Abstract
Cyclic adenosine monophosphate (cAMP) is a nearly ubiquitous signaling molecule important for numerous signaling pathways in human skin. We studied a novel class of mammalian adenylyl cyclase, the soluble adenylyl cyclase (sAC). We examined sAC localization in normal human skin and found it to be present in keratinocytes, melanocytes, mononuclear cells, eccrine ducts, and nerves. In normal skin, sAC keratinocyte staining was evenly distributed throughout the cell. However, in certain hyperproliferative disorders of the skin, including psoriasis, verruca vulgaris, and SCCIS on sun-damaged skin, sAC keratinocyte staining was predominantly nuclear. In contrast, in other hyperproliferative disorders, such as basal cell carcinoma, sAC staining was similar to normal human skin. Using a model of epithelial differentiation, we established that sAC migrates into the nucleus when differentiated cells are induced to reenter the cell cycle. Previous work had determined that nuclear sAC activates the cAMP-response-element-binding (CREB) transcription factor, and we found that in psoriasis lesions, nuclear sAC occurs concomitantly with activation of CREB. Hence, sAC may play a role in the pathogenesis of certain hyperproliferative skin disorders via modulation of gene expression.
INTRODUCTION
Epidermal hyperplasia can occur secondary to a number of stimuli. These stimuli can be separated into congenital genetic alterations, infectious, inflammatory, and cell-cycle/ apoptotic dysregulation as seen within the spectrum of epidermolytic hyperkeratosis, human papilloma virus (HPV), psoriasis, and skin cancer, respectively. Although each of these skin diseases is induced by a varied set of stimuli, they all are defined by the proliferation of keratinocytes. Keratinocyte proliferation requires alteration in programmed differentiation along with induction of the cell cycle. Cellular differentiation and cell cycle are modulated by numerous signaling pathways, and hyperstimulation or dysregulation of these pathways represents key events leading to many diseases of epidermal hyperplasia. The cyclic adenosine monophosphate (cAMP)-signaling pathway is integral to both cellular differentiation and proliferation, and has been implicated in the pathogenesis of diseases of epidermal hyperplasia such as psoriasis (Yoshikawa et al., 1975; Wadskov et al., 1979; Adachi et al., 1980; Voorhees, 1982; Grandjean-Laquerriere et al., 2005).
The signaling molecule cAMP has long been studied in the epidermis. cAMP and its effector proteins, such as protein kinase-A (PKA) and cAMP-response-elementbinding protein (CREB), have known roles in the cells of the epidermis and dermis, including keratinocytes, melanocytes, eccrine ductal cells, and fibroblasts (Slominski et al., 2006). In many cases the initiating stimulus for these cAMPdependent pathways are well established; for example, the melanocyte-stimulating hormone-induced cAMP pathways in melanocytes (Abdel-Malek et al., 2008). In other cases, the stimuli leading to cAMP signal transduction are less clear.
Mammalian cells contain two distinct classes of adenylyl cyclase, the transmembrane adenylyl cyclases (tmACs) and soluble adenylyl cyclase (sAC) (Buck et al., 1999). tmACs are more widely studied and are exclusively expressed at the plasma membrane. sAC, unlike tmACs, is not exclusively expressed at the plasma membrane, but instead is found in the cytoplasm, at the mitochondria, at the centriole, and within the nucleus (Zippin et al., 2003, 2004; Feng et al., 2005, 2006).
Because cAMP has an integral role in the proliferation, differentiation, and expression of key proteins in keratinocytes, we examined the expression and localization of sAC protein in normal human skin and disease. We found that sAC is upregulated in the nuclei of keratinocytes in certain hyperproliferative skin diseases, including psoriasis and squamous cell carcinoma (SCC) in situ. Interestingly, sAC is lost from the nucleus when a malignant epithelial tumor acquires invasive properties in the dermis. We have previously demonstrated that nuclear sAC is associated with the activated cAMP-dependent transcription factor CREB, and have found this trend to also exist in human psoriasis lesions. These data shed light on the complexity of cAMP signaling in skin disease and suggest sAC might represent a key player in the pathogenesis of hyperproliferative skin diseases.
