Summary
Background and objectives
Phosphate control impacts dialysis outcomes. Our aim was to define peritoneal phosphate transport in peritoneal dialysis (PD) and to explore its association with hyperphosphatemia, phosphate clearance (PPhCl), and PD modality.
Design, setting, participants, & measurements
Two hundred sixty-four patients (61% on continuous ambulatory PD [CAPD]) were evaluated at month 12. PPhCl was calculated from 24-hour peritoneal effluent. Phosphate (Ph) and creatinine (Cr) dialysate/plasma (D/P) were calculated at a 4-hour 3.86% peritoneal equilibration test.
Results
D/PPh correlated with D/PCr. PPhCl correlated better with D/PPh than with D/PCr. Prevalence of hyperphosphatemia (>5.5 mg/dl) was 30%. In a multiple regression analysis, only residual renal function was independently, negatively associated with hyperphosphatemia; in anuric patients, only D/PPh was an independent factor predicting hyperphosphatemia. D/PPh was 0.57 ± 0.10, and according to this, 16% of the patients were fast, 31% were fast-average, 35% were slow-average, and 17% were slow transporters. PPhCl was 37.5 ± 11.7 L/wk; it was lower in the slow transporter group (31 ± 14 L/wk). Among fast and fast-average transporters, PPhCl was comparable in both PD modalities. In comparison to automated PD, CAPD was associated with increased PPhCl among slow-average (36 ± 8 versus 32 ± 7 L/wk) and slow transporters (34 ± 15 versus 24 ± 9 L/wk).
Conclusions
In hyperphosphatemic, particularly anuric, patients, optimal PD modality should consider peritoneal phosphate transport characteristics. Increasing dwell times and transfer to CAPD are effective strategies to improve phosphate handling in patients with inadequate phosphate control on automated PD.
Introduction
Hyperphosphatemia is common among dialysis patients and is a major risk factor for cardiovascular mortality in both hemodialysis and peritoneal dialysis (PD) patients (1–7). In PD patients, phosphate control is known to deteriorate as residual renal function (RRF) declines (8,9). In fact, although the importance of RRF in maintaining serum phosphorus levels in PD patients has already been established (8), the role of peritoneal phosphate clearance in achieving adequate phosphate homeostasis has not been well studied. Dialytic phosphate clearance is the product of nonmodifiable peritoneal transport characteristics and the modifiable components of dialysis prescription. Whether creatinine may be used as a surrogate marker of phosphate transport is now under debate. Some authors consider that creatinine clearance measurements provide a good estimate of phosphorus clearance (10), whereas others showed that the peritoneal transport state defined by the creatinine equilibration pattern is poorly predictive of daily phosphate clearance (11). In fact, until now, no studies have tried to define peritoneal membrane phosphate transport status in an prevalent adult PD population. Finally, few studies have addressed the importance of PD modality concerning dialytic phosphate clearance (10,12).
Because of this, the objectives of our study were to define peritoneal membrane phosphate transport status in a large, prevalent adult PD population and to explore its association with hyperphosphatemia, phosphate peritoneal clearance, and PD modality.
