Summary
Background and objectives
New arteriovenous fistulas (AVF) are frequently unsuitable for hemodialysis because of AVF nonmaturation. Aggressive endovascular or surgical interventions are often undertaken to salvage nonmaturing AVFs. The effect of early interventions to promote AVF maturation on subsequent long-term AVF outcomes is unknown.
Design, setting, participants, & measurements
We evaluated 173 hemodialysis patients from two academic centers who received a new AVF. Of these, 96 (56%) required no further intervention, 54 (31%) required one intervention, and 23 (13%) required two or more interventions to achieve suitability for dialysis. We calculated AVF survival and frequency of postmaturation interventions in each group.
Results
Cumulative AVF survival (access cannulation to permanent failure) in patients with two or more versus one versus zero interventions before maturation was 68% versus 78% versus 92% at 1 year, 57% versus 71% versus 85% at 2 years, and 42% versus 57% versus 75% at 3 years. Using Cox regression analysis with interventions before maturation, age, sex, race, diabetes, peripheral vascular disease, access site, and obesity in the model, intervention before maturation (two or more) was the only factor associated with cumulative AVF survival. The number of interventions required to maintain patency after maturation was 3.51 ± 2.20 versus 1.37 ± 0.31 versus 0.76 ± 0.10 per year in patients with two or more versus one versus zero interventions before maturation.
Conclusions
Compared with AVF that mature without interventions, AVF that require interventions have decreased cumulative survival and require more interventions to maintain their patency for hemodialysis.
Introduction
Vascular access is truly the “lifeline” for the hemodialysis patient (1–4). Approximately one billion dollars are spent annually in the United States treating complications from vascular access dysfunction (4–7). The National Kidney Foundation Kidney Disease Quality Initiative guidelines for vascular access (8) and the Fistula First Breakthrough Initiative (9–11) have promoted the arteriovenous fistula (AVF) as the preferred vascular access of choice because of better long-term survival and fewer complications compared with arteriovenous grafts and tunneled catheters, if the AVF matures for dialysis (12,13). AVFs that fail to mature, because of either early thrombosis or failure to obtain suitability for dialysis use (4,14,15), are the major obstacle to increasing the proportion of dialysis patients with AVFs in the United States. Consequently, we have seen a major effort to aggressively treat and salvage nonfunctioning AVFs to improve AVF maturation outcomes (16–22). Although these interventions are beneficial in promoting AVF maturation and eventual suitability for dialysis, the biologic changes resulting from the interventions may have a deleterious effect on long-term AVF outcomes (23).
To evaluate this question, we compared the long-term outcomes of AVFs requiring interventions to achieve maturation with those obtained in a control group of AVFs not requiring such interventions. The primary clinical outcomes studied were (1) cumulative access survival (time from access cannulation to failure) and (2) the frequency of interventions to maintain access patency after first cannulation. As a secondary analysis, we compared AVF outcomes for endovascular versus surgical interventions in nonmaturing AVFs.
Materials and Methods
Study Population
Prospective access databases from the University of Cincinnati (UC) and University of Alabama at Birmingham (UAB) were queried to identify prevalent hemodialysis patients requiring a new AVF placement from 2005 to 2007. All of the patients were under the care of university nephrologists at their respective medical centers. At UC all of the vascular accesses are placed by one dedicated vascular access surgeon, and subsequent vascular access revisions or interventions are performed by the same vascular access surgeon or by interventional nephrologists at a dedicated outpatient vascular access center. At UAB, initial vascular access and subsequent vascular access placements are performed by a team of four transplant surgeons and interventional radiologists or nephrologists. The AVF prevalence at UC and UAB was approximately 35 and 40%, respectively, during 2007, in a largely inner-city population (comparable with the overall renal network prevalence for these regions at the time) (9).
Vascular Access Management
At UC, either preoperative ultrasound mapping or angiography is performed to assist the surgeon for vascular access surgery. When preoperative ultrasound mapping was performed, a minimum threshold of 2.5 mm for the vein and 2.0 mm for the artery was used to determine creation of an AVF (8). The patients are evaluated by the surgeon at 2- and 6-week clinic visits after creation of an AVF. If there was an abnormality detected on physical exam by the surgeon, the patient either had salvage procedures performed by the surgeon or was referred to interventional nephrology. These procedures could include endovascular (angioplasty) or surgical revisions to the AVF. AVFs are typically allowed to mature for 3 to 6 months before initial cannulation, and permission for initial AVF cannulation is given by the vascular access surgeon.
