Summary
Background and objectives
Hemoglobin (Hb) is an important nitric oxide (NO) buffer and a modulator of NO bioavailability. In addition, endothelial dysfunction is common in hypertensive patients, suggesting a pivotal role of hemoglobin concentration ([Hb]) in vascular function. To investigate the potential role of [Hb] in endothelium-dependent vasodilation, the relationship between Hb and endothelial function was tested in a group of patients with essential hypertension.
Design, setting, participants, & measurements
In this retrospective study, 174 nonsmoking, uncomplicated, never-treated hypertensives were enrolled. Endothelium-dependent and -independent vasodilation was assessed by measurement of forearm blood flow response during intra-arterial infusion of increasing doses of acetylcholine (ACh) and sodium nitroprusside (SNP) using strain-gauge plethysmography. Correlation with established risk factors of endothelial dysfunction was performed.
Results
The vasodilatory response to ACh was inversely (P < 0.001) related to [Hb], and this relationship was dose dependent (P < 0.001), being minimal at the lowest dose and maximal at the highest dose. No association was found between Hb and the vasodilatory response to SNP. In a multiple linear regression model adjusted for Framingham risk factors (age, sex, BP, cholesterol, body mass index, glucose) and emerging risk factors (homeostasis model assessment index, C-reactive protein, estimated GFR), [Hb] maintained a strong and independent link with the vasodilatory response to ACh (P < 0.001).
Conclusions
In a large group of nonsmoking untreated hypertensives, [Hb] is inversely related to forearm endothelium-dependent vasodilation. [Hb] should be taken into account, especially in conditions associated with low [Hb], when performing vascular function studies.
Introduction
It is well recognized that hypertensive patients have impaired endothelium-dependent vasodilation (1–3). Several hemodynamic and nonhemodynamic factors are involved in the reduced nitric oxide (NO) bioavailability in these patients, such as the presence of a genetic polymorphism (4–6), oxidative stress (7), vascular inflammation (8), and competitive endothelial NO synthase antagonists (9). Among the hemodynamic factors, a reduction in shear stress, the major endogenous physical stimulus of endothelial NO synthase, represents one of the most important mechanisms responsible for reduced endothelium-dependent vasodilation in essential hypertension.
Shear stress is directly related to blood velocity and viscosity and is inversely related to blood vessel diameter. Blood viscosity is affected by erythrocyte count, and this strongly influences the rheological properties of blood. Furthermore, these cells appear to contribute to the regulation of vascular tone by additional mechanisms that are dependent and independent of shear stress. Indeed, the oxygen carrier molecule, hemoglobin (Hb), by a series of biochemical processes including NO oxidation and nitrosylation of the iron molecule and sulfur-containing amino acids in the globin molecule, acts as a transient or permanent NO buffer modulating its bioavailability. The potential clinical relevance of the interaction between hemoglobin concentration ([Hb]) and NO bioavailability may be explained by the observation that high hematocrit and blood viscosity are negative independent predictors for cardiovascular events beyond blood viscosity in a high-risk condition such as hypercholesterolemia (10). On the other hand, [Hb] normalization is associated with a higher risk of death and cardiovascular events in patients with moderate to severe chronic kidney disease and ESRD (11), both conditions characterized by reduced NO synthesis (12).
As alluded to before, uncomplicated essential hypertension is characterized by the presence of different degrees of endothelial dysfunction underlying parallel changes in NO bioavailability. However, population-based studies have consistently demonstrated that, on average, [Hb] is raised in patients with essential hypertension (13). Thus, it appears plausible that Hb-dependent mechanisms influencing NO bioavailability contribute to endothelial dysfunction in hypertension. However, the relationship between circulating Hb levels and endothelial function has never been studied in this population. This association is present in the hematologic disease polycythemia vera (characterized by increased red cell mass and high blood viscosity), in which the endothelial response to vasodilatory stimuli is decreased (14). Therefore, we set out to study the relationship between [Hb] and endothelial function in a large series of nonsmoking patients with untreated and uncomplicated hypertension from a large database. In analyzing this relationship, we considered several potential confounders for the interpretation of the Hb-endothelial function relationship, including renal function, inflammation, insulin sensitivity, and the full series of Framingham risk factors.
Materials and Methods
Study Population
The hypertensive study population consisted of 174 outpatients (97 women and 77 men; mean age 48 ± 11 years; range 22 to 73 years) attending the Catanzaro University Hospital directly or as referrals from general practitioners for detection or investigation of cardiovascular risk factors. To participate in this retrospective study, subjects were required to have a clinic BP ≥140/90 mmHg on two separate visits. Clinic BP was measured at 3-minute intervals with a standard sphygmomanometer after a 10-minute rest period in the supine position and the average of three measurements was recorded. All patients were untreated for hypertension before the beginning of the study. We excluded patients who had experienced stroke, coronary events, or presented clinical evidence of peripheral artery disease, heart failure, overt renal dysfunction (serum creatinine >1.5 mg/dl), or diabetes mellitus. Secondary forms of hypertension were excluded by systematic testing using a standard clinical protocol including renal ultrasound studies, computed tomography, renal scan, catecholamines, plasma renin activity, and aldosterone measurements. To avoid a possible interaction between cigarette smoking and [Hb], we excluded current or previous smokers from this study. Serum creatinine was measured by an automated technique based on the measurement of Jaffe chromogen. Values of estimated GFR (eGFR) were calculated using the modified Modification of Diet in Renal Disease equation, as described by Levey et al. (15). C-reactive protein (CRP) was measured by a high-sensitivity turbidimetric immunoassay (Behring, Marburg, Germany). Insulin resistance was estimated by the homeostasis model assessment (HOMAIR) from the fasting glucose and insulin concentrations according to the equation HOMAIR = [insulin (μU/ml × glucose (mmol/L)]/22.5. [Hb] levels were measured in whole blood after hemolysis using photometry.
