Abstract
Repeated exposure to drugs of abuse enhances the motor-stimulant response to these drugs, a phenomenon termed behavioral sensitization. Animals that are extinguished from self-administration training readily relapse to drug, conditioned cue, or stress priming. The involvement of sensitization in reinstated drug-seeking behavior remains controversial. This review describes sensitization and reinstated drug seeking as behavioral events, and the neural circuitry, neurochemistry, and neuropharmacology underlying both behavioral models will be described, compared, and contrasted. It seems that although sensitization and reinstatement involve overlapping circuitry and neurotransmitter and receptor systems, the role of sensitization in reinstatement remains ill-defined. Nevertheless, it is argued that sensitization remains a useful model for determining the neural basis of addiction, and an example is provided in which data from sensitization studies led to potential pharmacotherapies that have been tested in animal models of relapse and in human addicts.
I. Introduction
Substance abuse is a chronic and enduring phenomenon. Extensive investigations regarding the underlying neural mechanisms of addiction have been ongoing for the past 3 decades. Despite these efforts, few effective treatment options are available to treat drug dependence. Animal models of substance abuse include both noncontingent (experimenter-administered) and contingent (self-administered) drug administration. Up until the late 1990s, studies on drug-induced neuroplasticity focused primarily on examining the effects of repeated noncontingent drug treatments (i.e., sensitization) on dopamine and glutamate systems (Kalivas and Stewart, 1991; Vanderschuren and Kalivas, 2000). Although noncontingent drug administration has provided a wealth of data on how repeated drug exposure alters neuronal function, it is not always accepted that these data predict the neuroplasticity associated with contingent drug administration. Therefore, recent studies have focused on contingent drug self-administration and, in particular, reinstatement of drug-seeking behavior. The choice of this model is based on the idea that the treatments of addiction will intervene to prevent relapse, which reinstatement behavior purports to model (Epstein et al., 2006). Both the sensitization and reinstatement models assess the impact of repeated drug exposure on neural function, the primary difference being how the drug is administered. Thus, the present review will compare and contrast the neural consequences of drug treatment in contingent and noncontingent behavioral models of addiction used to study the neural consequences of drug administration. We then explore questions regarding the interchangeability and validity of each model with a goal to determine the extent of predictive validity that behavioral sensitization resulting from repeated noncontingent drug administration provides for the neuroplasticity underlying the reinstatement of extinguished contingent drug self-administration. Furthermore, whether behavioral sensitization is a component of reinstatement of drug seeking behavior will also be considered.
II. Definitions
A. Sensitization
The enhanced response to a stimulus, after repeated exposure to that stimulus, is termed sensitization (Robinson and Becker, 1986; Kalivas and Stewart, 1991). Regarding drugs of abuse, behavioral sensitization is defined by the augmented motor-stimulant response that occurs with repeated, intermittent exposure to a specific drug. Behavioral sensitization is a long-lasting phenomenon; the enhanced behavioral response has been reported to persist for at least a year (Paulson et al., 1991). A number of factors, including number of treatments, interval between treatments, dose, sex, age, and genetics, can affect the strength of behavioral sensitization (Post and Contel, 1983). Behavioral sensitization has been reported to occur in response to cocaine, amphetamine, morphine, ethanol, nicotine, and Δ9-tetrahydrocannabinol (Joyce and Iversen, 1979; Robinson and Becker, 1986; Benwell and Balfour, 1992; Cunningham and Noble, 1992; Post et al., 1992; Cadoni et al., 2001). Furthermore, cross-sensitization between drugs has been shown. For example, animals repeatedly exposed to Δ9-tetrahydrocannabinol exhibited a sensitized behavioral response to morphine (Cadoni et al., 2001), whereas animals repeatedly exposed to ethanol were sensitized to cocaine and vice versa (Itzhak and Martin, 1999), and animals repeatedly exposed to amphetamine were sensitized to morphine (Lett, 1989; Vezina et al., 1989; Vezina and Stewart, 1990). In addition, animals with a history of repeated exposure to toluene, via inhalation, exhibited a behaviorally sensitized response to cocaine (Beyer et al., 2001). This suggests that common mechanisms underlie the development of behavioral sensitization, despite the fact that different classes of drug have distinct binding sites in the brain.
The development of behavioral sensitization can be separated into two phases: initiation and expression. Initiation is the immediate neural events that induce behavioral sensitization, and expression is the long-term consequences of these initial events (Kalivas and Stewart, 1991). Initiation is commonly linked to the ventral tegmental area (VTA1), and expression is associated with the nucleus accumbens. The environment can influence both the initiation and expression of behavioral sensitization. Thus, animals repeatedly exposed to drugs such as cocaine, amphetamine, or morphine during the initiation phase expressed more robust sensitization when re-exposed to drug in the same environment (paired) as previous drug exposure during the expression phase, compared with animals tested in an environment that differed (unpaired) from that used during the initiation phase (Vezina et al., 1989; Anagnostaras and Robinson, 1996; Wang and Hsiao, 2003; Mattson et al., 2008; Vezina and Leyton, 2009). Behavioral sensitization in the paired environment is commonly referred to as context-dependent sensitization as opposed to context-independent sensitization, which occurs in an unpaired paradigm. Context-dependent cross-sensitization has also been reported to occur (Vezina et al., 1989).
Behavioral sensitization is commonly assessed by monitoring motor activity. When monitoring motor activity, repeated exposure to drugs leads to an augmented motor-stimulant response. Sensitization to amphetamine-like psychostimulants can escalate to manifest as intensified stereotypic behavior that competes with locomotion. For example, when rats are sensitized to higher doses of amphetamine (e.g., 2.0 mg/kg i.p.), they show an initial reduction in activity in response to amphetamine challenge, followed by a delayed increase in locomotor activity (Leith and Kuczenski, 1982). Behavioral sensitization can also be assessed via conditioned place preference (CPP) or drug self-administration. In the CPP paradigm, sensitization is manifested as enhanced time spent in the drug-paired chamber. Thus cocaine, amphetamine, and morphine-induced CPP was enhanced in animals with a history of repeated exposure to these drugs (Lett, 1989; Shippenberg et al., 1996). The potential involvement of sensitization in drug self-administration is usually determined by the ability of repeated noncontingent drug exposure to enhance the acquisition of drug self-administration, using low drug doses, or by drug self-administration inducing locomotor sensitization in response to a noncontingent drug injection (Vezina, 2004). It was initially demonstrated that animals with a history of experimenter-administered amphetamine, which induced locomotor sensitization, subsequently showed an augmented acquisition of amphetamine self-administration (Piazza et al., 1990). Furthermore, pre-exposure to amphetamine, 3,4-methylenedioxy methamphetamine, or nicotine enhanced vulnerability to self-administration of cocaine (Horger et al., 1992; Fletcher et al., 2001). Subsequent studies demonstrated that animals with a history of intravenous cocaine or heroin self-administration demonstrate a sensitized motor response to a systemic challenge injection of the self-administered drug (Hooks et al., 1994; Phillips and Di Ciano, 1996; De Vries et al., 1998b; Zapata et al., 2003). In addition, it was shown that when motor activity was monitored during self-administration, sensitization to the stimulant properties of heroin developed (Marinelli et al., 1998). Animals that self-administered heroin also showed cross-sensitization to noncontingent amphetamine-induced locomotion (De Vries et al., 1998b). Finally, it has been reported that repeated, noncontingent administration of amphetamine enhances the reinforcing properties of amphetamine and cocaine, as assessed by a progressive ratio schedule of reinforcement (Mendrek et al., 1998; Suto et al., 2002; Vezina et al., 2002). Furthermore, studies have demonstrated that exposure to binge cocaine self-administration followed by abstinence also sensitizes the reinforcing properties of cocaine (Morgan and Roberts, 2004; Morgan et al., 2006).
B. Relapse/Reinstatement
The pharmacological studies outlined above are consistent with repeated noncontingent drug administration producing sensitization to drug-induced motor stimulation, conditioned place preference, and drug reinforcement. However, the most troublesome facet of addiction therapy is perhaps relapse prevention (O'Brien, 1997). Relapse has been modeled in animal studies via measures of reinstated drug-seeking in animals extinguished from drug self-administration training (for review, see Epstein et al., 2006). These studies typically involve establishing an operant response (i.e., lever press or nose poke) for drug, the subsequent extinction of this operand, and then reinstating this operant behavior. Three models are commonly used in reinstatement studies. These include 1) the between-session model, in which self-administration, extinction, and reinstatement occur on different days; 2) the within-session model, in which self-administration, extinction, and reinstatement occur on the same day; and 3) the between-within model, in which self-administration occurs on a day separate from extinction and reinstatement (Shalev et al., 2002). Using these models, it has been shown that after extinction of previously cocaine-reinforced lever pressing, this operant behavior can be reinstated by cocaine priming injections (de Wit and Stewart, 1981). Similar findings have been reported for heroin, nicotine, and amphetamine (de Wit and Stewart, 1983; Chiamulera et al., 1996; Ranaldi et al., 1999). In addition, like cross-sensitization, discussed in section II.A, reinstatement of previous cocaine-supported lever pressing can be induced by amphetamine and morphine (de Wit and Stewart, 1981). However, heroin and ethanol priming failed to reinstate cocaine self-administration (de Wit and Stewart, 1981). In addition to drug-primed reinstatement, re-exposure to the environment associated with drug self-administration in animals not undergoing extinction (context), cues previously paired with drug infusion, or acute stress can reinstate drug-seeking behavior (de Wit and Stewart, 1981; Shaham and Stewart, 1995; Meil and See, 1996; Crombag et al., 2002). Reinstatement can be influenced by a number of factors, including food training before drug training, use of noncontingent drug primes during training, amount of drug intake during training, and the length of withdrawal before reinstatement testing (Shalev et al., 2002).
In addition to the drug self-administration paradigm, the CPP paradigm has also been used to study reinstatement. In the CPP procedure, animals are repeatedly exposed to one distinct environment in the presence of drug and an alternative environment in the presence of vehicle. When given free access to both chambers, animals generally prefer the previously drug-paired environment. Past research has demonstrated that upon extinction, this preference can be reinstated by drug-priming injections with morphine, cocaine, methamphetamine, nicotine, and ethanol or by acute exposure to environmental stress (Mueller and Stewart, 2000; Parker and Mcdonald, 2000; Wang et al., 2000; Li et al., 2002; Sanchez et al., 2003; Biala and Budzynska, 2006). Furthermore, similar to sensitization, cross-reinstatement with cocaine or amphetamine injections reinstate morphine CPP or morphine injections reinstate nicotine CPP (Do Ribeiro Couto et al., 2005; Biala and Budzynska, 2006).
C. Behavioral Relationships between Sensitization and Relapse
Among the hypotheses proposed to explain drug relapse, the incentive motivation hypothesis posits that repeated drug use leads to greater salience being attached to drugs and drug-associated cues, suggesting that sensitization might play a role in reinstatement of drug seeking behavior (Robinson and Berridge, 2003). Although the studies outlined above would seem to largely support this contention, direct testing of this hypothesis has provided mixed results. Thus, after extinction of cocaine self-administration, priming injections of cocaine or amphetamine, but not heroin, successfully reinstate cocaine seeking. It is noteworthy that these same cocaine-trained animals also expressed locomotor sensitization to cocaine and amphetamine but not heroin (De Vries et al., 1998b). Animals trained to self-administer cocaine under a long-access paradigm (6 h/day) showed enhanced reinstated cocaine-seeking and locomotor responses to cocaine challenge compared with short-access animals (1 h/day) (Ferrario et al., 2005). In contrast to these findings, reports have demonstrated that both long- and short-access animals develop behavioral sensitization to cocaine or heroin, but only long-access animals exhibit reinstatement of drug-seeking behavior in response to drug-priming injections (Ahmed and Cador, 2006; Lenoir and Ahmed, 2007). However, these studies involved a within-session reinstatement paradigm with a 45-min extinction period, which could be problematic given that long-access animals exhibit enhanced resistance to extinction (Ahmed et al., 2000). In addition, Knackstedt and Kalivas (2007) have reported that the ability of cocaine priming to reinstate cocaine-seeking behavior is not dependent on escalation of drug-seeking behavior or behavioral sensitization. Taken together, the data suggest that sensitization develops to repeated drug exposure occurring during drug-self-administration but that locomotor sensitization does not always correlate with enhanced reinstatement of drug-seeking behavior.
