Summary
Background and objectives
Gitelman's syndrome (GS) is an autosomal recessive renal tubular disorder caused by mutations in the SLC12A3 gene encoding the thiazide-sensitive Na+-Cl− cotransporter (NCC). Despite meticulous sequencing of genomic DNA, approximately one-third of GS patients are negative or heterozygotes for the known mutations.
Design, Setting, Participants, & Measurements
Because blood leukocytes express NCC mRNA, we evaluate whether deep intronic mutations contribute to GS patients with uniallelic or undetectable SLC12A3 mutations. Twenty-nine patients with GS (men/women = 16/13), including eight negative and 21 uniallelic SLC12A3 mutations from 19 unrelated families, and normal controls were enrolled in an academic medical center. Analysis of cDNA from blood leukocytes, sequencing of the corresponding introns of genomic DNA for abnormal transcript, and analysis of NCC protein expression from renal biopsy were performed.
Results
We identified nine Taiwan aboriginal patients carrying c.1670–191C→T mutations in intron 13 and 10 nonaboriginal patients carrying c.2548+253C→T mutations in intron 21 from 14 families (14/19). These two mutations undetected in 100 healthy subjects created pseudoexons containing new premature termination codons. Haplotype analysis with markers flanking SLC12A3 revealed that both mutations did not have founder effects. Apical NCC expression in the DCT of renal tissue was markedly diminished in two patients carrying deep intronic mutations.
Conclusions
Deep intronic mutations in SLC12A3 causing defective NCC expression can be identified with the RNA-based approach in patients with GS. c.1670–191C→T and c.2548+253C→T are hot spot mutations that can be screened in GS patients with uniallelic or negative SLC12A3 mutations.
Introduction
Gitelman's syndrome (GS) (OMIM 263800) is an autosomal recessive renal tubular disorder characterized by hypokalemia, metabolic alkalosis, hypomagnesemia, and hypocalciuria (1). Clinically, salt craving, nocturia, tetanic episodes, paresthesias, and muscle weakness or paralysis are the most frequent symptoms of GS (2). At the molecular level, GS is caused by inactivating mutations in the SLC12A3 gene encoding the thiazide-sensitive Na+-Cl− cotransporter (NCC) in the luminal membrane of the distal convoluted tubule (DCT) (3,4). Nevertheless, some patients with classic Bartter's syndrome caused by mutations in CLCNKB that encode the basolateral Cl− channel (ClC-Kb) can exhibit a clinical picture similar to GS (5,6).
To date, more than 140 different mutations including missense, nonsense, frameshift, deletion, insertion, and splice-site mutations have been identified in patients with GS (3,6–11). The majority of GS patients are compound heterozygotes having two different mutations on the two alleles. However, 20 to 40% of patients with GS have only one heterozygous mutation (3,12–14). Because the mode of inheritance in GS is autosomal recessive, there may be an unidentified gene mutation on the other allele. Technical considerations of molecular analysis may provide some explanations for this issue (12,14). First, mutations can be missed by the widely performed PCR single-strand conformation polymorphism analysis. Second, detection analysis on the basis of individual exons will not identify large heterozygous deletions. Third, mutations may be present in gene-regulation fragments such as promoters or enhancers, intron sequences, or 5′ and 3′ noncoding regions, which have not been routinely screened.
As previously reported, we have found that approximately 30% of Chinese patients with GS had uniallelic or undetectable SLC12A3 mutations using genomic DNA analysis (11,15). Using multiplex ligation-dependent probe amplication analysis (16–18), the possibility of large deletions was excluded because differences in the copy numbers of the 26 exons was not detected (S. H. Lin et al., unpublished data). Over the past few years, deep intron mutations undetected by screening of exons and intron boundaries have increasingly been reported as an important cause of several inherited genetic disorders (19–21). Deep intron mutations can cause the abnormal inclusion of intron sequences in the mRNA, leading to frameshift or introduction of premature termination codons (PTC). Because blood leukocytes express NCC mRNA (22), it is feasible to detect deep intronic mutations using reverse transcriptase (RT)-PCR on blood leukocytes from GS patients.
Using RT-PCR for mRNA transcript, we therefore aimed to identify whether deep intronic mutations in the SLC12A3 were present in 29 GS patients carrying only uniallelic or undetectable SLC12A3 mutation belonging to 19 unrelated families. The results to be reported indicate that two deep recurrent mutations in intron 13 (c.1670–191C→T) and intron 21 (c.2548+253C→T) with the inclusion of cryptic exon in mRNA leading to a defective NCC expression in the DCT are identified in 19 patients from 14 families. Noticeably, c.1670–191C→T is a mutational hot spot in Taiwan aborigines with GS and may also be screened in Austronesian population with GS.
Materials and Methods
The study protocol was approved by the Ethics Committee on Human Studies at Tri-Service General Hospital, National Defense Medical Center, in Taiwan, R.O.C. The subjects were given a detailed description of the study before they provided informed consent.