Materials and Methods
This was a cross-sectional and retrospective study conducted at the La Paz University Hospital Home Dialysis Unit. From the 348 prevalent patients on PD that were treated in our unit from January 1992 until January 2009, 264 patients were included in this cross-sectional observational study. We excluded 84 patients with <1 year of PD treatment. Each patient included was evaluated at 12 months (1 year) after starting PD. Our patients were receiving standard prescriptions of continuous ambulatory peritoneal dialysis (CAPD) or automated peritoneal dialysis (APD), according to European best practice recommendations (13). Solutions with high calcium content (3.5 mEq/L) were standardly prescribed in the unit, unless the patient had sustained hypercalcemia. Phosphate binders were prescribed, as appropriate, to maintain serum phosphorus concentration between 3.5 and 5.5 mg/dl, according to the 2003 Kidney Disease Outcomes Quality Initiative guidelines for mineral metabolism (14). Residual renal function was calculated as the average of 24-hour urinary urea and creatinine clearances. Standard parameters of dialysis adequacy were determined by measuring total (renal and peritoneal) weekly urea clearance (Kt/V) and creatinine clearance using standard methods (15). Dialysate creatinine concentration was corrected for interference by glucose according to a reference formula determined by our laboratory. Protein equivalent nitrogen appearance (nPNA) was calculated using methods described by Randerson et al. (16) and normalized to actual weight. Phosphate renal and peritoneal clearances were calculated as follows: peritoneal phosphate clearance (L/wk per 1.73 m2) = (dialysate phosphate in mg/dl/plasma phosphate in mg/dl) × 24-hour effluent dialysate volume (L) × 7 (corrected for 1.73 m2 BSA); renal phosphate clearance (L/wk per 1.73 m2) = (urine phosphate in mg/dl/plasma phosphate in mg/dl) × 24-hour urinary volume (L) × 7 (corrected for 1.73 m2 BSA). Additionally, we performed a 3.86%, 4-hour glucose peritoneal equilibration test (PET). Dialysate/plasma (D/P) creatinine (Cr) ratio and D/P phosphate (Ph) ratio were measured at 4 hours. The 24-hour dialysate collection was performed exactly on the day before the realization of the PET for all patients. Concerning phosphate transport status, our patients were classified as slow (D/P Ph < 0.47), slow-average (0.47 ≤ D/P Ph <0.57), fast-average (0.57 ≤ D/P Ph <0.68), or fast (D/P Ph ≥ 0.68) transporters, according to the mean ± SD of the dialysate/plasma phosphate ratio (D/P Ph). We also divided our patients by the dialysate/plasma creatinine ratio (D/P Cr) as slow (D/P Cr ≤ 0.49), slow-average (0.50 ≤ D/P Cr ≤ 0.64), fast-average (0.65 ≤ D/P Cr ≤ 0.80), and fast (D/P Cr ≥ 0.81) transporters according to the criteria defined by Twardowski et al. (17).
Results were expressed as frequencies and percentages for categorical variables, mean ± SD for continuous variables, and median and interquartile range for nonparametric data. Correlations between two continuous variables were expressed as Pearson's or Spearman's correlations coefficients. Differences between hyperphosphatemic (serum phosphate > 5.5 mg/dl) and normophosphatemic (serum phosphate ≤ 5.5 mg/dl) patients were evaluated using unpaired t test, Mann-Whitney U test, and χ2 test as appropriate. In the subanalysis of anuric patients, we analyzed peritoneal phosphate clearance both as a continuous and a categorical variable, according to the mean value of peritoneal phosphate clearance (37.5 L/wk per 1.73 m2). Multivariate analysis using conditional logistic regression and multiple linear regression models (as appropriated) were performed to analyze the determinants of hyperphosphatemia (serum phosphate being analyzed both as a continuous and categorical variable) at 1 year of treatment, first in all of the patients and then focused on anuric patients. The following variables were included in the models: PD modality as a categorical variable (CAPD versus APD) and nPNA, peritoneal urea Kt/V, peritoneal creatinine clearance, peritoneal phosphate clearance, D/P Ph, D/P Cr, and RRF as continuous variables. We compared clearances of small solutes (urea, creatinine, and phosphate) according to PD modality (CAPD versus APD) using the t test and Mann-Whitney U test, as appropriate. Peritoneal phosphate transport was categorized according to mean ± SD of D/P Ph. We compared clearances of small solutes (urea, creatinine, and phosphate) across the four categories of peritoneal membrane phosphate transport status using one-way ANOVA and Kruskal-Wallis tests, as appropriate. We finally explored the adequacy and phosphate clearances according to PD modality, controlling for peritoneal phosphate transport status. P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS, version 15.0, for Windows software (SPSS, Chicago, IL).
Results
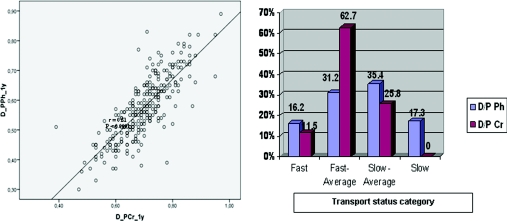
Patient baseline characteristics are listed in Table 1. The baseline evaluation (at 3 months) concerning peritoneal membrane small solute transport and residual renal function (data not shown) was not significantly different from that at 1 year. In the PET at 1 year, mean 4-hour D/P ratio was 0.70 ± 0.1 for creatinine and 0.57 ± 0.1 for phosphate. A close correlation was observed between D/P Ph and D/P Cr at 1 year (r = 0.81, P < 0.0001; Figure 1, left).