At UAB all of the patients receive preoperative ultrasound mapping before new vascular access evaluation with creation of an AVF requiring a minimum vein diameter of 2.5 mm and artery diameter of 2.0 mm (12,24,25). The patients were evaluated for 1 to 2 weeks after AVF placement by the surgeons and assessed clinically for maturation by dialysis nurses and nephrologists. If AVFs were felt to be unsuitable for cannulation or not maturating adequately, a postoperative ultrasound was ordered (25). The ultrasound was used to screen for remediable causes of AVF immaturity and was followed by specific surgical or endovascular salvage procedures (25). AVFs were typically cannulated at 8 to 12 weeks. Radiocephalic, brachicephalic, and basilic vein transpositions AVFs were the three types of fistulas created in our study population.
Data Collection and Analyses
Information related to access history, surgeries, procedures, and outcomes were collected from the access databases from both centers. The databases included information about vascular access placements and subsequent surgical or endovascular procedures.
From the respective access databases, we identified a comprehensive list of AVFs placed in prevalent hemodialysis patients over a 3-year period. We identified 221 patients (128 patients at UC and 93 patients at UAB) who had new AVFs placed and were on hemodialysis during this study period. After excluding primary failures from both centers, a total of 173 AVFs remained for analysis (108 from UC and 65 from UAB). The primary failure rate was 21% in the initial study population. Cumulative access survival was calculated from the time of access cannulation to permanent failure. Access cannulation was deemed successful when the patient's tunneled catheter was removed. All of the patients were dialyzing with tunneled catheters before AVF surgery. The clinical outcome of each AVF was determined from the databases.
Demographic and clinical information was collected using electronic medical records on each patient including sex, race, presence or absence of diabetes, peripheral vascular disease (PVD), BMI ≥30, and age ≥65. Institutional review board approval from both centers was obtained before initiation of this study.
Statistical Analyses
The data were reported as percentages (means ± SE) as appropriate. The clinical characteristics were analyzed using contingency table analysis, ANOVA, and t tests. A P value <0.05 was considered statistically significant. Cumulative access survival was plotted using Kaplan-Meier survival techniques with patients censored for death, kidney transplant, or end of follow-up, and the log-rank test was used to compare the survival between patient groups. A P value <0.05 was considered to be statistically significant. Univariable and multivariable Cox proportional hazard models were performed, and hazard ratios (HR) and their associated 95% confidence intervals (CIs) were computed. For the analysis comparing cumulative survival between angioplasty and surgical interventions, those patients who had both surgical and angioplasty (six in total) procedures to promote AVF maturation were placed in the angioplasty group for the survival analysis. All of the statistical analyses were performed using the JMP® 8.0 (Cary, NC) statistical software package.
Results
Patient Population
The study population was comprised of 173 patients. 74% of the patients were men, 75% were black, 50% had diabetes, and 20% had PVD. 68% of patients had upper arm AVFs placed. Only 28% of patients were ≥65 years of age, and 34% had BMI ≥30. Table 1 summarizes the demographic and clinical characteristics of the patient population by number of interventions before maturation. Diabetes, PVD, BMI ≥30, and female sex were associated with more interventions before AVF maturation (Table 1). Age ≥65, race, or access site did not differ by number of interventions before maturation (Table 1). The proportion of interventions was similar for groups with zero, one, and two or more interventions in both first and subsequent AVFs (Table 1). The median duration of dialysis treatment (dialysis vintage) was 251 days in the group with one intervention and 167 days in the group with two or more interventions.
Table 1.