Patients
We had collected 29 GS patients carrying heterozygous mutations in one allele or no detectable mutations in the SLC12A3 gene. They were 16 men and 13 women with a mean age of 26.7, belonging to 19 unrelated families. All had persistent hypokalemia (<3.5 mEq/L) with renal K+ wasting, persistently high urinary NaCl excretion, and normal BP. They did not take laxatives or diuretics. All but two had typical hypocalciuria (urinary calcium to creatinine ratio <0.04 mg/mg), and all but three had hypomagnesemia (<1.6 mg/dl). Eight patients from five unrelated families did not have any SLC12A3 mutations, and 21 patients from 14 families only had SLC12A3 mutations on one allele. The mutations on only one allele included the known T60M, T163M, L215P, IVS7–1G→A+971InsACCGAAAATTTT, R642C, R871H, W844X, D848N, 2881–2delAG (8,15), and a novel R83Q. Molecular analysis for CLCNKB, NKCC2, and ROMK mutations were all negative. Using multiplex ligation-dependent probe amplication analysis, none had a large mutation of SCL12A3.
Methods
RNA Expression Assay.
Total RNA obtained from peripheral blood leukocytes was isolated from all GS patients by using QIAamp RNA blood mini kit (Qiagen) and then was reverse-transcribed into cDNA using a high capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, California). cDNA regions were amplified by PCR with the exonic primer pairs (Table 1), and the PCR products were sequenced by using BigDye terminator v3.1 mix and subsequent analysis by capillary electrophoresis on an ABI Prism 3130 genetic analysis (PerkinElmer Applied Biosystems).
Table 1.
Primers used for RT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Exons 1 to 3 | CAGAACTGCCCACAACAGAG | TTGAGCATGCAACGAATCAT |
| Exons 3 to 7 | GTTGCATGCTCAACATTTGG | GGCAAAGGAGACCATGATGA |
| Exons 7 to 11 | AACTATTTAGTGGGGACGCTGA | CCATGCTCATGGTCTGGTAA |
| Exons 11 to 15 | CTCTGCTGCCAAAGTCTTCC | TGTGGTCTTCCACCTCATTG |
| Exons 15 to 20 | AAGAACTACCGCCCCCAGTG | GTCCTCCGCTGGGTCAAAC |
| Exons 20 to 24 | AGTCTCTGGCGCTTTGGAC | CCGAAGGGACTTGACTCTGT |
| Exons 25 to 26 | TGCTGGATTACTCCCGAGAC | TTTTCCTTGCAGCTCCATCT |
Genomic DNA Isolation and PCR.
Genomic DNA was isolated from peripheral blood of all patients and 100 normal control subjects. Intronic genomic DNA was amplified using primers located in introns 13 (forward, TACAGTTGTGCCTGGCTGAG, and reverse, GGCAGTCGAGCCTCTTTCTA) and 21 (forward, AGCAGGGCAAGAAGACCATA, and reverse, ACAGCTCCTACCTGGGGACT). The DNA mutations are numbered on the basis of cDNA sequence and intronic positions described in Ensembl.
Haplotype Analysis.
Haplotypes were derived by using microsatellite markers (D16S419, D16S3140, D16S408, and D16S389) flanking the SLC12A3 locus (3). Fragment sizes were analyzed with GENESCAN version 2.0 and GENOTYPER version 2.1 software. The most likely haplotypes were inferred by minimizing the number of cross-over events in each family.
NCC Expression in the Kidneys.
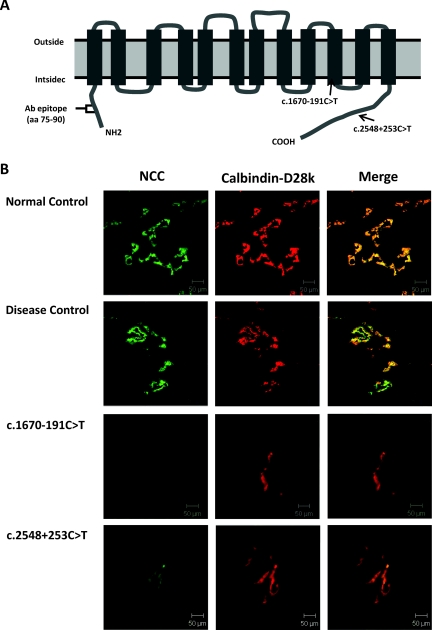
Renal biopsy was performed in one patient (patient 1) with homozygous mutation (c.1670–191C→T) and the other patient (patient 14) with compound heterozygous mutation (c.2548+253C→T/T60M). A 28-year-old male patient who had chronic hypokalemia and mild proteinuria caused by anorexia/bulimia nervosa was selected as disease control. Normal kidney tissue was obtained from a 52-year-old male patient with renal cell carcinoma undergoing total nephrectomy. An antibody-recognized amino-terminal portion (amino acids 75 to 90) of NCC (Chemicon, Temecula, California) was applied to detect NCC expression by immunofluorescence as described previously (23). An antibody to detect the expression of calbindin-D28k (Swant, Bellinzona, Switzerland) as a distal renal tubule marker was also used to localize the DCT.
