Abstract
Salvia divinorum is a perennial sage native to Oaxaca, Mexico, that has been used traditionally in divination rituals and as a treatment for the “semimagical” disease panzón de borrego. Because of the intense “out-of-body” experiences reported after inhalation of the pyrolized smoke, S. divinorum has been gaining popularity as a recreational hallucinogen, and the United States and several other countries have regulated its use. Early studies isolated the neoclerodane diterpene salvinorin A as the principal psychoactive constituent responsible for these hallucinogenic effects. Since the finding that salvinorin A exerts its potent psychotropic actions through the activation of KOP receptors, there has been much interest in elucidating the underlying mechanisms behind its effects. These effects are particularly remarkable, because 1) salvinorin A is the first reported non-nitrogenous opioid receptor agonist, and 2) its effects are not mediated by the 5-HT2A receptor, the classic target of hallucinogens such as lysergic acid diethylamide and mescaline. Rigorous investigation into the structural features of salvinorin A responsible for opioid receptor affinity and selectivity has produced numerous receptor probes, affinity labels, and tools for evaluating the biological processes responsible for its observed psychological effects. Salvinorin A has therapeutic potential as a treatment for pain, mood and personality disorders, substance abuse, and gastrointestinal disturbances, and suggests that nonalkaloids are potential scaffolds for drug development for aminergic G-protein coupled receptors.
I. Introduction
Psychoactive natural products have been used to study the intricate workings of various receptor systems in the central nervous system (CNS1). The isolation of morphine from Papaver somniferum by Friedrich Wilhelm Adam Sertürner in 1805 spawned global interest in elucidating its biological activity, ultimately leading to the discovery of opioid receptors and the development of a class of widely used analgesics (Sertürner, 1817). More significantly, Sertürner's work sparked global interest in the isolation of natural substances and is considered the beginning of pharmaceutical development. In the following years, more psychoactive agents were isolated from their natural sources, including the psychostimulants caffeine in 1819 (Runge, 1820) and cocaine from Erythroxylum coca in 1855 (Gaedcke, 1855).
Secondary metabolites themselves also have significant utility as lead compounds for biological probe development (Carlson, 2010). A recent survey reported that more than 40% of all new chemical entities submitted to the public domain for approval by the U.S. Food and Drug Administration are themselves natural products or derived from natural sources, highlighting the continued importance of this field despite the rise in combinatorial chemistry techniques (Newman and Cragg, 2007). Derivatization of the opioid receptor agonist morphine, for example, has produced oxycodone, the widely used analgesic, and buprenorphine, a promising alternative to methadone for the treatment of substance abuse (Casy and Parfitt, 1986; Schottenfeld et al., 2000). Likewise, isolation and identification of Δ9-tetrahydrocannabinol, the psychoactive constituent of Cannabis sativa, ultimately led to the identification of cannabinoid (CB) receptors, spawning many stimulating new areas of research into the therapeutic potential of CB receptor ligands (Gaoni and Mechoulam, 1964; Matsuda et al., 1990; Munro et al., 1993) and fatty acid amide hydrolase (Cravatt et al., 1996; Snider et al., 2010).
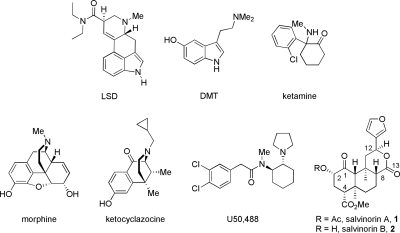
A much more recent example of phytochemical elucidation and pharmacological characterization involves the hallucinogenic sage Salvia divinorum. The early work of Ortega et al. (1982) and Valdés et al. (1984) to isolate and identify salvinorin A (1) (Fig. 1) as the bioactive constituent responsible for the psychotropic effects led to the revelation that activation of κ-opioid (KOP) receptors, and not serotonin-2A (5-HT2A) receptors, is responsible for this hallucinogenic activity (Roth et al., 2002). These results were remarkable, considering that salvinorin A shared little structural similarity to other known KOP receptor agonists, such as dynorphin A, yet produced hallucinatory effects similar to those produced by 5-HT2A receptor agonists lysergic acid diethylamide (LSD) and psilocin. This was also particularly intriguing because a basic amino substituent had long been considered a requirement for opioid receptor binding and efficacy (Rees and Hunter, 1990). In the 8 years since this seminal discovery, much work has gone into characterizing the structure-activity relationships (SAR) of salvinorin A, elucidating the binding mode of this unique structural scaffold, determining its pharmacokinetics and methods of inactivation, and evaluating the behavioral effects in vivo.