RESULTS
Examination of sAC immunostaining in normal human skin
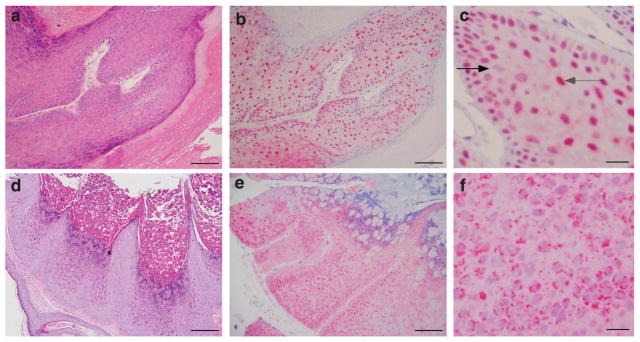
Using a previously described mouse monoclonal antibody against human sAC protein (Zippin et al., 2003, 2004), we examined sAC expression in normal human skin. sAC was present in multiple cell types within both epidermis and dermis (Figure 1a–f). All specific staining was absent if primary antibody was either incubated with blocking peptide (Figure 1c) or omitted (data not shown). In the epidermis, sAC was strongly expressed in keratinocytes (Figure 1b). Staining appeared evenly distributed throughout the cell, without specific localization. There were occasional cells with nuclear staining, but these cells represented a minority of the total keratinocytes. sAC was absent in the cornified cell layer. sAC protein was also present in melanocytes as confirmed by costaining with Melan-A (Figure 1d). sAC was present in a variety of mononuclear cells in the dermis (Figure 1b). Co-staining with different markers, such as CD3, CD20, CD1a, and CD56, established that these cells consisted of T-cells, macrophages, and dendritic cells (data not shown). sAC was also present in eccrine duct cells (Figure 1e). In addition, we found sAC protein in cutaneous nerve axons as confirmed by co-staining with PGP9.5 (Figure 1f).
Figure 1. Immunostaining of sAC in normal skin.
(a) Hematoxylin (blue) and eosin (red) staining of normal human skin. (b) Normal human skin immunostained with R21 (red) and hematoxylin (blue). (c) Normal human skin immunostained with R21+ blocking peptide (red) and hematoxylin (blue). (d) Normal epidermis immunostained with R21 (red), Melan-A (brown), and hematoxylin (blue). (e) Normal human skin eccrine duct immunostained with R21 (red) and hematoxylin (blue). (f) Normal human skin cutaneous nerve immunostained with R21 (red), PGP9.5 (brown), and hematoxylin (blue). Bars = 50 μm (a–c) and 10μm (d–f).
Examination of sAC immunostaining in common viral infections of the epidermis
In most lesions of verruca vulgaris (Figure 2a–c and Table 1), sAC cytoplasmic staining was significantly reduced as compared with normal epidermis (Figure 1b), and instead sAC was predominately nuclear throughout the lesion. As in normal epidermis, staining was mainly undetectable in the cornified layer. sAC was not exclusively present in the nucleus. While some cells had strong nuclear staining (Figure 2c, gray arrow), other nuclei had no sAC staining (Figure 2c, black arrow). In contrast to HPV-induced skin lesions, molluscum contagiosum virus (MCV) infection did not induce a predominance of sAC nuclear staining (Figure 2d–f and Table 1). In fact, it was rare to see a nucleus positive for sAC staining. Instead, sAC staining was granular in quality and perinuclear in localization (Figure 2f).
Figure 2. Immunostaining of sAC in viral infections of the epidermis.
(a) Hematoxylin (blue) and eosin (red) staining of a verruca vulgaris skin lesion. (b) Verruca vulgaris skin lesion immunostained with R21 (red) and hematoxylin (blue). (c) Verruca vulgaris skin lesion immunostained with R21 (red) and hematoxylin (blue). The black arrow demonstrates a nucleus negative for sAC. The gray arrow demonstrates a nucleus postive for sAC. (d) Hematoxylin (blue) and eosin (red) staining of a molluscum contagiosum skin lesion. (e) Molluscum contagiosum skin lesion immunostained with R21 (red) and hematoxylin (blue). (f) Molluscum contagiosum skin lesion immunostained with R21 (red) and hematoxylin (blue). Bars = 100 μm (a, b, d, e) and 10 mm (c, f).
Table 1.
Degree of nuclear sAC staining in examples of keratinocyte hyperproliferative skin disease
| Expression pattern | |||
|---|---|---|---|
| + | +/− | − | |
| Verruca vulgaris | 6 | 0 | 3 |
| Molluscum contagiosum | 0 | 0 | 7 |
| Seborrheic keratosis | 6 | 1 | 3 |
| Epidermolytic hyperkeratosis | 0 | 0 | 4 |
| Acanthosis nigricans | 0 | 3 | 2 |
| Actinic keratosis | 4 | 1 | 0 |
| Bowenoid papulosis with high-grade dysplasia, +HPV in situ | 1 | 3 | 7 |
| Squamous cell carcinoma in situ, no dermal involvement, sun-exposed sites | 9 | 1 | 3 |
| Squamous cell carcinoma, invasion of dermis, both sun-exposed and non-exposed sites | 3 | 1 | 2 |
| Basal cell carcinoma | 0 | 0 | 10 |
| Pityriasis rubra pilaris | 0 | 1 | 2 |
| Psoriasis vulgaris | 5 | 5 | 0 |
| Psoriasis pustular | 3 | 0 | 0 |
| Psoriasis guttate | 4 | 0 | 0 |
Abbreviation: sAC, soluble adenylyl cyclase.
−: Approximately 10% of keratinocytes have nuclei positive for sAC and sAC staining is strongly cytoplasmic (equivalent to normal skin); +/−: 10–70% of keratinocytes have nuclei positive for sAC and decreased cytoplasmic sAC staining in keratinocytes with sAC nuclear staining; +: >70% of keratinocytes have nuclei positive for sAC, with barely detectable cytoplasmic sAC staining.
