Abstract
Objective
Studies of folate intake and colorectal cancer risk have been inconsistent. We examined the relation with colon cancer risk in a series of 13 prospective studies.
Methods
Study- and sex-specific relative risks (RRs) were estimated from the primary data using Cox proportional hazards models and then pooled using a random-effects model.
Results
Among 725,134 participants, 5,720 incident colon cancers were diagnosed during follow-up. The pooled multivariate RRs (95% confidence interval [CI]) comparing the highest vs. lowest quintile of intake were 0.92 (95% CI 0.84–1.00, p-value, test for between-studies heterogeneity = 0.85) for dietary folate and 0.85 (95% CI 0.77–0.95, p-value, test for between-studies heterogeneity = 0.42) for total folate. Results for total folate intake were similar in analyses using absolute intake cutpoints (pooled multivariate RR = 0.87, 95% CI 0.78–0.98, comparing ≥560 mcg/days vs. <240 mcg/days, p-value, test for trend = 0.009). When analyzed as a continuous variable, a 2% risk reduction (95% CI 0–3%) was estimated for every 100 μg/day increase in total folate intake.
Conclusion
These data support the hypothesis that higher folate intake is modestly associated with reduced risk of colon cancer.
Keywords: Colon cancer, Folate, Cohort studies, Meta-analysis, Pooled analysis
Introduction
Much evidence suggests that folate intake might reduce the risk of some cancers, especially those of the colon and rectum [1]. In a case–control study among patients with ulcerative colitis, long-term users of folate supplements had less than half the rate of colonic neoplasia of nonusers [2]. Since then, at least 14 case–control studies [3–16] and 11 cohort studies [17–27] have examined the association between folate intake from dietary and/or supplemental sources and colorectal cancer risk. Results from the case–control studies have been inconsistent: a lower risk of colon cancer has been observed with higher folate intake in some studies [3–6, 10, 12, 14], but not in others [7–9, 11, 13, 15, 16]. Inverse associations have been more consistently observed in prospective studies, [17–23, 25–27] although in several studies, the relative risks were not statistically significant [17, 18, 20, 22, 23, 27]. A recent meta-analysis found a stronger inverse association for folate from dietary sources alone (summary RR comparing the highest vs. lowest quintile = 0.75, 95% CI 0.64–0.89; p-value, test for between-studies heterogeneity = 0.67; n = 5 studies) compared with folate intake from dietary and supplemental sources (summary RR = 0.95, 95% CI 0.81–1.11; p-value, test for between-studies heterogeneity = 0.33; n = 3 studies), with the difference in the results between dietary and total folate intake being marginally significant (p-value = 0.06) [28]. However, only one study [23] was included in both the dietary folate and total folate analyses.
Differences in the associations between folate intake and colon cancer risk according to sex [5, 21] and tumor site [7, 18, 26] have been reported. Some studies [4, 8, 17, 18, 21, 25], but not all [22, 23], have shown stronger inverse associations for combinations of high folate intake with high methionine and/or low alcohol intakes compared to high folate intake alone, supporting a role of methyl group availability as an underlying mechanism for an effect of folate on colorectal carcinogenesis [29].
Because prospective studies are less vulnerable to the selection and recall biases that can undermine the validity of case–control studies of diet–disease associations, we examined the association between folate intake and colon cancer risk by pooling the primary data from 13 prospective cohort studies that met predefined inclusion criteria.
Study population
The Pooling Project of Prospective Studies of Diet and Cancer has been described previously [30]. For the present analysis, we included prospective studies [17–20, 22–27, 31–33] that met the following predefined criteria: (1) at least 50 incident colorectal cancer cases; (2) assessment of usual dietary intake; and (3) a validation study of the dietary assessment method or a closely related instrument (Table 1). The Adventist Health Study [34], included in the Pooling Project, was excluded from this analysis because folate intake was not assessed at baseline. The Nurses’ Health Study was divided into two parts (1980–1986 and 1986–2000 follow-up periods). Following the underlying theory of survival data, blocks of person-time in different time periods are asymptotically uncorrelated, regardless of the extent to which they are derived from the same people [35], so pooling estimates from these two time periods provide the same information as using a single time period but takes advantage of the updated dietary assessment in 1986. In addition to the exclusion criteria originally applied in each individual study, we excluded participants whose loge-transformed energy intakes were beyond three standard deviations from the loge-transformed mean intake of the baseline population of each study or who had a history of cancer (except non-melanoma skin cancer) at baseline.
Table 1.
Characteristics of cohort studies included in the pooled analysis of folate intake and colon cancer risk
| Follow-up period | Baseline cohort (n)e | Colon cancer cases (n) | Prevalence of multivitamin use (%) | Median (10th–90th percentile) intake |
||
|---|---|---|---|---|---|---|
| Folate from foods (μg/day)a | Folate from foods and supplements (μg/day)a | |||||
| Alpha-Tocopherol Beta-Carotene Cancer Prevention Study | 1985–1999 | 26,987 | 187 | 8 | 256 (206–314) | 259 (207–329) |
| Breast Cancer Detection Demonstration Project Follow-up Cohort | 1987–1998 | 41,987 | 349 | 33 | 301 (183–502) | 381 (200–835) |
| Canadian National Breast Screening Studyb | 1980–2000 | 49,654 | 431 | – | 244 (169–343) | – |
| Cancer Prevention Study II Nutrition Cohort (men) | 1992–1998 | 66,071 | 467 | 33 | 309 (198–447) | 363 (212–777) |
| Cancer Prevention Study II Nutrition Cohort (women) | 1992–1999 | 74,046 | 349 | 42 | 271 (164–436) | 371 (181–779) |
| Health Professionals Follow-up Study | 1986–2000 | 47,766 | 456 | 43 | 353 (242–514) | 404 (255–827) |
| Iowa Women’s Health Study | 1986–2001 | 34,588 | 799 | 33 | 248 (169–364) | 281 (178–679) |
| Netherlands Cohort Study (men)c | 1986–1993 | 58,279 | 393 | 3 | 210 (158–295) | – |
| Netherlands Cohort Study (women)c | 1986–1993 | 62,573 | 353 | 6 | 184 (137–261) | – |
| New York State Cohort (men) | 1980–1987 | 30,363 | 335 | 38 | 409 (290–603) | 496 (307–874) |
| New York State Cohort (women) | 1980–1987 | 22,550 | 223 | 49 | 378 (263–552) | 501 (289–861) |
| New York University Women’s Health Study | 1985–1998 | 13,258 | 96 | 50 | 270 (155–453) | 447 (183–770) |
| Nurses’ Health Study (a) | 1980–1986 | 88,651 | 162 | 34 | 240 (151–379) | 277 (159–667) |
| Nurses’ Health Study (b) | 1986–2000 | 68,502d | 429 | 43 | 274 (190–397) | 322 (202–709) |
| Prospective Study on Hormones, Diet and Breast Cancer (ORDET)b | 1987–2001 | 9,027 | 43 | – | 259 (201–342) | – |
| Swedish Mammography Cohortb | 1987–2003 | 60,950 | 485 | – | 218 (170–277) | – |
| Women’s Health Study | 1993–2003 | 38,384 | 163 | 32 | 287 (201–409) | 327 (211–706) |
| Total | 725,134 | 5,720 | ||||
Calorie-adjusted median (10th–90th percentile) intake among baseline cohort or subcohort for the Canadian National Breast Screening Study and the Netherlands Cohort Study, adjusted to 2,100 kcal/day for men and 1,600 kcal/day for women
Information on supplementary folate intake was not available at baseline for this study
Folate was not contained in multivitamin supplements at the time of the baseline questionnaire for this study
These women are a subset of the women included in Nurses’ Health Study (a) and are not included in the total baseline cohort size
Cohort sizes after applying study-specific exclusion criteria and then excluding participants with loge-transformed energy intake values beyond three standard deviations from the study-specific mean and previous cancer diagnoses (other than nonmelanoma skin cancer); the Canadian National Breast Screening Study and the Netherlands Cohort Study are analyzed as case-cohort studies so their baseline cohort sizes do not reflect the above exclusions
Case definition and ascertainment
Each study ascertained incident colorectal cancers using follow-up questionnaires and subsequent medical record review [17, 19, 27, 33], linkage with a cancer registry [22, 24–26, 31, 32], or both [18, 20, 23]. In addition, some studies used linkage with a death registry [17–20, 23, 26, 27, 31, 32]. Follow-up for cancer is estimated to be >90% in each cohort. Because risk factors for colon cancer may differ from those for rectal cancer [36], we limited these analyses to colon cancer. We also examined cancers of the proximal colon (cecum, appendix, ascending colon, hepatic flexure, transverse colon, and splenic flexure) and distal colon (descending and sigmoid colon) separately. Colon tumors with unspecified or overlapping sites were excluded from the site-specific analyses but were included in the overall analyses.