Table 1.
Population baseline characteristics
| Total (n = 264) | |
|---|---|
| Sex (male) | 160 (60.6%) |
| Age (years) | 51.4 ± 16.0 |
| CAPD/APD | 160 (60.6%)/104 (39.4%) |
| Diabetes mellitus | 46 (17.4%) |
| Renal diagnosis | |
| Chronic glomerulonephritis | 47 (17.8%) |
| Tubulointersticial nephropathy | 47 (17.8%) |
| Diabetic nephropathy | 37 (14.0%) |
| Poliquistic autossomic dominant nephropathy | 29 (11.0%) |
| Hypertensive nephrosclerosis | 29 (11.0%) |
| Unknown | 38 (14.4%) |
| Others | 37 (14.0%) |
Figure 1.
(Left) Relation between D_P Ph_1y (dialysate to plasma phosphate ratio) and D_PCr_1y (dialysate to plasma creatinine ratio). (Right) Categorization of patients concerning phosphate peritoneal transport (according to mean ± SD of D/P Ph) and creatinine peritoneal transport (according to Twardowski et al.).
Correlation between Creatinine Transport and Phosphate Transport
Peritoneal phosphate clearance at 1 year correlated better with peritoneal creatinine clearance at 1 year than with peritoneal urea Kt/V at 1 year (r = 0.650, P < 0.0001 and r = 0.451, P < 0.0001, respectively). Peritoneal phosphate clearance at 1 year correlated better with D/P Ph at 1 year than with D/P Cr at 1 year (r = 0.490, P < 0.0001 and r = 0.400 P < 0.0001, respectively).
Hyperphosphatemia, RRF, and Phosphate Clearance
At 1 year of treatment, 79 patients (30%) had hyperphosphatemia (serum phosphate levels >5.5 mg/dl). Phosphate levels correlated negatively with residual renal function and renal phosphate clearance (r = −0.290, P < 0.0001 and r = −0.225, P = 0.008, respectively) but did not correlate with nPNA or with peritoneal small solute clearances. In a multiple regression analysis, only RRF was independently and negatively associated with hyperphosphatemia [Exp(B) = 0.75; 95% confidence interval, 0.64 to 0.89; P = 0.001]. The same results were obtained considering serum phosphate as a continuous variable (data not shown). In fact, serum phosphate levels were >5.5mg/dl in 43.6% of anuric patients (n = 78) versus 24.2% of patients with RRF (n = 186, P = 0.002). Among patients with RRF, those with hyperphosphatemia had significantly lower renal phosphate clearances than patients with serum phosphate ≤5.5mg/dl (28.2 ± 21.1 versus 45.5 ± 30.1 L/wk per 1.73 m2, P = 0.018), whereas peritoneal phosphate clearance was similar in the two groups (34.7 ± 10.7 versus 34.6 ± 10.9 L/wk per 1.73 m2; Table 2).
Table 2.
Dialysis adequacy, phosphate clearance, and peritoneal transport status in patients with a serum phosphorous level of ≤5.5 mg/dl versus >5.5 mg/dl and stratified according to residual renal function at 1 year of treatment
| Serum Phosphate |
All Patients n = 264 | ||||
|---|---|---|---|---|---|
| Patients with RRF |
Anuric Patients |
||||
| ≤5.5 mg/dl (n = 141) | >5.5 mg/dl (n = 45) | ≤5.5 mg/dl (n = 44) | >5.5 mg/dl (n = 34) | ||
| Total weekly urea Kt/V | 2.7 ± 0.7a | 2.1 ± 0.4a | 2.1 ± 0.3 | 2.0 ± 0.3 | 2.4 ± 0.7 |
| Peritoneal weekly urea Kt/V | 1.5 ± 0.4 | 1.4 ± 0.3 | 2.1 ± 0.3 | 2.0 ± 0.3 | 1.6 ± 0.4 |
| Renal weekly urea Kt/V | 1.2 ± 0.8a | 0.7 ± 0.5a | 0.8 ± 0.8 | ||
| Total weekly CCr (L/wk per 1.73 m2) | 93.7 ± 34.5a | 68.0 ± 20.4a | 52.6 ± 10.3 | 53.7 ± 7.8 | 79.0 ± 32.7 |
| Peritoneal weekly CCr (L/wk per 1.73 m2) | 35.5 ± 9.8 | 34.2 ± 7.3 | 52.6 ± 10.3 | 53.7 ± 10.1 | 39.4 ± 11.8 |