In vitro epithelial cell model of differentiation
There are key differences between the pathogenesis of HPV and MCV. HPV is known to exert its pathological effects on the epidermis mainly by inducing keratinocyte proliferation and entry of cells into S-phase of mitosis. Proliferation and host DNA synthesis are key since this virus replicates in the nucleus and requires the host DNA polymerase to synthesize new viral genomes (Tyring, 2000). MCV, like all pox viruses, replicates in the cytoplasm because its genome encodes for a viral DNA polymerase (Brown et al., 1981) and, therefore, it does not require the cell to enter the cell cycle to replicate. Because of this difference in viral pathogenesis between HPV and MCV, we hypothesized that sAC nuclear staining occurs in tandem with entry into the cell cycle.
Madin–Darby canine kidney (MDCK) cells (Figure 3), Caco-2 human colonic cells (data not shown), and human retinal pigment epithelial cells (data not shown) represent three of the best-characterized models of epithelial differentiation (Foerg et al., 2007). When these cell lines are grown to confluence, cellular division stops and cells develop tight junctions and other markers of epithelial differentiation. Eventually, these cells form the functional sheets of polarized epithelial cells. Once fully differentiated, sAC staining in the epithelial-like cells was exclusively within the cytoplasm of MDCK (Figure 3a, middle panel), Caco-2 (data not shown), and retinal pigment epithelial cells (data not shown). A key feature of these cell models is that differentiation is not permanent; simple removal of cellular contacts induces the cells to de-differentiate and resume proliferation. There are two established methods for disrupting cell-to-cell contacts of the epithelial sheets: clearing a line of cells by scraping with a metal spatula (sometimes referred to as wounding) or by simple trypsinization. After wounding (data not shown) or trypsinization (Figure 3b), sAC nuclear staining returned in MDCK (Figure 3b, middle panel), Caco-2 (data not shown), and retinal pigment epithelial cells (data not shown).
Figure 3. sAC is present in the nucleus when epithelial cells are proliferating and not when epithelial cells are differentiating.
(a) Differentiated MDCK cells stained with DAPI (left), anti-N-term sAC antibody (center), and overlay of DAPI and anti-N-term (right). (b) Proliferating undifferentiated MDCK cells stained with DAPI (left), anti-N-term sAC antibody (center), and overlay of DAPI and anti-N-term (right). Bar = 10 μm.
Examination of sAC immunostaining in UV-induced keratinocyte neoplasms
UV radiation is capable of inducing both benign and malignant neoplasms of the epidermis. Seborrheic keratoses are benign neoplasms; the exact genetic mutations remain unknown (Noiles and Vender, 2008). In the majority of seborrheic keratoses examined (see Supplementary Figure S1 online and Table 1), sAC staining was predominately nuclear, with a relatively decreased level of cytoplasmic staining as compared with normal skin (Figure 1b).
Actinic keratosis (AK), SCC in situ (SCCIS), and invasive SCC can be considered as a continuum of increasing pathogenecity. All three neoplasms occur secondary to UVinduced DNA damage and in most cases are typified by mutations in p53 (Criscione et al., 2009). In AKs, sAC staining was enriched in the nucleus and significantly decreased in the cytoplasm (Figure 4a and b, inset and Table 1). Nearly all SCCIS cases examined had a similar sAC staining pattern to AKs; that is, sAC was enriched in the nucleus and was relatively decreased in the cytoplasm as compared with normal skin (Figure 4c and d, inset and Table 1). sAC staining in invasive SCC revealed a more mosaic pattern. Approximately 50% of SCC samples examined (Table 1), regardless of subtype, had strong nuclear sAC staining in the SCCIS component, but no nuclear sAC staining in the invasive component (Figure 4e and f). In fact, in some of these SCC cases the invasive component was devoid of all sAC staining. The remaining SCC cases retained a predominant nuclear sAC staining pattern, with a relatively decreased cytoplasmic staining intensity (Figure 4g and h, and Table 1).
Figure 4. Immunostaining of sAC in AK and SCC.
(a) Hematoxylin (red) and eosin (blue) staining of AK. (b) AK immunostained with R21 (red) and hematoxylin (blue); inset, magnified view of the area in panel b. (c) Hematoxylin (red) and eosin (blue) staining of SCCIS. (d) SCCIS immunostained with R21 (red) and hematoxylin (blue); inset, magnified view of the area in panel d. (e) Hematoxylin (red) and eosin (blue) staining of SCC. (f) SCC immunostained with R21 (red) and hematoxylin (blue). (g) Hematoxylin (red) and eosin (blue) staining of SCC. (h) SCC immunostained with R21 (red) and hematoxylin (blue). Bars = 50 μm.