Fig. 1.
Chemical structures of salvinorins A (1) and B (2) and ligands with activity at 5-HT (LSD, DMT), NMDA (ketamine), MOP (morphine), and KOP (ketocyclazocine, U50,488) receptors.
This review is intended to be a comprehensive assessment of the insight we have gained regarding the medicinal chemistry, neuropharmacology, and therapeutic potential of S. divinorum. Several reviews have been published that describe some specific facets of the chemical derivatization (Prisinzano and Rothman, 2008) and psychopharmacology of salvinorin A (Prisinzano, 2005; Vortherms and Roth, 2006) as well as the abuse potential of S. divinorum (Babu et al., 2008; Griffin et al., 2008). In this review, we will describe in depth how S. divinorum advanced from being a hallucinogenic tool for divination rituals to being a source of therapeutic potential for the treatment of gastrointestinal disorders, pain, stimulant dependence, and mood disturbances.
II. Ethnopharmacology and Constituents of S. divinorum
S. divinorum Epling and Játiva-M. (Lamiaceae) is a perennial mint native to the Oaxaca region of Mexico and was discovered by Wasson and Hofman in 1962 (Hofmann, 1980). This herb is traditionally used in divination rituals of the Mazatec shamans to produce hallucinations (Valdés, 1994) and is also referred to as “ska Maria Pastora” because of their belief that the plant is the reincarnation of the Virgin Mary (Valdés et al., 1983). S. divinorum is traditionally used to treat a variety of conditions, including anemia, headache, rheumatism, diarrhea, and the “semimagical” disease panzón de borrego, the curse of a swollen belly believed to be caused by a sorcerer (Valdés et al., 1983).
By tradition, S. divinorum is ingested orally, either by chewing the leaves or pulverizing fresh leaves into a juice and drinking the resulting extract (Siebert, 1994). Smoke inhalation and chewing leaves are the most common routes of administration among recreational users (Giroud et al., 2000) and results in a rapid onset of hallucinatory effects. The hallucinatory action of salvinorin A can last for up to an hour after traditional oral administration, according to two early reports (Siebert, 1994; Valdés, 1994). Because of its intense “out-of-body” effects (González et al., 2006), the use of S. divinorum has been gaining popularity among teens and young adults (Lange et al., 2008, 2010). As of this writing, sale and possession of S. divinorum has been regulated in 20 countries, 13 of which have banned possession outright. Although not listed under the United States Controlled Substances Act, S. divinorum was identified as a “drug of concern” in 2003, and 23 states have enacted various degrees of control regarding its use and sale (Siebert, 2010). This has also recently been a topic of debate in the political and scientific community in the United Kingdom (Kalant, 2010).