Dietary assessment
Diet was assessed at baseline in each study with a study-specific food frequency questionnaire. We obtained intake data for the foods on the questionnaire and for several nutrients, including folate from foods (dietary folate) and folate from foods and supplements (total folate), if available, from each study. Because the New York State Cohort had only entered their supplement data as user vs. nonuser, we derived total folate intake for this study by assuming a frequency of one multivitamin per day among multivitamin users and by estimating the amount of folate in a multivitamin as 400 μg, the dose used in the Nurses’ Health Study for generic multivitamins. Nutrient intakes were energy adjusted by the residual method [37].
Statistical analysis
Each study was analyzed using the Cox proportional hazards model with SAS PROC PHREG [38]. The Canadian National Breast Screening Study and the Netherlands Cohort Study were analyzed as case–cohort studies [39].
We evaluated associations with energy-adjusted dietary and total folate intake. Study- and sex-specific quintiles or deciles were based on the distributions for the subcohort in the two case–cohort studies and on the baseline populations for the remaining studies. The Canadian National Breast Screening Study, Prospective Study on Hormones, Diet and Breast Cancer, and Swedish Mammography Cohort were not included in the total folate analyses because information on multivitamin use was not available at baseline in these studies. Although total folate intake was estimated in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study, this study also was not included in the quantile analyses for total folate intake because only 8% of the participants in this study reported using multivitamins, the main source of supplemental intake. Thus, the total folate intake in the higher categories in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study was not comparable to the other studies in which more than 30% of the study participants used multivitamins. Further, the Netherlands Cohort Study was not included in the quantile analyses for total folate intake because the multivitamins that were used in the Netherlands when the study was initiated did not include folate, so the folate intake in this study only comes from food sources. We also analyzed total folate intake using absolute intake cutpoints, which were identical across studies. These analyses included both the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and Netherlands Cohort Study because their lower total folate intake levels compared to the other studies could be taken into account using identical absolute intake cutpoints. If no participants diagnosed with colon cancer were in the highest intake category in a study, the relative risk could not be estimated for the highest category in that study and the noncases in the highest category in that study were included in the second highest intake category. To calculate the p-value for the test for trend across categories, participants were assigned the median value of their study’s category of intake, and this variable was entered as a continuous variable in the regression model.
For all studies, we included age at baseline and the year that the baseline questionnaire was returned as stratification variables. Person-years of follow-up were calculated from the date the baseline questionnaire was returned until the date of colon cancer diagnosis, loss to follow-up, if available, death, or end of follow-up, whichever came first. The Cancer Prevention Study II Nutrition Cohort, Netherlands Cohort Study, and the New York State Cohort were each analyzed as two separate cohorts of men and women. If there were missing data for a measured covariate within a study, an indicator variable was created for missing responses for that covariate, if applicable. Two-sided 95% CIs and p-values were calculated.
To combine the study-specific effects, we used the random-effects model [40, 41]; the study-specific effects were weighted by the inverse of the sum of their variance and the estimated between-studies variance component. We tested for the statistical significance of between-studies heterogeneity among the study-specific estimates using the Q statistic [40, 42]. We tested for effect modification by sex and smoking status using a meta-regression model [43]. We also evaluated whether the association between total folate intake and colon cancer risk varied by levels of alcohol and methionine intake. For these analyses, a cross-product term of total folate intake expressed as a continuous variable and the ordinal score of alcohol or methionine intake was included in the model. We tested the null hypothesis of no effect modification using a Wald test. When evaluating associations by tumor site (proximal colon vs distal colon cancer), we assessed the statistical significance of differences in the natural logarithm of the RRs by tumor site with a contrast test [44].
Results
A total of 5,720 individuals were diagnosed with incident colon cancers over follow-up times ranging from up to 7–20 years among the 229,466 men and 495,668 women in the thirteen cohort studies (Table 1). Median energy-adjusted dietary folate intake ranged from 184 to 409 μg/day across studies.
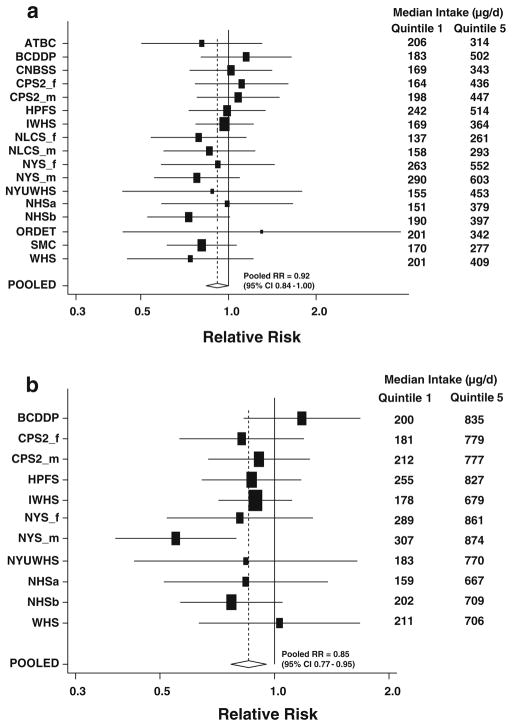
For both dietary and total folate intakes, the pooled age-adjusted RRs were similar to the pooled multivariate-adjusted estimates (Table 2). The pooled multivariate-adjusted RR for comparison of the highest vs lowest quintile of dietary folate intake was 0.92 (95% CI 0.84 to 1.00, p-value, test for trend = 0.07). For the highest quintile, the test for heterogeneity between studies was nonsignificant (p-value, test for heterogeneity = 0.85, Fig. 1a), indicating that the differences in RRs among the cohorts were compatible with random variation. Results were similar for men and women (p-value, test for between-studies heterogeneity due to sex for the highest quintile = 0.99). The results were similar when we further adjusted for dietary fiber intake (pooled multivariate RR comparing the highest versus lowest quintile of dietary folate intake = 0.91, 95% CI = 0.82–1.01). When we examined the association for dietary folate intake only in those studies that measured multivitamin use, we observed similar results (pooled multivariate RR = 0.92, 95% CI 0.83–1.01 comparing the highest vs. lowest quintile). Further, among individuals who did not use multivitamins (n = 10 studies, 3,314 colon cancer cases), the association was similar (pooled multivariate RR for highest vs lowest quintile = 0.91, 95% CI, 0.79–1.05, p-value, test for trend = 0.18). The association was also similar when only the placebo groups of the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and Women’s Health Study were analyzed rather than the entire study population (results not shown), as was done in our analyses of vitamins A, C, and E intake [84]. We observed a stronger inverse association for dietary folate in the European studies compared with the North American studies (in which participants were exposed to folate fortification from about 1997); however, the difference was not statistically significant (p-value, test for difference = 0.17). Further, among the North American studies, we did not observe attenuation of the effect estimates for dietary folate in analyses of the pre-fortification period. In analyses comparing the highest versus lowest decile of intake, the pooled multivariate RR was 0.90 (95% CI 0.79–1.02; p-value, test for between-studies heterogeneity = 0.70). The association was slightly stronger after we excluded cases diagnosed during the first 5 years of follow-up (pooled multivariate RR = 0.84 comparing highest vs lowest quintile, 95% CI 0.75–0.94) compared to that observed for the first 5 years of follow-up (pooled multivariate RR = 1.06, 95% CI 0.92–1.22, p-value test for difference by follow-up time = 0.01).