The study also included 30 normotensive subjects (15 men and 15 women; mean age 45 ± 7 years; range 32 to 48 years). Normalcy was determined by clinical history, physical examination, and laboratory analyses to exclude cardiovascular, hematologic, renal, or hepatic impairment. All subjects were nonsmokers with a BP ≤140/90 mmHg.
Forearm Blood Flow Measurements
All study measurements were performed by the same experienced investigators (R.M., A.S.) at 9:00 a.m. after overnight fasting with the subjects lying supine in a quiet air-conditioned room (22 to 24°C). Subjects were instructed to continue their regular diet but caffeine and alcohol were stopped at least 24 hours before study measurements. Forearm volume was determined by water displacement. Under local anesthetic and sterile conditions, a 20-gauge polyethylene catheter (Vasculon 2) was inserted into the brachial artery of the nondominant arm for evaluation of BP (Baxter Healthcare Corporation, Deerfield, IL) and drug administration. This arm was elevated above the level of the right atrium, and a mercury-filled silastic strain gauge was placed on the widest part of the forearm. The strain gauge was connected to a plethysmograph (model EC-4, Hokanson, Issaquah, WA) calibrated to measure the percent change in volume; this was connected to a chart recorder to obtain the forearm blood flow (FBF) measurements. A cuff placed on the upper arm was inflated to 40 mmHg with a rapid cuff inflator (model E-10, Hokanson, Issaquah, WA) to exclude venous outflow from the extremity. A wrist cuff was inflated to BP values 1 minute before each measurement to exclude the hand blood flow. The antecubital vein of the opposite arm was cannulated. The FBF was measured as the slope of the change in the forearm volume. The mean of at least three measurements was obtained at each time point. Forearm vascular resistance (VR), expressed in units (U), was calculated by dividing mean BP by FBF.
Vascular Function
The protocol previously described by Panza et al. (2) and subsequently used by our group (3,5,6,9) was used for the study presented here. All patients underwent measurement of FBF and BP during intra-arterial infusion of saline, acetylcholine (ACh), and sodium nitroprusside (SNP) at increasing doses. ACh (Sigma, Milan, Italy) was diluted with sodium chloride (0.9%) immediately before infusion. SNP (Malesci, Florence, Italy) was diluted in 5% glucose solution immediately before each infusion and protected from light with aluminum foil. All participants rested for 30 minutes after arterial cannulation so that a stable baseline could be reached before data collection; measurements of FBF and VR were repeated every 5 minutes until stable. Endothelium-dependent and -independent vasodilation were assessed by a dose-response curve to intra-arterial ACh infusions (7.5, 15, and 30 μg/ml per minute, each for 5 minutes) and SNP infusions (0.8, 1.6, and 3.2 μg/ml per minute, each for 5 minutes), respectively. The drug infusion rate, adjusted for the forearm volume of each subject, was 1 ml/min. The sequence of administration of ACh and SNP was randomized to avoid any bias related to the order of drug infusion. Inter- and intraobserver variability has been previously reported (16).
Statistical Analyses
Data are expressed as mean ± SD or as percentage frequency, and comparisons between groups were made using the t test or the χ2 test, as appropriate. Relationships between paired parameters were analyzed by Pearson product moment correlation coefficient. To test the independent relationship between FBF and [Hb], we constructed multivariate linear models on the basis of a series of hematologic parameters (hematocrit and mean corpuscular hemoglobin concentration [MCHC]) and traditional (age, gender, body mass index [BMI], systolic BP, and LDL-cholesterol and triglyceride levels) and emerging cardiovascular risk factors (eGFR, HOMAIR, and CRP). In the multivariate linear regression analysis, data are expressed as standardized regression coefficient (β) and semipartial r2 (17), this latter giving the proportion of variance of the dependent variable explained by each independent variable, and P value. The differences between curves were compared using the area under the curves. A P value <0.05 was considered statistically significant. All comparisons were performed using the statistical package SPSS for Windows version 10.0 (Chicago, IL). Data are expressed as β and P value.
Results
Baseline demographic, clinical, and hemodynamic characteristics of hypertensive subjects grouped on the basis of the median value of [Hb] and normotensive controls are summarized in Table 1. There were no significant differences in age, gender, heart rate, [Hb], or triglyceride levels between control subjects and hypertensive patients. All patients were normotolerant. Systolic and diastolic BP values were significantly (P = 0.0001) lower in normotensives compared with hypertensives. VR was higher in hypertensives than normotensive controls (35.7 ± 7.0 U versus 29.7 ± 5.3 U; P = 0.0001), whereas baseline FBF did not differ between the two groups (3.3 ± 0.6 versus 3.4 ± 0.5 ml/100 ml of tissue per minute; P = 0.390). We observed similar results in hypertensive patients matched for sex and gender (Table 2). In Table 3, we report data of study population divided for men and women. As evident, hypertensive and normotensive men had [Hb], hematocrit, and eGFR mean values higher than female groups.
Table 1.