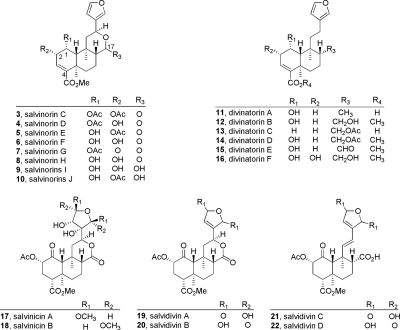
The principal active component of S. divinorum is the neoclerodane diterpene salvinorin A (Ortega et al., 1982; Valdés et al., 1984). Valdés et al. (1984) identified this bioactive constituent as divinorin A and described the deacetylated derivative divinorin B (Valdés et al., 1984), although Ortega et al. (1982) had already described these compounds as salvinorins A (1) and B (2). Other components of S. divinorum have been identified, namely salvinorins C to J (3–10) (Valdés et al., 2001; Munro and Rizzacasa, 2003; Lee et al., 2005b; Shirota et al., 2006; Ma and Lee, 2007; Kutrzeba et al., 2009a), divinatorins A to F (11–16) (Bigham et al., 2003; Lee et al., 2005b; Shirota et al., 2006), salvinicins A and B (17, 18) (Harding et al., 2005b), and salvidivins A to D (19–22) (Shirota et al., 2006) (Fig. 2), as well as other constituents of varying structural classes. Most recently, 4 and 5 have been described as a rapidly equilibrating mixture of acetyl regioisomers (Kutrzeba et al., 2010), and salvinorin J (10) has also been identified as a mixture of C-17 stereoisomers (Kutrzeba et al., 2009a). It has been shown biosynthetically that salvinorin A and its derivatives are probably produced through the 1-deoxy-d-xylulose pathway (Kutrzeba et al., 2007). Phytochemical investigations into the constituents of Salvia splendens Sellow ex Roem. and Schult. also identified several neoclerodanes that are structurally similar to those found in S. divinorum (Fontana et al., 2006), although these and most semisynthetic analogs (Li et al., 2007; Fontana et al., 2008, 2009) proved to be largely inactive at KOP receptors (see section IV.A).
Fig. 2.
Chemical structures of naturally occurring neoclerodane diterpenes isolated from S. divinorum (3–22).
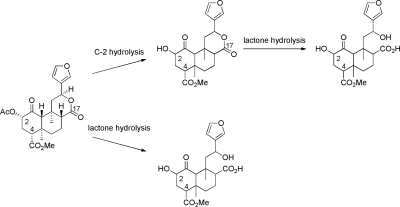
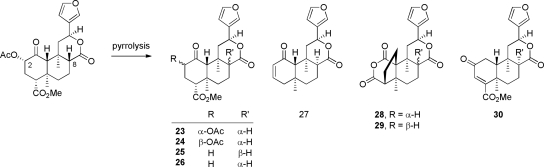
An area of recent interest has been determination of the composition of neoclerodane diterpenes present in the smoke of S. divinorum and the pyrolysis products of salvinorin A (23–30) (Ma et al., 2010a,b). As shown in Fig. 3, epimerization of the C-8 position is a prominent result of pyrolysis of salvinorin A and S. divinorum. As evidenced by products 27–29 (Fig. 4), the C-4 carbomethoxy substituent is labile under these conditions, resulting in the C-4-descarboxylated derivative 27 and the unique ring-constrained anhydride products 28 and 29. It is evident therefore that burning the leaves of S. divinorum has a potentially detrimental effect on the psychoactive potency of the material.
Fig. 3.
Hydrolysis products of salvinorin A. [Adapted from Tsujikawa K, Kuwayama K, Miyaguchi H, Kanamori T, Iwata YT, and Inoue H (2009) In vitro stability and metabolism of salvinorin A in rat plasma. Xenobiotica 39:391–398. Copyright © 2009 Informa Medical and Pharmaceutical Science. Used with permission.]
Fig. 4.
Pyrrolysis products of the smoke of salvinorin A (23–29) and S. divinorum (30).
Salvinorin A has been shown to exhibit short, extremely intense hallucinations with potency similar to that of LSD (Sheffler and Roth, 2003). A smoked dose of 200 to 500 μg of salvinorin A produces vivid hallucinations whose peak effects last 5 to 10 min, with lingering effects lasting approximately 1 h, according to early studies (Siebert, 1994; Valdés, 1994). Analysis of the X-ray crystal structure indicates that salvinorin A is structurally dissimilar to LSD as well as to other known hallucinogens such as N,N-dimethyltryptamine (DMT) and ketamine (Fig. 1) (Ortega et al., 1982; Valdés et al., 1984). Furthermore, it was found that salvinorin A did not exert its hallucinatory actions through 5-HT receptors (Siebert, 1994), including the molecular target presumed to be responsible for hallucinations, the 5-HT2A receptor (Titeler et al., 1988). Instead, a screen of 50 receptors and molecular targets performed by the National Institutes of Mental Health Psychoactive Drug Screening Program indicated that salvinorin A binds selectively to KOP receptors (Roth et al., 2002). This was in contrast to LSD, which was shown to bind with high affinity to many 5-HT receptors and monoamine transporters in this study. Again, salvinorin A is structurally dissimilar to other KOP receptor agonists ketocyclazocine and (−)-(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidiny)cyclohexyl]benzeneacetamide (U50,488; Fig. 1). Since these initial screens, other groups have reported allosteric interactions between salvinorin A and μ-opioid (MOP) receptors (Rothman et al., 2007) and partial agonism of dopamine D2High receptors (Seeman et al., 2009), although independent replication of these findings has not since been reported.