Table 2.
Pooled age-adjusted and multivariatea relative risks (95% confidence intervals (CI)) of colon cancer for quintiles of folate intake
| Quintile of intake |
p-Value, test for trend | p-Value, test for between-studies heterogeneity for quintile 5 | p-Value, test for between-studies heterogeneity due to sex for quintile 5 | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| Dietary folate | ||||||||
| Men and women | ||||||||
| Total number of cases | 1,165 | 1,198 | 1,097 | 1,120 | 1,140 | |||
| Pooled age-adjusted relative risk (95% CI) | 1.00 | 0.98 (0.91–1.07) | 0.88 (0.81–0.96) | 0.87 (0.80–0.94) | 0.87 (0.80–0.94) | <0.001 | 0.91 | 0.75 |
| Pooled multivariate relative risk (95% CI) | 1.00 | 1.01 (0.93–1.10) | 0.91 (0.82–1.01) | 0.91 (0.83–1.00) | 0.92 (0.84–1.00) | 0.07 | 0.85 | 0.99 |
| Men | ||||||||
| Total number of cases | 388 | 388 | 335 | 356 | 371 | |||
| Pooled multivariate relative risk (95% CI) | 1.00 | 0.96 (0.80–1.16) | 0.80 (0.63–1.02) | 0.86 (0.69–1.05) | 0.92 (0.78–1.07) | 0.40 | 0.65 | – |
| Women | ||||||||
| Total number of cases | 777 | 810 | 762 | 764 | 769 | |||
| Pooled multivariate relative risk (95% CI) | 1.00 | 1.03 (0.93–1.14) | 0.96 (0.86–1.06) | 0.93 (0.84–1.04) | 0.92 (0.82–1.02) | 0.10 | 0.72 | – |
| Total folate (includes dietary and supplementary sources)b | ||||||||
| Men and women | ||||||||
| Total number of cases | 801 | 808 | 757 | 752 | 710 | |||
| Pooled age-adjusted relative risk (95% CI) | 1.00 | 0.94 (0.85–1.04) | 0.85 (0.77–0.94) | 0.85 (0.77–0.94) | 0.78 (0.70–0.86) | <0.001 | 0.50 | 0.16 |
| Pooled multivariate relative risk (95% CI) | 1.00 | 0.98 (0.88–1.08) | 0.90 (0.81–1.00) | 0.92 (0.83–1.01) | 0.85 (0.77–0.95) | 0.02 | 0.42 | 0.30 |
| Men | ||||||||
| Total number of cases | 268 | 279 | 242 | 248 | 221 | |||
| Pooled multivariate relative risk (95% CI) | 1.00 | 0.99 (0.81–1.21) | 0.87 (0.72–1.03) | 0.90 (0.75–1.08) | 0.77 (0.57–1.03) | 0.06 | 0.09 | – |
| Women | ||||||||
| Total number of cases | 533 | 529 | 515 | 504 | 489 | |||
| Pooled multivariate relative risk (95% CI) | 1.00 | 0.97 (0.86–1.09) | 0.92 (0.81–1.04) | 0.92 (0.81–1.04) | 0.89 (0.78–1.01) | 0.13 | 0.75 | – |
Multivariate relative risks were adjusted for education (<high school graduate, high school graduate, >high school graduate), body mass index (<23, 23–<25, 25–<30, ≥30 kg/m2), height (among men:<1.70, 1.70–<1.75, 1.75–<1.80, 1.80–<1.85, ≥1.85 m); among women:<1.60, 1.60–<1.65, 1.65–<1.70, 1.70–<1.75, ≥1.75 m), smoking (never, past [<20, 20–<40, ≥40 years duration], current [<25 cigarettes/day and <40 years duration, ≥25 cigarettes/day and <40 years duration, <25 cigarettes/day and ≥40 years duration, ≥25 cigarettes/day and ≥40 years duration]), energy intake (continuous), alcohol intake (0,>0–<5, 5–<15, 15–<30, ≥30 g/day), red meat intake (quintiles), milk intake (quartiles), multivitamin use (3 categories [no,<6/week, ≥6/week]) were modeled for the Breast Cancer Detection Demonstration Project Follow-up Cohort, Health Professionals Follow-up Study, Iowa Women’s Health Study, New York University Women’s Health Study, Nurses’ Health Study (a), Nurses’ Health Study (b) and Women’s Health Study; 2 levels [no, yes] for the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study, Cancer Prevention Study II Nutrition Cohort, Netherlands Cohort Study, and New York State Cohort), family history of colorectal cancer (no, yes), use of non-steroidal anti-inflammatory drugs (no, yes), physical activity (low, medium, high), and among women only, history of oral contraceptive use (no, yes) and use of postmenopausal hormone therapy (premenopausal, ever, never). Age in years and year of questionnaire return were included as stratification variables
Multivitamin use was removed from the multivariate model with total folate intake as the exposure. The Canadian National Breast Screening Study, Netherlands Cohort Study, Prospective Study on Hormones, Diet and Breast Cancer, and Swedish Mammography Cohort were excluded from these analyses because data were not available on multivitamin use or folate was not included in the multivitamins that were available at baseline. The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study was excluded from these analyses because the prevalence of multivitamin use in this study was<8%; therefore, folate intake in this study was not comparable to intake levels in the other studies that had a higher prevalence of multivitamin use (>20%). Thus, the analyses of total folate intake included 3,828 cases
Fig. 1.
Study-specific and pooled multivariate relative risks and 95% confidence intervals of colon cancer for comparison of the highest vs lowest quintile of dietary (a) and total (b) folate intake. The black squares and horizontal lines correspond to the study-specific relative risks and 95% confidence intervals for the comparison of quintile 5 to quintile 1 of folate consumption. The relative risks were adjusted for the same covariates listed in Table 2. The area of the black squares reflects the study-specific weight (inverse of the variance). The diamond represents the pooled relative risk and 95% confidence interval. Study abbreviations are the following: ATBC Alpha-Tocopherol Beta-Carotene Cancer Prevention Study, BCDDP Breast Cancer Detection Demonstration Project Follow-Up Cohort, CNBSS Canadian National Breast Screening Study, CPS2_f Cancer Prevention Study II Nutrition Cohort, women, CPS2_m Cancer Prevention Study II Nutrition Cohort, men, HPFS Health Professionals Follow-up Study, IWHS Iowa Women’s Health Study, NLCS_f Netherlands Cohort Study, women, NLCS_m Netherlands Cohort Study, men, NYS_f New York State Cohort, women, NYS_m New York State Cohort, men, NYUWHS New York University Women’s Health Study, NHSa Nurses’ Health Study (a), and NHSb Nurses’ Health Study (b), ORDET Prospective Study on Hormones, Diet and Breast Cancer, SMC Swedish Mammography Cohort, WHS Women’s Health Study
The nonparametric regression analyses did not detect nonlinearity in the association between dietary folate intake and colon cancer risk (p-value, test for nonlinearity > 0.05). Therefore, we conducted additional analyses in which dietary folate intake was modeled as a continuous variable. The pooled multivariate RR for an increment of 100 μg/day was 0.98 (95% CI 0.95–1.01; p-value, test for between-studies heterogeneity = 0.31). When the analyses were restricted to only those studies that had assessed total folate intake, the pooled multivariate RR for dietary folate intake for the same increment was 0.99 (95% CI 0.96–1.02; p-value, test for between-studies heterogeneity = 0.51).