Baseline demographics by number of interventions to promote AVF maturation
| Zero Interventions | One Intervention | Two or More Interventions | P | |
|---|---|---|---|---|
| Patients (n = 173) | 96 (55.5%) | 54 (31.2%) | 23 (13.3%) | |
| Sex | 0.0107 | |||
| female | 17 (17.7%) | 16 (29.6%) | 11 (47.8%) | |
| male | 79 (82.3%) | 38 (70.4%) | 12 (52.2%) | |
| Race | 0.2664 | |||
| black | 71 (74.0%) | 38 (70.4%) | 20 (87.0%) | |
| white | 25 (26.0%) | 16 (29.6%) | 3 (13.0%) | |
| Diabetes | 0.0422 | |||
| yes | 41 (42.7%) | 30 (55.6%) | 16 (69.6%) | |
| no | 55 (57.3%) | 24 (44.4%) | 7 (30.4%) | |
| PVD | 0.0415 | |||
| yes | 18 (18.8%) | 7 (13.0%) | 9 (39.1%) | |
| no | 78 (81.2%) | 47 (87.0%) | 14 (60.9%) | |
| Access site | 0.7710 | |||
| upper arm | 66 (68.8%) | 38 (70.4%) | 14 (60.9%) | |
| forearm | 30 (31.3%) | 16 (29.6%) | 9 (39.1%) | |
| Age ≥65 | 0.4021 | |||
| yes | 24 (25%) | 16 (28.3%) | 9 (39.1%) | |
| no | 72 (75%) | 38 (71.7%) | 14 (60.9%) | |
| BMI ≥30 | 0.0491 | |||
| yes | 28 (29.2%) | 17 (31.5%) | 13 (56.5%) | |
| no | 68 (70.2%) | 37 (68.5%) | 10 (43.5%) | |
| First versus subsequent fistula | 0.1727 | |||
| first | 61 (63.5%) | 38 (70.4%) | 19 (82.6%) | |
| subsequent | 35 (36.5%) | 16 (29.6%) | 4 (17.4%) |
Cumulative Access Survival
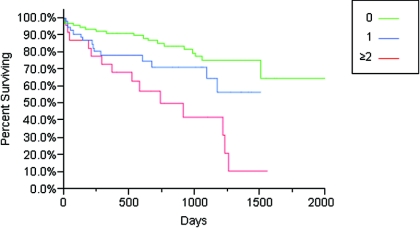
Cumulative survival, defined from the time of access cannulation to permanent failure, was shorter in patients who had two or more interventions before AVF maturation compared with those with zero interventions (HR, 2.07; 95% CI, 1.21 to 2.94; P = 0.0001) (Figure 1). When comparing cumulative survival among patients with one intervention to those with zero interventions before maturation, there was a trend toward worse cumulative survival in patients receiving one intervention before maturation (HR, 1.91; 05% CI, 0.944 to 3.81; P = 0.07) (Figure 1). Cumulative survival in patients with two or more versus one versus zero interventions before maturation was 68% versus 78% versus 92% at 1 year, 57% versus 71% versus 85% at 2 years, and 42% versus 57% versus 75% at 3 years (Figure 1). The median duration of follow-up was 672 days. There was no difference in cumulative survival by center.
Figure 1.
Cumulative access survival (time from fistula cannulation until failure) by number of interventions before manipulation (zero, one, or two or more). By log-rank test, P = 0.0001 for all three groups, P = 0.0620 for zero versus one intervention, and P < 0.0001 for zero versus two or more interventions.
After performing a Cox regression analysis adjusting for interventions before maturation, sex, race, diabetes, peripheral vascular disease, access site, age ≥65, and BMI ≥30, interventions before maturation (two or more) was the only factor associated with cumulative access failure (HR, 1.67; 95% CI, 1.01 to 2.70; P = 0.02; P = 0.004 for the overall model).
Number of Interventions to Maintain Access Patency after Dialysis Use
Patients who had two or more interventions before maturation required a significantly higher mean number of interventions/years after cannulation to maintain patency, as compared with those requiring one intervention (3.51 versus 1.37; P = 0.04) and no interventions before maturation (3.51 versus 0.755; P = 0.004) (Table 2). There was no difference in the number of interventions after AVF use when comparing those AVFs that had zero or one intervention before maturation (0.755 versus 1.37; P = 0.37) (Table 2).
Table 2.
Number of interventions after cannulation by number of interventions to promote AVF maturation
| Zero Interventions | One Intervention | Two or More Interventions | P | |
|---|---|---|---|---|
| Number of patients | 96 | 54 | 23 | |
| Mean number of interventions per year after AVF cannulation (± SE of mean) | 0.755 ± 0.0971 | 1.37 ± 0.308 | 3.51 ± 2.20a | 0.0152 |
When comparing: zero versus one intervention, P = 0.37; 0 versus two or more interventions, P = 0.004; one versus two or more interventions, P = 0.04.