Compared with SCC, basal cell carcinoma demonstrated a very different sAC immunostaining pattern. sAC staining was virtually absent from all nuclei; the frequency of nuclear staining was equivalent to the frequency in normal skin. sAC staining in BCC was intense and diffusely cytosolic, and this pattern was identical among all basal cells carcinomas analyzed regardless of pathologic subtype (Supplementary Figure S2a–c and Table 1). Of all the epidermal diseases examined in this study, sAC localization in BCC most closely resembled that in normal skin.
Examination of sAC immunostaining in virally induced malignant neoplasms
Although the vast majority of HPV infections develop into benign growths, a few HPV subtypes, namely 16, 18, 31, and 33, cause high-grade squamous proliferative lesions in the skin ranging from bowenoid papulosis to frank carcinoma (Jablonska and Majewski, 1999). If the viral genome integrates into the host chromosome, viral production stops and large amounts of viral E6 and E7 proteins are expressed, inducing the destruction of the tumor suppressor p53(Tyring, 2000). Histologically, lesions of bowenoid papulosis are nearly identical to SCCIS; however, from a clinical perspective they resemble common venereal warts and do not develop invasive properties; in most cases, although there are rare reports of invasion (Jablonska and Majewski, 1999). Although these neoplasms are caused by HPV infection, sAC localization in high-risk HPV infections did not match sAC localization in low-risk HPV infections (Figure 2a–c). Instead, sAC localization was absent from the nucleus and present in a perinuclear granular staining pattern (Supplementary Figure S2d–f).
Examination of sAC immunostaining in benign inflammatory proliferations of the epidermis
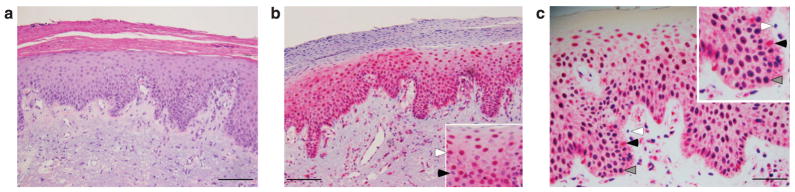
We found consistent sAC staining pattern in a variety of human psoriasis cases, including at least five examples of guttate, plaque-type, and pustular psoriasis (Table 1). Among all forms of psoriasis, within the area of epidermal thickening, sAC staining changed from predominately cytoplasmic (Figure 1b) to a distribution where sAC protein was almost exclusively nuclear (Figure 5b, inset, white arrow). sAC was not present in all nuclei (Figure 5b, inset, black arrow), nor did sAC expression extend into the area of parakeratosis.
Figure 5. Nuclear sAC is enhanced in psoriatic skin and is associated with phosphorylated CREB.
(a) Hematoxylin (red) and eosin (blue) staining of psoriatic lesional skin. (b) Psoriatic lesional skin immunostained with R21 (red) and hematoxylin (blue). The inset is a magnified view of a portion of the epidermis demonstrating that some nuclei are positive for sAC (white arrow) and some are negative for sAC (black arrow). (c) Psoriatic lesional skin immunostained with R21 (red) and an antibody against phosphorylated CREB (light blue). Panel c and inset: Nuclei positive for both sAC and phosphorylated CREB appear dark blue to purple (gray arrows). White arrows indicate nuclei positive for phosphorylated CREB only (light blue). Black arrows indicate nuclei positive for sAC only (red). Bars = 50 μm.
Pityriasis rubra pilaris is another disease of keratinocyte proliferation. Although its exact pathophysiological mechanism is not known, it is believed to occur secondary to an antigen-triggered immune response such as streptococcal infection (Mohrenschlager and Abeck, 2002). Unlike psoriasis, sAC staining in pityriasis rubra pilaris biopsies was predominately cytoplasmic, with little to no increase in nuclear staining (Table 1 and data not shown).
In psoriasis, nuclear sAC is associated with activated cAMP effector proteins
cAMP-dependent gene expression, mediated by the transcription factor CREB, is known to occur in psoriatic keratinocytes (Funding et al., 2007), and we previously demonstrated that nuclear sAC is capable of activating CREB by inducing a PKA-dependent phosphorylation (Zippin et al., 2004). We hypothesized that in cells with increased level of nuclear sAC, there would also be increased level of activated CREB. Staining psoriasis skin samples with an antibody that recognizes the phosphorylated (ie, activated) form of CREB demonstrated that a large number of keratinocyte nuclei were positive for phosphoCREB, and that the majority of keratinocytes that contain high levels of active CREB also contain high levels of nuclear sAC (Figure 5c).