The presence of salvinorin A in botanical samples has been evaluated quantitatively by several methods. An early study described the use of high-performance liquid chromatography (HPLC) for determining the composition of salvinorin A in plant materials using UV detection (Gruber et al., 1999). This group demonstrated that the concentration of salvinorin A is highly variable between samples, showing composition ranging from 0.089 to 0.37%, with an average of 0.245%. A follow-up study in 2006 reported similar findings in samples of S. divinorum purchased through the Internet, with samples ranging from 0.126 to 1.137 mg/g (Wolowich et al., 2006). Gas chromatography-MS methodology has also been developed (Pichini et al., 2005), as well as combined polymerase chain reaction/HPLC-mass spectrometry methodology, which can be used to identify S. divinorum in phytochemical and forensic applications (Bertea et al., 2006). Solid-phase extraction techniques coupled with liquid chromatography (LC)/electrospray ionization-MS are also applicable toward forensic analysis of botanical samples (McDonough et al., 2008). A complementary procedure was reported in 2009, where gas chromatography-MS was combined with thin-layer chromatography to provide another approach toward distinguishing between constituents of S. divinorum and cannabinoids, such as Δ9-tetrahydrocannabinol, using vanillin staining (Jermain and Evans, 2009). This method requires significant sample preparation, however, and a more direct mode of analysis of S. divinorum leaves was more recently published (Kennedy and Wiseman, 2010). This method uses desorption electrospray ionization-MS to identify the presence of salvinorin A as well as the salvinorins B to E and divinatorin B.
III. Metabolism and Pharmacokinetics of Salvinorin A
Once salvinorin A was identified as the principal bioactive constituent of S. divinorum in studies in vitro, research focused on investigating its physiological effects in vivo. As noted above, salvinorin A is a potent hallucinogen and selective KOP agonist that exerts its effects with a rapid onset of action and abrupt loss of activity shortly after administration. In light of this unique profile, much effort has been exerted toward identifying the physiological processes involved in the distribution and degradation of salvinorin A.
A. Metabolism of Salvinorin A
The chemical structure of salvinorin A contains several features that may be targets of enzymatic modification (Fig. 4). Ester hydrolysis of the C-2 acetate results in the C-2 hydroxyl derivative salvinorin B. Initial studies suggested that salvinorin B is an inactive metabolite of salvinorin A (Valdés et al., 2001; Chavkin et al., 2004), which was supported in subsequent studies (Chavkin et al., 2004). It was also demonstrated ex vivo that salvinorin B is the major metabolite of salvinorin A in nonhuman primates (Schmidt et al., 2005b). The concentration of salvinorin B was below the limit of quantitation in rhesus monkey plasma, however, suggesting that this metabolite is either cleared rapidly or accumulates in organs or tissues (Schmidt et al., 2005a). That salvinorin A is rapidly metabolized is supported by the finding that only approximately 0.8% of an administrated dose of salvinorin A (0.5 mg) was extracted from urine in human volunteers (Pichini et al., 2005).
