed that a 0.1-mg/dl increase in serum bilirubin decreased CV events and all-cause mortality by 9% and 10%, respectively. In the same multivariable-adjusted model, individuals with genotype 7/7 had approximately one tenth the risk for CV events and one fourth the risk for all-cause mortality compared with carriers of the 6 allele, which resulted in hazard ratios of 0.12 (95% CI, 0.04 to 0.43) and 0.25 (95% CI, 0.08 to 0.79), respectively. Compared with subjects with genotype 6/6, 7/7 homozygotes also had a significantly lower risk for CVD events (hazard ratio 0.10 [95% CI, 0.03 to 0.36]) and death (hazard ratio 0.23 [95% CI, 0.07 to 0.75]).
Figure 6.
Association of baseline serum bilirubin and the UGT1A1*28 genotype with fatal and nonfatal CV events and all-cause mortality after a follow-up of 12 years using multivariate Cox proportional hazards analysis.
Discussion
In the prospective cohort study, we first reported a graded, reverse association between serum bilirubin and adverse clinical outcomes among HD patients. Moreover, there was a significantly decreased risk of CVD and all-cause mortality for subjects homozygous for the 7 allele and such effect might be conveyed through its influence on increasing serum bilirubin levels.
CVD remains the major cause of death among the HD population, and its incidence has not decreased over the years, although major improvements in medications and dialysis technology have been made. Apart from the traditional risk factors, a series of nontraditional risk factors (2,3,29,30) including calcium and phosphate abnormalities, oxidative stress, inflammation, and malnutrition may render ESRD patients more prone to develop excess risks of CV events and death. New strategies for identifying risk factors, pathophysiologic pathways, and targets for intervention are needed in such high-risk populations. Bilirubin is a protective factor with antioxidant (4,5) and anti-inflammatory (6,7) properties. Cumulative evidence indicates that serum bilirubin concentrations are inversely related to CAD, peripheral vascular disease, and stroke (13–15) in retrospective and prospective studies among individuals without evidence of kidney disease. Information regarding the association of serum bilirubin with adverse outcomes in patients undergoing HD is scarce.
In the study presented here, serum bilirubin was found to be negatively correlated with inflammatory marker (hsCRP) and positively with nutritional index (serum albumin), indicating that serum bilirubin is a surrogate for malnutrition-inflammatory complex in HD patients (29,30). We further found that the lower the serum bilirubin, the less the hemoglobin response and the greater the increase of serum ferritin. Our data corroborate the previous findings (30) that patients in malnutrition-inflammation status exhibited poor epoetin response and had abnormally high serum ferritin. The most compelling observation of this study is that for each 0.1-mg/dl decrease in bilirubin levels, risks for CV events and overall mortality increased 9% and 10%, respectively. Unconjugated and conjugated bilirubins have been shown to be efficient co-antioxidants for α-tocopherol in inhibiting lipid peroxidation of plasma LDL (8,9). In the study presented here, it is inferred that serum bilirubin could be a surrogate for unmeasured oxidative stress markers or it might act through nonoxidative stress related mechanisms (e.g., inflammation) to reduce the risk of CVD. In addition to providing possible pathophysiologic insight, our findings suggest that serum bilirubin may provide useful prognostic information in chronic HD patients.
As compared with the general population, patients undergoing HD are known to have a higher annual mortality rate from CVD; these diseases account for more than 40% of mortality (1,24). Oxidative stress and inflammation-mediated atherosclerosis are extremely complex. However, this approach may be one way of investigating the gene's moderating effect on such disorders in the context that there is direct or indirect metabolic activity by the gene. Previous studies suggest that the TA repeats might be the key polymorphism within the UGT1A1 gene controlling bilirubin levels in people free of kidney failure (16,17). In the study presented here, for the first time, we demonstrated that UGT1A1*28 polymorphism may modify serum bilirubin levels and associate with CV events and mortality among patients undergoing chronic HD. Mean serum bilirubin in our cohort was highest in 7/7 carriers, intermediate in 7/6 carriers, and lowest in 6/6 carriers, which is in accordance with previous studies in nonkidney disease subjects. Although most previous studies found an association between bilirubin levels and atherosclerosis outcome, only the prospective Framingham Heart Study (22) found an association between the polymorphism and CV endpoints. The major difference between these studies is that the Framingham Study (22) was a prospective population-based cohort study in which subjects were followed for 24 years, whereas the other studies (23–26) were retrospective cross-sectional case-control studies.