DISCUSSION
sAC is a newly described signaling molecule, which is capable of regulating a variety of cellular processes such as apoptosis (Kumar et al., 2009), gene expression (Zippin et al., 2004), neutrophil activation (Han et al., 2005), and sperm motility (Hess et al., 2005). We examined the localization of sAC in normal human skin and found the protein to be diffusely expressed in keratinocytes and melanocytes of the epidermis, eccrine ductal cells, mononuclear cells, and cutaneous nerves. sAC staining in eccrine ductal cells is particularly interesting because of the established localization and physiology of sAC in kidney and epididymis (Pastor-Soler et al., 2003; Paunescu et al., 2008; Brown et al., 2009), and similarities in physiology between eccrine ducts, kidney, and epididymis (Granger et al., 2002). In kidney and epididymis, sAC associates with and stimulates proton flux through vacuolar–ATPases (V-ATPase), which are integral to pH regulation of these organs (Pastor-Soler et al., 2003; Paunescu et al., 2008). V-ATPases are also present in eccrine ductal cells where they are important for the proper secretion of sweat (Granger et al., 2002). It would be interesting to determine whether sAC activity is involved in sweat production in mammals. The observed cutaneous nerve staining is also interesting given that previous reports have demonstrated the presence of sAC in dorsal root ganglion cells of the rat and its involvement in axonal guidance (Wu et al., 2006).
Nuclear sAC staining has been observed in mammalian liver (Zippin et al., 2004). sAC staining was not present in all nuclei, but instead was detected in the nucleus of a small subset of hepatocytes where it was hypothesized to reflect the turnover of individual hepatocytes. In this study, we confirmed this staining pattern in normal skin, and have established that sAC is nuclear during cellular proliferation and is absent from the nucleus when cells differentiate. As the skin loses dead cells from the cornified layer, new cells are replaced from below. In normal skin, cell division occurs both at the basal cell layer and immediately above the basal cell layer, which consists of a group of cells called transient amplifying cells (Iizuka et al., 2004). Consistent with this fact, we find sAC nuclear staining in normal skin to occur primarily in the lower levels of the epidermis (Figure 1). While basal cell layer stem cells can divide forever, their rate of division is very slow compared with that of transient amplifying cells, which have a finite proliferative potential. In psoriasis, the transient amplifying cell layer is thought to represent the group of keratinocytes, which respond to inflammatory cytokines and lead to skin lesions (Iizuka et al., 2004). Therefore, one would expect to detect the majority of sAC nuclear staining above the basal cell layer in psoriasis, and consistent with this hypothesis, we found the strongest sAC nuclear staining in psoriasis lesions in a band of keratinocytes above the basal cell layer and below the upper layers of the epidermis where cells are beginning to differentiate (Figure 5b).
We previously demonstrated that when sAC is nuclear it stimulates the phosphorylation and activation of the CREB transcription factor. We have also shown that in mammalian tissues, such as the liver, when sAC is present in the nucleus, CREB protein tends to be phosphorylated, and when sAC is absent from the nucleus, CREB tends to not be phosphorylated (Zippin et al., 2004). In this report, we confirmed the association between nuclear sAC and active CREB in psoriasis skin lesions. CREB activity in keratinocytes has been recently linked to psoriasis pathogenesis. Recent studies have found increased level of phosphorylated CREB in both psoriatic epidermis and in psoriatic keratinocytes in culture (Funding et al., 2007). Although T-cells and the immune system clearly have an important role in the pathogenesis of psoriasis, recent work has demonstrated that alteration in the expression of key keratinocyte transcription factors, c-Jun and JunB, is sufficient to induce psoriasis-like effects in mice (Zenz et al., 2005). Keratinocyte c-Jun and JunB are both regulated by CREB and are upregulated in psoriatic lesional skin as compared with perilesional normal skin, suggesting a role for keratinocyte cAMP signaling in psoriasis pathogenesis (Zenz and Wagner, 2006).
Although there are many reports suggesting a role for cAMP signaling in psoriasis pathogenesis, this area of research remains a topic of debate. Most of the debate stems from conflicting data regarding cAMP signaling in psoriatic keratinocytes. Whereas some laboratories have recorded elevation of adenylyl cyclase activity and cAMP levels in psoriasis, others report a decrease (Yoshikawa et al., 1975; Wadskov et al., 1979; Adachi et al., 1980; Voorhees, 1982). In addition, other groups have found highly decreased binding of cAMP to PKA in erythrocytes membranes, which was specific for active psoriasis (Raynaud et al., 1989; Schopf et al., 2002). Of note, all of these studies were performed with reagents, which activate or inhibit only the tmAC class of mammalian adenylyl cyclases; therefore, the role of sAC in psoriasis pathogenesis has not been fully examined. Following the discovery and characterization of sAC, our laboratory has generated a cadre of reagents, which distinguish between sAC- and tmAC-generated cAMP. We have used these reagents both in vivo and in vitro to determine the relative contribution of each class of cyclase in physiological pathways such as insulin release, mitochondrial respiration (Acin-Perez et al., 2009), nerve cell migration (Wu et al., 2006), and kidney ion channel regulation (Paunescu et al., 2008). We intend to readdress the role of cAMP in the pathogenesis of psoriasis both in vivo and in vitro using reagents that can distinguish between tmAC- and sAC-generated cAMP.