Results
Deep Intronic Mutations in SLC12A3
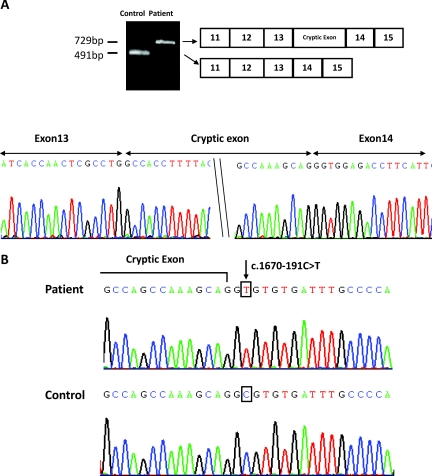
We identified two deep intronic mutations in 19 patients from 14 of 19 families. As shown in Figure 1, complete mRNA transcript analysis from blood leukocytes of patient 1, who had unknown SLC12A3 mutations, demonstrated a larger band (729 bp), compared with the relatively small band (491 bp) in normal control, using exon 11 to 15 primers. Sequencing of this larger mRNA transcript demonstrated a 238-bp insertion of cryptic exon between exon 13 and 14. Direct sequencing of the entire intron 13 showed a homozygous single base substitution of C to T at position −191 just before 1670 coding DNA (c.1670–191C→T). This C→T point mutation located at +2 after the insertion sequence created a novel donor splicing site for the 238-bp cryptic exon inclusion (Figure 1). This homozygous mutation was found in seven patients with undetectable SLC12A3 mutations from four different families and two patients with heterozygous mutations from two different families. Of interest, all of the patients harboring this mutation were Taiwanese aborigines. Nonaboriginal patients with GS and 100 unrelated healthy subjects did not carry this mutation.
Figure 1.
The identification of deep intronic mutation c.1670–191C→T in intron 13 of SLC12A3. (A) RT-PCR analysis using exon 11 to 15-specific primers in patient 1 shows a comparatively large band (729 bp) in contrast to a relatively small band (491 bp) in normal control. Sequencing of this larger mRNA transcript demonstrated a 238-bp insertion of cryptic exon between exons 13 and 14. (B) Direct sequencing of intron 13 demonstrates a homozygous single base substitution of C to T at the position −191 just before 1670 coding DNA (c.1670–191C→T).
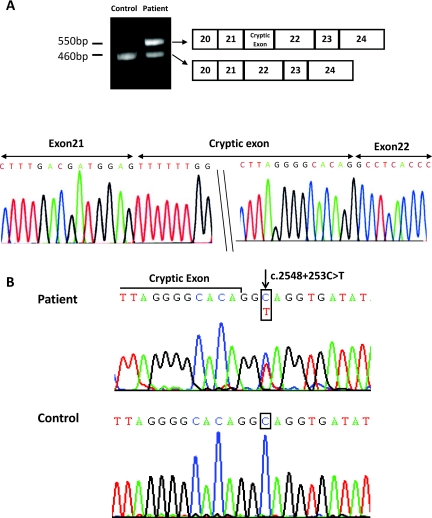
As shown in Figure 2, complete mRNA transcript analysis on patient 10, who had a heterozygous T163M mutation (Table 2), revealed a larger band (550 bp) compared with a relatively small band (460 bp) in normal control, using exon 20 to 24 primers. Sequencing of this larger mRNA transcript and the corresponding intron showed that a new donor splicing site for a 90-bp cryptic exon between exons 21 and 22 was created by a novel point mutation (c.2548+253C→T) deep in intron 21 (Figure 2). Similar to the 238-bp pseudoexon causing a translation frameshift with introduction of a premature termination codon (TGA) after 10 amino acids, the 90-bp cryptic exon introduced a premature termination codon (TAA) after four amino acids (Figure 3). This heterozygous mutation was found in 10 patients with heterozygous SLC12A3 mutation from eight families including family K carrying two SLC12A3 mutations on one allele. As shown in Figure 4, T163M and R871H on one allele of patient 15 from family K was transmitted from the father, and (c.2548+253C→T) on the other allele came from the mother. The c.2548+253C→T mutation was also not detected in 100 unrelated healthy subjects. Nevertheless, 10 patients from five unrelated families including one negative and four uniallelic SLC12A3 mutations (T60M, T163M, IVS7–1G→A+971InsACCGAAAATTTT, and D848N) were not found to have deep intron mutations. Exons 7 and 8 skipping from RT-PCR products in two male patients carrying heterozygous IVS7–1G→A+ 971InsACCGAAAATTTT mutation were identified.
Figure 2.
The identification of a deep intronic mutation c.2854+253C→T in the intron 21. (A) Electrophoresis of the RT-PCR products by using exon 20 to 24-specific primers in patient 10 shows the incorporation of a 90-bp cryptic exon following exon 21. (B) Direct sequencing of intron 21 revealed a heterozygous single base substitution of C to T at position 253 just after 2548 coding DNA (c.2548+253C→T).
Table 2.