Demographic, hemodynamic, and biochemical data in hypertensive patients and control subjects
| Hypertensive Patients |
Normotensives | P | ||||
|---|---|---|---|---|---|---|
| All | [Hb] < 14 g/dl | [Hb] ≥ 14 g/dl | P | |||
| Age, years | 48 ± 11 | 47 ± 11 | 48 ± 10 | 0.531 | 45 ± 7 | 0.151 |
| Male gender (%) | 77 (44%) | 33 (38%) | 44 (50%) | 0.127 | 15 (50%) | 0.700 |
| BMI, kg/m2 | 27.4 ± 3.7 | 27.1 ± 3.7 | 27.6 ± 3.6 | 0.368 | 24.2 ± 2.3 | 0.0001 |
| Systolic BP, mmHg | 153 ± 15 | 153 ± 15 | 153 ± 16 | 0.999 | 132 ± 4 | 0.0001 |
| Diastolic BP, mmHg | 94 ± 10 | 95 ± 10 | 93 ± 11 | 0.211 | 80 ± 4 | 0.0001 |
| Heart rate, bpm | 73 ± 9 | 73 ± 9 | 73 ± 10 | 0.999 | 71 ± 9 | 0.262 |
| [Hb], g/dl | 13.8 ± 1.1 | 12.9 ± 0.6 | 14.8 ± 0.5 | 0.0001 | 13.4 ± 0.6 | 0.054 |
| Serum iron, μg/dl | 90 ± 28 | 85 ± 30 | 94 ± 25 | 0.032 | 87 ± 26 | 0.585 |
| Hematocrit, % | 43 ± 4 | 41 ± 3 | 45 ± 2 | 0.001 | 41 ± 2 | 0.008 |
| MCHC, g/dl | 32.6 ± 1.7 | 32.1 ± 1.6 | 33.0 ± 1.6 | 0.0001 | 33.7 ± 1.1 | 0.0001 |
| Cholesterol, mg/dl | 206 ± 32 | 205 ± 31 | 207 ± 33 | 0.681 | 186 ± 12 | 0.0001 |
| LDL-cholesterol, mg/dl | 129 ± 33 | 128 ± 34 | 131 ± 32 | 0.550 | 114 ± 24 | 0.018 |
| HDL-cholesterol, mg/dl | 52 ± 13 | 53 ± 12 | 52 ± 13 | 0.599 | 57 ± 11 | 0.048 |
| Triglyceride, mg/dl | 118 ± 40 | 115 ± 41 | 121 ± 40 | 0.330 | 103 ± 28 | 0.050 |
| Glucose, mg/dl | 95 ± 11 | 94 ± 11 | 97 ± 12 | 0.087 | 87 ± 7 | 0.0001 |
| Insulin, U/L | 14.1 ± 7.2 | 12.7 ± 6.8 | 15.5 ± 7.4 | 0.010 | 7.7 ± 3.4 | 0.0001 |
| HOMAIR | 3.3 ± 1.9 | 3.0 ± 1.7 | 3.7 ± 2.0 | 0.014 | 1.7 ± 0.7 | 0.0001 |
| Creatinine, mg/dl | 0.92 ± 0.17 | 0.91 ± 0.18 | 0.92 ± 0.16 | 0.699 | 0.79 ± 0.10 | 0.0001 |
| eGFR, ml/min per 1.73 m2 | 84 ± 21 | 83 ± 21 | 85 ± 21 | 0.620 | 98 ± 18 | 0.0001 |
| CRP, mg/L | 4.2 ± 2.5 | 3.8 ± 2.2 | 4.6 ± 2.8 | 0.038 | 1.5 ± 0.6 | 0.0001 |
| FBF, % increase | ||||||
| ACh | 328 ± 216 | 398 ± 253 | 267 ± 150 | 0.0001 | 664 ± 108 | 0.0001 |
| SNP | 320 ± 114 | 328 ± 113 | 313 ± 114 | 0.385 | 348 ± 97 | 0.206 |
Data are expressed as mean ± SD unless otherwise stated.
Table 2.
Demographic, hemodynamic, and biochemical data in hypertensive patients and control subjects matched for age and gender
| Hypertensives | Normotensives | P | |
|---|---|---|---|
| Age, years | 45 ± 7 | 45 ± 7 | 0.977 |
| Male gender (%) | 15 (50%) | 15 (50%) | |
| BMI, kg/m2 | 27.0 ± 3.3 | 24.2 ± 2.3 | 0.0001 |
| Systolic BP, mmHg | 155 ± 13 | 132 ± 4 | 0.0001 |
| Diastolic BP, mmHg | 95 ± 9 | 80 ± 4 | 0.0001 |
| Heart rate, bpm | 72 ± 9 | 71 ± 9 | 0.669 |
| Hb, g/dl | 13.7 ± 1.0 | 13.4 ± 0.6 | 0.164 |
| Hematocrit, % | 42 ± 4 | 41 ± 2 | 0.087 |
| MCHC, g/dl | 32.7 ± 1.6 | 33.7 ± 1.1 | 0.008 |
| Serum iron, mcg/dl | 88 ± 27 | 87 ± 25 | 0.917 |
| Cholesterol, mg/dl | 206 ± 32 | 186 ± 12 | 0.002 |
| LDL-cholesterol, mg/dl | 130 ± 34 | 114 ± 24 | 0.045 |
| HDL-cholesterol, mg/dl | 52 ± 13 | 57 ± 11 | 0.099 |
| Triglyceride, mg/dl | 117 ± 38 | 103 ± 28 | 0.135 |
| Glucose, mg/dl | 94 ± 11 | 87 ± 7 | 0.003 |
| Insulin, U/L | 16.6 ± 7.5 | 7.7 ± 3.4 | 0.0001 |
| HOMAIR | 3.9 ± 1.9 | 1.7 ± 0.7 | 0.0001 |
| Creatinine, mg/dl | 0.91 ± 0.16 | 0.79 ± 0.10 | 0.002 |
| eGFR, ml/min per 1.73 m2 | 85 ± 18 | 98 ± 18 | 0.010 |
| CRP, mg/L | 4.1 ± 2.3 | 1.5 ± 0.6 | 0.0001 |
| FBF, % increase | |||
| ACh | 296 ± 207 | 664 ± 108 | 0.0001 |
| SNP | 309 ± 114 | 348 ± 97 | 0.163 |
Data are expressed as mean ± SD unless otherwise stated.