Aside from inducing DNA mutation, UV radiation, via a cAMP-dependent mechanism, alters the cytokine expression profile of keratinocytes leading to local immune suppression, a risk factor for development of a skin cancer (Grandjean- Laquerriere et al., 2003, 2005). Studies examining UVinduced cytokine expression in keratinocytes are particularly interesting because these data clearly demonstrate that the effects of UV radiation are PKA-dependent; however, the source of cAMP mediating these effects remains elusive (Grandjean-Laquerriere et al., 2005). Nuclear sAC is induced in UV-dependent neoplasms and likely leads to activation of CREB. It would be interesting to determine whether sAC might be a source of cAMP responsible for UV-induced PKA activation and immune-suppressive cytokine expression.
MATERIALS AND METHODS
Antibodies
R21 and anti-N-term (produced in the Laboratory of Drs Levin and Buck); phosphoCREB (Cell Signaling Technologies, Danvers, MA); A103/Melan-A, CD20, and CD68 (Dako, Carpinteria, CA); CD1a and CD56 (Novocastra, Deerfield, IL); CD3 (Neomarkers, Fremont, CA); CD123 (BD Biosciences, San Jose, CA); and PGP9.5 (Abcam, Cambridge, MA).
Immunohistochemistry of human tissue
Cases were retrospectively identified using archival tissue from the database of the Division of Dermatopathology, Weill Medical College of Cornell University. All cases were interpreted by C Magro. The cases chosen included verruca vulgaris (9), MCV (7), seborrheic keratosis (10), epidermolytic hyperkeratosis (4), acanthosis nigricans (5), AKs (5), bowenoid papulosis (11), SCC both in situ and invasive (19), basal cell carcinoma (10), pityriais rubra pilaris (3), and psoriasis (17). For normal skin unremarkable sections of skin from excision specimens were chosen. Immunostaining of patient samples was approved under IRB protocol number 0710009479, Weill Cornell Medical Center, New York, NY. The study was conducted according to the Declaration of Helsinki Principles.
All steps were performed using the Leica Microsystems BondMax Autostainer (Bannockburn, IL). Formalin-fixed, paraffinembedded samples were first baked at 60 °C for 30 minutes followed by a dewaxing procedure. Slides were treated with a Leica Microsystems Dewax solution (part number AR922) for 3 minutes at 72 °C, then a Dewax solution wash at 72 °C, and finally a Dewax solution wash at ambient temperature. This was followed by three washes with Ethyl Alcohol 200 proof (Pharmco-Aaper, Brookfield, CT, cat. number 111000200) and three washes with Leica Microsystems Wash buffer (part number AR9590).
All sections were treated as follows for sAC immunostaining: following the dewaxing procedure, the samples were pretreated by two washes in Leica Microsystems HIER1 (part number AR9961), followed by HIER1 pretreatment for 30 minutes at 100 °C, and then HIER1 pretreatment for 12 minutes at ambient temperature. Before immunostaining, the sections were blocked using the Dako Dual Endogneous Enzyme Block (part number S2003) for 5 minutes followed by three washes with Bond Wash Solution. The wash buffer (Bond Wash Solution) is used in all washing steps described below unless otherwise noted.
R21 is a mouse monoclonal antibody directed against amino acids 203–216 of human sACfl protein (Zippin et al., 2003). The primary antibody (3mgml−1, 1:500) was applied for 25 minutes in a buffered Primary Antibody Diluent (AR9352) from Leica Microsystems. Following this step the sections were treated by a post primary AP step for 20 minutes for signal amplification as part of the procedure detailed in the Leica Microsystems Bond Polymer AP Red Detection kit (part number DS9305). The amplification polymer was then added for 30 minutes followed by two washes in wash buffer and one in deionized water. Finally, the mixed red substrate was applied for 10 minutes followed by an additional 10 minutes with new substrate, three washes in deionized water only, and, finally, mounting with coverslip.
When blocking peptide was used, the antibody was pre-diluted in Bond Primary Antibody Diluent with and without blocking peptide (100 molar excess) and rocked at room temperature overnight. These pre-diluted solutions were used for immunostaining as above.
When R21 was immunostained alone, hematoxylin (part of Bond Polymer Define Detection kit) co-stain was used to highlight the nuclei. The stain was incubated on the slide for 5 minutes followed by one wash in 70% alcohol, three washes in 100% alcohol, two washes in Citrasolv (Fisherbrand 22–143975), and mounting with coverslip.
For co-staining of R21 stained sections with phosphorylated CREB antibody (1:500) antibody, the sample was pretreated by two washes of Leica Microsystems HIER1 (part number AR9961), followed by HIER1 pretreatment for 30 minutes at 100 °C, and then HIER1 pretreatment for 12 minutes at ambient temperature. Before immunostaining, the sections were blocked using the Dako Dual Endogneous Enzyme Block (part number S2003) for 5 minutes.
The primary antibody was applied for 30 minutes in Bond Primary Antibody Diluent. Following this step the sections were treated with a post primary AP step for 20 minutes for signal amplification as part of the procedure detailed in the Leica Microsystems Bond Polymer AP Red Detection kit (part number DS9305). The amplification polymer was then added for 30 minutes followed by three washes. Finally the Alkaline Phosphotase Substrate kit III Vector (part number SK-5300) was applied for 10 minutes followed by an additional 10 minutes with new substrate, three washes, and, finally, mounting with coverslip.