| Renal weekly CCr (L/wk per 1.73 m2) | 58.2 ± 37.1a | 33.8 ± 22.2a | 39.6 ± 38.2 | ||
| Total weekly CPh (L/wk per 1.73 m2) | 80.1 ± 29.9a | 62.9 ± 21.5a | 46.6 ± 10.6 | 43.4 ± 11.3 | 61.6 ± 27.2 |
| Peritoneal weekly CPh (L/wk per 1.73 m2) | 34.6 ± 10.9 | 34.7 ± 10.7 | 46.6 ± 10.6 | 43.4 ± 11.3 | 37.5 ± 11.7 |
| Renal weekly CPh (L/wk per 1.73 m2) | 45.5 ± 30.1a | 28.2 ± 21.1a | 22.2 ± 29.6 | ||
| RRF (ml/min per 1.73 m2) | 5.8 ± 3.7a | 3.7 ± 2.5a | 3.8 ± 3.8 | ||
| D/P Cr | 0.69 ± 0.1 | 0.70 ± 0.1 | 0.73 ± 0.1 | 0.71 ± 0.1 | 0.70 ± 0.09 |
| D/P Ph | 0.56 ± 0.1 | 0.58 ± 0.1 | 0.63 ± 0.1b | 0.57 ± 0.1b | 0.57 ± 0.11 |
| nPNA (g/kg per day) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.2 | 1.2 ± 0.3 |
Values are expressed as mean ± SD. CCr, creatinine clearance; CPh phosphate clearance.
P < 0.05 patients with RRF and serum phosphorous ≤5.5 mg/dl versus patients with RRF and serum phosphorous >5.5 mg/dl, according to independent sample t test.
P < 0.05 anuric patients with serum phosphorous ≤5.5 mg/dl versus anuric patients with phosphorous >5.5 mg/dl, according to independent sample t test.
Anuric Patients
Anuric hyperphosphatemic patients had lower mean levels of phosphate peritoneal clearance and a statistically significant slower membrane phosphate transport rate (Table 2). Concerning adequacy parameters among anuric patients, we observed that 97.2% had a peritoneal Kt/V of 1.7 or greater and 83.3% had a peritoneal creatinine clearance of 45 L/wk per 1.73 m2 or greater. However, only 68.8% had a peritoneal phosphate clearance of 37.5 L/wk per 1.73 m2 or greater (equal or greater than the mean peritoneal phosphate clearance of the population in this analysis). In fact, serum phosphate levels were >5.5mg/dl in 62.5% of anuric patients with peritoneal phosphate clearance <37.5 L/wk per 1.73 m2 (n = 24) versus 33.9% of anuric patients with a peritoneal phosphate clearance of ≥37.5 L/wk per 1.73 m2 (n = 54, P = 0.019). In a multiple regression analysis, only D/P Ph was independently and negatively associated with hyperphosphatemia in anuric patients [Exp(B) = 0.003, 95% confidence interval, 0.001 to 0.50; P = 0.026]. We obtained the same results considering serum phosphate as a continuous variable (data not shown).
Modality of PD Controlling for Peritoneal Membrane Phosphate Transport
To compare phosphate clearances according to PD modality, also controlling for peritoneal membrane phosphate transport rate, we studied 260 patients (we excluded 4 patients without 1-year PET evaluation): 157 (60.4%) were treated with CAPD and 103 (39.6%) with APD. We detected a different categorization of patients concerning phosphate and creatinine peritoneal transports. Relative to peritoneal phosphate transport, 42 (16.2%) patients were classified as fast transporters (D/P Ph ≥ 0.68), 81 (31.2%) as fast-average transporters (0.57 ≤ D/P Ph <0.68), 92 (35.4%) as slow-average transporters (0.47 ≤ D/P Ph < 0.57), and 45 (17.3%) as slow transporters (D/P Ph < 0.47). Concerning peritoneal creatinine transport, 30 patients (11.5%) were classified as fast transporters (D/P Cr ≥ 0.81), 163 (62.7%) as fast-average transporters (0.65 ≤ D/P Cr ≤ 0.80), 67 (25.8%) as slow-average transporters (0.50 ≤ D/P Cr ≤ 0.64), and 0 as slow transporters (D/P Cr ≤ 0.49; Table 3; Figure 1, right).