Demographics and laboratory findings in GS patients with deep intronic mutations in NCC
| Family | Patient | Gender | Age | Location | Mutation | Laboratory Findings |
|||
|---|---|---|---|---|---|---|---|---|---|
| Plasma K+ (mEq/liter) | Plasma HCO3− (mEq/liter) | Plasma Mg2+ (mg/dl) | Urine Ca2+/Cr (mg/mg) | ||||||
| A | 1 | M | 35 | IVS13 | c.1670−191C→T (Homo) | 1.7 | 29.2 | 1.4 | 0.02 |
| 2 | F | 32 | IVS13 | c.1670−191C→T (Homo) | 2.4 | 31.4 | 1.3 | 0.01 | |
| B | 3 | M | 37 | IVS13 | c.1670−191C→T (Homo) | 1.8 | 28.8 | 1.2 | 0.03 |
| 4 | F | 50 | IVS13 | c.1670−191C→T (Homo) | 2.6 | 29.6 | 1.5 | 0.02 | |
| C | 5 | M | 18 | IVS13 | c.1670−191C→T (Homo) | 1.8 | 20.5 | 1.3 | 0.01 |
| D | 6 | M | 22 | Exon5/IVS13 | L215P/c.1670−191C→T | 1.8 | 29.4 | 1.2 | 0.01 |
| E | 7 | F | 28 | Exon1/IVS13 | R83Q/c.1670−191C→T | 2.2 | 31.1 | 1.3 | 0.04 |
| F | 8 | F | 30 | IVS13 | c.1670−191C→T (Homo) | 2.6 | 28.4 | 1.1 | 0.01 |
| 9 | M | 27 | IVS13 | c.1670−191C→T (Homo) | 1.5 | 30.2 | 1.8 | 0.02 | |
| G | 10 | M | 15 | Exon3/IVS21 | T163M/c.2548+253C→T | 2.0 | 29.7 | 1.5 | 0.07 |
| 11 | M | 15 | Exon3/IVS21 | T163M/c.2548+253C→T | 1.8 | 29.7 | 1.5 | 0.02 | |
| H | 12 | M | 18 | Exon24/IVS21 | 2881–2delAG/c.2548+253C→T | 1.9 | 31.7 | 1.2 | 0.01 |
| I | 13 | M | 20 | Exon1/IVS21 | T60M/c.2548+253C→T | 2.2 | 32.8 | 1.9 | 0.02 |
| J | 14 | M | 26 | Exon1/IVS21 | T60M/c.2548+253C→T | 1.9 | 31.6 | 1.4 | 0.01 |
| K | 15 | M | 32 | Exon3,22/IVS21 | T163M,R871H/c.2548+253C→T | 1.6 | 33.2 | 1.1 | 0.03 |
| L | 16 | F | 28 | Exon21/IVS21 | W844X/c.2548+253C→T | 2.5 | 30.1 | 1.6 | 0.01 |
| M | 17 | F | 31 | Exon1/IVS21 | T60M/c.2548+253C→T | 2.4 | 31.8 | 1.5 | 0.03 |
| N | 18 | M | 23 | Exon15/IVS21 | R642C/c.2548+253C→T | 1.8 | 31.7 | 1.4 | 0.01 |
| 19 | F | 21 | Exon15/IVS21 | R642C/c.2548+253C→T | 2.4 | 30.9 | 1.2 | 0.02 | |
M, male; F, female.
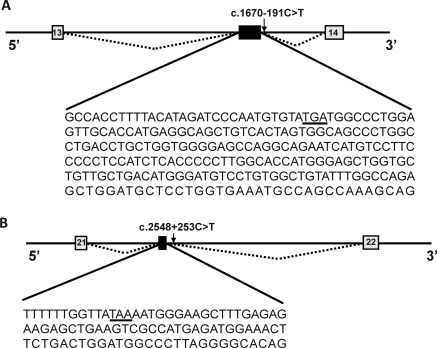
Figure 3.
Cryptic exons created by two deep intron mutations causing translation frameshift with introduction of premature termination codons TGA after 10 amino acids (A) and TAA after 4 amino acid (B), respectively, as shown with underlining. Constitutive and cryptic exons are represented by light gray and dark boxes, respectively. Mutated nucleotides are indicated by arrows.
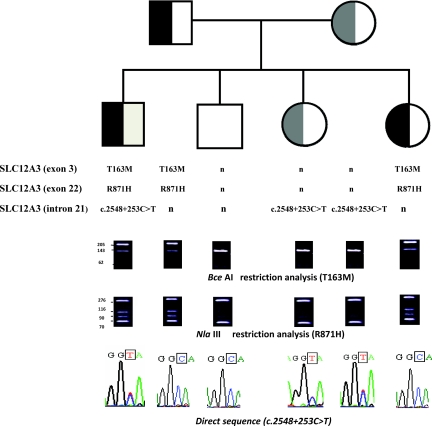
Figure 4.
Pedigree of family K. Men are indicated by squares, and women are indicated by circles. T163M (exon 3) and R871H (exon 22) were transmitted from the father, and (c.2548+253C→T) in intron 21 was transmitted from the mother.
The demographics and laboratory findings of the 19 patients carrying these two deep intronic mutations are shown in Table 2. They were 12 men and seven women. One man (patient 10) did not have hypocalciuria, and two other men (patient 9 and 13) did not present with hypomagnesemia. Of note, severe hypokalemia with muscle paralysis was often the primary presenting symptom in affected men. Dizziness, palpitations, and tetany were common symptoms in women who had moderate to severe hypokalemia.
Halpotype Analysis
Given the high frequency of these two deep intronic mutations among GS patients carrying uniallelic or negative SLC12A3 mutations (19/29, 65%), we wondered whether these two mutations had a common origin or just reflected a mutational hot spot in SLC12A3. Four microsatellite markers (D16S419, D16S3140, D16S408, and D16S389) flanking SLC12A3 were performed in these patients. Patients with these two mutations did not share identical CA or GT repeat numbers (Table 3), suggesting that c.1670–191C→T and c.2548+253C→T mutations were recurrent hot spots rather than founder effects arising from a common ancestor.