A stronger association was observed for total folate intake, which included intake from both foods and supplements (pooled multivariate RR for highest vs lowest quintile = 0.85, 95% CI 0.77–0.95, p-value, test for trend = 0.02). A statistically significant RR comparing the top versus bottom quintile was observed in only the male cohort of the New York State Cohort (Fig. 1b). Although the associations for men and women were not significantly different from each other (p-value, test for between-studies heterogeneity due to sex for the highest quintile = 0.30), a stronger association was observed among men (pooled multivariate RR for highest vs. lowest quintile = 0.77, 95% CI 0.57–1.03) than women (pooled multivariate RR = 0.89, 95% CI 0.78–1.01). In the analysis comparing the highest vs lowest decile of intake, there was only a slightly stronger reduction in risk (pooled multivariate RR = 0.81, 95% CI 0.69–0.95, p-value, test for trend = 0.01) compared to that for the overall quintile analysis. We also conducted analyses for total folate intake using categories based on identical absolute intake cutpoints based on multiples of 80 μg above the average US intake of approximately 240 μg/day [45]. Results for total folate intake were similar in these analyses (pooled multivariate RR = 0.87, 95% CI 0.78–0.98 comparing ≥560 vs. <240 mcg/days, p-value, test for trend = 0.009). Analyses excluding the New York State Cohort, which used regression weight methods to calculate nutrient intakes to compensate for their shorter dietary questionnaire [46], yielded similar results (data not shown). The nonparametric regression curve and a formal test showed that the association between total folate intake and colon cancer risk was consistent with a linear association (p-value, test for nonlinearity > 0.1). In the analysis of total folate intake as a continuous variable, a 2% risk reduction (95% CI 0–3%; p-value = 0.03) was estimated for every 100 μg/day increase in total folate intake.
Similar associations were observed for total folate intake by follow-up period. The pooled multivariate RRs (95% CI) comparing the highest vs lowest quintile of total folate intake were 0.87 (0.76–1.00) when cases diagnosed during the first 5 years of follow-up were excluded and 0.83 (0.70–0.97) for only the first 5 years of follow-up (p-value, test for difference by follow-up = 0.65). There was no material difference in the association of dietary or total folate intake with colon cancer risk in individuals <65 versus ≥65 years of age (p-value, test for difference by age group > 0.55).
The RRs comparing the highest vs lowest quintile of total folate intake were not materially changed after further adjustment for intakes of each of the following: total vitamin D, dietary β-carotene, total calcium, methionine, and dietary fiber intakes, but the association was slightly attenuated when adjusted for total vitamin A, vitamin C, or vitamin E intakes. When we examined associations with total folate intake in subgroups restricted to the highest quintiles of intake of these micronutrients, the pattern of the association between total folate consumption and colon cancer risk was generally similar, although the association for total folate intake became nonsignificant in each model due to the reduced number of cases (pooled multivariate RRs [95% CI] for the highest vs lowest quintile of total folate intake = 0.81 [0.58–1.11] within the highest quintile of total vitamin C intake, 0.81 [0.60–1.09] within the highest quintile of total vitamin E intake, and 0.78 [0.56–1.08] within the highest quintile of total calcium intake). However, within the highest quintile of total vitamin D intake (n = 536 colon cancer cases among 6 studies), the association was stronger (pooled multivariate RR for the highest vs. lowest quintile of total folate intake = 0.58, 95% CI 0.38–0.89).
When we analyzed the effect of supplemental folate alone, the pooled multivariate-adjusted RR comparing the highest versus lowest tertile of supplemental folate intake was 0.90 (95% CI 0.79–1.02). Further adjustment for dietary folate intake did not materially change the results for supplemental folate intake (results not shown). Similarly, the results for dietary folate intake were not materially changed when adjusted for supplemental folate intake (results not shown).
Although there was a suggestion that the inverse association with dietary and total folate intake was stronger for cancers of the distal colon than for those of the proximal colon, the difference was not statistically significant (Table 3).
Table 3.
Pooled multivariatea relative risks of colon cancer (and 95% confidence intervals) for quintiles of folate intake by tumor siteb
| Quintile of intake |
p-Value, test for trend | p-Value, test for between-studies heterogeneity for quintile 5 | p-Value, test for between-studies heterogeneity due to sex for quintile 5 | p-Value, test for common effects by tumor site for quintile 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||
| Dietary folate | |||||||||
| Proximal colon | 1.00 | 1.03 (0.91–1.17) | 0.95 (0.83–1.10) | 0.97 (0.86–1.09) | 0.93 (0.82–1.05) | 0.25 | 0.88 | 0.19 | 0.61 |
| Distal colon | 1.00 | 0.95 (0.83–1.08) | 0.87 (0.76–0.99) | 0.84 (0.73–0.96) | 0.88 (0.77–1.01) | 0.13 | 0.56 | 0.35 | |
| Total folatec | |||||||||
| Proximal colon | 1.00 | 1.05 (0.90–1.23) | 0.94 (0.82–1.09) | 0.92 (0.80–1.06) | 0.87 (0.76–1.01) | 0.03 | 0.88 | 0.39 | 0.71 |
| Distal colon | 1.00 | 0.92 (0.76–1.11) | 0.89 (0.75–1.06) | 0.90 (0.77–1.06) | 0.83 (0.69–1.01) | 0.20 | 0.24 | 0.09 | |
Relative risks were adjusted for the same covariates as listed in Table 2
The analyses of dietary folate intake included 2995 proximal colon cancer cases and 2268 distal colon cancer cases; the analyses of total folate intake included 2041 proximal colon cancer cases and 1520 distal colon cancer cases
The Canadian National Breast Screening Study, Netherlands Cohort Study, Prospective Study on Hormones, Diet and Breast Cancer, and Swedish Mammography Cohort were excluded from these analyses because data were not available on multivitamin use or folate was not included in the multivitamins that were available at baseline. The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study was excluded from these analyses because the prevalence of multivitamin use in this study was<8%; therefore, total folate intake in this study was not comparable to intake levels in the other studies that had a higher prevalence of multivitamin use (>20%)
We examined whether associations between total folate intake and colon cancer risk differed by levels of alcohol consumption, methionine intake, and smoking status (Table 4). A stronger inverse association for total folate intake was observed among individuals who drank at least 15 g/day of alcohol compared with nondrinkers, although the interaction was not statistically significant (p-value test for interaction = 0.22). A statistically significant inverse association was observed among current smokers but not among never or past smokers; however, the interaction was not statistically significant (p-value test for interaction = 0.08). The association with total folate intake did not differ by methionine intake (p-value test for interaction = 0.72).
Table 4.