The first investigation dedicated to tracking the metabolism of salvinorin A in vitro was conducted by Tsujikawa et al. (2009). By monitoring rat plasma samples, the contribution of several esterases relevant to human subjects could be monitored, including acetylcholinesterase, butyrylcholinesterase, arylesterase, and carboxylesterase. Monitoring samples by LC-tandem MS identified several metabolites that were hypothesized to be products of C-2 deacetylation (salvinorin B) and Ca2+-dependent lactonase-mediated hydrolysis of the C-ring lactone (Fig. 3). Accordingly, degradation of salvinorin A was inhibited by esterase inhibitor NaF as well as the carboxylesterase-selective inhibitor bis-p-nitrophenyl phosphate. It is noteworthy that other inhibitors specific to acetylcholinesterase, butyrylcholinesterase, and arylesterase failed to inhibit salvinorin A degradation, highlighting an apparent lack of involvement of these enzymes in the metabolism of salvinorin A.
The metabolic processes involved in inactivation of salvinorin A were further clarified after a study by Teksin et al. (2009) designed to elucidate specific CYP450 isoforms that cause significant degradation in vitro. A screen of 10 isoforms identified four that produce degradation of salvinorin A at 50 μM: CYP2D6, CYP1A1, CYP2C18, and CYP2E1. Furthermore, these CYP450 isoforms produced a higher degree of degradation at 5 μM, indicating that such CYP450-mediated degradation follows Michaelis-Menten kinetics. This study was also the first to investigate the role of glucuronidation in salvinorin A metabolism. This study also found a decrease in salvinorin A of 7% (±5.60) at 50 μM (p < 0.05), 18.1% (±5.20) at 10 μM (p < 0.05), and 51% (±4.00) at 5 μM (p < 0.05) when incubated with UGT2B7, the major enzyme involved in glucuronidation of most drugs (King et al., 1996; Coffman et al., 1997, 1998). As was the case for cytochrome P450 isoforms, UGT-mediated metabolism of salvinorin A is saturable at high concentrations, showing Michaelis-Menten kinetics. Although the identities of the metabolites produced were not determined, several key enzymes were identified that may be responsible for the rapid loss of activity seen with salvinorin A in vivo. Furthermore, as many of these enzymes are also involved in the metabolism of other widely abused substances, this study highlights the potential for drug-drug interactions with S. divinorum.
A recent study sought to delineate more potential routes of metabolism in vitro using microbes as a model for mammalian metabolism (Kutrzeba et al., 2009b). Thirty fungal species were screened, and it was found that the principal metabolite of all species was salvinorin B. Although the efficiency of metabolism varied greatly among species—from 10 to 100% conversion over a period of 14 days—no products other than salvinorin B were found. It should be noted, however, that microbial systems primarily produce functionalized products similar to phase I metabolism: hydroxylation, oxidation, reduction, and epoxidation (Hanson, 1992). That no other metabolites were noted over the long duration of this study (14 days) suggests a large degree of metabolic stability inherent in the tricyclic trans-decalin core of salvinorin A.
Combined, these studies indicate that the labile C-2 acetate of salvinorin A is preferentially hydrolyzed in vitro by esterases and several cytochrome P450 isoforms to the pharmacologically inactive salvinorin B. Other enzymes, such as lactonases and UGT2B7, may be involved in producing increasingly hydrophilic byproducts that aid in clearance and elimination. Studies that aimed to identify metabolites of salvinorin A failed to find evidence of hydrolysis of the C-4 carbomethoxy moiety, suggesting that this group may be too sterically hindered for enzymatic modification. The stability of this group was also noted during attempts to modify salvinorin A using synthetic methods, because vigorous conditions are necessary to produce the C-18 acid (Tidgewell et al., 2004; Lee et al., 2005a; Munro et al., 2005a,b). Only one group (Kutrzeba et al., 2009b) reported screening specifically for epimerization of the C-8 position; however, no evidence of epimerization was found.
B. Pharmacokinetic Properties of Salvinorin A
Several investigations explored the pharmacokinetic profile of salvinorin A, including several LC-MS studies that identified samples of salvinorin A found in plasma samples (Chavkin et al., 2004; Schmidt et al., 2005b; Barnes et al., 2006). One study (Chavkin et al., 2004) reported the identification of [M+23] precursor-related ions using electrospray ionization–high-resolution MS, whereas a later report identified two major product ions (m/z 295 and 373, [M−60] and [M−138], respectively) produced by collision-induced dissociation (Barnes et al., 2006). Schmidt et al. (2005b) presented a method that demonstrated sensitivity 3 orders of magnitude greater than previously reported HPLC methods for the identification of salvinorin A in plants (Gruber et al., 1999).