The lack of a clear-cut correlation between locomotor sensitization and reinstatement of drug-seeking behavior might call into question the validity of the sensitization model. When considering face validity, reinstatement is clearly a more valid model than sensitization, given that animals self-administer the drug in the former whereas the experimenter delivers the drug in the latter model. However, the construct validity of the reinstatement model has also been questioned (Epstein et al., 2006). Thus, the reasons to cease drug use, the contingencies associated with the priming of reinstatement, and the time course of vulnerability to reinstatement differ between animals and humans (Epstein et al., 2006). Furthermore, reinstatement studies typically include extinction procedures that are not common with human drug users (Fuchs et al., 2006). In addition to differences in route of administration, reinstatement and sensitization differ in the amount of drug to which animals are exposed as well as the rapidity with which drug enters the central nervous system. Although both models have shortcomings, as will be discussed below, there seems to be a remarkable overlap between the neurochemical circuitries of behavioral sensitization and reinstated drug-seeking behavior, suggesting that the behavioral sensitization model has construct validity relative to the reinstatement model. Therefore, one could argue that results generated from sensitization studies would, at the very least, be predictive of the impact of repeated contingent drug exposure on central nervous system function. In addition, the possibility remains that sensitization is one factor in reinstatement. In this regard, a recent report suggests that interoceptive stimuli of cocaine can acquire discriminative stimulus properties that would thereby facilitate reinstated drug seeking (Keiflin et al., 2008). When examining the discrepancies in the literature, it should be noted that sensitization of incentive motivation, not locomotion, is important to addiction (Robinson and Berridge, 2008). Thus, it is possible that although the motor circuit might reach a maximum level of sensitization, the motivation circuit continues to sensitize. In this case, long-access animals would exhibit similar levels of locomotor sensitization but greater levels of reinstatement of drug-seeking behavior.
III. Neurocircuitry
A. Neurons versus Circuits
When studying the role of various brain regions in behavioral sensitization or reinstatement, experiments rely on temporary or permanent lesions or injection of receptor agonists or antagonists. Results from these studies can be interpreted to indicate the involvement of neurons in these regions in the behaviors under examination. However, one must remember that the brain regions discussed below work in concert as a circuit to control behavioral responses to drugs. Thus, after the presentation of data that support or refute the involvement of specific brain regions in sensitization or reinstatement, a discussion of the interactions of the various brain regions, based on known anatomical connections, is provided.
B. Sensitization
When discussing the neural circuits that underlie sensitization, one must consider that the augmented motor-stimulant response has two distinct phases: initiation and expression. Based on intracranial amphetamine injections, it was initially determined that the actions of drugs on the VTA were responsible for the initiation of sensitization, whereas actions within the nucleus accumbens were responsible for the expression of sensitization (Kalivas and Weber, 1988). Lesion studies have also implicated the medial prefrontal cortex (mPFC) in the development of behavioral sensitization. For example, ibotenate lesions of the mPFC, which encompasses both the prelimbic and infralimbic regions, disrupted the induction of sensitization to cocaine and amphetamine (Wolf et al., 1995; Cador et al., 1999; Li et al., 1999). Furthermore, discrete lesions of the prelimbic region of the mPFC with the excitotoxin quinolinic acid also blocked the induction of cocaine-induced sensitization (Tzschentke and Schmidt, 1998, 2000). However, these same authors reported that neither large nor discrete mPFC quinolinic acid lesions altered the induction of sensitization to amphetamine, suggesting that differences exist between cocaine- and amphetamine-induced sensitization (Tzschentke and Schmidt, 2000).
In addition to the mesocorticolimbic system (i.e., VTA, nucleus accumbens, and mPFC) the ventral pallidum, hippocampus, amygdala, laterodorsal tegmentum (LDT), and the paraventricular nucleus (PVN) of the thalamus have been suggested to play a role in the development of sensitization. Thus, lesion studies demonstrated that the LDT and PVN, but not the hippocampus, play a role in the development of sensitization (Wolf et al., 1995; Young and Deutch, 1998; Nelson et al., 2007). However, other experiments showed that inactivation of the dorsal but not the ventral hippocampus blocked the expression of amphetamine sensitization (Degoulet et al., 2008). In contrast to this finding, a recent study demonstrated that amphetamine sensitization depends on enhanced activity of ventral hippocampal neurons that increases activity of VTA dopamine neurons (Lodge and Grace, 2008). Ibotenate lesions of the amygdala have been reported to either block or have no effect on the development of amphetamine sensitization (Wolf et al., 1995; Cador et al., 1999). Finally, studies with μ-opioid agonists and antagonists suggest that ventral palladium is involved in both the initiation and expression of behavioral sensitization to morphine (Mickiewicz et al., 2009). As will be discussed further below, it is likely that each of these brain regions affects the development of sensitization by influencing the mesocorticolimbic dopamine system.
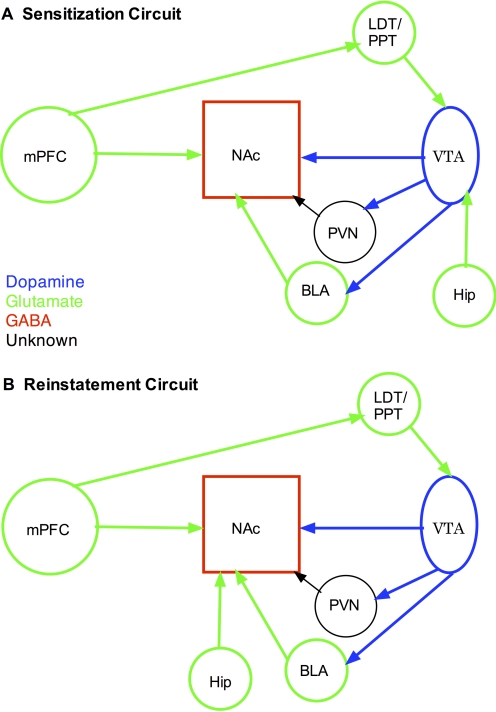
The common circuitry for behavioral sensitization includes dopamine projections from the VTA to the nucleus accumbens and glutamate projections from the mPFC to the nucleus accumbens (Pierce and Kalivas, 1997a). In addition to dopamine, GABAergic neurons project from the VTA to the nucleus accumbens and mPFC, whereas dopamine terminals located in the nucleus accumbens also release glutamate (Van Bockstaele and Pickel, 1995; Tecuapetla et al., 2010). Furthermore, this circuit can be activated by projections from the mPFC to the VTA (Li et al., 1999), probably via the LDT (Omelchenko and Sesack, 2005, 2007). In addition to direct connections, the VTA probably indirectly influences the nucleus accumbens and mPFC via projections to the basolateral amygdala and PVN (Oades and Halliday, 1987; Kita and Kitai, 1990; Takada et al., 1990; Shinonaga et al., 1994; Moga et al., 1995). Finally, the hippocampus is likely to impact the mesolimbic dopamine system via inputs to the VTA (Lodge and Grace, 2008). Thus, as illustrated in Fig. 1A, the mPFC provides excitatory input, either directly or indirectly via the LDT, to the VTA, which provides dopaminergic influence to the nucleus accumbens, either directly or indirectly via the basolateral amygdala and PVN. This circuit can also be influenced by direct hippocampal input to the VTA and mPFC input to the nucleus accumbens. The neurochemistry and neuropharmacology of this circuit will be discussed below.
Fig 1.
Comparison of the neurocircuitry involved in sensitization and reinstatement behaviors. The nucleus accumbens (NAc) serves as an output to motor circuits. A, in the case of sensitization, the nucleus accumbens is modulated by dopamine input from the VTA that either directly innervates this target or does so indirectly via the PVN and basolateral amygdala (BLA). The nucleus accumbens also receives direct glutamate inputs from the mPFC as well as indirect projections via the LDT/PPT and VTA. Finally, the VTA also receives glutamate input from the hippocampus (Hip). B, the circuitry underlying reinstatement is nearly identical to that of sensitization except that the Hip affects the circuit via the NAc rather than the VTA.
C. Relapse/Reinstatement
Studies exploring the neurocircuitry of reinstatement of drug-seeking behavior have typically involved measurement of Fos synthesis in response to drug, cue, or stress priming or have used lesions or reversible inactivation of select targets to alter reinstatement. Cocaine priming increases Fos synthesis in the VTA, caudate nucleus, and central nucleus of the amygdala, whereas cue priming produces an increase in Fos in the nucleus accumbens, basolateral amygdala, and hippocampus, and both cocaine and cue priming increased Fos in the anterior cingulate cortex (Neisewander et al., 2000). Inactivation of the VTA, nucleus accumbens core, ventral pallidum, and dorsal mPFC all prevent cocaine-primed reinstatement (McFarland and Kalivas, 2001; Capriles et al., 2003). Furthermore, injection of cocaine into the dorsal mPFC or morphine into the VTA reinstates drug-seeking behavior (Stewart, 1984; Park et al., 2002). In addition to the structures discussed with drug-primed reinstatement (Capriles et al., 2003; McLaughlin and See, 2003; Fuchs et al., 2004; McFarland et al., 2004; Chaudhri et al., 2008), the basolateral amygdala is implicated in cue-induced reinstatement of cocaine- and heroin-seeking behavior (Meil and See, 1997; Kruzich and See, 2001; Fuchs and See, 2002; Kantak et al., 2002; McLaughlin and See, 2003), whereas the dorsal and ventral hippocampus have been implicated in context- and cue-dependent reinstatement, respectively (Sun and Rebec, 2003; Fuchs et al., 2005). Finally, the PVN has been implicated in context-dependent reinstatement of beer-seeking (Hamlin et al., 2009). Brain regions shown to be involved in stress-primed reinstatement include the nucleus accumbens shell, central amygdala, bed nucleus of the stria terminalis, and lateral tegmental nuclei in addition to those regions previously discussed for drug-primed reinstatement (Shaham et al., 2000a,b; McFarland et al., 2004). In addition to previously discussed regions, the LDT and pedunculopontine tegmentum (PPT) have been recently shown to be involved in drug-primed reinstatement, although their role in stress- and cue-induced reinstatement has yet to be explored (Schmidt et al., 2009). Thus, although a shared core circuit for reinstatement exists, stress, cues, and context affect this circuitry via additional inputs.
Previous studies suggest that the reinstatement circuit includes a motor subcircuit and limbic subcircuit (Kalivas and McFarland, 2003). The motor subcircuit is the projection from the dorsal mPFC to the core of the nucleus accumbens, which is proposed to be a final common pathway for reinstatement. As illustrated in Fig. 1B, the limbic subcircuit consists of the VTA, basolateral amygdala, the LDT/PPT, hippocampus, and the extended amygdala, which includes the nucleus accumbens shell, central nucleus of the amygdala, and the bed nucleus of the stria terminalis. As described earlier, reinstatement of drug seeking generally involves priming the animals with drug, cue, or stress, and specific neuroanatomical regions of the limbic subcircuit subserve each of these forms of reinstatement. Thus, the mPFC projects to the VTA directly or via the LDT/PPT and, in the case of stress, via the extended amygdala (Shaham et al., 2000b; Kalivas and McFarland, 2003; McFarland et al., 2004; Schmidt et al., 2009). The VTA projects back to the dorsal mPFC either directly, or in the case of cues, via the basolateral amygdala, (Kalivas and McFarland, 2003). Finally, the dorsal hippocampus and PVN provide contextual information to the limbic circuit by impinging on the basolateral amygdala and nucleus accumbens shell, respectively (Fuchs et al., 2007; Hamlin et al., 2009). It should be noted, however, that much of the literature on the neurocircuitry of reinstatement is based on studies with cocaine. A recent report exploring the neural circuits of heroin-seeking behavior largely confirmed the findings with cocaine but indicated an expanded circuitry for reinstatement of heroin self-administration (Rogers et al., 2008). As with sensitization, a discussion on the neurochemistry and neuropharmacology of reinstatement is provided in section IV.