There are several possible reasons for the significant association between UGT1A1*28 polymorphism and outcomes in our study in a follow-up of 12 years. First of all, genetic factors for outcomes may be easier to detect in a prospective study with long-term follow up than in a study with short follow-up, which might be significantly affected by many other factors, including environmental factors. Second, it has been shown that inflammation and oxidative stress are markedly increased in ESRD. It is postulated that the lower bilirubin level in HD patients imposed by a decrease in gene transcription of the 6 allele may act synergistically with other risk factors to increase the vulnerability to development of adverse outcomes. Third, our study included people with genetic backgrounds different from those subjects of previous studies that were mainly performed in Western countries. Of note, ethnic differences do exist in the UGT1A1*28 polymorphism. The prevalence of the 7/7 homozygous variant is significantly greater in African populations (12% to 27%) and in Caucasian populations (5% to 15%) than in Southeast Asian populations (1.2% to 5%) (21). Finally, only a small population of chronic kidney disease patients will live long enough to develop ESRD and commence HD. Those survive the conventional risk factors throughout the progression of chronic kidney disease without fatal events. The “survival bias” (31) might lead to the particular effect of the genotype in HD patients being different from the general population. These distinctive features may explain the discrepancy in the results between our study and others (23–26).
In conclusion, we found an independent relation between lower levels of serum bilirubin and adverse outcomes among subjects undergoing chronic HD. Moreover, the UGT1A1 gene polymorphism had strong effects on bilirubin levels and the 7/7 genotype might have an important effect on preventing the development of CVD and death. Accurate risk stratification that takes into account serum bilirubin and genetic information would allow recognition of high-risk groups. Close follow-up and more aggressive treatment of remediable risk factors might improve outcome in such high-risk populations.
Disclosures
None.
Acknowledgments
This study was supported by grants from the National Science Council (NSC 94-2314-B010-033 and 96-2628-B010-001-MY3) and the Taipei Veterans General Hospital (V96ER2-012, V99S5-002, and V99C1-121) and by the Ministry of Education, Aim for the Top University Plan. We are extremely grateful to Drs. Brian Chen, F.G. Hsieh, H.H. Liou, and N.Y. Hsiao for their kind help in the collection of samples at their HD facilities. We are also deeply indebted to Miss P.C. Lee for her expert secretarial assistance and graphic design.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De Meester J, Wetzels JF, Rosendaal FR, Dekker FW: Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302: 1782–1789, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Rattazzi M, Puato M, Faggin E, Bertipaglia B, Grego F, Pauletto P: New markers of accelerated atherosclerosis in end-stage renal disease. J Nephrol 16: 11–20, 2003 [PubMed] [Google Scholar]
- 3. Clermont G, Lecour S, Lahet J, Siohan P, Vergely C, Chevet D, Rifle G, Rochette L: Alteration in plasma antioxidant capacities in chronic renal failure and haemodialysis patients: A possible explanation for the increased cardiovascular risk in these patients. Cardiovasc Res 47: 618–623, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Stocker R, Yamamoto Y, McDonagh A, Glazer A: Bilirubin is an antioxidant of possible physiological importance. Science 235: 1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Stocker R, Keaney JF, Jr: Role of oxidative modifications in atherosclerosis. Physiol Rev 84: 1381–1478, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Willis D, Moore AR, Frederik R, Willoughby DA: Heme oxygenase: A novel target for the modulation of the inflammatory response. Nat Med 2: 87–90, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Nakagami T, Toyomura K, Kinoshita T, Morisawa S: A beneficial role of bile pigments as an endogenous tissue protector: Anticomplement effects of biliverdin and conjugated bilirubin. Biochim Biophys Acta 1158: 189–193, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Neuzil J, Stocker R: Free and albumin-bound bilirubin are efficient co-antioxidants for α-tocopherol, inhibiting plasma low density lipoprotein lipid peroxidation. J Biol Chem 269: 16712–16719, 1994 [PubMed] [Google Scholar]