Table 3.
Demographic, hemodynamic, and biochemical data in hypertensive patients and normotensive subjects according to gender
| Data | Hypertensives |
Normotensives |
||||||
|---|---|---|---|---|---|---|---|---|
| All | Men | Women | P | All | Men | Women | P | |
| Age, years | 48 ± 11 | 47 ± 9 | 48 ± 12 | 0.600 | 45 ± 7 | 45 ± 7 | 45 ± 7 | 0.901 |
| Male gender (%) | 77 (44%) | 77 | 97 | 15 (50%) | 15 | 15 | ||
| BMI, kg/m2 | 27.4 ± 3.7 | 27.3 ± 3.1 | 27.3 ± 4.0 | 0.999 | 24.2 ± 2.3 | 24.2 ± 2.6 | 24.3 ± 2.0 | 0.965 |
| Systolic BP, mmHg | 153 ± 15 | 150 ± 16 | 154 ± 15 | 0.081 | 132 ± 4 | 131 ± 4 | 133 ± 3 | 0.417 |
| Diastolic BP, mmHg | 94 ± 10 | 94 ± 10 | 94 ± 10 | 0.697 | 80 ± 4 | 80 ± 4 | 80 ± 4 | 0.932 |
| Heart rate, bpm | 73 ± 9 | 72 ± 10 | 74 ± 9 | 0.281 | 71 ± 9 | 72 ± 10 | 74 ± 7 | 0.530 |
| [Hb], g/dl | 13.8 ± 1.1 | 14.2 ± 1.0 | 13.6 ± 1.0 | 0.0001 | 13.4 ± 0.6 | 14.0 ± 0.7 | 13.3 ± 0.8 | 0.019 |
| Hematocrit, % | 43 ± 4 | 43 ± 3 | 42 ± 4 | 0.007 | 41 ± 2 | 42 ± 2 | 40 ± 2 | 0.007 |
| MCHC, g/dl | 32.6 ± 1.7 | 32.8 ± 1.5 | 32.5 ± 1.8 | 0.252 | 33.7 ± 1.1 | 33.7 ± 0.89 | 33.6 ± 1.3 | 0.915 |
| Serum iron, μg/dl | 90 ± 28 | 91 ± 26 | 88 ± 29 | 0.499 | 87 ± 26 | 88 ± 27 | 86 ± 25 | 0.835 |
| Cholesterol, mg/dl | 206 ± 32 | 203 ± 32 | 208 ± 32 | 0.307 | 186 ± 12 | 184 ± 14 | 189 ± 10 | 0.356 |
| LDL-cholesterol, mg/dl | 129 ± 33 | 127 ± 33 | 132 ± 32 | 0.314 | 114 ± 24 | 109 ± 25 | 119 ± 22 | 0.285 |
| HDL-cholesterol, mg/dl | 52 ± 13 | 51 ± 11 | 53 ± 14 | 0.443 | 57 ± 11 | 58 ± 10 | 56 ± 13 | 0.601 |
| Triglyceride, mg/dl | 118 ± 40 | 121 ± 41 | 115 ± 40 | 0.340 | 103 ± 28 | 107 ± 32 | 99 ± 24 | 0.449 |
| Glucose, mg/dl | 95 ± 11 | 98 ± 11 | 93 ± 11 | 0.006 | 87 ± 7 | 87 ± 4 | 87 ± 9 | 0.993 |
| Insulin, U/L | 14.1 ± 7.2 | 15.1 ± 6.8 | 13.2 ± 7.4 | 0.081 | 7.7 ± 3.4 | 8.4 ± 3.7 | 7.1 ± 3.0 | 0.303 |
| HOMAIR | 3.3 ± 1.9 | 3.6 ± 1.7 | 3.0 ± 1.9 | 0.032 | 1.7 ± 0.7 | 1.8 ± 0.8 | 1.5 ± 0.7 | 0.332 |
| Creatinine, mg/dl | 0.92 ± 0.17 | 0.93 ± 0.16 | 0.90 ± 0.17 | 0.136 | 0.79 ± 0.10 | 0.82 ± 0.10 | 0.76 ± 0.09 | 0.080 |
| eGFR, ml/min per 1.73 m2 | 84 ± 21 | 95 ± 19 | 75 ± 17 | 0.0001 | 98 ± 18 | 108 ± 17 | 88 ± 14 | 0.002 |
| CRP, mg/L | 4.2 ± 2.5 | 4.0 ± 2.4 | 4.0 ± 2.4 | 0.524 | 1.5 ± 0.6 | 1.6 ± 0.5 | 1.3 ± 0.7 | 0.366 |
| FBF, % increase | ||||||||
| ACh | 328 ± 216 | 287 ± 167 | 359 ± 167 | 0.028 | 664 ± 108 | 634 ± 100 | 655 ± 101 | 0.561 |
| SNP | 320 ± 114 | 321 ± 122 | 320 ± 107 | 0.916 | 348 ± 97 | 323 ± 96 | 373 ± 94 | 0.156 |
Data are expressed as mean ± SD unless otherwise stated.