The earliest report investigating the pharmacokinetic properties of salvinorin A monitored a single intravenous bolus dose of salvinorin A (0.032 mg/kg i.v.) in male and female rhesus monkeys (Schmidt et al., 2005a). Although the overall elimination t1/2 was 56.6 ± 24.8 min, distinct gender differences were observed: in male monkeys, there was a rapid t1/2 for distribution, elimination t1/2 was 37.9 ± 5.6 min, and the area under the dose-response curve was 572 ± 133 ng · min−1 · ml−1, whereas in female monkeys, t1/2 for distribution was slower (0.95 ± 0.2 min), the t1/2 for elimination was 80.0 ± 13.1 min, and the area under the dose-response curve was 1087 ± 46 ng · min−1 · ml−1. This is similar to other observations of the antinociceptive effects of opioids (Negus et al., 2002, 2004), as well as the effects of synthetic selective KOP agonists (Negus and Mello, 1999; Craft, 2003), in rats and rhesus monkeys. Although the principal metabolite of salvinorin A has been reported to be salvinorin B in several studies (see section III.A), plasma levels of salvinorin B in this study were below the limit of detection (50–1000 ng/ml for a 0.5-ml sample). It has been proposed that the more hydrophilic salvinorin B is rapidly cleared from plasma or accumulating in tissues, although this hypothesis remains to be rigorously investigated.
Hooker et al. (2008) were the first to describe in detail the effect of a 11C-labeled derivative of salvinorin A in the brains and peripheral organs of female baboons. After incorporating a radiolabeled 11C-acetate moiety into salvinorin B, the central distribution of [11C]salvinorin A was monitored using positron emission spectroscopy. Before in vivo studies, physiochemical analysis revealed a logD, pH 7.4, of 2.34 ± 0.09 with plasma protein binding of 16.1 ± 0.24%, suitable for blood-brain barrier penetration. After administration, it was found that the maximum central concentration was 0.0175% injected dose per cubic centimeter, with the highest concentrations in the cerebellum (0.016 ± 0.002 injected dose/cm3), notable for its role in integrating sensory perception with motor control. The hallucinogenic properties of salvinorin A may occur through the striatal (visual) cortex, where a significant level of salvinorin A was also detected. Maximum brain concentrations were reached within 40 s, nearly an order of magnitude faster than in an earlier study using [11C]cocaine (Volkow et al., 1997). Indirect actions on dopaminergic (Zhang et al., 2005), noradrenergic (Grilli et al., 2009), and endocannabinoid (Braida et al., 2007, 2008) systems have also been characterized. Combined, the potential for a rapid release of dopamine (DA) within the reward circuitry of the nucleus accumbens might suggest the potential for drug reinforcement behavior with salvinorin A. Brain clearance was also quite rapid, however, with 25% of maximum concentration reached in less than 30 min (t1/2 from peak, 8 min). This is consistent with the reported maximal activity in humans lasting less than 10 min (Siebert, 1994).
These results, in conjunction with metabolism studies that suggested that cleavage of the C-2 acetate function of salvinorin A produces an inactive metabolite (salvinorin B), prompted investigation into the pharmacokinetic profiles of hydrolytically stable C-2 analogs. In the rat hot-plate test, a 2-methoxymethyl-salvinorin B analog (94; see section IV.A) exhibited a dose-dependent increase in Emax (0.5–5 mg/kg i.p.) over 120 min, maximal effects occurring at 30 min. In the same test, salvinorin A (10 mg/kg i.p.) was unable to produce antinociception, probably because of metabolism of the C-2 acetate. Combined, these results suggest that the metabolic stability of 94 is responsible for an increased duration of action in vivo (Wang et al., 2008). A second study aimed to monitor brain and plasma exposure of a 11C-labeled analog, 2-ethoxymethyl-salvinorin B ([11C]95), with the use of positron emission spectroscopy analysis (Hooker et al., 2009). [11C]95 was determined to have a similar logD as salvinorin A (2.46 ± 0.01 versus 2.34 ± 0.09, respectively), with diminished degree of plasma protein binding (23.4 versus 16.1% unbound for salvinorin A). As expected, metabolism of [11C]95 was greatly diminished: 50% of [11C]95 remained unchanged at 30 min, whereas it took only 5 min to eliminate 50% of [11C]salvinorin A (Hooker et al., 2008). However, slowed metabolism did not produce a similar increase in brain exposure, suggesting that C-2 metabolism alone does not account for the rapid loss of hallucinogenic activity of salvinorin A. These results were initially unexpected in light of those seen for 94; ultimately, it was found that route of administration played a significant role in the observed pharmacokinetics of [11C]95, because intraperitoneal administration of both [11C]95 and [11C]salvinorin A showed a nearly 3-fold higher proportion of [11C]95 remaining in brain homogenates after 60 min.