D. Neural Circuitry Relationships between Sensitization and Relapse
It is apparent in Fig. 1 that there is significant overlap in the circuits involved in sensitization and reinstatement behaviors. The mesocorticolimbic dopamine system seems to be the primary circuit, the mPFC projecting to VTA via the LDT/PPT, which then projects to the nucleus accumbens. Projections from the mPFC directly to the nucleus accumbens are also common for reinstatement and sensitization. The PVN and basolateral amygdala can serve as an intermediary destination between the VTA and the nucleus accumbens. The involvement of the hippocampus seems to differ for the two models, with input to the shell of the nucleus accumbens being important for reinstatement and input to the VTA being important for sensitization. A couple of important points should be noted with regard to studies determining the circuits involved in drug-evoked behavior. First, most studies on behavioral sensitization involved lesions before initial drug exposure (i.e., during the initiation of drug-induced neuroplasticity), whereas studies on reinstatement involve interventions that occurred after a history of drug taking (i.e., during the expression of drug-induced neuroadaptations). Second, the studies on sensitization have not isolated subcircuits that might contribute to context-dependent sensitization or stress cross-sensitization between drugs of abuse. Nevertheless, there is remarkable overlap in the neural circuits believed to underlie behavioral sensitization and reinstatement of drug-seeking behavior. These data provide additional construct validity for the sensitization model in that they seem to accurately predict the neural circuitry of substance abuse. Thus, it is possible that repeated exposure of the circuits described above to drug, be it contingent or noncontingent, produce adaptations that can promote relapse to drug seeking.
IV. Neurochemistry/Neuropharmacology
A. Sensitization
1. Dopamine.
Early studies suggest that enhanced dopamine transmission in the mesoaccumbens system underpinned locomotor sensitization. Thus, reports demonstrated that cocaine-evoked dopamine release was either enhanced or unaltered in the VTA of sensitized animals (Kalivas and Duffy, 1993; Parsons and Justice, 1993). However, dopamine neuron excitability is enhanced in the VTA in animals with a history of repeated exposure to amphetamine, cocaine, or ethanol (White and Wang, 1984; Henry et al., 1989; Brodie, 2002). Animals behaviorally sensitized to cocaine, amphetamine, nicotine, or morphine (Robinson et al., 1988; Kalivas and Duffy, 1990; Johnson and Glick, 1993; Parsons and Justice, 1993; Shoaib et al., 1994), but not ethanol (Zapata et al., 2006), show enhanced dopamine release in the nucleus accumbens in response to challenge drug exposure. In addition to the mesoaccumbens circuit, more recent studies focused on the mesocortical dopamine system. Most studies have been conducted with cocaine and have shown that repeated cocaine administration produces time-dependent alterations in mPFC dopamine transmission, early withdrawal being associated with decreased cocaine-mediated dopamine transmission and prolonged withdrawal being associated with enhanced dopamine transmission (Sorg et al., 1997; Chefer et al., 2000; Wu et al., 2003; Williams and Steketee, 2005). Amphetamine-induced sensitization was found to be associated with no changes in mPFC dopamine transmission after short withdrawal, but testing at longer withdrawals was not conducted (Peleg-Raibstein and Feldon, 2008). In contrast to these findings, repeated nicotine administration enhances nicotine-evoked dopamine release in the mPFC 24 h after daily treatment (Nisell et al., 1996). Taken together, these data suggest that, in general, behavioral sensitization is associated with augmented dopamine transmission in the mesocorticolimbic dopamine system. However, it should be noted that studies in both human and nonhuman primates indicate that minimal or no sensitization of dopamine release is occurring in cocaine-dependent individuals or monkeys trained to self-administer cocaine (for review, see Bradberry, 2007). This may highlight a difference in dosing in that both humans and primate models tend to involve substantially more cocaine intake than the rodent experiments outlined above. In addition, whereas the primate and human studies involve contingent drug use, the rodent studies are largely with noncontingent drug use, although a study examining a cocaine challenge in rats trained to self-administer cocaine found sensitized dopamine release in parallel with augmented locomotor behavior (Hooks et al., 1994). In contrast to these findings, human volunteers who received three noncontingent injections of amphetamine in a laboratory setting displayed enhanced dopamine release in the nucleus accumbens in response to an amphetamine challenge injection 2 weeks and 1 year later as measured by decreased [11C]raclopride binding (Boileau et al., 2006). In addition, a recent report demonstrated that in nondependent human cocaine users, intranasal cocaine administration increased dopamine release in the ventral striatum (Cox et al., 2009). Unlike previous studies in humans and nonhuman primates, cocaine-evoked dopamine release was conducted in the presence of drug-associated cues (Cox et al., 2009). It is noteworthy that the degree of dopamine release was positively correlated with history of psychostimulant use, suggesting an enhanced dopamine response with repeated drug use (Cox et al., 2009).
2. Dopamine Receptors.
In addition to changes in neurotransmitter release, dopamine binding to its receptors plays a key role in behavioral sensitization. For example, enhanced excitability of VTA dopamine neurons that occurs with repeated cocaine is associated with decreased dopamine D2 autoreceptor sensitivity (White and Wang, 1984; Henry et al., 1989). In addition, repeated intra-VTA injections of low doses of the D2 antagonist eticlopride, which are presumably autoreceptor-selective, enhanced subsequent stimulant responses to amphetamine (Tanabe et al., 2004). Blockade of dopamine D1 receptors in the VTA during the initiation phase prevents the development of amphetamine but not cocaine sensitization (Stewart and Vezina, 1989; Vezina, 1996; Steketee, 1998). Furthermore, repeated injections of 2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazepine (SKF38393), a D1 receptor agonist, into the VTA induced sensitization to cocaine (Pierce et al., 1996b). Taken together, these data suggest that reduced autoreceptor inhibition of dopamine neurons in the VTA allows for enhanced dopamine stimulation of D1 receptors in this region that is capable of increasing glutamate release and thus further activation of dopamine projection neurons (Kalivas and Duffy, 1995; Wolf and Xue, 1998).
In the nucleus accumbens, repeated cocaine has been shown to increase dopamine D1 receptor sensitivity (Henry and White, 1991). In addition, injections of (±)-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetra-hydro-1H-benzazepine (SKF82958), a dopamine D1 receptor agonist, into the nucleus accumbens in combination with systemic cocaine, enhanced the development of cocaine sensitization (De Vries et al., 1998a). Previous studies have demonstrated that injections of (±)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide (SKF81297) or SKF38393 into the mPFC blocked the expression of cocaine or amphetamine sensitization (Sorg et al., 2001; Moro et al., 2007), whereas other studies have not found an effect (Beyer and Steketee, 2002). The ability of intra-mPFC quinpirole, a D2 agonist, to inhibit cocaine-induced motor activity was reduced in sensitized animals, whereas repeated injections of the D2 antagonist sulpiride into the mPFC induced sensitization to cocaine (Beyer and Steketee, 2002; Steketee and Walsh, 2005). Although the role of mPFC dopamine D1 receptors in sensitization remains to be clarified, the data above suggest that dopamine D2 receptor function in the mPFC is reduced, that is supported by other studies (Bowers et al., 2004).
3. Glutamate.
Initial reports demonstrated that in amphetamine- or cocaine-sensitized animals, dopamine neurons in the VTA were more responsive to glutamate, which involved increased α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor sensitivity (White et al., 1995b; Zhang et al., 1997). Microdialysis studies have revealed that short-term amphetamine administration produces a delayed increase in glutamate levels in the VTA that is not enhanced by repeated exposure, whereas cocaine increases glutamate levels only in the VTA of sensitized animals (Kalivas and Duffy, 1998; Wolf and Xue, 1998). Ibotenate lesions of the mPFC prevent amphetamine from increasing VTA glutamate levels (Wolf and Xue, 1999). In addition, repeated injections of the glutamate uptake inhibitor l-trans-pyrollidine-2,4-dicarboxylic acid into the VTA induced behavioral sensitization to amphetamine, an effect blocked by the N-methyl-d-aspartate (NMDA) receptor antagonist 3-(2-carboxy-piperazin-4-yl)-propyl-1-phosphonic acid (Aked et al., 2005). Thus, repeated increases in glutamate levels in the VTA seem to be capable of inducing a sensitized behavioral response to amphetamine or cocaine. These results are consistent with findings that repeated injections of amphetamine or morphine into the VTA also induce sensitization (Vezina et al., 1987; Kalivas and Weber, 1988).
Recent electrophysiological studies have demonstrated that single or repeated noncontingent exposure to drugs of abuse, including cocaine, amphetamine, ethanol, morphine, and nicotine, induce an NMDA receptor-dependent APMA receptor-induced long-term potentiation (LTP) of VTA dopamine neurons (Ungless et al., 2001; Saal et al., 2003; Borgland et al., 2004). This LTP, which is dependent on activation of dopamine D1 receptors, is of short duration after noncontingent cocaine administration but lasts at least 3 months after contingent cocaine self-administration (Saal et al., 2003; Schilström et al., 2006; Chen et al., 2008). Additional studies have shown that cocaine-induced LTP in the VTA results from insertion of AMPA receptors lacking the GluR2 subunit, which imparts increased Ca2+ permeability to these receptors (Bellone and Lüscher, 2005; Mameli et al., 2007; Argilli et al., 2008). Stress, which is known to cross-sensitize with drugs of abuse (Kalivas and Stewart, 1991), also induces LTP in VTA dopamine neurons (Saal et al., 2003). Corticotropin-releasing factor (CRF), which is released in the VTA in response to stress (Wang et al., 2005), potentiates NMDA receptor-mediated transmission, and this potentiation is enhanced in mice with a history of repeated cocaine exposure (Hahn et al., 2009). It is noteworthy that CRF potentiates AMPA receptor transmission in animals repeatedly exposed to cocaine but not cocaine-naive animals (Hahn et al., 2009). It is postulated that the enhanced response of AMPA receptors to CRF produces enhanced dopamine transmission in the mesocorticolimbic dopamine system (Ungless et al., 2010). Finally, excitatory modulation of VTA dopamine neurons can be further enhanced by CRF-induced attenuation of inhibitory modulation produced by dopamine D2 and GABA-B receptors (Beckstead et al., 2009). Taken together, these data provide a physiological basis for enhanced mesocorticolimbic dopamine transmission associated with expression of behavioral sensitization to drugs of abuse and stress.
Unlike the VTA, repeated cocaine decreases, rather than increases, neuronal responsiveness to glutamate in the nucleus accumbens (White et al., 1995a). Behavioral sensitization to cocaine is associated with reduced basal levels of glutamate in the nucleus accumbens, whereas challenge injections of cocaine or amphetamine produce increased glutamate release in this region (Pierce et al., 1996a; Reid and Berger, 1996; Baker et al., 2002; Kim et al., 2005). It is noteworthy that one report demonstrated that cocaine-induced increases in nucleus accumbens glutamate release occurred only in animals that developed sensitization (Pierce et al., 1996a). In addition, restoration of basal glutamate levels with N-acetylcysteine prevented the expression of behavioral sensitization (Madayag et al., 2007). Thus the reduced basal levels of glutamate seen in sensitized animals seems to allow for enhanced glutamate release in response to drug challenge and thus the expression of sensitization (Xi et al., 2002). Additional studies demonstrated that animals withdrawn from repeated cocaine exhibit an increased AMPA/NMDA receptor ratio in the nucleus accumbens, which is indicative of LTP (Kourrich et al., 2007). It is noteworthy that upon challenge with cocaine to test for the expression of sensitization, animals exhibit a decrease in the AMPA/NMDA receptor ratio 24 h later, suggesting a long-term depression–like state of nucleus accumbens neurons (Thomas et al., 2001; Kourrich et al., 2007). Taken together, these data suggest the possibility that reduced basal levels of glutamate produce a compensatory LTP state that is reversed by the enhanced release of glutamate that occurs in response to cocaine (Thomas et al., 2008).