Indicates which group differs from others.
Surgical versus Endovascular Intervention to Promote AVF Maturation
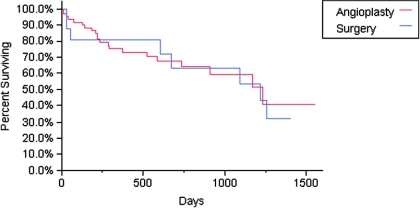
Among the 77 patients who received interventions to promote AVF maturation, 55 received endovascular interventions, 16 received surgical revisions, and six patients received both surgical and endovascular interventions. The six patients who required both surgical and endovascular interventions were placed in the endovascular group for the purposes of the analysis. There was no difference in cumulative survival when comparing patients who had endovascular versus surgery to promote AVF maturation (P = 0.8298) (Figure 2).
Figure 2.
Cumulative access survival (time from fistule cannulation until access failure) comparing angioplasties versus surgery before AVF use. P = 0.8298 by log-rank test. Patients who received both angioplasty and surgery to promote AVF maturation were placed into the angioplasty group for analysis.
Discussion
In an effort to improve vascular access outcomes, both the Fistula First Initiative (9–11) and National Kidney Foundation Kidney Disease Quality Initiative guidelines (8) have promoted increased AVF use in hemodialysis patients. In one respect, these initiatives have been hugely successful, resulting in a progressive increase in AVF use over the past few years, which currently exceeds 50% in the United States hemodialysis population (26). Unfortunately, there has been a concurrent increase in AVFs that fail to mature for dialysis (14,24,27–29), which was as high as 60% in a recent large, multi-center, randomized clinical trial (14). Although there is not a standard definition for AVF nonmaturation, the recently published Dialysis Access Consortium study considered nonmaturation as AVFs not cannulated by two needles with optimal dialysis blood flow within 4 to 5 months after AVF creation (14). The most common etiology for AVF nonmaturation is a lack of vein dilation or aggressive neointimal hyperplasia (4). Nonmaturing AVFs frequently have identifiable anatomic abnormalities (most commonly peri-anastomotic stenosis), which can be recognized by physical examination (evaluation of pulse, thrill, and augmentation) (30), postoperative ultrasound (31,32), or angiogram (19,20,33,34). Targeted percutaneous or surgical interventions to repair these abnormalities are often successful in salvaging nonmaturing AVFs to make them suitable for dialysis (19–21,35–37).
The few published studies evaluating long term outcomes in AVFs requiring interventions to promote maturation reported cumulative survival rates of 68 to 82% at 1 year (19,20,35,36) and 62% at 2 years (35), similar to the rates observed in our investigation. Unfortunately, previous studies did not provide a comparison with a concurrent control group of AVFs not requiring such interventions. The current study evaluated the association between the number of interventions required to promote maturation (zero, one, or two or more) and cumulative fistula survival and observed significantly inferior long-term AVF survival in patients requiring two or more interventions to achieve AVF maturation, as compared with those requiring zero or one interventions. Moreover, AVFs requiring two or more interventions to promote maturation also required more interventions to maintain long-term patency after dialysis use.
Why might interventions to promote fistula maturation be associated with shortened AVF survival and a greater need for future AVF interventions? One possible explanation is that these interventions, particularly endovascular procedures, induce endothelial injury that leads to aggressive neointimal hyperplasia, rapid restenosis, and access failure. In support of this hypothesis, Chang et al. (23) observed that restenotic lesions in AVF after angioplasty had greater cellular proliferation activity within the intima and media, as compared with AVFs with primary stenosis. Likewise, in cardiovascular models of vascular injury after coronary interventions, a sequence of inflammation, granulation, extracellular matrix remodeling, smooth muscle cell proliferation, and migration occurs, leading to neointimal thickening and restenosis, as well as the inability of the vessels to undergo dilation after injury (38–41). An alternative hypothesis is that AVFs that require interventions to achieve maturation are simply created from “poor quality vessels,” which in turn leads to shortened cumulative AVF survival.