Pooled multivariatea relative risks (95% confidence intervals) of colon cancer for a 100 mcg/d increment in total folate intake by alcohol consumption, methionine intake, and smoking statusb
| Number of cases | RR (95% CI) | p-Value, test for interaction | |
|---|---|---|---|
| Alcohol consumption (g/d)c | |||
| 0 | 1,681 | 1.00 (0.98–1.02) | 0.22 |
| >0– <15 | 2,136 | 0.98 (0.96–1.00) | |
| ≥15 | 838 | 0.96 (0.92–1.00) | |
| Methionine intake | |||
| Tertile 1 | 1,639 | 0.99 (0.96–1.02) | 0.72 |
| Tertile 2 | 1,542 | 0.97 (0.95–1.00) | |
| Tertile 3 | 1,484 | 0.98 (0.96–1.01) | |
| Smoking status | |||
| Neverd | 2,009 | 0.99 (0.97–1.01) | 0.08 |
| Pastd | 1,775 | 0.99 (0.96–1.01) | |
| Current | 815 | 0.94 (0.90–0.98) | |
Relative risks were adjusted for the same covariates as listed in Table 2
The Canadian National Breast Screening Study, Prospective Study on Hormones, Diet and Breast Cancer, and Swedish Mammography Cohort were excluded from these analyses because data were not available on multivitamin use at baseline
The New York University Women’s Health Study was excluded from these analyses because this study did not assess alcohol consumption
The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study was not included in this stratum because this study only includes current smokers
Discussion
In this pooled analysis of 13 prospective cohort studies, dietary folate intake was associated with a marginally significant reduction in colon cancer risk. However, a stronger statistically significant inverse association was observed for folate intake from dietary and supplemental sources. A stronger inverse association for the highest quintile of total folate intake compared with dietary folate intake may be due not only to the higher intakes in supplement users but also because folic acid in supplements (pteroyl-monoglutamic acid) is more bioavailable [47] than folate from foods (polyglutamates), which is vulnerable to substantial losses during cooking [48] and intestinal absorption [49].
Although results from case–control studies [3–16] have been inconsistent regarding the association between folate intake and colorectal cancer risk, most prospective studies have reported inverse associations [17–27]. Ten of the 11 published prospective studies were included in the current pooled analysis [17–20, 22–27]. The NHANES I Epidemiologic Follow-up Study [21], not included in our analysis because that study used a single 24-h recall to measure folate intake instead of a food frequency questionnaire, reported a 43% risk reduction (RR = 0.57, 95% CI 0.34–0.97) for colon cancer for the highest vs. lowest quartile of dietary folate intake during 20 years of follow-up among 10,011 participants. Total folate intake was not evaluated in that study.
In the studies included in our analyses, folate intake assessed by food frequency questionnaires has been compared with intake measured by dietary records [18, 26, 50–52] or multiple 24-h recalls [53, 54], with correlation coefficients ranging from 0.43 to 0.71. In addition, the correlation between folate intake assessed by food frequency questionnaire and erythrocyte folate level, regarded as a good indicator of body stores of folate [49], was 0.56 in the Health Professionals Follow-up Study and 0.55 in the Nurses’ Health Study [51]. A similar correlation (r = 0.52) between total folate intake and serum folate levels was also reported in the New York University Women’s Health Study [20]. Thus, folate levels in these cohorts estimated by food frequency questionnaires appear to agree well with other measures of folate intake, including biomarkers. However, because folate intake is measured with error, the magnitude of the inverse associations that we observed for dietary and total folate intake and colon cancer risk is likely to have been underestimated. Because the critical period for folate intake in colon carcinogenesis is unclear, a one-time measurement of folate intake or supplement use at baseline is also likely to underestimate the relation between folate intake and colon cancer risk, as was observed in an earlier report from the Nurses’ Health Study for multivitamin use [19].
Consumption of vegetables [55], a major contributor to dietary folate intake [45], and multivitamin use [56, 57] have been shown to be correlated with other lifestyle factors that may also be associated with colon cancer risk. In this analysis, however, simultaneous adjustment for multiple potential confounding factors, including other dietary factors, resulted in minimal attenuation of the age-adjusted RRs for dietary and total folate intake, suggesting that residual confounding by other lifestyle factors is not likely to substantially confound the association.
We observed slightly attenuated associations with total folate intake after further adjustment for intakes of some micronutrients contained in multivitamin supplements, such as vitamins A, C, and E. This finding may raise the issue of whether the effect of folate intake on colon cancer risk is independent, but we also observed a weak, marginally significant association between dietary folate intake and colon cancer risk among non-users of multivitamins and an inverse association with total folate intake among participants in the highest quintile of intake of these other micronutrients. The high correlation between intakes of micronutrients in multivitamins among multivitamin users limits our ability to distinguish between them in observational studies of their associations with health outcomes.
Several hypotheses have been suggested by which folate may prevent colorectal carcinogenesis [58–60]. Folate is critical for the synthesis and regeneration of S-adenosyl-methionine that serves as the essential methyl donor for over 100 biochemical reactions, including the methylation of DNA [59]. Consequently, low plasma folate levels may lead to global hypomethylation of DNA, an early event in colorectal carcinogenesis [61, 62]. In human feeding trials, moderately folate-deficient diets induced genomic hypomethylation in lymphocyte DNA [63, 64], whereas oral supplementation with folate can reverse genomic hypomethylation [63] and decrease cell proliferation [65].
Low folate intake can also cause misincorporation of uracil during DNA synthesis [66, 67], potentially leading to DNA double-strand breaks [68], which in turn cause chromosome aberrations and neoplastic transformation [69]. Blount et al [70] demonstrated that both uracil mis-incorporation into DNA and high micronucleus frequency (a measure of chromosome breaks) in white blood cells from folate-deficient persons were markedly reduced after 8 weeks of folate supplementation. Several studies also have shown an association between a functional polymorphism in the methyltetrahydrofolate reductase gene and colorectal cancer risk that further supports a specific role of folate in colorectal carcinogenesis [71].
Our result of a stronger association with total folate intake compared to dietary folate intake differs from that of a recent meta-analysis that showed a larger reduction in risk associated with folate from dietary sources alone compared with folate from dietary and supplemental sources. Although the meta-analysis included three prospective studies [23, 24, 36] of which only 1 study [23] presented estimates for both dietary and total folate intake separately, we were able to include in our analysis eight studies that each had data on both dietary and supplemental folate intake. For these eight studies that assessed both dietary and total folate intake, the pooled RR comparing the highest vs lowest quintile of total folate intake (pooled multivariate RR = 0.85, 95% CI 0.77–0.95) was stronger than that for dietary folate intake (pooled multivariate RR = 0.93, 95% CI 0.85–1.03).
High levels of alcohol consumption interfere with folate utilization and increase folate requirements [72], and serum folate levels are lower in smokers than in non-smokers [73]. The inverse association between folate intake and colon cancer risk was stronger among individuals who drank alcohol and among smokers, both independent risk factors for colorectal cancer [74–76], although neither interaction reached statistical significance. In rats fed a diet with standard folate levels, alcohol administration increased intracolonic acetaldehyde levels and significantly decreased colonic mucosal folate levels by 48% [77], possibly due to cleavage of folate by acetaldehyde [72]. Components of cigarette smoke are known to convert some forms of folate into biologically inactive compounds [78, 79]. Thus, folate supplementation may be even more important among regular alcohol consumers and smokers.
A recent intervention trial of high-dose folic acid supplementation showed an increased risk of recurrence of colorectal adenomas, precursor lesions of colorectal cancer, in the intervention compared to the placebo group [80]. This result, in combination with some experimental studies [81] and a recent report showing the reversal of downward trends in colorectal cancer incidence in the United States and Canada after folate fortification [82], has raised the issue of whether folate supplementation may have different effects depending on whether preneoplastic changes have already been initiated [83]. Our study, based on incident colon cancer as the outcome with a long follow-up time, does not appear to support the findings from the trial within the range of intakes in these populations as we did not observe an increased risk of colon cancer during the first 5 years of follow-up, when the individuals who developed colon cancer may have had preclinical lesions at baseline.