For co-staining with Melan-A (1:50), PGP9.5 (1:2500), CD1a (1:20), CD3 (1:100), CD20 (1:200), CD56 (1:50), CD68 (1:300), and CD123 (1:50), following staining with R21, the samples were blocked using the Peroxidase Block (part of the Leica Biosystems Bond Polymer Define Detection kit, part number DS9713) for 5 minutes. Sections were then pretreated in HIER2 for 20 or 30 minutes and washed as above, depending on the antibody, as per manufacturer’s instructions. This was followed by three washes at 35 °C and one wash at ambient temperature.
The primary antibody was applied for 25 minutes in Bond Primary Antibody Diluent followed by three washes. Following this step the sections were treated with a post primary step for 15 minutes for signal amplification as part of the procedure detailed in the Leica Microsystems Bond Polymer Define Detection kit (part number DS9713) followed by three washes in wash buffer. The amplification polymer was then added for 30 minutes followed by two washes in wash buffer and one in deionized water. Finally, the Mixed Diaminobenzidine (DAB) Define was applied for 10 minutes. Counterstaining was accomplished by adding hematoxylin for 5 minutes.
Cell culture and immunocytochemistry
MDCK cells were cultured in DMEM+ 10% fetal calf serum and grown to confluence on glass coverslips. Confluent cultures were fed daily over 1 week to allow complete differentiation of the cells. At this point, some coverslips were immunostained while others were trypsinized and split on fresh coverslips at a lower density to induce proliferation. These cells were immunostained within 24 hours of trypsinization. For immunostaining, coverslips were washed in phosphate-buffered saline, fixed for 30 minutes in 4% paraformaldehyde, and permeabilized in 0.1% Triton X-100, and then blocked in 2% bovine serum albumin for at least 1 hour. Cells were stained with anti-sAC rabbit polyclonal antibody overnight in 2% BSA/0.01% Triton X-100, washed three times over 10 minutes in 2% BSA/0.01% Triton X-100, stained for 1 hour at room temperature with goat-anti-rabbit Alexa Fluor-488 (Molecular Probes, Eugene, OR), treated with DAPI for 5 minutes, and then washed and mounted with gelvatol/DABCO (Sigma, St Louis, MO).
Supplementary Material
Acknowledgments
This work was supported by NIH grants (GM62328, HD059913, and NS55255 to JB and LRL).
Abbreviations
- AK
actinic keratosis
- cAMP
cyclic adenosine monophosphate
- CREB
cAMP response element binding
- HPV
human papilloma virus
- MCV
molluscum contagiosum
- MDCK
Madin–Darby canine kidney
- PKA
protein kinase-A
- sAC
soluble adenylyl cyclase
- SCC
squamous cell carcinoma
- SCCIS
squamous cell carcinoma in situ
- tmAC
transmembrane adenylyl cyclase
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL: Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
References
- Abdel-Malek ZA, Knittel J, Kadekaro AL, et al. The melanocortin 1 receptor and the UV response of human melanocytes—a shift in paradigm. Photochem Photobiol. 2008;84:501–8. doi: 10.1111/j.1751-1097.2008.00294.x. [DOI] [PubMed] [Google Scholar]
- Acin-Perez R, Salazar E, Kamenetsky M, et al. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–76. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi K, Iizuka H, Halprin KM, et al. Epidermal cyclic AMP is not decreased in psoriasis lesions. J Invest Dermatol. 1980;74:74–6. [PubMed] [Google Scholar]
- Brown D, Paunescu TG, Breton S, et al. Regulation of the V-ATPase in kidney epithelial cells: dual role in acid–base homeostasis and vesicle trafficking. J Exp Biol. 2009;212:1762–72. doi: 10.1242/jeb.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ST, Nalley JF, Kraus SJ. Molluscum contagiosum. Sex Transm Dis. 1981;8:227–34. doi: 10.1097/00007435-198107000-00012. [DOI] [PubMed] [Google Scholar]
- Buck J, Sinclair ML, Schapal L, et al. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523–30. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- Feng Q, Zhang Y, Li Y, et al. Two domains are critical for the nuclear localization of soluble adenylyl cyclase. Biochimie. 2006;88:319–28. doi: 10.1016/j.biochi.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Feng QP, Zuo J, Meng Y, et al. Nuclear localization region in soluble adenylyl cyclase. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005;27:280–4. [PubMed] [Google Scholar]
- Foerg C, Ziegler U, Fernandez-Carneado J, et al. Differentiation restricted endocytosis of cell penetrating peptides in MDCK cells corresponds with activities of Rho-GTPases. Pharm Res. 2007;24:628–42. doi: 10.1007/s11095-006-9212-1. [DOI] [PubMed] [Google Scholar]
- Funding AT, Johansen C, Kragballe K, et al. Mitogen- and stressactivated protein kinase 2 and cyclic AMP response element binding protein are activated in lesional psoriatic epidermis. J Invest Dermatol. 2007;127:2012–9. doi: 10.1038/sj.jid.5700821. [DOI] [PubMed] [Google Scholar]