Table 3.
Peritoneal membrane transport status and solute clearances according to PD modality, at 1 year of treatment
| Variable | CAPD | APD | P |
|---|---|---|---|
| Peritoneal urea Kt/V | 1.45 ± 0.3 | 1.71 ± 0.5 | <0.0001a |
| Peritoneal creatinine clearance (L/wk per 1.73 m2) | 38.7 ± 9.1 | 40.0 ± 13.5 | 0.527 |
| Peritoneal phosphate clearance (L/wk per 1.73 m2) | 36.5 ± 9.2 | 36.7 ± 12.1 | 0.928 |
| D/P Cr | 0.69 ± 0.1 | 0.71 ± 0.1 | 0.319 |
| fast transporter | 17/30 (56.7%) | 13/30 (43.3%) | |
| fast-average transporter | 98/163 (60.1%) | 65/163 (39.9%) | |
| slow-average transporter | 42/67 (62.7%) | 25/67 (37.3%) | |
| slow transporter | 0 | 0 | |
| D/P Ph | 0.61 ± 0.1 | 0.61 ± 0.1 | 0.884 |
| fast transporter | 23/42 (54.8%) | 13/42 (45.2%) | |
| fast-average transporter | 49/81 (60.5%) | 32/81 (39.5%) | |
| slow-average transporter | 52/92 (56.5%) | 40/92 (43.5%) | |
| slow transporter | 33/45 (73.3%) | 12/45 (27.7%) |
Values are expressed as frequencies and percentages for categorical variables, mean ± SD for continuous variables. Patients are categorized, concerning creatinine transport status, as slow (D/P Cr ≤ 0.49), slow-average (0.50 ≤ D/P Cr ≤ 0.64), fast-average (0.65 ≤ D/P Cr ≤ 0.80), and fast (D/P Cr ≥ 0.81) transporters, according to the criteria defined by Twardowski et al.. Patients are categorized, concerning phosphate transport status, as slow (D/P Ph < 0.47), slow-average (0.47 ≤ D/P Ph < 0.57), fast-average (0.57 ≤ D/P Ph < 0.68), or fast (D/P Ph ≥ 0.68) transporters, according to mean ± SD of dialysate/plasma phosphate ratio (D/P Ph).
P < 0.005 comparisons between patients in CAPD versus APD using the t test if data were parametric and the Mann-Whitney U test if nonparametric.
According to PD modality, patients treated with APD had higher peritoneal urea Kt/V than patients treated with CAPD (1.71 ± 0.5 versus 1.45 ± 0.3, P < 0.0001), but there was no difference in peritoneal creatinine clearance or in peritoneal phosphate clearance between those treated with APD or CAPD (Table 3). Patients with fast-transport membranes had a higher peritoneal urea Kt/V, a higher peritoneal creatinine clearance, and a higher peritoneal phosphate clearance than the rest of categories (Table 4).
Table 4.
Solute clearances comparison across peritoneal membrane phosphate transport status, at 1 year of treatment
| Variable | Fast | Fast-Average | Slow-Average | Slow | P |
|---|---|---|---|---|---|
| Peritoneal urea Kt/V | 1.87 ± 0.5 | 1.63 ± 0.5 | 1.58 ± 0.4 | 1.51 ± 0.4 | 0.016a |
| Peritoneal creatinine clearance (L/wk per 1.73 m2) | 49.3 ± 12.2 | 41.8 ± 13.9 | 37.1 ± 8.8 | 34.3 ± 12.2 | 0.005a |
| Peritoneal phosphate clearance (L/wk per 1.73 m2) | 47.4 ± 12.6 | 39.4 ± 9.9 | 34.0 ± 7.6 | 31.4 ± 14.3 | <0.0001a |
Values expressed as mean ± SD. Patients classified as slow, slow-average, fast-average. or fast transporters, according to mean ± SD of dialysate plasma phosphate ratio (D/P Ph) at a 4-hour, 3.86% glucose PET.
P < 0.005 intergroup solute clearances comparison across the four transport groups using one-way ANOVA if data were parametric and Kruskal-Wallis test if nonparametric.