Hypertensive patients with a [Hb] above the median had higher fasting insulin, HOMAIR, and CRP values. No significant differences were observed in age, BMI, heart rate, lipid profile, fasting glucose, creatinine, and eGFR. There were no significant differences in baseline FBF values (3.2 ± 0.6 versus 3.3 ± 0.5 ml/100 ml of tissue per minute; P = 0.233).
Endothelium-Dependent Vasodilation
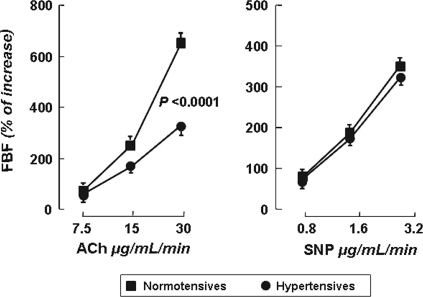
Intra-arterial infusion of ACh caused a significant dose-dependent increase in FBF and decrease in forearm VR in both groups. The FBF values at the three incremental doses of ACh were 2.3 ± 0.6 (+69%), 8.6 ± 2.2 (+256%), and 22.5 ± 6.3 ml/100 ml of tissue per minute (+664%) and 1.9 ± 1.3 (+58%), 5.5 ± 3.9 (+169%), and 10.6 ± 7.2 ml/100 ml of tissue per minute (+328%) for normotensive and hypertensive patients, respectively (Figure 1). Thus, ACh-stimulated FBF was significantly reduced in hypertensive subjects compared with normotensive control subjects (P = 0.0001). In both groups, BP and heart rate remained unmodified during intra-arterial infusion of ACh.
Figure 1.
FBF dose-response curves to ACh and SNP in normotensive subjects and hypertensive patients. Values are reported as mean ± SEM.
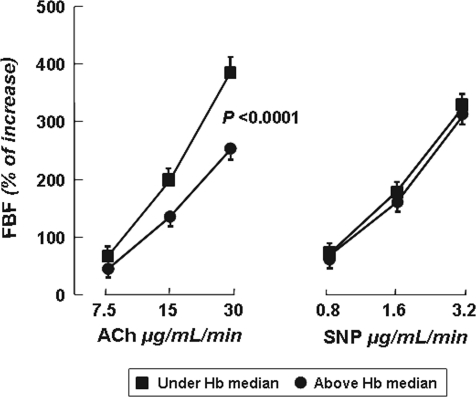
Interestingly, ACh-stimulated vasodilation was significantly higher in hypertensive patients with [Hb] below the median value (Figure 2). On the contrary, we did not observe any significant difference in ACh-stimulated vasodilation (peak increase 660 ± 100% versus 669 ± 119%; P = 0.824) by dividing normotensive controls on the basis of median [Hb].
Figure 2.
FBF dose-response curves to ACh and SNP in hypertensive patients divided by median [Hb] of 14 mg/dl. Values are reported as mean ± SEM.
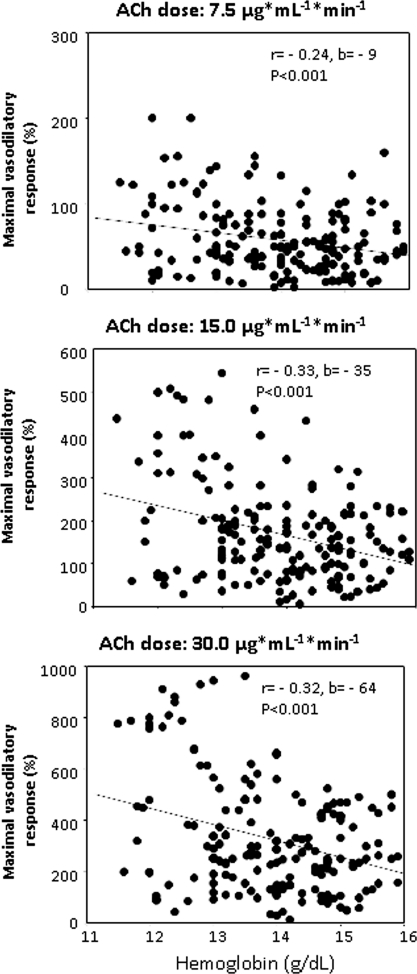
In Figure 3 we illustrate the detailed analysis of the relationship between the vasodilatory responses to three ACh doses and [Hb] values. Remarkably, the vascular response to each dose was directly related to [Hb], and the steepness of these relationships was highest at the maximal ACh dose (−64%/g Hb), intermediate (−35%/g Hb) at the second dose, and minimal at the lowest dose (−9%/g Hb) (P < 0.001). The hematocrit-vasodilatory response to ACh was similar to that observed with [Hb] (data not shown).
Figure 3.
Relationship between Hb and FBF response to three incremental doses of ACh: (top) 7.5, (middle) 15.0, and (bottom) 30.0 μg/ml per minute.
Endothelium-Independent Vasodilation
The FBF values at three incremental doses of SNP were 2.5 ± 0.7 (+74%), 6.2 ± 2.4 (+185%), and 11.6 ± 3.6 ml/100 ml of tissue per minute (+348%) and 2.3 ± 0.7 (+70%), 5.5 ± 2.1 (+171%), and 10.4 ± 3.9 ml/100 ml of tissue per minute (+320%) in normotensive controls and in hypertensive patients, respectively. Similarly, no significant difference was observed in the vasodilatory responses to SNP infusion in the two groups of hypertensive patients (Figure 2). Intra-arterial infusion of SNP caused no changes in BP or heart rate.