Like the VTA, neurons in the mPFC show an increased responsiveness to glutamate after repeated exposure to amphetamine (Peterson et al., 2000). Furthermore, K+-stimulated glutamate efflux in the mPFC was enhanced in animals after repeated methamphetamine exposure (Stephans and Yamamoto, 1995). Repeated cocaine was shown to produce time-dependent increases in cocaine-mediated mPFC glutamate overflow, short (but not long) withdrawals from repeated cocaine exposure being associated with cocaine-induced increases in extracellular glutamate concentrations in the mPFC (Williams and Steketee, 2004). It is noteworthy that, as was seen previously in the nucleus accumbens (Pierce et al., 1996a), cocaine only increased mPFC levels of glutamate in animals that expressed behavioral sensitization to cocaine (Williams and Steketee, 2004). These data contrast with those from an earlier study, in which a challenge injection of cocaine failed to effect mPFC glutamate levels in sensitized animals (Hotsenpiller and Wolf, 2002). Finally, physiological studies demonstrate that repeated cocaine exposures induce LTP in the mPFC that is dependent on dopamine D1 receptors and results from a reduction in GABA-A receptor-mediated inhibition of pyramidal neurons (Huang et al., 2007a). In general, the data are supportive of the idea that enhanced glutamate transmission in the mPFC is associated with behavioral sensitization.
4. Glutamate Receptors.
As mentioned above, the enhanced glutamate-induced excitability of VTA dopamine neurons seen in behaviorally sensitized animals involves AMPA receptors (Zhang et al., 1997). In support of this, repeated injections of AMPA into the VTA induced cocaine sensitization (Dunn et al., 2005), whereas intra-VTA AMPA injections in sensitized animals increased dopamine and glutamate release in the nucleus accumbens and glutamate release in the VTA (Giorgetti et al., 2001). Studies on the effects of repeated drug exposure on the expression of AMPA receptor subunits in the VTA has been mixed with increased GluR1 levels or no change in GluRs (Fitzgerald et al., 1996; Churchill et al., 1999; Bardo et al., 2001; Lu et al., 2002). On the other hand, more recent studies have shown that repeated cocaine promotes increased insertion of GluR2-lacking AMPA receptors in VTA dopamine neurons, leading to the increased AMPA/NMDA receptor ratio associated with LTP (Bellone and Lüscher, 2005; Mameli et al., 2007; Argilli et al., 2008). With regard to NMDA receptors, coadministration of the competitive NMDA receptor antagonist 3-(2-carboxy-piperazin-4-yl)-propyl-1-phosphonic acid into the VTA with systemic cocaine or VTA amphetamine prevents the development of behavioral sensitization (Kalivas and Alesdatter, 1993; Cador et al., 1999). Systemic injection of NMDA antagonists selective for NMDA receptors containing NR2 subunits also prevents the development of cocaine sensitization as well as the cocaine-induced increase in AMPA/NMDA receptor ratios seen in the VTA (Schumann et al., 2009). Furthermore, sensitization to cocaine or morphine is associated with increased expression of NMDA NR1 subunits (Fitzgerald et al., 1996; Churchill et al., 1999). Finally, injection of a nonselective metabotropic glutamate receptor (mGluR) antagonist into the VTA prevents amphetamine sensitization, whereas repeated administration of a nonselective mGluR agonist induces sensitization to cocaine (Kim and Vezina, 1998; Dunn et al., 2005).
Injections of AMPA into the nucleus accumbens produced greater motor activity in cocaine-sensitized animals compared with controls, whereas injection of the AMPA antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo{f}quinoxaline-2,3-dione blocked the development of cocaine sensitization (Pierce et al., 1996a; Ghasemzadeh et al., 2003). Intra-accumbens administration of 2-[(1S,2S)-2-carboxycyclopropyl]-3-(9H-xanthen-9-yl)-d-alanine (LY341495), a group II mGluR antagonist, induces hyperactivity in amphetamine-sensitized rats but not in control rats (Chi et al., 2006). Repeated exposure to cocaine, followed by withdrawal, increases the levels of GluR1 and AMPA receptor surface expression in the nucleus accumbens after prolonged withdrawal, whereas repeated amphetamine has no effect on AMPA receptor surface expression (Churchill et al., 1999; Boudreau and Wolf, 2005; Boudreau et al., 2007; Boudreau et al., 2009; Ghasemzadeh et al., 2009b; Nelson et al., 2009; Schumann and Yaka, 2009). On the other hand, NMDA receptor subunits have been reported to be increased or decreased in sensitized animals, and surface expression is unaltered (Yamaguchi et al., 2002; Nelson et al., 2009; Schumann and Yaka, 2009). It is noteworthy that the long-term depression induced by a challenge injection of cocaine in sensitized animals that was discussed in section IV.A.3 is paralleled by a decrease in the surface expression of AMPA receptors in the nucleus accumbens (Thomas et al., 2001; Boudreau et al., 2007; Kourrich et al., 2007), which reverses back to the cocaine-induced enhanced surface expression after withdrawal (Ferrario et al., 2010). Taken together, these data suggest that behavioral sensitization is associated with enhanced glutamate transmission via AMPA receptors in the nucleus accumbens that might be reduced by group II mGluRs. In support of the latter point, repeated cocaine administration has been reported to decrease group II mGluR function and expression in the nucleus accumbens, which allows for increased glutamate release in this region (Xi et al., 2002; Ghasemzadeh et al., 2009b). In addition, systemic administration of (1R,4R,5S,6R)-4-amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY379268), a group II mGluR agonist, prevents the enhanced nucleus accumbens glutamate overflow seen in response to an amphetamine challenge in amphetamine-sensitized animals as well as the enhanced cocaine self-administration seen in these animals (Kim et al., 2005).
Studies of the involvement of mPFC glutamate receptors have revealed that the ability of the group II mGluR agonist (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate to the block the expression of behavioral sensitization when injected into this region is reduced after prolonged withdrawal from cocaine (Xie and Steketee, 2009a). Furthermore, infusions of LY341495, a group II mGluR antagonist, enhanced mesocorticolimbic glutamate transmission in control but not cocaine-sensitized rats, suggesting that repeated cocaine administration reduces tonic inhibition of glutamate transmission (Xie and Steketee, 2009b). In support of this, repeated cocaine administration uncouples group II mGluRs from their G proteins in the mPFC (Xi et al., 2002; Bowers et al., 2004). In addition, repeated cocaine reduced group II mGluR-mediated long-term depression in the mPFC (Huang et al., 2007b). Taken together, these data suggest that long-term cocaine sensitization is associated with a reduction in mPFC group II mGluR function. Finally, long-term withdrawal from repeated cocaine is associated with increased phosphorylation of GluR1 subunits and increased synaptic localization of AMPA and NMDA receptors (Zhang et al., 2007; Ghasemzadeh et al., 2009a). Together, these changes in metabotropic and ionotropic receptors would be expected increase the excitability of mPFC projection neurons.
The involvement of glutamate in other brain regions that form the sensitization circuit has received limited attention. Morphine sensitization is associated with decreased GluR2 expression in both the amygdala and the dorsal hippocampus with no changes in GluR1 levels in the hippocampus (Grignaschi et al., 2004; Sepehrizadeh et al., 2008). This could lead to enhanced levels of calcium-permeable AMPA receptors thought to underlie synaptic plasticity. Injection of AMPA into the LDT increases glutamate in the VTA, which is enhanced in amphetamine-sensitized animals (Nelson et al., 2007). Thus, glutamate, at various levels of the neural circuitry described in section III, seems to be involved in the development of sensitization.
5. Summary.
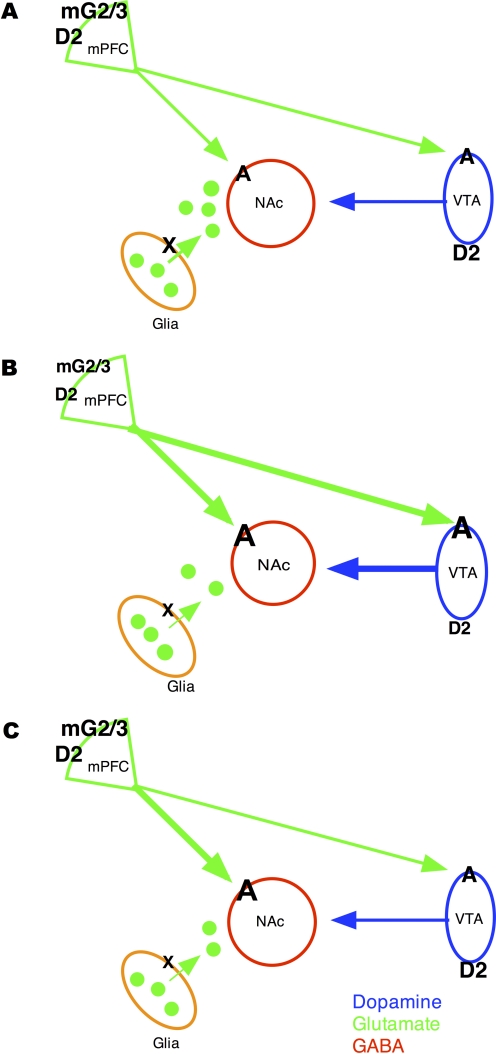
As shown in Fig. 2B and summarized in Table 1, repeated exposure to drugs of abuse produces transient increases in somatodendritic dopamine release in the VTA (Kalivas and Duffy, 1993) as a result of decreased autoreceptor sensitivity (White and Wang, 1984; Henry et al., 1989), which can act on D1 receptors to enhance glutamate release from mPFC projecting neurons and to induce LTP in dopamine neurons (Kalivas and Duffy, 1995; Ungless et al., 2001; Saal et al., 2003). The increase in released glutamate can then act on AMPA receptors to promote dopamine release in the nucleus accumbens (Karreman et al., 1996; Ungless et al., 2010). Long-term, enhanced nucleus accumbens dopamine release occurs as a result of synaptic plasticity at the level of the nucleus accumbens, in particular via an increase in calcium/calmodulin-dependent protein kinase II activity (Pierce and Kalivas, 1997b). Enhanced dopamine release in the nucleus accumbens can act on D1 receptors to promote behavioral sensitization (Giorgetti et al., 2001). The mPFC projections to the VTA are either direct or indirect via glutamate inputs from the LDT (Omelchenko and Sesack, 2005, 2007; Nelson et al., 2007). Furthermore, within the mPFC, D2 and group II mGluR receptor function is reduced (Beyer and Steketee, 2002; Bowers et al., 2004), allowing for increased excitability of projection neurons. In the nucleus accumbens, basal glutamate levels are reduced, thereby attenuating autoreceptor regulation of release that promotes enhanced drug-induced glutamate release from mPFC neurons in sensitized animals (Pierce et al., 1998; Baker et al., 2002; Xi et al., 2002). The reduced basal glutamate levels are believed to enhance AMPA receptor function (Boudreau and Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007). Increased glutamate release in the nucleus accumbens can act on these enhanced AMPA receptors to also promote behavioral sensitization (Pierce et al., 1996a; Ghasemzadeh et al., 2003). Finally, studies indicating the possibility that repeated cocaine produces an increase in calcium-permeable AMPA receptors in the hippocampus and amygdala suggest that neurotransmission within the mesocorticolimbic circuit can be further enhanced by glutamate input to the VTA and nucleus accumbens from the hippocampus and amygdala, respectively (Shinonaga et al., 1994; Lodge and Grace, 2008; Sepehrizadeh et al., 2008).