Table 3.
Haplotype analysis in GS patient with deep intronic mutations in SLC12A3
| Family | Patient | D16S419 | D16S3140 | D16S408 | D16S389 |
|---|---|---|---|---|---|
| c.1670–191C→T | |||||
| A | 1 (Homo) | 8, 9 | 8, 11 | 2, 3 | 4, 4 |
| 2 (Homo) | 8, 9 | 8, 11 | 2, 3 | 4, 4 | |
| B | 3 (Homo) | 4, 9 | 18, 18 | 3, 4 | 4, 8 |
| 4 (Homo) | 4, 9 | 16, 18 | 2, 3 | 4, 8 | |
| C | 5 (Homo) | 9, 9 | 4, 4 | 3, 4 | 4, 4 |
| D | 6 | 4, 9 | 9, 18 | 3, 3 | 4, 8 |
| E | 7 | 9, 9 | 4, 4 | 3, 4 | 4, 10 |
| F | 8 (Homo) | 4, 9 | 18, 18 | 3, 4 | 4, 8 |
| 9 (Homo) | 4, 9 | 18, 18 | 3, 4 | 4, 8 | |
| c.2548+253C→T | |||||
| G | 10 | 8, 8 | 15, 19 | 1, 3 | 4, 4 |
| 11 | 8, 8 | 15, 19 | 1, 3 | 4, 4 | |
| H | 12 | 8, 10 | 4, 18 | 1, 5 | 4, 7 |
| I | 13 | 7, 8 | 13, 20 | 1, 5 | 4, 8 |
| J | 14 | 8, 9 | 17, 18 | 1, 3 | 4, 4 |
| K | 15 | 9, 10 | 12, 17 | 1, 5 | 4, 5 |
| L | 16 | 9, 10 | 14, 19 | 1, 3 | 4, 6 |
| M | 17 | 5, 9 | 10, 11 | 1, 3 | 4, 4 |
| N | 18 | 4, 8 | 9, 20 | 1, 4 | 5, 5 |
| 19 | 4, 8 | 9, 20 | 1, 4 | 5, 5 |
The intermarker distances are: D16S419 – 3.4 Mb – D16S3140 – 0.009 Mb – D16S408 – 0.57 Mb – SLC12A3 – 4.98 Mb – D16S389.
NCC Expression in Human Kidney Tissue
As shown in Figure 5B, there was distinct NCC expression along the apical membranes of the DCT in the normal control and disease control. In contrast, NCC expression in the DCT from two patients with different deep intronic mutations was markedly attenuated despite positive staining of calbindin-D28k. This finding suggested that these two deep intron mutations may cause markedly diminished expression of NCC in the DCT, leading to defective NCC function.
Figure 5.
Immunofluorescence of NCC and calbindin-D28k in renal biopsy tissue. (A) The position of each mutation in the predicted topology of human NCC is represented by arrows. An antibody recognized amino-terminal (amino acids 75 to 90) of NCC was applied to detect NCC protein expression. (B) Representative immunofluorescence micrographs of NCC (green, left panels), calbindin-D28k (red, middle panels), and merged (right panels) in renal tissue from normal control, disease control, and GS patients with homozygous c.1670–191C→T and heterozygous c.2548+253C→T mutations. The scale bars indicate 50 μm. aa, amino acids.
Discussion
In this study, we identified two recurrent mutations deep in introns 13 and 21 (c.1670–191C→T and c.2548+253C→T) in 19 of 29 patients with GS carrying undetectable or uniallelic SLC12A3 mutations from 14 out of 19 families. These two deep intronic mutations created 238- and 90-bp cryptic exons, both of which contained new PTC-containing transcripts (TGA and TAA, respectively). Renal tissue from one patient with homozygous mutation (c.1670–191C→T) and the other with compound heterozygous mutation (c.2548+253C→T) showed markedly diminished NCC expression in the DCT. These findings indicated that deep intronic mutations in SLC12A3 can lead to GS.
The previously reported intronic mutations in SLC12A3 are almost localized in the consensus donor or acceptor splicing sites (3,8,22,24–27) and easily detected by the analysis of exon and intron boundaries of genomic DNA. However, mutations deep in the introns are usually not screened, and mutations there may be often missed unless cDNA analysis is applied (28). Using the RNA-based approach from blood leukocytes expressing NCC mRNA, we were able to identify two recurrent deep intronic mutations in approximately 65% (19 of 29) of GS patients with previously undetectable or uniallelic SLC12A3 mutations. The identification of deep intronic mutations in SLC12A3 may account for a substantial portion of the “missing 20 to 40%” of GS patients as mentioned above. In fact, cDNA analysis using various tissues such as urine epithelial tubular cells, kidney tissue, or skin tissue has been increasingly reported to detect and confirm mutations in the introns causing splicing abnormalities including deep intronic mutations in inherited renal tubular diseases such as Alport's syndrome, Dent's disease, adult polycystic kidney disease, and Bartter's syndrome (21,29–32). cDNA analysis often revealed exon skipping in GS patients with the previously reported splice site mutations in the introns rather than cryptic pseudoexon insertion from the introns in patients with deep intronic mutations as shown in our study.