- Grandjean-Laquerriere A, Le Naour R, Gangloff SC, et al. Differential regulation of TNF-alpha, IL-6 and IL-10 in UVB-irradiated human keratinocytes via cyclic AMP/protein kinase A pathway. Cytokine. 2003;23:138–49. doi: 10.1016/s1043-4666(03)00224-2. [DOI] [PubMed] [Google Scholar]
- Grandjean-Laquerriere A, Le Naour R, Gangloff SC, et al. Contribution of protein kinase A and protein kinase C pathways in ultraviolet B-induced IL-8 expression by human keratinocytes. Cytokine. 2005;29:197–207. doi: 10.1016/j.cyto.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Granger D, Marsolais M, Burry J, et al. V-type H+-ATPase in the human eccrine sweat duct: immunolocalization and functional demonstration. Am J Physiol Cell Physiol. 2002;282:C1454–60. doi: 10.1152/ajpcell.00319.2001. [DOI] [PubMed] [Google Scholar]
- Han H, Stessin A, Roberts J, et al. Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils. J Exp Med. 2005;202:353–61. doi: 10.1084/jem.20050778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–59. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka H, Takahashi H, Ishida-Yamamoto A. Psoriatic architecture constructed by epidermal remodeling. J Dermatol Sci. 2004;35:93–9. doi: 10.1016/j.jdermsci.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Jablonska S, Majewski S. Bowenoid papulosis transforming into squamous cell carcinoma of the genitalia. Br J Dermatol. 1999;141:576–7. doi: 10.1046/j.1365-2133.1999.03064.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kostin S, Flacke JP, et al. Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J Biol Chem. 2009;284:14760–8. doi: 10.1074/jbc.M900925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrenschlager M, Abeck D. Further clinical evidence for involvement of bacterial superantigens in juvenile pityriasis rubra pilaris (PRP): report of two new cases. Pediatr Dermatol. 2002;19:569. doi: 10.1046/j.1525-1470.2002.00236_5.x. [DOI] [PubMed] [Google Scholar]
- Noiles K, Vender R. Are all seborrheic keratoses benign? Review of the typical lesion and its variants. J Cutan Med Surg. 2008;12:203–10. doi: 10.2310/7750.2008.07096. [DOI] [PubMed] [Google Scholar]
- Pastor-Soler N, Beaulieu V, Litvin TN, et al. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278:49523–9. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu TG, Da Silva N, Russo LM, et al. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol. 2008;294:F130–8. doi: 10.1152/ajprenal.00406.2007. [DOI] [PubMed] [Google Scholar]
- Raynaud F, Gerbaud P, Enjolras O, et al. A cAMP binding abnormality in psoriasis. Lancet. 1989;1:1153–6. doi: 10.1016/s0140-6736(89)92748-7. [DOI] [PubMed] [Google Scholar]
- Schopf RE, Langendorf Y, Benz RE, et al. A highly decreased binding of cyclic adenosine monophosphate to protein kinase A in erythrocyte membranes is specific for active psoriasis. J Invest Dermatol. 2002;119:160–5. doi: 10.1046/j.1523-1747.2002.01808.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Zmijewski M, et al. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–48. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring SK. Human papillomavirus infections: epidemiology, pathogenesis, and host immune response. J Am Acad Dermatol. 2000;43:S18–26. doi: 10.1067/mjd.2000.107807. [DOI] [PubMed] [Google Scholar]
- Voorhees JJ. “Psoriasis as a possible defect of the adenyl cyclase-cyclic AMP Cascade” by Voorhees and Duell, October 1971. Commentary: cyclic adenosine monophosphate regulation of normal and psoriatic epidermis. Arch Dermatol. 1982;118:862–74. [PubMed] [Google Scholar]
- Wadskov S, Kassis V, Sondergaard J. Cyclic AMP and psoriasis once more. Acta Derm Venereol. 1979;59:525–7. [PubMed] [Google Scholar]
- Wu KY, Zippin JH, Huron DR, et al. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci. 2006;9:1257–64. doi: 10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K, Adachi K, Halprin KM, et al. Is the cyclic AMP in psoriatic epidermis low? Br J Dermatol. 1975;93:253–8. doi: 10.1111/j.1365-2133.1975.tb06490.x. [DOI] [PubMed] [Google Scholar]
- Zenz R, Eferl R, Kenner L, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–75. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- Zenz R, Wagner EF. Jun signalling in the epidermis: from developmental defects to psoriasis and skin tumors. Int J Biochem Cell Biol. 2006;38:1043–9. doi: 10.1016/j.biocel.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Zippin JH, Chen Y, Nahirney P, et al. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–4. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- Zippin JH, Farrell J, Huron D, et al. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004;164:527–34. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.