Fig 2.
A, the neurochemistry and neuropharmacology of the sensitization and reinstatement circuits compared with normal circuits. B, sensitization is associated with enhanced dopamine transmission from the VTA to the nucleus accumbens that results from reduced D2 autoreceptor function and enhanced AMPA (A) receptor function. Enhanced glutamate transmission from the mPFC to the nucleus accumbens and VTA is also seen in sensitized animals and is thought to result in part from reduced inhibitory modulation produced by D2 receptors and group II mGluRs (mG2/3). In addition, reduced function of the cystine/glutamate antiporter (X) produces lower basal levels of glutamate in the nucleus accumbens that leads to increased AMPA receptor function in this region. Finally, reduced basal levels of glutamate would produce reduced activation of inhibitory group II mGluRs in the nucleus accumbens that would allow for increased evoked release of glutamate in this region (not illustrated). Like sensitization (C), reinstatement is also associated with reduced basal glutamate levels in the nucleus accumbens and enhanced AMPA receptor function in this region along with enhanced drug-evoked glutamate release. Whether other drug-induced changes seen in sensitization are also apparent during reinstatement is not known.
TABLE 1.
Summary of drug-induced changes associated with behavioral sensitization and reinstatement
See text for relevant citations.
| Brain Region | Sensitization | Reinstatement |
|---|---|---|
| VTA | ||
| Dopamine release | Increased | Unknown |
| Autoreceptor function | Decreased | Unknown |
| Glutamate release | Increased | Unknown |
| AMPA function | Increased | Increased |
| LTP | Transient | Prolonged |
| Nucleus accumbens | ||
| Dopamine release | Increased | Increased or decreased |
| Basal glutamate | Decreased | Decreased |
| Glutamate release | Increased | Increased |
| AMPA function | Increased | Increased |
| mGluR2/3 function | Decreased | Decreased |
| mPFC | ||
| Dopamine release | Increased (Delayed) | Unknown |
| D2 function | Decreased | Unknown |
| Glutamate release | Increased (Transient) | Unknown |
| Group II mGluR function | Decreased | Unknown |
B. Reinstatement
1. Dopamine.
Studies on the role of dopamine in reinstatement of drug-seeking behavior have focused primarily on the nucleus accumbens and have produced mixed results. Thus, cocaine- or amphetamine-primed reinstatement is associated with reduced (Neisewander et al., 1996) or enhanced (Ranaldi et al., 1999; Di Ciano et al., 2001) dopamine release in the nucleus accumbens. Intra-accumbens injections of amphetamine, which would increase dopamine release, have been reported to reinstate ethanol-seeking behavior (Samson et al., 1999). Cue-induced reinstatement of amphetamine- or ethanol-seeking behavior is associated with either no change or reduced dopamine release, respectively (Katner and Weiss, 1999; Di Ciano et al., 2001). Both D1 and D2 receptors in the nucleus accumbens seem to play a role in reinstatement behavior, although the results are not always consistent. Injection of (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH23390) into the nucleus accumbens reduces cocaine-primed reinstatement of cocaine seeking or context-primed reinstatement of ethanol seeking (Anderson et al., 2003; Bachtell et al., 2005; Chaudhri et al., 2009; Madayag et al., 2010). In contrast, intra-accumbens SKF81297 reinstates cocaine-seeking behavior (Bachtell et al., 2005; Schmidt et al., 2006). Similar findings have been reported for D2 receptors. Thus, intra-accumbens quinpirole or 7-hydroxy-2-dipropylaminotetralin reinstates cocaine-seeking behavior, whereas eticlopride or sulpiride prevents cocaine-primed reinstatement (Bachtell et al., 2005; Anderson et al., 2006; Schmidt et al., 2006). Although the data seem to consistently show a general role for nucleus accumbens dopamine receptors in reinstatement, discrepancies rise as to the specific involvement of the shell (Anderson et al., 2003, 2006; Schmidt et al., 2006) versus the core (McFarland and Kalivas, 2001; Bachtell et al., 2005; Madayag et al., 2010). Thus, although D1 antagonist microinjection into the shell inhibits reinstated cocaine seeking, similar treatment into the core is ineffective.
In addition to the nucleus accumbens, some reports have also implicated other dopamine systems in reinstatement behavior. Injections of the nonselective dopamine antagonist fluphenazine into the dorsal mPFC prevented cocaine- or stress-primed reinstatement, whereas injections of dopamine reinstated cocaine seeking (McFarland and Kalivas, 2001; McFarland et al., 2004). SCH23390 in the dorsal mPFC blocks cocaine- or heroin-primed reinstatement of drug seeking and cocaine- and stress-primed reinstatement of CPP (Sanchez et al., 2003; Sun and Rebec, 2005; See, 2009). Intra-mPFC microinjection of the D1 agonist SKF81297 induced reinstatement to cocaine seeking (Schmidt et al., 2009). As well, intra-mPFC microinjection of the D2 antagonist eticlopride reduced cocaine-primed reinstatement (Sun and Rebec, 2005). Reinstatement of cocaine-seeking behavior is associated with enhanced cocaine-mediated dopamine release in the amygdala (Tran-Nguyen et al., 1998). Injection of amphetamine into the basolateral amygdala enhanced cue-induced reinstatement, whereas SCH23390 delivered to this same region prevented cue- and cocaine-induced reinstatement of cocaine seeking (See et al., 2001; Ledford et al., 2003; Alleweireldt et al., 2006). Taken together, the data suggest that dopamine stimulation of D1 receptors (in the mPFC, nucleus accumbens shell, and amygdala) and D2 receptors (in the nucleus accumbens) can modulate reinstatement of drug seeking behavior.
2. Glutamate.
As with dopamine, the majority of studies conducted on the involvement of glutamate in the reinstatement of drug seeking focused on the nucleus accumbens. Repeated exposure to cocaine reduces basal glutamate levels in the nucleus accumbens core, which, when returned to normal levels by pretreatment with N-acetylcysteine, prevents reinstatement of cocaine seeking (Baker et al., 2003). Injection of (S)-4-carboxyphenylglycine, which inhibits the cystine/glutamate antiporter, into the nucleus accumbens core blocks the actions of N-acetylcysteine (Kau et al., 2008). Cocaine-primed reinstatement is associated with increased glutamate release in the core of the nucleus accumbens that can be prevented by inactivation of the dorsal mPFC (McFarland et al., 2003). Similar findings have been reported for both heroin and cue-induced reinstatement of heroin seeking behavior (LaLumiere and Kalivas, 2008). Injections of AMPA into the core or shell of the nucleus accumbens can reinstate cocaine seeking, an effect that can be blocked by injection of antisense for the GluR1 subunit into these same regions (Cornish et al., 1999; Ping et al., 2008). Cocaine self-administration produces an increase in surface expression of GluR2-lacking AMPA receptors in nucleus accumbens neurons, which are likely to produce an LTP state, as demonstrated by physiological studies (Conrad et al., 2008; Mameli et al., 2009; Moussawi et al., 2009). 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX) in the core or shell prevents cocaine-, heroin, or cue-induced reinstatement (Cornish and Kalivas, 2000; Park et al., 2002; Bäckström and Hyytiä, 2007; Famous et al., 2008; LaLumiere and Kalivas, 2008). Disruption of trafficking of GluR2 subunits to the membrane in the core or shell also prevents cocaine-primed reinstatement (Famous et al., 2008). It is noteworthy that, like sensitization, conditioned cues reduce AMPA receptor surface expression 24 h after exposure to these cues (Conrad et al., 2008). In contrast to the consistent findings regarding nucleus accumbens AMPA receptors and reinstatement, injection of the NMDA agonist 1-aminocyclobutane-cis-1,3-dicarboxylic acid into the core reinstates cocaine seeking, whereas injection of 2-amino-5-phosphonovaleric acid, an NMDA antagonist, into the core or shell either blocks, has no effect on, or reinstates cocaine seeking (Cornish et al., 1999; Cornish and Kalivas, 2000; Bäckström and Hyytiä, 2007; Famous et al., 2007). Finally, injections of the group II mGluR agonist LY379268 into the nucleus accumbens blocks 1) context-induced heroin seeking and 2) cocaine-primed drug seeking (Bossert et al., 2006; Peters and Kalivas, 2006). Together, these data suggest that cocaine self-administration reduces basal glutamate levels in the nucleus accumbens that in turn reduce group II mGluR modulation of glutamate release (Moran et al., 2005), thus allowing for increased release of glutamate and subsequent stimulation of AMPA receptors that promotes reinstatement. The reduced basal levels of glutamate are offset by increased responsivity of AMPA receptors that can be reversed during reinstatement (Conrad et al., 2008; Mameli et al., 2009; Moussawi et al., 2009). These effects can be prevented by normalizing basal glutamate, stimulating group II mGluRs, or blocking AMPA receptors (Cornish and Kalivas, 2000; Park et al., 2002; Bossert et al., 2006; Peters and Kalivas, 2006; Bäckström and Hyytiä, 2007; Famous et al., 2008; Kau et al., 2008; LaLumiere and Kalivas, 2008).
In addition to the nucleus accumbens, studies have shown that blocking ionotropic receptors with kynurenic acid or CNQX in the VTA blocks cocaine-induced reinstatement, whereas injections of LY379268 prevented context-induced heroin seeking (Bossert et al., 2004; Sun et al., 2005; Schmidt et al., 2009). CNQX in the LDT/PPT also has been reported to inhibit cocaine-seeking behavior (Schmidt et al., 2009). The mGluR1 agonist (3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone (JNJ16259685) in the dorsal hippocampus blocks context-dependent cocaine reinstatement (Xie et al., 2010). In contrast, neither CNQX nor 2-amino-5-phosphonovaleric acid in the basolateral amygdala disrupts cue-induced cocaine seeking (See et al., 2001). Thus, inhibition of glutamate transmission in the VTA or dorsal hippocampus, but not the basolateral amygdala, can affect reinstated drug seeking. Finally, infralimbic AMPA administration suppresses cocaine seeking by activating an infralimbic-cortex–to–nucleus-accumbens shell circuit during extinction (Peters et al., 2008). This suggests that activation of this circuit plays a role in learning to inhibit drug seeking behavior when drug is no longer available.
3. Summary
As seen in Fig. 2C and summarized in Table 1, reinstatement primarily involves enhanced glutamate transmission from the dorsal mPFC to the core of the nucleus accumbens that results from reduced basal levels of glutamate in the nucleus accumbens that allows for reduced autoreceptor control of glutamate release and thereby increased drug-induced glutamate release that activate AMPA receptors (Cornish et al., 1999; Cornish and Kalivas, 2000; Baker et al., 2003; McFarland et al., 2003; Moran et al., 2005; Bäckström and Hyytiä, 2007; Famous et al., 2008; LaLumiere and Kalivas, 2008). These AMPA receptors have been shown to have increased function that is reduced by exposure to previously drug-paired cues (Conrad et al., 2008). The mPFC also affects reinstatement via glutamatergic projections to the LDT/PPT and VTA acting on AMPA receptors in both sites (Schmidt et al., 2009). Dopamine projections from the VTA to the nucleus accumbens, mPFC, and basolateral amygdala also seem to be involved in reinstatement. Specifically, dopamine acting on D1 receptors would be capable of stimulating glutamate projection neurons in the amygdala and mPFC (See et al., 2001; Sun and Rebec, 2005; Alleweireldt et al., 2006; Schmidt et al., 2009; See, 2009), whereas dopamine acting on both D1 and D2 receptors in the nucleus accumbens shell (Anderson et al., 2003; Bachtell et al., 2005; Anderson et al., 2006; Schmidt et al., 2006) could act in concert with AMPA receptors to reduce GABAergic transmission to the ventral pallidum, which has been shown to be important in reinstatement (O'Connor, 2001; Torregrossa et al., 2008).