The type of vascular intervention may affect long-term fistula survival. Some have speculated that the injury resulting from angioplasty is greater than that obtained with surgical revision. Previous retrospective studies comparing surgical revision and angioplasty of previously functional forearm AVFs that had developed stenosis provided conflicting results, with one study showing improved postintervention AVF patency in the surgery group versus the angioplasty group (42) and another demonstrating no difference in postintervention patency between the two types of intervention (43). Unlike this study, the interventions in these prior studies were used to treat stenosis in functional forearm AVF, rather than to salvage immature AVFs before dialysis use. However, our small sample size precluded definitive conclusions about the relative effect of surgical versus endovascular intervention to promote AVF maturation. We continue to believe, however, that a randomized study examining this issue is desperately needed.
At present, there are no effective pharmacologic treatments to promote AVF maturation, largely due to our limited understanding of the pathophysiology of AVF maturation (1–3,17,44,45). In this regard, a large randomized, double-blinded clinical trial found that clopidogrel significantly reduced early AVF thrombosis but failed to decrease AVF nonmaturation (14). Until effective pharmacologic interventions are established, the mainstay approach to salvaging nonmaturing AVF remains the performance of endovascular or surgical interventions. Whereas such interventions are clearly beneficial in converting immature AVFs to ones that are suitable for dialysis, the use of such interventions is associated with shortened cumulative AVF survival and the need for frequent interventions to maintain their patency.
Finally, we would like to emphasize that we are not arguing against the use of endovascular or surgical intervention for enhancing AVF maturation. In particular, we are completely cognizant of the fact that a lack of intervention would likely have resulted in primary AVF failure in the patients in the intervention group (46). However, we do want to bring to the attention of the dialysis access community the fact that multiple interventions may have at least some negative effect in the long term, both on survival and on the number of interventions required to maintain patency. The latter is likely to significantly influence overall cost (which could become an important determinant of practice patterns in the context of a possible future bundling of dialysis access within overall dialysis care).
This study has some limitations. First, it was a retrospective study. However, both participating centers used similar prospective access databases with all procedures performed at a single hospital or outpatient interventional nephrology center. Thus, we have a high degree of confidence that the access events captured were accurate and comprehensive. Second, due to the retrospective study design, we cannot determine whether the shortened cumulative AVF survival was a consequence of the interventions performed to achieve maturation or whether the need for two or more interventions to achieve AVF maturation was simply a marker for “poor vessels.” Third, we included in our analysis only those AVFs that successfully matured for dialysis. However, our intention was to specifically evaluate access survival after successful cannulation for dialysis, because the major advantages of AVFs over arteriovenous grafts are their longer cumulative survival and lower frequency of interventions, once primary failures are excluded (12,47). Finally, our study evaluated only prevalent dialysis patients; therefore, our results may not be applicable to an incident population. A major strength of our study is that it is multi-center, from two academic centers with large dialysis patient populations. Thus, our results are likely to be broadly applicable to other dialysis centers.
Conclusions
Our results suggest that repeated interventions to promote AVF maturation are associated with shorter long-term AVF survival and an increase in interventions to maintain access patency after successful dialysis use. Furthermore, our results (1) emphasize the importance for further research evaluating the mechanisms of injury associated with interventions to promote maturation and (2) underscore the need for development of novel pharmacologic therapies to enhance cumulative AVF survival in patients whose AVFs require interventions to achieve maturation and decrease the number of interventions to maintain access patency. Thus, we hope that in the future it may be possible to combine novel anti-stenotic therapies and devices with surgical or endovascular interventions to enhance AVF maturation. Unfortunately, we do not have access to such interventions at present, hence the need to quantify the effect of repeated endovascular and surgical interventions on AVF survival.
Disclosures
Dr. Lee is a consultant for Proteon Therapeutics. Dr. Roy-Chaudhury is on the advisory board/consultant for Pervasis Therapeutics, Inc., Proteon Therapeutics, WL Gore, Bioconnect Systems, Philometron, and NanoVasc and receives research support from BioConnect Systems and WL Gore. Dr. Allon is a consultant for CorMedix. These funding sources had no involvement in the design or execution of this study.