- 9. Wu TW, Fung KP, Wu J, Yang CC, Weisel RD: Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol 51: 859–862, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Schwertner HA: Association of smoking and low serum bilirubin antioxidant concentrations. Atherosclerosis 136: 383–387, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Vítek L, Jirsa M, Brodanová M, Kalab M, Marecek Z, Danzig V, Novotný L, Kotal P: Gilbert syndrome and ischemic heart disease: A protective effect of elevated bilirubin levels. Atherosclerosis 160: 449–456, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Schwertner HA, Jackson WG, Tolan G: Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem 40: 18–23, 1994 [PubMed] [Google Scholar]
- 13. Djoussé L, Levy D, Cupples LA, Evans JC, D'Agostino RB, Ellison RC: Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am J Cardiol 87: 1196–1200, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Perlstein TS, Pande R, Beckman JA, Creager MA: Serum total bilirubin level and prevalent lower-extremity peripheral arterial disease: National Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arteriocler Thromb Vasc Biol 28: 166–172, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Kimm H, Yun JE, Jo J, Jee SH: Low serum bilirubin level as an independent predictor of stroke incidence: A prospective study in Korean men and women. Stroke 40: 3422–3427, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Lin JP, Cupples LA, Wilson PW, Heard-Costa N, O'Donnell CJ: Evidence for a gene influencing serum bilirubin on chromosome 2q telomere: A genomewide scan in the Framingham study. Am J Hum Genet 72: 1029–1034, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kronenberg F, Coon H, Gutin A, Abkevich V, Samuels ME, Ballinger DG, Hopkins PN, Hunt SC: A genome scan for loci influencing anti-atherogenic serum bilirubin levels. Eur J Hum Genet 10: 539–546, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Bosma PL, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GNJ, Hansen PLM, Oude Elferick RPJ, Chowdhury NR: The genetic basis of the reduced expression of bilirubin UDP glucuronosyltransferase in Gilbert's syndrome. N Engl J Med 333: 1171–1175, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Monaghan G, Ryan M, Seddon R, Burchell B: Genetic variation in bilirubin UDP-glucuronosyltransferase gene promoter and Gilbert syndrome. Lancet 347: 578–581, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Beutler E, Gelbart T, Demina A: Racial variability in the UDP glucuronosyltransferase 1 (UGT1A1) promoter: A balanced polymorphism for regulation of bilirubin metabolism. Proc Natl Acad Sci U S A 95: 8170–8174, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Premawardhena A, Fisher CA, Liu YT, Verma IC, de Silva S, Arambepola M, Clegg JB, Weatherall DJ: The global distribution of length polymorphisms of the promoters of the glucuronosyltransferase 1 gene (UGT1A1): Hematologic and evolutionary implications. Blood Cells Mol Dis 31: 98–101, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lin JP, O'Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, Yang S, Kronenberg F: Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation 114: 1476–1481, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Rantner B, Kollerits B, Anderwald-Stadler M, Klein-Weigel P, Gruber I, Gehringer A, Haak M, Schnapka-Köpf M, Fraedrich G, Kronenberg F: Association between the UGT1A1 TA-repeat polymorphism and bilirubin concentration in patients with intermittent claudication: Results from the CAVASIC Study. Clin Chem 54: 851–857, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Lingenhel A, Kollerits B, Schwaiger JP, Hunt SC, Gress R, Hopkins PN, Schoenborn V, Heid IM, Kronenberg F: Serum bilirubin levels, UGT1A1 polymorphisms and risk for coronary artery disease. Exp Gerontol 43: 1102–1107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bosma PJ, van der Meer IM, Bakker CT, Hofman A, Paul-Abrahamse M, Witteman JC: UGT1A1*28 allele and coronary heart disease: The Rotterdam Study. Clin Chem 49: 1180–1181, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Gajdos V, Petit FM, Perret C, Mollet-Boudjemline A, Colin P, Capel L, Nicaud V, Evans A, Arveiler D, Parisot F, Francoual J, Genin E, Cambien F, Labrune P: Further evidence that the UGT1A1*28 allele is not associated with coronary heart disease: The ECTIM Study. Clin Chem 52: 2313–2314, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Daugirdas JT: Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of errors. J Am Soc Nephrol 4: 1205–1213, 1993 [DOI] [PubMed] [Google Scholar]
- 28. U.S. Renal Data System: USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2008 [Google Scholar]
- 29. Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Gunnell J, Jane Y, Yeun JY, Thomas A, Depner TA, George A, Kaysen GA: Acute phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis 33: 63–72, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD: Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63: 793–808, 2003 [DOI] [PubMed] [Google Scholar]