NCC staining of human renal tissue from previous GS patients has found that abnormal intracellular processing is the most common mechanism of diminished or absent NCC expression on the apical membrane of DCT (33,34). We evaluated NCC expression consequent to these two deep intron mutations in human renal tissues and also found markedly diminished NCC expression in the DCT. Both mutations generated cryptic exons containing PTCs, which likely caused the production of abnormally truncated protein or rapid degradation of the nonsense transcript by the nonsense-mediated decay surveillance mechanism, leading to a marked decrease in NCC expression and loss of NCC function.
The identification of two deep intronic mutations in SLC12A3 in 19 GS patients from 14 unrelated Taiwanese families suggested that these two mutations may have a common origin or just reflect the existence of a mutational hot spot in SLC12A3. Halpotype analysis with markers flanking the SLC12A3 demonstrated that c.1670–191C→T and c.2548+253C→T mutations are located on mutational hot spots rather than founder effect. Of interest, this c.1670–191C→T was only identified in Taiwan aborigines and identical to one reported from Japan and inherited from a Filipino maternal allele (35). Taiwan has been suggested to be the homeland of the Austronesian language family (36,37). It is possible that this deep intronic mutation be a recurrent hot spot not only for Taiwan aborigines but the Austronesian ethnic group. Screening for this deep intron mutation may provide an early molecular diagnosis of GS in patients of this ancestry.
The majority of patients with GS are compound heterozygotes having two different mutations on the two alleles. However, it has been reported that 7% of GS patients carry three or more different NCC mutations (8,14). This means that the identification of two different SLC12A3 mutations will not always be on separate alleles without complete genetic analysis of the GS family pedigree. This is exemplified by one patient who carried T163M and R871H on one allele from the father and c.2548+253C→T in intron 21 on the other from the mother.
Five families with negative or uniallelic SLC12A3 mutations who did not have deep intronic mutations are still missing a genetic explanation for their GS. Looking back at the technical limitations of the present technique, there may be a gene mutation still undetectable, such as gene-regulation fragments in the promoter, enhancer, and 5′ and 3′ noncoding regions (9,38). Screening of other potential candidate gene mutations causing GS-like phenotypes may be also considered. STE20 (sterile 20)/SPS1-related proline/alanine-rich kinase (SPAK) (39), an upstream regulator of NCC, is a possible candidate gene for these GS patients because SPAK−/− mice have been found to develop a GS phenotype (40).
With respect to phenotype, we have shown that the GS phenotype may vary widely, with a male propensity for increased severity. More broadly, the nature and position of SLC12A3 mutation may also be important factors in determining the severity of GS. A recent study reported that GS patients with truncated mutations or splicing variants in at least one allele tended to manifest more severe symptoms (16). We found that the reported female patient from Japan and our female patients carrying deep intronic mutation appeared to exhibit moderate to severe symptoms (35). Whether the phenotypes in GS are modified by the deep intronic mutations merits further investigation.
Mutation-specific approaches for the different classes of mutations are currently under investigation as novel therapies for genetic disorders (41). Alleles with intronic mutations activating cryptic splicing sites are potentially correctable with antisense oligonucleotides to prevent the splicing machinery from recognizing the cryptic exon and promote normal splicing, as successfully shown in several genetic disorders (42–44). Similar antisense oligonucleotides may also apply to GS patients bearing deep intronic mutations and can be first tested on the leukocytes expressing NCC mRNA.
In conclusion, we have found two recurrent mutations deep in introns 13 (c.1670–191C→T) and 21 (c.2548+253C→T) that cause in-frame cryptic exons containing stop codons in GS patients with previously negative or uniallelic SLC12A3 mutations. These two deep intronic mutations lead to marked attenuation of NCC expression in the DCT. The c.1670–191C→T mutation may be a “hot spot” not only for Taiwan aborigines but related ethnic groups throughout Austronesia. Our results indicate that analysis of cDNA from blood leukocytes to detect deep intronic mutations must be considered for GS patients who have otherwise undetectable or uniallelic SLC12A3 mutations.
Disclosures
None.