Acknowledgments
Dr. Lee is supported by NIH Grant 5K23DK083528-02 and a National Kidney Foundation Franklin McDonald/Fresenius Medical Care Young Investigator Clinical Research Award. Dr. Roy-Chaudhury is supported by NIH Grants 5U01-DK82218, NIH 5U01-DK82218S (ARRA), NIH 5R01-EB004527, and NIH 1R21-DK089280-01 and a VA Merit Review and industry grants from Bioconnect Systems, WL Gore, and Shire. Portions of this manuscript were presented in abstract form at the American Society of Nephrology Renal Week, October 29, 2009, San Diego, California. Some initial work on the concepts outlined in this manuscript was undertaken by Dr. Tony Samaha and Dr. Syed Ali.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Fistulas First—But Can They Last?” on pages 463–464.
References
- 1. Lee T, Roy-Chaudhury P: Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv Chronic Kidney Dis 16: 329–338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roy-Chaudhury P, Lee TC: Vascular stenosis: Biology and interventions. Curr Opin Nephrol Hypertens 16: 516–522, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Roy-Chaudhury P, Spergel LM, Besarab A, Asif A, Ravani P: Biology of arteriovenous fistula failure. J Nephrol 20: 150–163, 2007 [PubMed] [Google Scholar]
- 4. Roy-Chaudhury P, Sukhatme VP, Cheung AK: Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J Am Soc Nephrol 17: 1112–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Feldman HI, Held PJ, Hutchinson JT, Stoiber E, Hartigan MF, Berlin JA: Hemodialysis vascular access morbidity in the United States. Kidney Int 43: 1091–1096, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Feldman HI, Kobrin S, Wasserstein A: Hemodialysis vascular access morbidity. J Am Soc Nephrol 7: 523–535, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Beathard GA: Strategy for maximizing the use of arteriovenous fistulae. Semin Dial 13: 291–296, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Clinical Practice Guidelines for Vascular Access. Am J Kidney Dis 48: S176–S273, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Fistula First National Access Improvements Initiative. Available at: www.fistulafirst.org/ Accessed July 20, 2010
- 10. Gold JA, Hoffman K: Fistula First: The National Vascular Access Improvement Initiative. Wmj 105: 71–73, 2006 [PubMed] [Google Scholar]
- 11. Peters VJ, Clemons G, Augustine B: “Fistula First” as a CMS breakthrough initiative: Improving vascular access through collaboration. Nephrol Nurs J 32: 686–687, 2005 [PubMed] [Google Scholar]
- 12. Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI. for the Dialysis Access Consortium Study G : Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dember LM, Kaufman JS, Beck GJ, Dixon BS, Gassman JJ, Greene T, Himmelfarb J, Hunsicker LG, Kusek JW, Lawson JH, Middleton JP, Radeva M, Schwab SJ, Whiting JF, Feldman HI: Design of the Dialysis Access Consortium (DAC) clopidogrel prevention of early AV fistula thrombosis trial. Clin Trials 2: 413–422, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Asif A, Lenz O, Merrill D, Cherla G, Cipleu CD, Ellis R, Francois B, Epstein DL, Pennell P: Percutaneous management of perianastomotic stenosis in arteriovenous fistulae: Results of a prospective study. Kidney Int 69: 1904–1909, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Asif A, Roy-Chaudhury P, Beathard GA: Early arteriovenous fistula failure: A logical proposal for when and how to intervene. Clin J Am Soc Nephrol 1: 332–339, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Beathard GA: Angioplasty for arteriovenous grafts and fistulae. Semin Nephrol 22: 202–210, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Beathard GA, Arnold P, Jackson J, Litchfield T: Aggressive treatment of early fistula failure. Kidney Int 64: 1487–1494, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Beathard GA, Settle SM, Shields MW: Salvage of the nonfunctioning arteriovenous fistula. Am J Kidney Dis 33: 910–916, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Nassar GM, Nguyen B, Rhee E, Achkar K: Endovascular treatment of the “failing to mature” arteriovenous fistula. Clin J Am Soc Nephrol 1: 275–280, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Beathard GA: Fistula salvage by endovascular therapy. Adv Chronic Kidney Dis 16: 339–351, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Chang CJ, Ko PJ, Hsu LA, Ko YS, Ko YL, Chen CF, Huang CC, Hsu TS, Lee YS, Pang JH: Highly increased cell proliferation activity in the restenotic hemodialysis vascular access after percutaneous transluminal angioplasty: Implication in prevention of restenosis. Am J Kidney Dis 43: 74–84, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Kats M, Hawxby AM, Barker J, Allon M: Impact of obesity on arteriovenous fistula outcomes in dialysis patients. Kidney Int 71: 39–43, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Clinical Indicators & Preventive Health. Am J Kidney Dis 55: S259–S268, 2010 [Google Scholar]