C. Neurochemical/Neuropharmacological Relationships between Sensitization and Relapse
Many similarities are noted in a comparison of the neurochemistry and neuropharmacology of behavioral sensitization and relapse. In both cases, basal glutamate levels in the nucleus accumbens are reduced (Baker et al., 2002, 2003) but re-exposure to drug increases glutamate in this region (Pierce et al., 1996a; Moran et al., 2005), which can then act on AMPA receptors to promote the behavioral response (Pierce et al., 1996a; Cornish et al., 1999). Both contingent and noncontingent drug administration induce LTP in nucleus accumbens neurons that involves insertion of GluR2-lacking AMPA receptors into the membrane, which can be temporarily reversed by re-exposure to drug or drug-paired cues (Thomas et al., 2001; Boudreau and Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007; Conrad et al., 2008; Mameli et al., 2009; Moussawi et al., 2009; Ferrario et al., 2010). Loss or reduction of glutamate input from the mPFC to the nucleus accumbens prevents the expression of sensitization and reinstatement (Pierce et al., 1998; McFarland et al., 2003). In addition, mPFC glutamate projections to the VTA via the LDT/PPT that act on AMPA receptors have also been shown to be important for both behavioral responses (Nelson et al., 2007; Schmidt et al., 2009). Although the role of glutamate systems in reinstatement and sensitization seem to mirror each other, the role of dopamine systems does not. For example, sensitization, but not reinstatement, is associated with transient reductions in autoreceptor sensitivity in the VTA that allows, in part, for increased excitability of dopamine neurons (White and Wang, 1984; Henry et al., 1989). However, these VTA dopamine responses to repeated cocaine exposure are typically thought to underlie the initiation rather that the expression of sensitization. In addition, although injections of a D1 agonist into the mPFC reinstated cocaine-seeking behavior, it did not lead to the expression of sensitization (Beyer and Steketee, 2002; Schmidt et al., 2009). Taken together, the data suggest that glutamate circuits are the key player in uniting sensitization with relapse. Furthermore, these data point to the need to use the reinstatement model to verify the importance of findings from the behavioral sensitization model in relapse to drug seeking.
V. The Role of Sensitization in Relapse to Drug-Seeking Behavior
The neurocircuitry that underlies behavioral sensitization and relapse to drug seeking behavior are similar, as are the neurochemical and neuropharmacological mechanisms. However, studies have been less convincing that behavioral sensitization plays a role in reinstatement. Thus, drugs that can reinstate drug seeking in animals extinguished from cocaine self-administration have been shown also to produce a sensitized behavioral response in these same animals, whereas drugs that do not induce reinstatement do not produce sensitization (De Vries et al., 1998b, 2002). Other studies have compared the impact on reinstatement of short-access versus long-access drug self-administration with mixed results (Ferrario et al., 2005; Ahmed and Cador, 2006; Knackstedt and Kalivas, 2007; Lenoir and Ahmed, 2007). Hence, long-access animals escalate their intake of cocaine and exhibit a more robust reinstatement response compared with short-access animals, but differences in behavioral sensitization are not always apparent. It is possible that the lack of difference in behavioral sensitization could result from a ceiling effect. Thus, use of a training regimen that minimizes the amount of drug exposure before testing for sensitization or reinstatement would presumably allow one to determine whether sensitization plays a role in reinstatement. In this regard, a previous study demonstrated that previous experimenter-administered amphetamine that produced behavioral sensitization enhanced not only the reinforcing effects of cocaine but also reinstatement induced by intra-accumbens administration of AMPA (Suto et al., 2004). In this same study, it was shown that with extended withdrawal from cocaine self-administration differences between amphetamine-presensitized animals and controls were no longer apparent. Taken together, these data suggest that presensitized individuals [such presensitization might occur as a result of genetics or exposure to environmental stressors (Altman et al., 1996) or with a limited history of drug use] would be more vulnerable to relapsing to drug seeking. Thus, sensitization is likely to play a role in reinstatement in the early stages of substance abuse. With more prolonged drug exposure, regardless of whether the short- or long-access paradigm is used, other factors, such as the interoceptive stimuli of cocaine becoming discriminative stimuli, could facilitate reinstatement behavior above and beyond that produced by sensitization (Keiflin et al., 2008).
VI. Therapeutic Implications
The present review discusses research efforts that have revealed a neurocircuitry that seems to underlie behavioral sensitization and reinstatement of drug-seeking behavior, at least in rodent models. Moreover, recently reviewed imaging data in human studies, although limited, is largely in agreement with the animal research (Koob and Volkow, 2010). Thus, methylphenidate-induced craving that occurs in cocaine abusers in the presence of cues, activates prefrontal cortical regions, including the orbital and medial prefrontal cortices (Volkow et al., 2005). In addition, imaging research has demonstrated that cues activate both the hippocampus and amygdala in experienced drug users (Grant et al., 1996; Childress et al., 1999; Kilts et al., 2001; Volkow et al., 2004). Given the importance of alterations of glutamate systems in sensitization and reinstatement behaviors (see above), further exploration of neurocircuitry underlying relapse in human drug abusers awaits development of appropriate ligands for the glutamate systems (Koob and Volkow, 2010).
Although behavioral studies have not firmly established a role for sensitization in the relapse to drug-seeking behavior, experiments have demonstrated a remarkable overlap in the neurocircuitry. Thus, by providing more efficient experimental throughput, sensitization could serve as a useful model to determine the mechanisms by which repeated drug exposure alters brain function to enhance behavioral responses to drugs of abuse. Positive results for these studies would then be confirmed in more time-consuming and technically challenging reinstatement studies. As an example, sensitization studies revealed a deficit in basal glutamate levels in the nucleus accumbens that was confirmed in animals with a history of cocaine self-administration. This led to experiments demonstrating that restoring basal glutamate levels in the nucleus accumbens could reduce relapse to drug-seeking behavior in animal models and humans (Baker et al., 2003; Mardikian et al., 2007; Zhou and Kalivas, 2008; Knackstedt et al., 2009, 2010; Sari et al., 2009). This type of progression from studies with behavioral sensitization to validation using models of reinstated drug-seeking and to clinical trials is especially useful to consider for potential pharmacotherapeutic targets identified from genomic and proteomic screens, which do not provide a priori evidence for the involvement of a drug-induced change in addiction.
Acknowledgments
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA023215] (to J.D.S.) [Grants DA015369, DA012513, DA003906] (to P.W.K.).
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Steketee and Kalivas.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.109.001933.
- AMPA
- α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione
- CPP
- conditioned place preference
- CRF
- corticotropin-releasing factor
- GluR
- glutamate receptor
- JNJ16259685
- (3,4-dihydro-2H-pyrano[2,3-b] quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone
- LDT
- laterodorsal tegmentum
- LTP
- long-term potentiation
- LY341495
- 2-[(1S,2S)-2-carboxycyclopropyl]-3-(9H-xanthen-9-yl)-d-alanine
- LY379268
- (1R,4R,5S,6R)-4-amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid
- mGluR
- metabotropic glutamate receptor
- mPFC
- medial prefrontal cortex
- NMDA
- N-methyl-d-aspartate
- PPT
- pedunculopontine tegmentum
- PVN
- paraventricular nucleus
- SCH23390
- (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- SKF 81297
- (±)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide
- SKF38393
- 2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazepine
- SKF82958
- (±)-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetra-hydro-1H-benzazepine
- VTA
- ventral tegmental area.
References
- Ahmed SH, Cador M. (2006) Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology 31:563–571 [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413–421 [DOI] [PubMed] [Google Scholar]
- Aked J, Coizet V, Clark D, Overton PG. (2005) Local injection of a glutamate uptake inhibitor into the ventral tegmental area produces sensitization to the behavioural effects of d-amphetamine. Neuroscience 134:361–367 [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. (2006) Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology 31:363–374 [DOI] [PubMed] [Google Scholar]
- Altman J, Everitt BJ, Glautier S, Markou A, Nutt D, Oretti R, Phillips GD, Robbins TW. (1996) The biological, social and clinical bases of drug addiction: commentary and debate. Psychopharmacology (Berl) 125:285–345 [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. (1996) Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci 110:1397–1414 [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. (2003) Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 168:132–138 [DOI] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Pierce RC. (2006) Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology 31:1452–1461 [DOI] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. (2008) Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci 28:9092–9100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. (2005) Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 183:41–53 [DOI] [PubMed] [Google Scholar]
- Bäckström P, Hyytiä P. (2007) Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 192:571–580 [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. (2003) Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6:743–749 [DOI] [PubMed] [Google Scholar]
- Baker DA, Shen H, Kalivas PW. (2002) Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids 23:161–162 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Robinet PM, Mattingly BA, Margulies JE. (2001) Effect of 6-hydroxydopamine or repeated amphetamine treatment on mesencephalic mRNA levels for AMPA glutamate receptor subunits in the rat. Neurosci Lett 302:133–136 [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, Williams JT. (2009) CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacology 34:1926–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. (2005) mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci 21:1280–1288 [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105:849–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer CE, Stafford D, LeSage MG, Glowa JR, Steketee JD. (2001) Repeated exposure to inhaled toluene induces behavioral and neurochemical cross-sensitization to cocaine in rats. Psychopharmacology (Berl) 154:198–204 [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. (2002) Cocaine sensitization: modulation by dopamine D2 receptors. Cereb Cortex 12:526–535 [DOI] [PubMed] [Google Scholar]
- Biala G, Budzynska B. (2006) Reinstatement of nicotine-conditioned place preference by drug priming: effects of calcium channel antagonists. Eur J Pharmacol 537:85–93 [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. (2006) Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry 63:1386–1395 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. (2004) Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci 24:7482–7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. (2004) A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci 24:10726–10730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. (2006) The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res 173:148–152 [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME. (2009) Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. J Neurochem 110:363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. (2007) Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci 27:10621–10635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. (2005) Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci 25:9144–9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. (2004) Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron 42:269–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW. (2007) Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology (Berl) 191:705–717 [DOI] [PubMed] [Google Scholar]
- Brodie MS. (2002) Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res 26:1024–1030 [DOI] [PubMed] [Google Scholar]
- Cadoni C, Pisanu A, Solinas M, Acquas E, Di Chiara G. (2001) Behavioural sensitization after repeated exposure to Delta 9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology (Berl) 158:259–266 [DOI] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Cailhol S, Stinus L. (1999) D-amphetamine-induced behavioral sensitization: Implication of a glutamatergic medial prefrontal cortex-ventral tegmental area innervation. Neuroscience 94:705–721 [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. (2003) A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 168:66–74 [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH. (2008) Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci 28:2288–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. (2009) Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 207:303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Morón JA, Hope B, Rea W, Shippenberg TS. (2000) Kappa-opioid receptor activation prevents alterations in mesocortical dopamine neurotransmission that occur during abstinence from cocaine. Neuroscience 101:619–627 [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. (2008) Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron 59:288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Jang JK, Kim JH, Vezina P. (2006) Blockade of group II metabotropic glutamate receptors in the nucleus accumbens produces hyperlocomotion in rats previously exposed to amphetamine. Neuropharmacology 51:986–992 [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Borgo C, Falchetto S, Valerio E, Tessari M. (1996) Nicotine reinstatement of nicotine self-administration after long-term extinction. Psychopharmacology (Berl) 127:102–107 [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Swanson CJ, Urbina M, Kalivas PW. (1999) Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem 72:2397–2403 [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. (1999) A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience 93:1359–1367 [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. (2000) Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20:RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SM, Benkelfat C, Dagher A, Delaney JS, Durand F, McKenzie SA, Kolivakis T, Casey KF, Leyton M. (2009) Striatal dopamine responses to intranasal cocaine self-administration in humans. Biol Psychiatry 65:846–850 [DOI] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. (2002) Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology 27:1006–1015 [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. (1992) Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav 43:307–313 [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Cools AR, Shippenberg TS. (1998a) Infusion of a D-1 receptor agonist into the nucleus accumbens enhances cocaine-induced behavioural sensitization. Neuroreport 9:1763–1768 [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. (1998b) Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci 10:3565–3571 [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Raasø H, Vanderschuren LJ. (2002) Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology 26:18–26 [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 75:134–143 [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. (1983) Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology (Berl) 79:29–31 [DOI] [PubMed] [Google Scholar]