When peritoneal phosphate clearance by modality was examined for each phosphate membrane category, there was no significant difference in phosphate clearance between the modalities in the fast and fast-average transport categories (Table 5). However, in the slow-average and slow categories, treatment with CAPD was associated with significantly higher phosphate clearance than with APD (Table 5). We did not found any significant difference in RRF or in renal phosphate clearance between the four groups (slow, slow-average, fast-average, and fast transporters) that could indirectly interfere on peritoneal phosphate transport. There was no significant difference in peritoneal creatinine clearance between the two PD modalities across any of the membrane transport categories (Table 5).
Table 5.
Peritoneal urea, creatinine, and phosphate clearances according to peritoneal membrane phosphate transport status and PD modality, at 1 year of treatment
| CAPD | APD | P | |
|---|---|---|---|
| Peritoneal Kt/V | |||
| fast | 1.47 ± 0.3 | 1.99 ± 0.4 | 0.026a |
| fast-average | 1.74 ± 0.51 | 1.56 ± 0.5 | 0.054 |
| slow-average | 1.66 ± 0.2 | 1.46 ± 0.4 | 0.242 |
| slow | 1.58 ± 0.3 | 1.44 ± 0.3 | 0.720 |
| Peritoneal creatinine clearance | |||
| fast | 49.6 ± 8.8 | 54.4 ± 12.8 | 0.249 |
| fast-average | 41.0 ± 14.2 | 42.3 ± 14.0 | 0.775 |
| slow-average | 41.1 ± 6.2 | 37.1 ± 11.5 | 0.968 |
| slow | 37.2 ± 5.1 | 31.8 ± 13.2 | 0.279 |
| Peritoneal phosphate clearance | |||
| fast | 46.9 ± 12.6 | 48.1 ± 13.0 | 0.755 |
| fast-average | 39.3 ± 10.4 | 39.6 ± 9.3 | 0.865 |
| slow-average | 35.9 ± 7.8 | 31.6 ± 6.6 | 0.006a |
| slow | 33.9 ± 15.2 | 24.5 ± 9.0 | 0.049a |
Values expressed as mean ± SD. Patients classified as slow, slow-average, fast-average, or fast transporters, according to mean ± SD of dialysate plasma phosphate ratio (D/P Ph) at a 4-hour, 3.86% glucose PET.
P < 0.005 intergroup comparisons between patients in CAPD versus APD using the t test if data were parametric and the Mann-Whitney U test if nonparametric.
Discussion
Our study highlighted that, other than RRF, peritoneal phosphate transport rate and clearance evaluations are additional parameters that can be used to optimize phosphorus control in PD patients.
Hyperphosphatemia is highly prevalent in PD patients and is a strong predictor of overall and cardiovascular mortality (1–4). Although elimination of inorganic phosphate by dialysis is a cornerstone in the management of hyperphosphatemia, the subject of phosphate handling by PD is scarcely addressed and has not been completely studied. Phosphate transport across the peritoneum is influenced by osmotic, chemical, and electrical gradients, as well as by transmembranous active phosphate transporters and thus is more complex than peritoneal urea or creatinine transport (18–20). As in Sedlacek et al. (10), our study showed that peritoneal phosphate clearance is more closely related with peritoneal creatinine clearance than with peritoneal urea clearance. This finding must be related to some special features of the phosphate molecule, because the molecular weight of phosphate (96 Da) lies right between those of urea (60 Da) and creatinine (130 Da), and the molecular radius of phosphate (2.8 Å) is closer to that of creatinine (3.0 Å) than urea (1.8 Å). However, concerning peritoneal membrane transport status, we showed a closer relation with peritoneal phosphate clearance and D/P Ph than with D/P Cr in a 4-hour, 3.86% glucose PET. This finding is even more important once residual renal function is lost. In that circumstance, the peritoneal membrane phosphate transport status plays the most important role in the management of hyperphosphatemia by PD. Hence, when we divided patients according to the classical Twardowski categories of peritoneal membrane transport status, we found that only 67 patients (25.8%) had a slow-average transport status, and none fell into the slow transport status category. However, when divided according to mean ± SD of D/P Ph, 137 patients (52.7%) had a slow-average/slow transport status, and these patients had significantly lower values of peritoneal small solute clearances than the patients in the fast/fast-average transport category. This means that D/P Cr is not a sufficiently accurate measure to classify patients for peritoneal membrane phosphate transport status. The aqueous layer that surrounds the phosphate molecule, increasing its effective molecular weight, may be one of the reasons why creatinine apparently reaches a more rapid equilibration across the peritoneal membrane than phosphate (18,20,21).