In summary, we observed a modest, inverse association between folate intake and risk of colon cancer. These findings were consistent across studies and among men and women. A stronger inverse association was observed for the top quintile of total folate compared to dietary folate, largely related to a lower risk among users of multivitamin supplements, as reported in our accompanying article [84]. Because multivitamins were the primary source of supplemental folic acid, as well as other vitamins, in our study, we cannot exclude the possibility that other constituents of multivitamins contribute to a lower risk of colon cancer. However, our findings do support the hypothesis that high folate/folic acid intake reduces the risk of colon cancer.
Acknowledgments
Supported by research grant CA55075 from the National Institutes of Health and by the National Colorectal Cancer Research Alliance.
Contributor Information
Dong-Hyun Kim, Department of Social and Preventive Medicine, College of Medicine, Hallym University, Chunchon, Korea.
Stephanie A. Smith-Warner, Email: pooling@hsphsun2.harvard.edu, Department of Nutrition, Harvard School of Public Health, 665 Huntington Ave., Boston, MA 02115, USA. Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA
Donna Spiegelman, Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA. Department of Biostatistics, Harvard School of Public Health, Boston, MA, USA.
Shiaw-Shyuan Yaun, Department of Nutrition, Harvard School of Public Health, 665 Huntington Ave., Boston, MA 02115, USA.
Graham A. Colditz, Institute for Public Health, Washington University School of Medicine, St. Louis, MO, USA
Jo L. Freudenheim, Department of Social and Preventive Medicine, University at Buffalo, State University of New York, Buffalo, NY, USA
Edward Giovannucci, Department of Nutrition, Harvard School of Public Health, 665 Huntington Ave., Boston, MA 02115, USA. Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA. Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
R. Alexandra Goldbohm, Department of Prevention and Health, TNO Quality of Life, Leiden, The Netherlands.
Saxon Graham, Department of Social and Preventive Medicine, University at Buffalo, State University of New York, Buffalo, NY, USA.
Lisa Harnack, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Eric J. Jacobs, Epidemiology and Surveillance Research, American Cancer Society, Atlanta, GA, USA
Michael Leitzmann, Institute of Epidemiology and Preventive Medicine, University of Regensburg, Regensburg, Germany.
Satu Mannisto, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, Helsinki, Finland.
Anthony B. Miller, Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
John D. Potter, Division of Public Health Services, Fred Hutchinson Cancer Research Center, Seattle, WA, USA
Thomas E. Rohan, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA
Arthur Schatzkin, Nutritional Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, DHHS, Bethesda, MD, USA.
Frank E. Speizer, Department of Environmental Health, Harvard School of Public Health, Boston, MA, USA. Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Victoria L. Stevens, Epidemiology and Surveillance Research, American Cancer Society, Atlanta, GA, USA
Rachael Stolzenberg-Solomon, Nutritional Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Paul Terry, Department of Epidemiology, Emory University, Rollins School of Public Health, Atlanta, GA, USA.
Paolo Toniolo, Department of Environmental Medicine, New York University, New York, NY, USA.
Matty P. Weijenberg, Department of Epidemiology, School for Oncology and Developmental Biology (GROW), Maastricht University, Maastricht, The Netherlands
Walter C. Willett, Department of Nutrition, Harvard School of Public Health, 665 Huntington Ave., Boston, MA 02115, USA. Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA. Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Alicja Wolk, Division of Nutritional Epidemiology, The National Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
Anne Zeleniuch-Jacquotte, Department of Environmental Medicine, New York University, New York, NY, USA.
David J. Hunter, Department of Nutrition, Harvard School of Public Health, 665 Huntington Ave., Boston, MA 02115, USA. Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA. Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
References
- 1.Glynn SA, Albanes D. Folate and cancer: a review of the literature. Nutr Cancer. 1994;22(2):101–119. doi: 10.1080/01635589409514336. [DOI] [PubMed] [Google Scholar]
- 2.Lashner BA, Heidenreich PA, Su GL, Kane SV, Hanauer SB. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis. A case–control study. Gastroenterology. 1989;97(2):255–259. doi: 10.1016/0016-5085(89)90058-9. [DOI] [PubMed] [Google Scholar]
- 3.Benito E, Stiggelbout A, Bosch FX, Obrador A, Kaldor J, Mulet M, et al. Nutritional factors in colorectal cancer risk: a case–control study in Majorca. Int J Cancer. 1991;49:161–167. doi: 10.1002/ijc.2910490202. [DOI] [PubMed] [Google Scholar]
- 4.Freudenheim JL, Graham S, Marshall JR, Haughey BP, Cholewinski S, Wilkinson G. Folate intake and carcinogenesis of the colon and rectum. Int J Epidemiol. 1991;20:368–374. doi: 10.1093/ije/20.2.368. [DOI] [PubMed] [Google Scholar]
- 5.Meyer F, White E. Alcohol and nutrients in relation to colon cancer in middle-aged adults. Am J Epidemiol. 1993;138:225–236. doi: 10.1093/oxfordjournals.aje.a116851. [DOI] [PubMed] [Google Scholar]
- 6.Ferraroni M, La Vecchia C, D’Avanzo B, Negri E, Franceschi S, Decarli A. Selected micronutrient intake and the risk of colorectal cancer. Br J Cancer. 1994;70(6):1150–1155. doi: 10.1038/bjc.1994.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutron-Ruault MC, Senesse P, Faivre J, Couillault C, Belghiti C. Folate and alcohol intakes: related or independent roles in the adenoma-carcinoma sequence? Nutr Cancer. 1996;26(3):337–346. doi: 10.1080/01635589609514489. [DOI] [PubMed] [Google Scholar]
- 8.Slattery ML, Schaffer D, Edwards SL, Ma KN, Potter JD. Are dietary factors involved in DNA methylation associated with colon cancer? Nutr Cancer. 1997;28(1):52–62. doi: 10.1080/01635589709514553. [DOI] [PubMed] [Google Scholar]
- 9.La Vecchia C, Braga C, Negri E, Franceschi S, Russo A, Conti E, et al. Intake of selected micronutrients and risk of colorectal cancer. Int J Cancer. 1997;73:525–530. doi: 10.1002/(sici)1097-0215(19971114)73:4<525::aid-ijc12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.White E, Shannon JS, Patterson RE. Relationship between vitamin and calcium supplement use and colon cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(10):769–774. [PubMed] [Google Scholar]
- 11.Levi F, Pasche C, Lucchini F, La Vecchia C. Selected micronutrients and colorectal cancer.a case–control study from the canton of Vaud, Switzerland. Eur J Cancer. 2000;36(16):2115–2119. doi: 10.1016/s0959-8049(00)00195-7. [DOI] [PubMed] [Google Scholar]
- 12.La Vecchia C, Negri E, Pelucchi C, Franceschi S. Dietary folate and colorectal cancer. Int J Cancer. 2002;102(5):545–547. doi: 10.1002/ijc.10720. [DOI] [PubMed] [Google Scholar]