- Degoulet M, Rouillon C, Rostain JC, David HN, Abraini JH. (2008) Modulation by the dorsal, but not the ventral, hippocampus of the expression of behavioural sensitization to amphetamine. Int J Neuropsychopharmacol 11:497–508 [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. (2001) Changes in dopamine efflux associated with extinction, CS-induced and d-amphetamine-induced reinstatement of drug-seeking behavior by rats. Behav Brain Res 120:147–158 [DOI] [PubMed] [Google Scholar]
- Do Ribeiro Couto B, Aguilar MA, Rodríguez-Arias M, Miñarro J. (2005) Cross-reinstatement by cocaine and amphetamine of morphine-induced place preference in mice. Behav Pharmacol 16:253–259 [DOI] [PubMed] [Google Scholar]
- Dunn JM, Inderwies BR, Licata SC, Pierce RC. (2005) Repeated administration of AMPA or a metabotropic glutamate receptor agonist into the rat ventral tegmental area augments the subsequent behavioral hyperactivity induced by cocaine. Psychopharmacology (Berl) 179:172–180 [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. (2008) Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci 28:11061–11070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famous KR, Schmidt HD, Pierce RC. (2007) When administered into the nucleus accumbens core or shell, the NMDA receptor antagonist AP-5 reinstates cocaine-seeking behavior in the rat. Neurosci Lett 420:169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. (2005) Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry 58:751–759 [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. (2010) The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology 35:818–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. (1996) Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmenta area: common adaptions among cross-sensitizing agents. J Neurosci 16:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Robinson SR, Slippoy DL. (2001) Pre-exposure to (±)3,4-methylenedioxy-methamphetamine (MDMA) facilitates acquisition of intravenous cocaine self-administration. Neuropsychopharmacology 25:195–203 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. (2006) Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci 26:3584–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. (2007) Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci 26:487–498 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. (2004) Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 176:459–465 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. (2002) Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 160:425–433 [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Permenter LK, Lake R, Worley PF, Kalivas PW. (2003) Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur J Neurosci 18:1645–1651 [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C. (2009a) Locomotor sensitization to cocaine is associated with distinct pattern of glutamate receptor trafficking to the postsynaptic density in prefrontal cortex: early versus late withdrawal effects. Pharmacol Biochem Behav 92:383–392 [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Windham LK, Lake RW, Acker CJ, Kalivas PW. (2009b) Cocaine activates Homer1 immediate early gene transcription in the mesocorticolimbic circuit: differential regulation by dopamine and glutamate signaling. Synapse 63:42–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti M, Hotsenpiller G, Ward P, Teppen T, Wolf ME. (2001) Amphetamine-induced plasticity of AMPA receptors in the ventral tegmental area: effects on extracellular levels of dopamine and glutamate in freely moving rats. J Neurosci 21:6362–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. (1996) Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93:12040–12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignaschi G, Burbassi S, Zennaro E, Bendotti C, Cervo L. (2004) A single high dose of cocaine induces behavioural sensitization and modifies mRNA encoding GluR1 and GAP-43 in rats. Eur J Neurosci 20:2833–2837 [DOI] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. (2009) Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci 29:6535–6544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. (2009) Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci 29:802–812 [DOI] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. (1989) Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther 251:833–839 [PubMed] [Google Scholar]
- Henry DJ, White FJ. (1991) Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther 258:882–890 [PubMed] [Google Scholar]
- Hooks MS, Duffy P, Striplin C, Kalivas PW. (1994) Behavioral and neurochemical sensitization following cocaine self-administration. Psychopharmacology (Berl) 115:265–272 [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. (1992) Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology (Berl) 107:271–276 [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Wolf ME. (2002) Extracellular glutamate levels in prefrontal cortex during the expression of associative responses to cocaine related stimuli. Neuropharmacology 43:1218–1229 [DOI] [PubMed] [Google Scholar]
- Huang CC, Lin HJ, Hsu KS. (2007a) Repeated cocaine administration promotes long-term potentiation induction in rat medial prefrontal cortex. Cereb Cortex 17:1877–1888 [DOI] [PubMed] [Google Scholar]
- Huang CC, Yang PC, Lin HJ, Hsu KS. (2007b) Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J Neurosci 27:2958–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. (1999) Effects of cocaine, nicotine, dizocipline and alcohol on mice locomotor activity: cocaine-alcohol cross-sensitization involves upregulation of striatal dopamine transporter binding sites. Brain Research 818:204–211 [DOI] [PubMed] [Google Scholar]
- Johnson DW, Glick SD. (1993) Dopamine release and metabolism in nucleus accumbens and striatum of morphine-tolerant and nontolerant rats. Pharmacol Biochem Behav 46:341–347 [DOI] [PubMed] [Google Scholar]
- Joyce EM, Iversen SD. (1979) The effect of morphine applied locally to mesencephalic dopamine cell bodies on spontaneous motor activity in the rat. Neurosci Lett 14:207–212 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Weber B. (1988) Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther 245:1095–1102 [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. (1993) Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther 267:486–495 [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. (1990) Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse 5:48–58 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. (1993) Time course of extracellular dopamine and behavioral sensitization to cocaine. II. Dopamine perikarya. J Neurosci 13:276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. (1995) D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci 15:5379–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. (1998) Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem 70:1497–1502 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168:44–56 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev 16:223–244 [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. (2002) Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci 22:1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karreman M, Westerink BH, Moghaddam B. (1996) Excitatory amino acid receptors in the ventral tegmental area regulate dopamine release in the ventral striatum. J Neurochem 67:601–607 [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. (1999) Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res 23:1751–1760 [PubMed] [Google Scholar]
- Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. (2008) Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience 155:530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiflin R, Isingrini E, Cador M. (2008) Cocaine-induced reinstatement in rats: evidence for a critical role of cocaine stimulus properties. Psychopharmacology (Berl) 197:649–660 [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. (2001) Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58:334–341 [DOI] [PubMed] [Google Scholar]
- Kim JH, Austin JD, Tanabe L, Creekmore E, Vezina P. (2005) Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci 21:295–300 [DOI] [PubMed] [Google Scholar]
- Kim JH, Vezina P. (1998) Metabotropic glutamate receptors are necessary for sensitization by amphetamine. Neuroreport 9:403–406 [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. (1990) Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol 298:40–49 [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. (2007) Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther 322:1103–1109 [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. (2009) The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 65:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. (2010) Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry 67:81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. (2007) Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci 27:7921–7928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. (2001) Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci 21:RC155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. (2008) Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci 28:3170–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford CC, Fuchs RA, See RE. (2003) Potentiated reinstatement of cocaine-seeking behavior following D-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology 28:1721–1729 [DOI] [PubMed] [Google Scholar]
- Leith NJ, Kuczenski R. (1982) Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacology (Berl) 76:310–315 [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. (2007) Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology 32:616–624 [DOI] [PubMed] [Google Scholar]
- Lett BT. (1989) Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 98:357–362 [DOI] [PubMed] [Google Scholar]
- Li SM, Ren YH, Zheng JW. (2002) Effect of 7-nitroindazole on drug-priming reinstatement of D-methamphetamine-induced conditioned place preference. Eur J Pharmacol 443:205–206 [DOI] [PubMed] [Google Scholar]
- Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. (1999) Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse 34:169–180 [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. (2008) Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci 28:7876–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Monteggia LM, Wolf ME. (2002) Repeated administration of amphetamine or cocaine does not alter AMPA receptor subunit expression in the rat midbrain. Neuropsychopharmacology 26:1–13 [DOI] [PubMed] [Google Scholar]
- Madayag A, Kau KS, Lobner D, Mantsch JR, Wisniewski S, Baker DA. (2010) Drug-induced plasticity contributing to heightened relapse susceptibility: neurochemical changes and augmented reinstatement in high-intake rats. J Neurosci 30:210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. (2007) Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci 27:13968–13976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Balland B, Luján R, Lüscher C. (2007) Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317:530–533 [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. (2009) Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci 12:1036–1041 [DOI] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. (2007) An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry 31:389–394 [DOI] [PubMed] [Google Scholar]
- Marinelli M, Le Moal M, Piazza PV. (1998) Sensitization to the motor effects of contingent infusions of heroin but not of kappa agonist RU 51599. Psychopharmacology (Berl) 139:281–285 [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Koya E, Simmons DE, Mitchell TB, Berkow A, Crombag HS, Hope BT. (2008) Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur J Neurosci 27:202–212 [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. (2004) Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24:1551–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. (2003) Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 168:57–65 [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. (1996) Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol 7:754–763 [PubMed] [Google Scholar]
- Meil WM, See RE. (1997) Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res 87:139–148 [DOI] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. (1998) Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl) 135:416–422 [DOI] [PubMed] [Google Scholar]
- Mickiewicz AL, Dallimore JE, Napier TC. (2009) The ventral pallidum is critically involved in the development and expression of morphine-induced sensitization. Neuropsychopharmacology 34:874–886 [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. (1995) Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 359:221–238 [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. (2005) Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 25:6389–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Liu Y, Roberts DC. (2006) Rapid and persistent sensitization to the reinforcing effects of cocaine. Neuropsychopharmacology 31:121–128 [DOI] [PubMed] [Google Scholar]
- Morgan D, Roberts DC. (2004) Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev 27:803–812 [DOI] [PubMed] [Google Scholar]
- Moro H, Sato H, Ida I, Oshima A, Sakurai N, Shihara N, Horikawa Y, Mikuni M. (2007) Effects of SKF-38393, a dopamine D1 receptor agonist on expression of amphetamine-induced behavioral sensitization and expression of immediate early gene arc in prefrontal cortex of rats. Pharmacol Biochem Behav 87:56–64 [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Stewart J. (2000) Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res 115:39–47 [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. (2000) Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, O'Dell LE, Tran-Nguyen LT, Castañeda E, Fuchs RA. (1996) Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology 15:506–514 [DOI] [PubMed] [Google Scholar]
- Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME. (2009) Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J Neurochem 109:35–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CL, Wetter JB, Milovanovic M, Wolf ME. (2007) The laterodorsal tegmentum contributes to behavioral sensitization to amphetamine. Neuroscience 146:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Hertel P, Panagis G, Svensson TH. (1996) Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse 22:369–381 [DOI] [PubMed] [Google Scholar]
- O'Brien CP. (1997) A range of research-based pharmacotherapies for addiction. Science 278:66–70 [DOI] [PubMed] [Google Scholar]
- O'Connor WT. (2001) Functional neuroanatomy of the ventral striopallidal GABA pathway. New sites of intervention in the treatment of schizophrenia. J Neurosci Methods 109:31–39 [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. (1987) Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res 434:117–165 [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. (2005) Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol 483:217–235 [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. (2007) Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience 146:1259–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. (2002) Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci 22:2916–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Mcdonald RV. (2000) Reinstatement of both a conditioned place preference and a conditioned place aversion with drug primes. Pharmacol Biochem Behav 66:559–561 [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr (1993) Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J Neurochem 61:1611–1619 [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. (1991) Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 103:480–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Feldon J. (2008) Effects of withdrawal from an escalating dose of amphetamine on conditioned fear and dopamine response in the medial prefrontal cortex. Behav Brain Res 186:12–22 [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. (2006) The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology 186:143–149 [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28:6046–6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. (2000) Altered responsiveness of medial prefrontal cortex neurons to glutamate and dopamine after withdrawal from repeated amphetamine treatment. Synapse 36:342–344 [DOI] [PubMed] [Google Scholar]