Acknowledgments
This study was supported in part by grants from the National Science Council, Taiwan (NSC 98-2314-B-016-002-MY2) and the Research Fund of Tri-Service General Hospital (TSGH-C-96-53, C-98-81, C100-023). We are indebted to the participating patients and their families for their cooperation and thank the physicians and nephrologists for the contribution to this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Gitelman HJ, Graham JB, Welt GL: A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 79: 211–235, 1996 [PubMed] [Google Scholar]
- 2. Cruz DN, Shaer AJ, Bia MJ, Lifton RP, Simon DB: Gitelman's syndrome revisited: An evaluation of symptoms and health-related quality of life. Kidney Int 59: 710–717, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP: Gitelman's variant of Bartter's syndrome, inherited hypokalemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Mastroianni N, Bettinelli A, Bianchetti M, Colussi G, De Fusco M, Sereni F, Ballabio A, Casari G: Novel molecular variants of the Na-Cl cotransporter gene are responsible for Gitelman syndrome. Am J Hum Genet 59: 1019–1026, 1996 [PMC free article] [PubMed] [Google Scholar]
- 5. Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, Jonh E, Lifton RP: Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet 17: 171–178, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Knoers NV, Levtchenko EN: Gitelman syndrome. Orphanet J Rare Dis 3: 22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zelikovic I, Szargel R, Hawash A, Labay V, Hatib I, Cohen N, Nakhoul F: A novel mutation in the chloride channel gene, CLCNKB, as a cause of Gitelman and Bartter syndromes. Kidney Int 63: 24–32, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Lemmink HH, Knoers NV, Karolyi L, van Dijk H, Niaudet P, Antignac C, Guay-Woodford LM, Goodyer PR, Carel JC, Hermes A, Seyberth HW, Monnens LA, van den Heuvel LP: Novel mutations in the thiazide-sensitive NaCl cotransporter gene in patients with Gitelman syndrome with predominant localization to the C-terminal domain. Kidney Int 54: 720–730, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Monkawa T, Kurihara I, Kobayashi K, Hayashi M, Saruta T: Novel mutations in thiazide-sensitive Na-Cl cotransporter gene of patients with Gitelman's syndrome. J Am Soc Nephrol 11: 65–70, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Peters M, Jeck N, Reinalter S, Leonhardt A, Tonshoff B, Klaus GG, Konrad M, Seyberth HW: Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med 112: 183–190, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Lin SH, Cheng NL, Hsu YJ, Halperin ML: Intrafamilial phenotype variability in patients with Gitelman syndrome having the same mutations in their thiazide-sensitive sodium/chloride cotransporter. Am J Kidney Dis 43: 304–312, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Reissinger A, Ludwig M, Utsch B, Promse A, Baulmann J, Weisser B, Vetter H, Kramer HJ, Bokemeyer D: Novel NCCT gene mutations as a cause of Gitelman's syndrome and a systematic review of mutant and polymorphic NCCT alleles. Kidney Blood Press Res 25: 354–362, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Shao L, Ren H, Wang W, Zhang W, Feng X, Li X, Chen N: Novel SLC12A3 mutations in Chinese patients with Gitelman's syndrome. Nephrol Physiol 108: 29–36, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Gamba G: Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Lin SH, Shiang JC, Huang CC, Yang SS, Hsu YJ, Cheng CJ: Phenotype and genotype analysis in Chinese patients with Gitelman's syndrome. J Clin Endocrinol Metab 90: 2500–2507, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Riveira-Munoz E, Chang Q, Godefroid N, Hoenderop JG, Bindels RJ, Dahan K, Devuyst O: Transcriptional and functional analyses of SLC12A3 mutations: New clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol 18: 1271–1283, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Nozu K, Krol RP, Nakanishi K, Yoshikawa N, Nozu Y, Ohtsuka Y, Iijima K, Matsuo M: Detection by multiplex ligation-dependent probe amplification of large deletion mutations in the COL4A5 gene in female patients with Alport syndrome. Pediatr Nephrol 24: 1773–1774, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Huang CH, Chang YY, Chen CH, Kuo YS, Hwu WL, Gerdes T, Ko TM: Copy number analysis of survival motor neuron genes by multiplex ligation-dependent probe amplication. Genet Med 9: 241–248, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Coutinho G, Xie J, Du L, Brusco A, Krainer AR, Gatti RA: Functional significance of a deep intronic mutation in the ATM gene and evidence for an alternative exon 28a. Hum Mutat 25: 118–124, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Davis RL, Homer VM, George PM, Brennan SO: A deep intronic mutation in FGB creates a consensus exonic splicing enhancer motif that results in afibrinogenemia caused by aberrany mRNA splicing, which can be corrected in vitro with antisense oligonucleotide treatment. Hum Mutat 30: 221–227, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Nozu K, Iijima K, Kawai K, Nozu Y, Nishida A, Takeshima Y, Fu XJ, Hashimura Y, Kaito H, Nashimura Y, Kaito H, Nakanishi K, Yoshikawa N, Matsuo M: In vivo and in vitro splicing assay of SLC12A1 in an antenatal salt-losing tubulopathy patient with an intronic mutation. Hum Genet 126: 533–538, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Abuladze N, Yanagawa N, Lee I, Jo OD, Newman D, Hwang J, Uyemura K, Pushkin A, Modlin RL, Kurtz I: Peripheral blood mononuclear cells express mutated NCCT mRNA in Gitelman's syndrome: Evidence for abnormal thiazide-sensitive NaCl cotransport. J Am Soc Nephrol 9: 819–826, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Yang SS, Lo YF, Yu IS, Lin SW, Chang TH, Hsu YJ, Chao TK, Sytwu HK, Uchida S, Sasaki S, Lin SH: Generation and analysis of the thiazode-sensitive Na+-Cl−-cotransporter S707X knockin mice as a model of Gitelman's syndrome. Hum Mutat 2010, in press [DOI] [PubMed] [Google Scholar]