Correlation Analysis
In Table 4 we reported the results of univariate linear analysis between ACh-stimulated vasodilation and different covariates in hypertensive patients and in normotensive controls. In particular, peak percent increase in ACh-stimulated vasodilation in the hypertensive group was inversely related to CRP (r = −0.440; P < 0.0001), accounting for 19.4% of its variation; HOMAIR (r = −0.391; P < 0.0001), accounting for 15.3% of its variation; [Hb] (r = −0.323; P < 0.0001), accounting for 10.4% of its variation; age (r = −0.288; P < 0.0001), accounting for 8.2% of its variation; eGFR (r = −0.270; P < 0.0001), accounting for 7.3% of its variation; BMI (r = −0.227; P < 0.001), accounting for 5.1% of its variation; and MCHC (r = 0.132; P = 0.041), accounting for 1.4% of its variation. On the contrary, in normotensive controls, ACh-stimulated vasodilation was inversely related to HOMAIR (r = −0.433; P = 0.008), accounting for 18.7% of its variation, and directly related to gender (r = 0.380; P = 0.019), accounting for 14.4% of the variation.
Table 4.
Independent predictors of peak increase in ACh-stimulated FBF in hypertensive patients and control subjects
| Variables | Hypertensives |
Normotensives |
||
|---|---|---|---|---|
| r | P | R | P | |
| CRP | −0.440 | 0.0001 | −0.266 | 0.078 |
| HOMAIR | −0.391 | 0.0001 | −0.433 | 0.008 |
| Hb | −0.323 | 0.0001 | −0.128 | 0.251 |
| Age (years) | −0.288 | 0.0001 | 0.182 | 0.168 |
| eGFR | −0.270 | 0.0001 | 0.184 | 0.166 |
| BMI | −0.227 | 0.001 | −0.230 | 0.111 |
| MCHC | 0.132 | 0.041 | 0.019 | 0.845 |
| HDL-cholesterol | 0.102 | 0.090 | 0.179 | 0.172 |
| Total cholesterol | 0.025 | 0.371 | 0.195 | 0.150 |
| Serum iron | 0.083 | 0.273 | 0.031 | 0.320 |
| Systolic BP | −0.094 | 0.109 | 0.023 | 0.453 |
| Diastolic BP | −0.026 | 0.365 | −0.231 | 0.110 |
| Triglyceride | −0.057 | 0.229 | −0.187 | 0.161 |
| LDL-cholesterol | 0.001 | 0.493 | 0.158 | 0.203 |
To further analyze the independent contribution of each independent factor to the peak FBF response to ACh, we constructed different multiple regression models. At first, we tested a baseline model including Framingham risk factors, eGFR, [Hb], and MCHC. Subsequent improvements in the model fitting were tested by entering, one at time, the HOMAIR and CRP values.
In the first model, [Hb], eGFR, gender, age, and BMI were retained as independent predictors of ACh-stimulated FBF, explaining the 30.3% of its variation. After the addition of HOMAIR, the model retained as independent predictors HOMAIR, eGFR, gender, [Hb], and age, explaining the 34.5% of the variation. When we also entered CRP in the analysis, the model retained it as the strongest predictor of the vascular dysfunction (Table 5).
Table 5.
Multiple regression models of peak increase in ACh-stimulated FBF
| Partial r2 (%) | Total r2 (%) | β | P | |
|---|---|---|---|---|
| Model 1 | ||||
| Hb | 10.5 | 10.5 | −0.230 | 0.0001 |
| eGFR | 8.2 | 18.7 | 0.360 | 0.0001 |
| gender | 6.5 | 25.2 | 0.285 | 0.0001 |
| age | 2.8 | 28.0 | −0.169 | 0.015 |
| BMI | 2.3 | 30.3 | −0.154 | 0.020 |
| Model 2 | ||||
| HOMAIR | 15.3 | 15.3 | −0.269 | 0.0001 |
| eGFR | 6.6 | 21.9 | 0.349 | 0.0001 |
| gender | 7.4 | 29.3 | 0.242 | 0.002 |
| hemoglobin | 3.6 | 32.9 | −0.203 | 0.003 |
| age | 1.6 | 34.5 | −0.138 | 0.041 |
| Model 3 | ||||
| CRP | 19.3 | 19.3 | −0.308 | 0.0001 |
| HOMAIR | 11.3 | 30.6 | −0.260 | 0.0001 |
| Hb | 4.7 | 35.3 | −0.187 | 0.004 |
| eGFR | 2.4 | 37.7 | 0.280 | 0.002 |
| age | 3.4 | 41.1 | 0.224 | 0.011 |
Model 1 = Framingham variables plus eGFR and Hb; model 2 = model 1 plus HOMAIR; model 3 = model 2 plus CRP.
Of interest and clinically relevant, [Hb], eGFR, gender, and age remained in all models, suggesting that the pathogenetic mechanisms involved in the endothelial damage are different. Analyses based on hematocrit produced similar results (data not shown).