- Phillips AG, Di Ciano P. (1996) Behavioral sensitization is induced by intravenous self-administration of cocaine by rats. Psychopharmacology (Berl) 124:279–281 [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. (1990) Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res 514:22–26 [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. (1996a) Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci 16:1550–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Born B, Adams M, Kalivas PW. (1996b) Repeated intra-ventral tegmental area administration of SKF-38393 induces behavioral and neurochemical sensitization to a subsequent cocaine challenge. J Pharmacol Exp Ther 278:384–392 [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. (1997a) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev 25:192–216 [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. (1997b) Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci 17:3254–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Reeder DC, Hicks J, Morgan ZR, Kalivas PW. (1998) Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience 82:1103–1114 [DOI] [PubMed] [Google Scholar]
- Ping A, Xi J, Prasad BM, Wang MH, Kruzich PJ. (2008) Contributions of nucleus accumbens core and shell GluR1 containing AMPA receptors in AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior. Brain Res 1215:173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Contel NR. (1983) Human and animal studies of cocaine: implications for develpment of behavioral pathology, in Stimulants: Neurochemical, Behavioral, and Clinical Perspectives (Creese I. ed) pp 169–203, Raven Press, New York [Google Scholar]
- Post RM, Weiss SR, Pert A. (1992) Sensitization and kindling effects of chronic cocaine administration, in Cocaine: Pharmacology, Physiology and Clinical Strategies (Lakoski JM, Galloway MP, White FJ. eds) pp 115–161, CRC Press, Ann Arbor, MI [Google Scholar]
- Ranaldi R, Pocock D, Zereik R, Wise RA. (1999) Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous D-amphetamine self-administration. J Neurosci 19:4102–4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Berger SP. (1996) Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport 7:1325–1329 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res 396:157–198 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. (2003) Addiction. Annu Rev Psychol 54:25–53 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. (2008) Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363:3137–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM. (1988) Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res 462:211–222 [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. (2008) The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience 151:579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. (2003) Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37:577–582 [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Slawecki C, Hodge C. (1999) The effects of microinjection of d-amphetamine into the n. accumbens during the late maintenance phase of an ethanol consumption bout. Pharmacol Biochem Behav 63:159–165 [DOI] [PubMed] [Google Scholar]
- Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA. (2003) Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience 119:497–505 [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. (2009) Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci 29:9239–9243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilström B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, et al. (2006) Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci 26:8549–8558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. (2006) Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci 23:219–228 [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Famous KR, Pierce RC. (2009) The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci 30:1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Matzner H, Michaeli A, Yaka R. (2009) NR2A/B-containing NMDA receptors mediate cocaine-induced synaptic plasticity in the VTA and cocaine psychomotor sensitization. Neurosci Lett 461:159–162 [DOI] [PubMed] [Google Scholar]
- Schumann J, Yaka R. (2009) Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci 29:6955–6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. (2009) Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol 12:431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. (2001) Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 154:301–310 [DOI] [PubMed] [Google Scholar]
- Sepehrizadeh Z, Bahrololoumi Shapourabadi M, Ahmadi S, Hashemi Bozchlou S, Zarrindast MR, Sahebgharani M. (2008) Decreased AMPA GluR2, but not GluR3, mRNA expression in rat amygdala and dorsal hippocampus following morphine-induced behavioural sensitization. Clin Exp Pharmacol Physiol 35:1321–1330 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. (2000a) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev 33:13–33 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. (2000b) Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci 12:292–302 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. (1995) Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 119:334–341 [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42 [DOI] [PubMed] [Google Scholar]
- Shinonaga Y, Takada M, Mizuno N. (1994) Topographic organization of collateral projections from the basolateral amygdaloid nucleus to both the prefrontal cortex and nucleus accumbens in the rat. Neuroscience 58:389–397 [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Heidbreder C. (1996) κ-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther 276:545–554 [PubMed] [Google Scholar]
- Shoaib M, Benwell ME, Akbar MT, Stolerman IP, Balfour DJ. (1994) Behavioural and neurochemical adaptations to nicotine in rats: influence of NMDA antagonists. Br J Pharmacol 111:1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Davidson DL, Kalivas PW, Prasad BM. (1997) Repeated daily cocaine alters subsequent cocaine-induced increase of extracellular dopamine in the medial prefrontal cortex. J Pharmacol Exp Ther 281:54–61 [PubMed] [Google Scholar]
- Sorg BA, Li N, Wu WR. (2001) Dopamine D1 receptor activation in the medial prefrontal cortex prevents the expression of cocaine sensitization. J Pharmacol Exp Ther 297:501–508 [PubMed] [Google Scholar]
- Steketee JD. (1998) Injection of SCH 23390 into the ventral tegmental area blocks the development of neurochemical but not behavioral sensitization to cocaine. Behav Pharmacol 9:69–76 [PubMed] [Google Scholar]
- Steketee JD, Walsh TJ. (2005) Repeated injections of sulpiride into the medial prefrontal cortex induces sensitization to cocaine in rats. Psychopharmacology (Berl) 179:753–760 [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BY. (1995) Effect of repeated methamphetamine administrations on dopamine and glutamate efflux in rat prefrontal cortex. Brain Res 700:99–106 [DOI] [PubMed] [Google Scholar]
- Stewart J. (1984) Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacol Biochem Behav 20:917–923 [DOI] [PubMed] [Google Scholar]
- Stewart J, Vezina P. (1989) Microinjections of Sch-23390 into the ventral tegmental area and substantia nigra pars reticulata attenuate the development of sensitization to the locomotor activating effects of systemic amphetamine. Brain Res 495:401–406 [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. (2005) Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology 30:2073–2081 [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. (2003) Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci 23:10258–10264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. (2005) The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 177:315–323 [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P. (2002) Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology 27:970–979 [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. (2004) Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology 29:2149–2159 [DOI] [PubMed] [Google Scholar]
- Takada M, Campbell KJ, Moriizumi T, Hattori T. (1990) On the origin of the dopaminergic innervation of the paraventricular thalamic nucleus. Neurosci Lett 115:33–36 [DOI] [PubMed] [Google Scholar]
- Tanabe LM, Suto N, Creekmore E, Steinmiller CL, Vezina P. (2004) Blockade of D2 dopamine receptors in the VTA induces a long-lasting enhancement of the locomotor activating effects of amphetamine. Behav Pharmacol 15:387–395 [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, et al. (2010) Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci 30:7105–7110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. (2001) Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci 4:1217–1223 [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. (2008) Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol 154:327–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Tang XC, Kalivas PW. (2008) The glutamatergic projection from the prefrontal cortex to the nucleus accumbens core is required for cocaine-induced decreases in ventral pallidal GABA. Neurosci Lett 438:142–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O'Dell LE, Neisewander JL. (1998) Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology 19:48–59 [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. (1998) Discrete quinolinic acid lesions of the rat prelimbic medial prefrontal cortex affect cocaine- and MK-801-, but not morphine- and amphetamine-induced reward and psychomotor activation as measured with the place preference conditioning paradigm. Behav Brain Res 97:115–127 [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. (2000) Differential effects of discrete subarea-specific lesions of the rat medial prefrontal cortex on amphetamine- and cocaine-induced behavioural sensitization. Cereb Cortex 10:488–498 [DOI] [PubMed] [Google Scholar]
- Ungless MA, Argilli E, Bonci A. (2010) Effects of stress and aversion on dopamine neurons: implications for addiction. Neurosci Biobehav Rev 35:151–156 [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411:583–587 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. (1995) GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res 682:215–221 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151:99–120 [DOI] [PubMed] [Google Scholar]
- Vezina P. (1996) D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci 16:2411–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev 27:827–839 [DOI] [PubMed] [Google Scholar]
- Vezina P, Giovino AA, Wise RA, Stewart J. (1989) Environment-specific cross-sensitization between the locomotor activating effects of morphine and amphetamine. Pharmacol Biochem Behav 32:581–584 [DOI] [PubMed] [Google Scholar]
- Vezina P, Kalivas PW, Stewart J. (1987) Sensitization occurs to the locomotor effects of morphine and the specific mu opioid receptor agonist, DAGO, administered repeatedly to the ventral tegmental area but not to the nucleus accumbens. Brain Res 417:51–58 [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. (2009) Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology 56 (Suppl 1):160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. (2002) Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci 22:4654–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. (1990) Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine: lack of conditioned effects. Brain Res 516:99–106 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. (2004) The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology 47 (Suppl 1):3–13 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. (2005) Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci 25:3932–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Luo F, Zhang WT, Han JS. (2000) Stress or drug priming induces reinstatement of extinguished conditioned place preference. Neuroreport 11:2781–2784 [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. (2005) Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci 25:5389–5396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Hsiao S. (2003) Amphetamine sensitization: nonassociative and associative components. Behav Neurosci 117:961–969 [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. (1984) Electrophysiological evidence for A10 dopamine autoreceptor subsensitivity following chronic d-amphetamine treatment. Brain Res 309:283–292 [DOI] [PubMed] [Google Scholar]
- White FJ, Hu HT, Henry DJ, Zhang XF. (1995a) Neurophysiological alterations in the mesocorticolimbic dopamine system with repeated cocaine administration, in The Neurobiology of Cocaine: Cellular and Molecular Mechanisms (Hammer RP., Jr ed) pp 99–119, CRC Press, New York [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. (1995b) Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther 273:445–454 [PubMed] [Google Scholar]
- Williams JM, Steketee JD. (2004) Cocaine increases medial prefrontal cortical glutamate overflow in cocaine-sensitized rats: a time course study. Eur J Neurosci 20:1639–1646 [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. (2005) Time-dependent effects of repeated cocaine administration on dopamine transmission in the medial prefrontal cortex. Neuropharmacology 48:51–61 [DOI] [PubMed] [Google Scholar]
- Wolf ME, Dahlin SL, Hu XT, Xue CJ, White K. (1995) Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: comparison with N-methyl-d-aspartate antagonists. Neuroscience 69:417–439 [DOI] [PubMed] [Google Scholar]
- Wolf ME, Xue CJ. (1998) Amphetamine and D1 dopamine receptor agonists produce biphasic effects on glutamate efflux in rat ventral tegmental area: modification by repeated amphetamine administration. J Neurochem 70:198–209 [DOI] [PubMed] [Google Scholar]
- Wolf ME, Xue CJ. (1999) Amphetamine-induced glutamate efflux in the rat ventral tegmental area is prevented by MK-801, SCH 23390, and ibotenic acid lesions of the prefrontal cortex. J Neurochem 73:1529–1538 [DOI] [PubMed] [Google Scholar]
- Wu WR, Li N, Sorg BA. (2003) Prolonged effects of repeated cocaine on medial prefrontal cortex dopamine response to cocaine and a stressful predatory odor challenge in rats. Brain Res 991:232–239 [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. (2002) Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther 303:608–615 [DOI] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. (2010) Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 208:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Steketee JD. (2009a) Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology (Berl) 203:501–510 [DOI] [PubMed] [Google Scholar]
- Xie X, Steketee JD. (2009b) Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology (Berl) 203:501–510 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Abe S, Hori T, Kurita H, Asada T, Okado N, Arai H. (2002) Repeated cocaine administration differentially affects NMDA receptor subunit (NR1, NR2A-C) mRNAs in rat brain. Synapse 46:157–169 [DOI] [PubMed] [Google Scholar]
- Young CD, Deutch AY. (1998) The effects of thalamic paraventricular nucleus lesions on cocaine-induced locomotor activity and sensitization. Pharmacol Biochem Behav 60:753–758 [DOI] [PubMed] [Google Scholar]
- Zapata A, Chefer VI, Ator R, Shippenberg TS, Rocha BA. (2003) Behavioural sensitization and enhanced dopamine response in the nucleus accumbens after intravenous cocaine self-administration in mice. Eur J Neurosci 17:590–596 [DOI] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. (2006) Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology 31:396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lee TH, Davidson C, Lazarus C, Wetsel WC, Ellinwood EH. (2007) Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology 32:377–387 [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. (1997) Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther 281:699–706 [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. (2008) N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry 63:338–340 [DOI] [PMC free article] [PubMed] [Google Scholar]