- 24. Syren ML, Tedeschi S, Cesareo L, Bellantuono R, Colussi G, Procaccio M, Alì A, Domenici R, Malberti F, Sprocati M, Sacco M, Miglietti N, Edefonti A, Sereni F, Casari G, Coviello DA, Bettinelli A: Identification of fifteen novel mutations in the SLC12A3 gene encoding the Na-Cl co-transporter in Italian patients with Gitelman syndrome. Hum Mutat 20: 78, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Coto E, Rodriguez J, Jeck N, Alvarez V, Stone R, Loris C, Rodriguez LM, Fischbach M, Seyberth HW, Santos F: A new mutation (intron 9 +1 G>T) in the SLC12A3 gene is linked to Gitelman syndrome in Gypsies. Kidney Int 65: 25–29, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Maki N, Komatsuda A, Wakui H, Ohtani H, Kigawa A, Aiba N, Hamai K, Motegi M, Yamaguchi A, Imai H, Sawada K: Four novel mutations in the thiazide-sensitive Na-Cl co-transporter gene in Japanese patients with Gitelman's syndrome. Nephrol Dial Transplant 19: 1761–1766, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Fava C, Montagnana M, Rosberg L, Burri P, Jönsson A, Wanby P, Wahrenberg H, Hulthén UL, Aurell M, Guidi GC, Melander O: Novel mutations in the SLC12A3 gene causing Gitelman's syndrome in Swedes. DNA Seq 18: 395–399, 2007 [DOI] [PubMed] [Google Scholar]
- 28. King K, Flinter FA, Nihalani V, Green PM: Unuusal deep intronic mutations in the COL4A5 gene cause X-linked Alport syndrome. Hum Genet 111: 548–554, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Kaito H, Nozu K, Fu XJ, Kamioka I, Fujita T, Kanda K, Krol RP, Suminaga R, Ishida A, Iijima K, Matsuo M: Detection of a transcript abnormality in mRNA of the SLC12A3 gene extracted from urinary sediment cells of a patient with Gitelman's syndrome. Pediatr Res 61: 502–505, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Igarashi T, Inatomi, Ohara T, Kuwahara T, Shimadzu M, Thakker RV: Clinical and genetic studies of CLCN5 mutations in Japanese families with Dent's disease. Kidney Int 58: 520–527, 2000 [DOI] [PubMed] [Google Scholar]
- 31. King K, Flinter FA, Green PM: A two-tier approach to mutation detection in the COL4A5 gene for Alport syndrome. Hum Mutat 27: 1061, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Michel-Calemard L, Dijoud F, Till M, Lambert JC, Vercherat M, Tardy V, Coubes C, Morel Y: Pseudoexon activation in the PKHD1 gene: A French founder intronic mutation IVS+653A>G causing severe autosomal recessive polycystic kidney disease. Clin Genet 75: 203–206, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Jang HR, Lee JW, Oh JW, Oh YK, Na KY, Joo KW, Jeon US, Cheong HI, Kim J, Han JS: From bench to bedside: Diagnosis of Gitelman's syndrome: Defect of sodium-chloride cotransporter in renal tissue. Kidney Int 70: 813–817, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Joo KW, Lee JW, Jang HR, Heo NJ, Jeon US, Oh YK, Lim CS, Na KY, Kim J, Cheong HI, Han JS: Reduced urinary excretion of thiazide-sensitive Na-Cl cotransporter in Gitelman syndrome: Preliminary data. Am J Kidney Dis 50: 765–773, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Nozu K, Iijima K, Nozu Y, Ikegami E, Imai T, Fu XJ, Kaito H, Nakanishi K, Yoshikawa N, Matsuo M: A deep intronic mutation in the SLC12A3 gene leads to Gitelman syndrome. Pediatr Res 66: 590–593, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Melton T, Clifford S, Martinson J, Batzer M, Stoneking M: Genetic evidence for the proto-Austronesian homeland in Asia: mtDNA and nuclear DNA variation in Taiwanese aboriginal tribes. Am J Hum Genet 63: 1807–1823, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trejaut JA, Kivisild T, Loo JH, Lee CL, He CL, Hsu CJ, Lee ZY, Lin M: Traces of archaic mitochondrial lineages persist in Austronesian-speaking Formosan populations. PLoS Biol 3: 1362–1372, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Jong JC, Van Der Vliet WA, Van Den Heuvel LP, Willems PH, Knoers NV, Bindels RJ: Functional expression of mutations in the human NaCl cotransporter: Evidence for impaired routing mechanisms in Gitelman's syndrome. J Am Soc Nephrol 13: 1442–1448, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Knodo Y, Sasaki S, Uchida S: Molecular pathogenesis of pseudohypoaldosteronism type II: Generation and analysis of a Wnk4 (D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH: SPAK knockout mice manifest Gitelman's syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Deutekom JC, Bremmer-Bout M, Janson AA, Ginjaar IB, Baas F, den Dunnen JT, van Ommen GJ: Antisense-induced exon skipping restores dystrophin expression in DMD patient derived muscle cells. Hum Mol Genet 10: 1547–1554, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Ugarte M, Aguado C, Desviat LR, Sanchez-Alcudia R, Rincon A, Perez B: Propionic and methylmalonic acidemia: Antisense therapeutics for intronic variations causing aberrantly splicing messenger RNA. Am J Hum Genet 81: 1262–1268, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gurvish OL, Tuohy TM, Howard MT, Finkel RS, Medne L, Anderson CB, Weiss RB, Wilton SD, Flanigan KM: DMD pseudoexon mutations: Splicing efficiency, phenotype, and potential therapy. Ann Neurol 63: 81–89, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Pros E, Fernandez-Rodriguez J, Canet B, Benito L, Sanchez A, Benavides A, Ramos FJ, Lopez-Ariztegui MA, Capella G, Blanco I, Serra E, Lazaro C: Antisense therapeutics for neurofibromatosis type 1 caused by deep intronic mutations. Hum Mutat 30: 454–462, 2009 [DOI] [PubMed] [Google Scholar]