Discussion
In this study we show that hypertensive patients had a reduced response to the endothelium-dependent agent ACh compared with normotensive controls, whereas the response to SNP was preserved, confirming that only the endothelium-mediated vasodilation is impaired in essential hypertension. We also found that among hypertensive patients, those with a [Hb] above the median had a blunted ACh-stimulated vasodilatory response compared with those with a [Hb] below the median. In normotensive control subjects, [Hb] influences neither the response to ACh nor that to SNP. Thus, the main and new finding of this study is the demonstration of an inverse and consistent relationship between [Hb] and ACh-stimulated vasodilation in a group of never-treated hypertensive patients, supporting the multifactorial pathogenesis of hypertension-related endothelial dysfunction. In fact, in hypertensive patients, endothelial damage may be induced by hemodynamic and nonhemodynamic factors. Several emerging factors are being considered as causative of endothelial dysfunction. For example, we recently demonstrated a significant relationship between ACh-stimulated vasodilation and asymmetric dimethylarginine levels (9), creatinine (18), and uric acid (19). Data obtained in this study are particularly relevant because they further explain the role, even if weak, of hemorheological modifications in endothelial function and suggest that [Hb] affects NO bioavailability. Although previous in vitro data demonstrated an inverse correlation between [Hb] and NO bioactivity, the interaction between NO and [Hb] is not well characterized in normal physiologic conditions in humans. Gradual NO scavenging by [Hb] is probably a mechanism so finely tuned as to be able to outbalance changes in [Hb], even within the normal range (20–23) just as documented in our patients. With regards to the interaction between [Hb] and SNP in vascular function, it is useful to remark that nitrite induced vasodilation by activating soluble guanylate cyclase. This vasodilatory activity was associated with the conversion of nitrite to NO by deoxyhemoglobin, suggesting that Hb possesses a physiologic nitrite reductase activity that may participate especially in hypoxic vasodilation.
A pivotal mechanism involved in the interaction of [Hb] with NO is represented by the reaction of NO with oxyhemoglobin to produce methemoglobin and nitrate. This reaction inactivates NO and impairs endothelium-dependent vasodilation as a consequence of a reduced NO bioavailability. The fast and irreversible reaction between NO and intravascular oxyhemoglobin causes immediate NO inactivation and, consequently, a reduced paracrine diffusion from endothelium to vascular smooth muscle cells (24–26). This mechanism is particularly important in the impairment of NO-mediated vasodilation that occurs during intravascular hemolysis (27). The speed and irreversibility of this reaction is such that even a relatively small degree of hemolysis can completely inhibit NO and reduce endothelium-dependent vasodilation. Because Hb is released into the plasma as a consequence of the lysis of erythrocytes, the ability of [Hb] to react with endothelial NO is limited by its compartmentalization inside of erythrocytes. The confinement of Hb within erythrocytes reduces the rate of the reaction between [Hb] and NO by a factor ≥1000 because the cellular membrane creates a barrier to the diffusion of NO (24,26). Another postulated mechanism by which NO bioactivity is reduced is via its binding to cysteinebeta-93, forming S-nitrohemoglobin, which possesses some biologic activities (23). However, this reaction mainly occurs in ischemic tissues because a high oxygen tension favors S-nitrosylation of Hb and a low oxygen tension favors NO release with associated vasodilation (22,23). These mechanisms contribute to explain the observed association between [Hb] and endothelial dysfunction in some clinical conditions. Reiter and coworkers reported an increased destruction of bioactive NO by [Hb] in sickle cell anemia (28). In addition, other clinical manifestations can be attributed to [Hb] release in various acquired and iatrogenic hemolytic disorders, suggesting that hemolysis and [Hb] should be considered as a novel mechanism of human disease (29). Natali et al. recently demonstrated an inverse relationship between hematocrit and [Hb] and endothelium-dependent and -independent vasodilation in diabetic patients (30). These findings are in contrast with those successively reported by Madsen and coworkers (31) who did not document any significant correlation between endothelium-dependent vasodilation and [Hb] and hematocrit in patients with type 2 diabetes.
In our population, we found that [Hb] in hypertensive patients interferes only with ACh-stimulated FBF, whereas no association was demonstrated in SNP-stimulated vascular reactivity. The discordance between our data and those from Natali is probably attributable to a difference in study populations. Our hypertensives were newly diagnosed, never-treated nonsmokers without clinical evidence of vascular complications. Natali's patients were diabetics with a long history of different degrees of heart failure or past myocardial infarction, all conditions affecting endothelial function. Nevertheless, possible different pathogenetic mechanisms involved in the endothelial dysfunction seen in diabetics or hypertensives cannot be excluded. A lower NO bioavailability may be due to reduced NO production, its increased degradation due to oxidative stress, or both. In addition, reduced smooth muscle cell sensitivity to the action of NO may be present; therefore, an altered sensitivity of soluble guanylate cyclase in hypertensives cannot be excluded. Of interest, a dysfunctioning endothelium also becomes a source of other substances and mediators (endothelins, angiotensin II, prostanoids, superoxide anions) that are detrimental to the arterial wall, promoting various proatherosclerotic features including a vasoconstricting action (32). In normotensive subjects, we did not observe any significant relationship between [Hb] and endothelium-dependent or -independent vascular reactivity. These results are in agreement with data reported by Madsen, who found no significant correlation between [Hb] and flow-mediated dilation evaluated in the brachial artery (31).
Although the range of [Hb] values explored by us (11.5 to 16.0 g/dl) was narrow, the Hb-vasodilatory response to ACh was progressively steeper from the lowest to the highest ACh dose. This link was unaffected by traditional confounders (age, gender, fasting glucose, lipid profile, and BP values) and emerging risk factors such as insulin resistance, eGFR, and CRP.
We observed that [Hb], even if in the normal range, is associated with impaired endothelium-dependent vasodilation in hypertensives. These effects, probably due to the buffering action of Hb on NO, extend the knowledge of potential pathophysiological mechanisms involved in endothelial dysfunction, which is an independent predictor of adverse cardiovascular outcomes. The findings presented here indicate that [Hb] should be taken into account in studies of vascular function in human diseases, particularly in clinical conditions characterized by alterations in erythrocyte mass.