- 13.Le Marchand L, Donlon T, Hankin JH, Kolonel LN, Wilkens LR, Seifried A. B-vitamin intake, metabolic genes, and colorectal cancer risk (United States) Cancer Causes Control. 2002;13(3):239–248. doi: 10.1023/a:1015057614870. [DOI] [PubMed] [Google Scholar]
- 14.Pufulete M, Al-Ghnaniem R, Leather AJ, Appleby P, Gout S, Terry C, et al. Folate status, genomic DNA hypomethylation, and risk of colorectal adenoma and cancer: a case control study. Gastroenterology. 2003;124(5):1240–1248. doi: 10.1016/s0016-5085(03)00279-8. [DOI] [PubMed] [Google Scholar]
- 15.Satia-Abouta J, Galanko JA, Martin CF, Potter JD, Ammerman A, Sandler RS. Associations of micronutrients with colon cancer risk in African Americans and whites: results from the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev. 2003;12(8):747–754. [PubMed] [Google Scholar]
- 16.Otani T, Iwasaki M, Hanaoka T, Kobayashi M, Ishihara J, Natsukawa S, et al. Folate, vitamin B6, vitamin B12, and vitamin B2 intake, genetic polymorphisms of related enzymes, and risk of colorectal cancer in a hospital-based case–control study in Japan. Nutr Cancer. 2005;53(1):42–50. doi: 10.1207/s15327914nc5301_5. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Alcohol, low-methionine-low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst. 1995;87:265–273. doi: 10.1093/jnci/87.4.265. [DOI] [PubMed] [Google Scholar]
- 18.Glynn SA, Albanes D, Pietinen P, Brown CC, Rautalahti M, Tangrea JA, et al. Colorectal cancer and folate status: A nested case–control study among male smokers. Cancer Epidemiol Biomarkers Prev. 1996;5:487–494. [PubMed] [Google Scholar]
- 19.Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, et al. Multivitamin use, folate and colon cancer in women in the Nurses’ Health Study. Ann Int Med. 1998;129:517–524. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Kato I, Dnistrian AM, Schwartz M, Toniolo P, Koenig K, Shore RE, et al. Serum folate, homocysteine and colorectal cancer risk in women: a nested case–control study. Br J Cancer. 1999;79(11–12):1917–1922. doi: 10.1038/sj.bjc.6690305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su LJ, Arab L. Nutritional status of folate and colon cancer risk: evidence from NHANES I epidemiologic follow-up study. Ann Epidemiol. 2001;11(1):65–72. doi: 10.1016/s1047-2797(00)00188-5. [DOI] [PubMed] [Google Scholar]
- 22.Terry P, Jain M, Miller AB, Howe GR, Rohan TE. Dietary intake of folic acid and colorectal cancer risk in a cohort of women. Int J Cancer. 2002;97(6):864–867. doi: 10.1002/ijc.10138. [DOI] [PubMed] [Google Scholar]
- 23.Flood A, Caprario L, Chaterjee N, Lacey JV, Jr, Schairer C, Schatzkin A. Folate, methionine, alcohol, and colorectal cancer in a prospective study of women in the United States. Cancer Causes Control. 2002;13(6):551–561. doi: 10.1023/a:1016330609603. [DOI] [PubMed] [Google Scholar]
- 24.Harnack L, Jacobs DR, Jr, Nicodemus K, Lazovich D, Anderson K, Folsom AR. Relationship of folate, vitamin B-6, vitamin B-12, and methionine intake to incidence of colorectal cancers. Nutr Cancer. 2002;43(2):152–158. doi: 10.1207/S15327914NC432_5. [DOI] [PubMed] [Google Scholar]
- 25.Konings EJ, Goldbohm RA, Brants HA, Saris WH, van den Brandt PA. Intake of dietary folate vitamers and risk of colorectal carcinoma: results from The Netherlands Cohort Study. Cancer. 2002;95(7):1421–1433. doi: 10.1002/cncr.10866. [DOI] [PubMed] [Google Scholar]
- 26.Larsson SC, Giovannucci E, Wolk A. A prospective study of dietary folate intake and risk of colorectal cancer: modification by caffeine intake and cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2005;14(3):740–743. doi: 10.1158/1055-9965.EPI-04-0581. [DOI] [PubMed] [Google Scholar]
- 27.Zhang SM, Moore SC, Lin J, Cook NR, Manson JE, Lee IM, et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. Am J Epidemiol. 2006;163(2):108–115. doi: 10.1093/aje/kwj016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. 2005;113(5):825–828. doi: 10.1002/ijc.20648. [DOI] [PubMed] [Google Scholar]
- 29.Cravo ML, Mason JB, Dayal Y, Hutchinson M, Smith D, Selhub J, et al. Folate deficiency enhances the development of colonic neoplasia in dimethylhydrazine-treated rats. Cancer Res. 1992;52(18):5002–5006. [PubMed] [Google Scholar]
- 30.Smith-Warner S, Spiegelman D, Ritz J, Albanes D, Beeson W, Bernstein L, et al. Methods for pooling results of epidemiologic studies: the pooling project of prospective studies of diet and cancer. Am J Epidemiol. 2006;163(11):1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 31.Bandera EV, Freudenheim JL, Marshall JR, Zielezny M, Priore RL, Brasure J, et al. Diet and alcohol consumption and lung cancer risk in the New York State Cohort (United States) Cancer Causes Control. 1997;8:828–840. doi: 10.1023/a:1018456127018. [DOI] [PubMed] [Google Scholar]
- 32.Sieri S, Krogh V, Muti P, Micheli A, Pala V, Crosignani P, et al. Fat and protein intake and subsequent breast cancer risk in postmenopausal women. Nutr Cancer. 2002;42(1):10–17. doi: 10.1207/S15327914NC421_2. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs EJ, Connell CJ, Chao A, McCullough ML, Rodriguez C, Thun MJ, et al. Multivitamin use and colorectal cancer incidence in a US cohort: does timing matter? Am J Epidemiol. 2003;158(7):621–628. doi: 10.1093/aje/kwg190. [DOI] [PubMed] [Google Scholar]
- 34.Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol. 1998;148(8):761–774. doi: 10.1093/oxfordjournals.aje.a009697. [DOI] [PubMed] [Google Scholar]
- 35.Rothman KJ. Modern epidemiology. Little Brown and Company; Boston: 1986. [Google Scholar]
- 36.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108(3):433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 38.SAS/STAT Software. The PHREG procedure: preliminary documentation. SAS Institute; Cary: 1991. [Google Scholar]
- 39.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11. [Google Scholar]
- 40.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 41.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 42.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 43.Stram DO. Meta-analysis of published data using a linear mixed-effects model. Biometrics. 1996;52:536–544. [PubMed] [Google Scholar]
- 44.Anderson TW. Introduction to multivariate statistics. 2. Wiley; New York: 1984. [Google Scholar]
- 45.Subar AF, Block G, James LD. Folate intake and food sources in the US population. Am J Clin Nutr. 1989;50(3):508–516. doi: 10.1093/ajcn/50.3.508. [DOI] [PubMed] [Google Scholar]
- 46.Byers T, Marshall J, Fiedler R, Zielezny M, Graham S. Assessing nutrient intake with an abbreviated dietary interview. Am J Epidemiol. 1985;122(1):41–50. doi: 10.1093/oxfordjournals.aje.a114085. [DOI] [PubMed] [Google Scholar]
- 47.Suitor CW, Bailey LB. Dietary folate equivalents: interpretation and application. J Am Diet Assoc. 2000;100(1):88–94. doi: 10.1016/S0002-8223(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 48.Herbert V. Recommended dietary intakes (RDI) of folate in humans. Am J Clin Nutr. 1987;45(4):661–670. doi: 10.1093/ajcn/45.4.661. [DOI] [PubMed] [Google Scholar]
- 49.Herbert V. Making sense of laboratory tests of folate status: folate requirements to sustain normality. Am J Hematol. 1987;26(2):199–207. doi: 10.1002/ajh.2830260211. [DOI] [PubMed] [Google Scholar]