Previous studies that made direct comparisons of phosphate clearance between CAPD and APD have not shown significant differences in peritoneal phosphate clearance (10,22) or worse peritoneal phosphate clearance on CAPD (23). These studies were limited by small sample size and no adjustment for peritoneal membrane transport category. Sedlaceak et al. (10) and Badve et al. (12) reported that phosphate clearance is increased in fast transporters compared with slow transporters in CAPD and APD when peritoneal transport status is defined according to Twardowski categories of D/P Cr in a 4-hour, 3.86% glucose PET. Our study expands on the work of these groups because we compared phosphate clearance between CAPD and APD by adjusting for peritoneal membrane phosphate transport category. In fact, when we compared modalities across each peritoneal phosphate transport category, we found that PD modality is a significant determinant of phosphate handling, in subgroups of patients, with superior peritoneal phosphate clearance associated with CAPD treatment among slow and slow-average transporters. Thus, our data suggest that peritoneal membrane phosphate transport status should also be considered when optimizing the PD prescription, especially among anuric patients. Like Wang et al. (8), we showed that anuric PD patients have poorer phosphate control than those with residual renal function, and in these patients, serum phosphate is an inverse function of the peritoneal phosphate clearance. Our findings that treatment with CAPD was associated with an increase in phosphate clearance of 13.6% among slow-average transporters and 38.4% among slow transporters, compared with APD, suggest that PD prescription may play an important role in phosphate handling, especially among anuric patients with slow transport categories.
The International Society for Peritoneal Dialysis guidelines on PD adequacy recommend a urea Kt/V target of ≥1.7 and a separate target of ≥45 L/wk per 1.73 m2 of creatinine clearance (13). Our data suggest that focusing only on urea and creatinine for assessment of dialysis adequacy may result in inadequate clearance of phosphate. In fact, although the majority of our anuric patients accomplished those adequacy targets, 43.6% of them had hyperphosphatemia. From these, 44% had a peritoneal phosphate clearance <37.5 L/wk per 1.73 m2, which might be amenable by adjusting PD prescription to peritoneal membrane phosphate transport rate, because 80% of them were slow/slow-average phosphate transporters, and 50% of these patients were on APD.
To our knowledge, this is the first study that defines peritoneal membrane phosphate transport status in a large, prevalent adult PD population and explores its association with hyperphosphatemia and peritoneal phosphate clearance.
One limitation is that this is a cross-sectional and observational study. Although we assessed a cross-section of the PD population who were receiving adequate dialysis on CAPD or APD, we did not attempt to assess the effect of tidal regimens, dwell times, or number of cycles on phosphate clearance. Another limitation of our study is that the phosphate binding prescription was not included. However, the lack of correlation between indirect protein intake (nPNA) with serum phosphate that we found, the universal uncertainty about phosphate binder accomplishment, and the fact that we focused our study on peritoneal phosphate elimination, may neutralize the effect of these limitations.
Conclusions
This study contributes to our understanding of peritoneal phosphate clearance by highlighting the importance of establishing peritoneal membrane phosphate transport status. In hyperphosphatemic and/or anuric patients, the decision on the optimal PD modality should also take into account peritoneal phosphate transport characteristics and not just urea Kt/V or peritoneal creatinine clearance. Increasing dwell times or transfer to CAPD could be effective strategies to improve phosphate handling in patients with inadequate phosphate control on APD.
Disclosures
This study has been performed partially with the help of Instituto de Salud Carlos III and Fondos FEDER (REDinREN, RETICS 06/0016) and FIS 09/00641 to R.S.