Study Limitations
An important limitation is the fact that Hb species (nitrosyl-hemoglobin and S-nitrosohemoglobin) were not measured. Similarly, ferritin and transferrin values were not available for many patients because only a small sample of patients had [Hb] values <12g/dl. Therefore, we have not determined these parameters.
Disclosures
None.
Acknowledgments
English language assistance was provided by Neil Reynolds of inScience Communications, funded by Pfizer.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Luscher TF, Vanhoutte PM: The Endothelium: Modulator of Cardiovascular Function. Boca Raton, FL, CRC Press, 1990, pp 1–215 [Google Scholar]
- 2. Panza JA, Garcia CE, Kilcoyne CM, Quyyumi AA, Cannon RO: Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation 91: 1732–1738, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G: Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Hingorani AD: Endothelial nitric oxide synthase polymorphisms and hypertension. Curr Hypertens Rep 5: 19–25, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Perticone F, Ceravolo R, Maio R, Ventura G, Zingone A, Perrotti N, Mattioli PL: Angiotensin-converting enzyme gene polymorphism is associated with endothelium-dependent vasodilation in never treated hypertensive patients. Hypertension 31: 900–905, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Perticone F, Sciacqua A, Barlassina C, Del Vecchio L, Signorello MC, Dal Fiume C, Andreozzi F, Sesti G, Cusi D: Gly460Trp alpha-adducin gene polymorphism and endothelial function in untreated hypertensive patients. J Hypertens 25: 2234–2239, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A: Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 97: 2222–2229, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Teoh H, Quan A, Lovren F, Wang G, Tirgari S, Szmitko PE, Szalai AJ, Ward ME, Verma S: Impaired endothelial function in C-reactive protein overexpressing mice. Atherosclerosis 201: 318–325, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, Tripepi G, Sesti G, Zoccali C: Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol 46: 518–523, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Lowe G, Rumley A, Norrie J, Ford I, Shepherd J, Cobbe S, Macfarlane P, Packard C: Blood rheology, cardiovascular risk factors, and cardiovascular disease: The West of Scotland Coronary Prevention Study. Thromb Haemost 84: 553–558, 2000 [PubMed] [Google Scholar]
- 11. Strippoli GF, Tognoni G, Navaneethan SD, Nicolucci A, Craig JC: Haemoglobin targets: We were wrong, time to move on. Lancet 369: 346–350, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Zoccali C, Kielstein JT: Asymmetric dimethylarginine: A new player in the pathogenesis of renal disease? Curr Opin Nephrol Hypertens 15: 314–320, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Cirillo M, Laurenzi M, Trevisan M, Stamler J. Hematocrit, blood pressure, and hypertension. The Gubbio Population Study. Hypertension 20: 319–326, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Neunteufl T, Heher S, Stefenelli T, Pabinger I, Gisslinger H: Endothelial dysfunction in patients with polycythaemia vera. Br J Haematol 115: 354–359, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Bosch JP, Breyer LJ, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Sciacqua A, Scozzafava A, Pujia A, Maio R, Borrello F, Andreozzi F, Vatrano M, Cassano S, Perticone M, Sesti G, Perticone F: Interaction between vascular dysfunction and cardiac mass increases the risk of cardiovascular outcomes in essential hypertension. Eur Heart J 26: 921–927, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Kleinbaum DG, Kupper LL, Muller KE, Nizam A: Applied Regression Analysis and Other Multivariable Methods, 3rd Ed., Washington, DC, Duxbury Press, 2000 [Google Scholar]
- 18. Perticone F, Maio R, Tripepi G, Zoccali C: Endothelial dysfunction and mild renal insufficiency in essential hypertension. Circulation 110: 821–852, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Zoccali C, Maio R, Mallamaci F, Sesti G, Perticone F: Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol 17: 1466–1471, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Gow AJ, Luchsinger BP, Pawloski JR, Singel DJ, Stamler JS: The oxyhemoglobin reaction of nitric oxide. Proc Natl Acad Sci U S A 96: 9027–9032, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butler AR, Megson IL, Wright PG: Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim Biophys Acta 1425: 168–176, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Jia L, Bonaventura C, Bonaventura J, Stamler JS: S-Nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature 380: 221–226, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA: Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034–2037, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Huang KT, Han TH, Hyduke DR, Vaughn MW, Van Herle H, Hein TW, Kuo L, Liao JC: Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci U S A 98: 11771–11776, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McMahon TJ, Pawloski JR, Hess DT, Piantadosi CA, Luchsinger BP, Dingel DJ, Stamler JJ: S-Nitrosohemoglobin is distinguished from other nitrosovasodilators by unique oxygen-dependent responses that support an allosteric mechanism of action. Blood 102: 410–411, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Schechter AN, Gladwin MT: Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med 348: 1483–1485, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB: Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest 115: 3409–3417, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT: Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 8: 1383–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Rother RP, Bell L, Hillmen P, Gladwin MT: The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA 293: 1653–1662, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Natali A, Toschi E, Baldeweg S, Casolaro A, Baldi S, Sironi AM, Yudkin JS, Ferrannini E: Haematocrit, type 2 diabetes, and endothelium-dependent vasodilatation of resistance vessels. Eur Heart J 26: 464–471, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Madsen PL, Scheuermann Freestone M, Neubauer S, Channon K, Clarke K: Haemoglobin and flow-mediated vasodilation. Clin Sci (Lond) 110: 467–473, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S: Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol 157: 527–536, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]