- 50.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 51.Giovannucci E, Stampfer MJ, Colditz GA, Rimm EB, Trichopoulos D, Rosner BA, et al. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst. 1993;85:875–884. doi: 10.1093/jnci/85.11.875. [DOI] [PubMed] [Google Scholar]
- 52.Feskanich D, Marshall J, Rimm EB, Litin LB, Willett WC. Simulated validation of a brief food frequency questionnaire. Ann Epidemiol. 1994;4:181–187. doi: 10.1016/1047-2797(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 53.Munger RG, Folsom AR, Kushi LH, Kaye SA, Sellers TA. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol. 1992;136(2):192–200. doi: 10.1093/oxfordjournals.aje.a116485. [DOI] [PubMed] [Google Scholar]
- 54.Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort Food Frequency Questionnaire. Epidemiology. 2000;11(4):462–468. doi: 10.1097/00001648-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 55.Serdula MK, Byers T, Mokdad AH, Simoes E, Mendlein JM, Coates RJ. The association between fruit and vegetable intake and chronic disease risk factors. Epidemiology. 1996;7:161–165. doi: 10.1097/00001648-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Block G, Cox C, Madans J, Schreiber GB, Licitra L, Melia N. Vitamin supplement use, by demographic characteristics. Am J Epidemiol. 1988;127(2):297–309. doi: 10.1093/oxfordjournals.aje.a114805. [DOI] [PubMed] [Google Scholar]
- 57.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 58.Mason JB. Folate and colonic carcinogenesis: searching for a mechanistic understanding. J Nutr Biochem. 1994;5(4):170–175. [Google Scholar]
- 59.Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem. 1999;10:66–88. doi: 10.1016/s0955-2863(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 60.Duthie SJ. Folic acid deficiency and cancer: mechanisms of DNA instability. Br Med Bull. 1999;55(3):578–592. doi: 10.1258/0007142991902646. [DOI] [PubMed] [Google Scholar]
- 61.Goelz SE, Vogelstein B, Hamilton SR, Feinberg AP. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985;228(4696):187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- 62.Zingg JM, Jones PA. Genetic and epigenetic aspects of DNA methylation on genome expression, evolution, mutation and carcinogenesis. Carcinogenesis. 1997;18(5):869–882. doi: 10.1093/carcin/18.5.869. [DOI] [PubMed] [Google Scholar]
- 63.Jacob RA, Gretz DM, Taylor PC, James SJ, Pogribny IP, Miller BJ, et al. Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. J Nutr. 1998;128(7):1204–1212. doi: 10.1093/jn/128.7.1204. [DOI] [PubMed] [Google Scholar]
- 64.Rampersaud GC, Kauwell GP, Hutson AD, Cerda JJ, Bailey LB. Genomic DNA methylation decreases in response to moderate folate depletion in elderly women. Am J Clin Nutr. 2000;72(4):998–1003. doi: 10.1093/ajcn/72.4.998. [DOI] [PubMed] [Google Scholar]
- 65.Khosraviani K, Weir HP, Hamilton P, Moorehead J, Williamson K. Effect of folate supplementation on mucosal cell proliferation in high risk patients for colon cancer. Gut. 2002;51(2):195–199. doi: 10.1136/gut.51.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sedwick WD, Kutler M, Brown OE. Antifolate-induced misincorporation of deoxyuridine monophosphate into DNA: inhibition of high molecular weight DNA synthesis in human lymphoblastoid cells. Proc Natl Acad Sci U S A. 1981;78(2):917–921. doi: 10.1073/pnas.78.2.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eto I, Krumdieck CL. Role of vitamin B12 and folate deficiencies in carcinogenesis. Adv Exp Med Biol. 1986;206:313–330. doi: 10.1007/978-1-4613-1835-4_23. [DOI] [PubMed] [Google Scholar]
- 68.James SJ, Yin L. Diet-induced DNA damage and altered nucleotide metabolism in lymphocytes from methyl-donor-deficient rats. Carcinogenesis. 1989;10(7):1209–1214. doi: 10.1093/carcin/10.7.1209. [DOI] [PubMed] [Google Scholar]
- 69.Borek C, Ong A, Morgan WF, Cleaver JE. Morphological transformation of 10T1/2 mouse embryo cells can be initiated by DNA double-strand breaks alone. Mol Carcinog. 1991;4(3):243–247. doi: 10.1002/mc.2940040311. [DOI] [PubMed] [Google Scholar]
- 70.Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94(7):3290–3295. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharp L, Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review. Am J Epidemiol. 2004;159(5):423–443. doi: 10.1093/aje/kwh066. [DOI] [PubMed] [Google Scholar]
- 72.Shaw S, Jayatilleke E, Herbert V, Colman N. Cleavage of folates during ethanol metabolism role of acetaldehyde/xanthine oxidase-generated superoxide. Biochem J. 1989;257(1):277–280. doi: 10.1042/bj2570277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen AT, Reidy JA, Annest JL, Welty TK, Zhou HG. Increased chromosome fragility as a consequence of blood folate levels, smoking status, and coffee consumption. Environ Mol Mutagen. 1989;13(4):319–324. doi: 10.1002/em.2850130407. [DOI] [PubMed] [Google Scholar]
- 74.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Kearney J, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in US men. J Natl Cancer Inst. 1994;86:183–191. doi: 10.1093/jnci/86.3.183. [DOI] [PubMed] [Google Scholar]
- 75.Kune GA, Vitetta L. Alcohol consumption and the etiology of colorectal cancer: a review of the scientific evidence from 1957 to 1991. Nutr Cancer. 1992;18:97–111. doi: 10.1080/01635589209514210. [DOI] [PubMed] [Google Scholar]
- 76.Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Int Med. 2004;140(8):603–613. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 77.Homann N, Tillonen J, Salaspuro M. Microbially produced acetaldehyde from ethanol may increase the risk of colon cancer via folate deficiency. Int J Cancer. 2000;86(2):169–173. doi: 10.1002/(sici)1097-0215(20000415)86:2<169::aid-ijc4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 78.Francis KT, Thompson RW, Krumdieck CL. Reaction of tetrahydrofolic acid with cyanate from urea solutions: formation of an inactive folate derivative. Am J Clin Nutr. 1977;30(12):2028–2032. doi: 10.1093/ajcn/30.12.2028. [DOI] [PubMed] [Google Scholar]
- 79.Abu Khaled M, Watkins CL, Krumdieck CL. Inactivation of B12 and folate coenzymes by butyl nitrite as observed by NMR: implications on one-carbon transfer mechanism. Biochem Biophys Res Commun. 1986;135(1):201–207. doi: 10.1016/0006-291x(86)90963-0. [DOI] [PubMed] [Google Scholar]
- 80.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 81.Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen. 2004;44(1):10–25. doi: 10.1002/em.20025. [DOI] [PubMed] [Google Scholar]
- 82.Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1325–1329. doi: 10.1158/1055-9965.EPI-07-0329. [DOI] [PubMed] [Google Scholar]
- 83.Ulrich CM, Potter JD. Folate and cancer—timing is everything. JAMA. 2007;297(21):2408–2409. doi: 10.1001/jama.297.21.2408. [DOI] [PubMed] [Google Scholar]
- 84.Park Y, Spiegelman D, Hunter DJ, Albanes D, Bergkvist L, Buring JE, Freudenheim JL, Giovannucci E, Goldbohm RA, Harnack L, Kato I, Krogh V, Leitzmann MF, Limburg PJ, Marshall JR, McCullough ML, Miller AB, Rohan TE, Schatzkin A, Shore R, Sieri S, Stampfer MJ, Virtamo J, Weijenberg M, Willett WC, Wolk A, Zhang SM, Smith-Warner SA. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010 doi: 10.1007/s10552-010-9549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]