Acknowledgments
A.P.B. is grateful to Dr. Ernesto Rocha for the opportunity to collaborate with the Home Dialysis Unit of La Paz University Hospital.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Krediet RT, Balafa O: Cardiovascular risk in the peritoneal dialysis patient. Nat Rev Nephrol 6: 451–460, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Ansell D: Serum phosphate and outcomes in PD patients. Nephrol Dial Transplant 22: 667–668, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Noordzij M, Korevaar JC, Bos WJ, Boeschoten EW, Dekker FW, Bossuyt PM, Krediet RT: Mineral metabolism and cardiovascular morbidity and mortality risk: Peritoneal dialysis patients compared with haemodialysis patients. Nephrol Dial Transplant 21: 2513–2520, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Melamed ML, Eutace JA, Plantiga L, Jaar BG, Fink NE, Klag MJ, Powe NR: Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Kidney Int 70: 351–357, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hamodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Wang AY, Woo J, Sea MM, Law MC, Lui SF, Li PK: Hyperphosphatemia in Chinese peritoneal dialysis patients with and without residual kidney function: What are the implications? Am J Kidney Dis 43: 712–720, 2004 [PubMed] [Google Scholar]
- 9. Pag DE, Knoll GA, Cheung V: The relationship between residual renal function, protein catabolic rate, and phosphate and magnesium levels in peritoneal dialysis patients. Adv Perit Dial 18: 189–191, 2002 [PubMed] [Google Scholar]
- 10. Sedlacek M, Dimaano F, Uribarrri J: Relationship between phosphorus and creatinine clearance in peritoneal dialysis: Clinical implications. Am J Kidney Dis 36: 1020–1024, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Schmitt CP, Borzych D, Nau B, Wuhl E, Zurowska A, Schaefer F: Dialytic phosphate removal: A modifiable measure of dialysis efficacy in automated peritoneal dialysis. Perit Dial Int 29: 465–471, 2009 [PubMed] [Google Scholar]
- 12. Badve SV, Zimmerman DL, Knoll GA, Burns KD, McCormick BB: Peritoneal phosphate clearance is influenced by peritoneal dialysis modality, independent of peritoneal transport characteristics. Clin J Am Soc Nephrol 3: 1711–1717, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dombros N, Dratwa M, Feriani M, Gokal R, Heimburger O, Krediet R, Plum J, Rodrigues A, Selgas R, Struijk D, Verger C: EBPG Expert Group on Peritoneal Dialysis: European best practice guidelines for peritoneal dialysis. Adequacy of peritoneal dialysis. Nephrol Dial Transplant Suppl 9: ix24–ix27, 2005 [DOI] [PubMed] [Google Scholar]
- 14. National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 15. Nolph KD, Moore HL, Twardowski ZJ, Khanna R, Prowant B, Meyer M, Ponferrada L: Cross-sectional assessment of weekly urea and creatinine clearances in patients on continuous ambulatory peritoneal dialysis. ASAIO J 38: M139–M141, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Randerson DH, Chapman GV, Farrell PC: Amino acid and dietary status in CAPD patients. In: Peritoneal Dialysis, edited by Atkins RC, Farrell PC, Thomson N. Edinburgh, Scotland, Churchill-Livingstone, 1981, pp 180–191 [Google Scholar]
- 17. Twardowski Z, Nolph KD, Khanna R, Prowant BF, Ryan LP, Moore HL, Nielsen MP: Peritoneal equilibration test. Perit Dial Int 7: 138–148, 1987 [Google Scholar]
- 18. Graff J, Fugleberg S, Brahm J, Fogh-Andersen N: The transport of phosphate between the plasma and dialysate compartments in peritoneal dialysis is influenced by an electric potential difference. Clin Physiol 16: 291–300, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Rippe B, Venturoli D, Simonsen O, Arteaga J: Fluid and electrolyte transport across the peritoneal membrane during CAPD according to the three-pore model. Perit Dial Int 24: 10–27, 2004 [PubMed] [Google Scholar]
- 20. Kuhlmann MK: Phosphate elimination in modalities of hemodialysis and peritoneal dialysis. Blood Purif 29: 137–144, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Badve S, McCormick BB: Phosphate balance on peritoneal dialysis. Perit Dial Int 28[Suppl 2]: S26–S32, 2008 [Google Scholar]
- 22. Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y: Superior dialytic clearance of beta 2-microglobulin and p-cresol by high-flux hemodialysis as compared to peritoneal dialysis. Kidney Int 70: 794–799, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Gallar P, Ortega O, Gutierrez M, Munoz M, Hilara L, Oliet A, Rodrígues I, Giménez E, Vigil A: Influencing factors in the control of phosphorous in peritoneal dialysis. Therapeutic options. Nefrologia 20: 355–361, 2000 [PubMed] [Google Scholar]