- 27. Allon M, Ornt DB, Schwab SJ, Rasmussen C, Delmez JA, Greene T, Kusek JW, Martin AA, Minda S: Factors associated with the prevalence of arteriovenous fistulas in hemodialysis patients in the HEMO study: Hemodialysis (HEMO) Study Group. Kidney Int 58: 2178–2185, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, Allon M: Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int 56: 275–280, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Beathard GA: An algorithm for the physical examination of early fistula failure. Semin Dial 18: 331–335, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Singh P, Robbin ML, Lockhart ME, Allon M: Clinically immature arteriovenous hemodialysis fistulas: Effect of US on salvage. Radiology 246: 299–305, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, Allon M: Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 225: 59–64, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Asif A, Cherla G, Merrill D, Cipleu CD, Briones P, Pennell P: Conversion of tunneled hemodialysis catheter-consigned patients to arteriovenous fistula. Kidney Int 67: 2399–2406, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Faiyaz R, Abreo K, Zaman F, Pervez A, Zibari G, Work J: Salvage of poorly developed arteriovenous fistulae with percutaneous ligation of accessory veins. Am J Kidney Dis 39: 824–827, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Shin SW, Do YS, Choo SW, Lieu WC, Choo IW: Salvage of immature arteriovenous fistulas with percutaneous transluminal angioplasty. Cardiovasc Intervent Radiol 28: 434–438, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Clark TW, Cohen RA, Kwak A, Markmann JF, Stavropoulos SW, Patel AA, Soulen MC, Mondschein JI, Kobrin S, Shlansky-Goldberg RD, Trerotola SO: Salvage of nonmaturing native fistulas by using angioplasty. Radiology 242: 286–292, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Turmel-Rodrigues L, Mouton A, Birmele B, Billaux L, Ammar N, Grezard O, Hauss S, Pengloan J: Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol Dial Transplant 16: 2365–2371, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Inoue T, Node K: Molecular basis of restenosis and novel issues of drug-eluting stents. Circ J 73: 615–621, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN: Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation 104: 2228–2235, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Nakatani M, Takeyama Y, Shibata M, Yorozuya M, Suzuki H, Koba S, Katagiri T: Mechanisms of restenosis after coronary intervention: difference between plain old balloon angioplasty and stenting. Cardiovasc Pathol 12: 40–48, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Libby P, Tanaka H: The molecular bases of restenosis. Prog Cardiovasc Dis 40: 97–106, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Tessitore N, Mansueto G, Lipari G, Bedogna V, Tardivo S, Baggio E, Cenzi D, Carbognin G, Poli A, Lupo A: Endovascular versus surgical preemptive repair of forearm arteriovenous fistula juxta-anastomotic stenosis: Analysis of data collected prospectively from 1999 to 2004. Clin J Am Soc Nephrol 1: 448–454, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Lipari G, Tessitore N, Poli A, Bedogna V, Impedovo A, Lupo A, Baggio E: Outcomes of surgical revision of stenosed and thrombosed forearm arteriovenous fistulae for haemodialysis. Nephrol Dial Transplant 22: 2605–2612, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Diskin CJ: Novel insights into the pathobiology of the vascular access: Do they translate into improved care? Blood Purif 29: 216–229, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Dixon BS: Why don't fistulas mature? Kidney Int 70: 1413–1422, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Miller GA, Goel N, Khariton A, Friedman A, Savransky Y, Trusov I, Jotwani K, Savransky E, Preddie D, Arnold WP: Aggressive approach to salvage non-maturing arteriovenous fistulae: A retrospective study with follow-up. J Vasc Access 10: 183–191, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Lee T, Barker J, Allon M: Comparison of survival of upper arm arteriovenous fistulas and grafts after failed forearm fistula. J Am Soc Nephrol 18: 1936–1941, 2007 [DOI] [PubMed] [Google Scholar]