Another study observed the pharmacokinetic parameters of salvinorin A in Sprague-Dawley rats using noncompartmental modeling after a single dose (10 mg/kg i.p.) (Teksin et al., 2009). Consistent with previous studies, salvinorin A had a rapid uptake in plasma (tmax = 15 min) and relatively fast elimination t1/2 (75.4 min). Elimination from brain was faster still, displaying a tmax of 10 min and elimination t1/2 of 36.1 min. There was a large volume of distribution (Vd = 47.1 l/kg); however, the brain-to-plasma ratio was very low, ranging from 0.092 to 0.074 over 60 min. This work also evaluated the activity of salvinorin A as a substrate for the xenobiotic efflux transporter P-glycoprotein (P-gp). It was found that, over a range of 5 to 10 μM, salvinorin A significantly (p < 0.01) increased P-gp-mediated ATPase activity, a phenomenon common among opioid ligands (Dagenais et al., 2004; Mercer et al., 2007, 2008; Cunningham et al., 2008). It was also determined that salvinorin A is not a P-gp inhibitor, suggesting that salvinorin A may be rapidly removed from the CNS by active transport mechanisms. However, it is well established that highly lipophilic molecules are able to easily diffuse through the blood-brain barrier by passive mechanisms (Polli et al., 2001). Because of the lipophilicity and rapid onset of action of salvinorin A, it is therefore anticipated that the net effect of P-gp on brain exposure may be diminished (Teksin et al., 2009).
IV. Effects of Salvinorin A In Vitro
A. Structure-Activity Relationships of Analogs
There has been considerable interest toward determining the structural features of salvinorin A required for activity at KOP receptors. One particularly interesting facet of the salvinorin A skeleton is the absence of a basic amino substituent that would carry a positive charge at physiological pH. Before the finding that salvinorin A binds with high affinity and selectivity at KOP receptors (Roth et al., 2002), the presence of a cationic nitrogen substituent was considered a stringent requirement for interaction with opioid receptors (Rees and Hunter, 1990).
The naturally occurring constituents of S. divinorum have been examined for opioid activity (1–14) (Fig. 2). Salvinorin A (1) exhibits high binding affinity for KOP receptors and is devoid of activity at δ-opioid (DOP) receptors. A study in 2007 found that 1 is an allosteric modulator of MOP receptors (Rothman et al., 2007), although these results have yet to be independently replicated. Removal of the C-2 acetate results in a product (salvinorin B; 2) that is devoid of affinity at MOP, DOP, and KOP receptors (Chavkin et al., 2004). Other secondary metabolites are generally devoid of opioid activity (Ki > 10,000 nM) (Table 1), although there are several notable exceptions. The C-1 acetylated derivative salvinorin C (3) was found to possess 250-fold diminished affinity for KOP receptors compared with salvinorin A (Ki = 1022 nM versus Ki = 4 nM). Divinatorins D (14) and E (15), analogs that do not contain a C-ring lactone, also were found to exhibit reduced affinity compared with salvinorin A (Ki = 230 and 418 nM, respectively, versus 1 nM), indicating that an intact C ring is not a stringent requirement for KOP receptor binding. Salvinorin G (7) was also shown to have modest binding affinity (Lee et al., 2005b), which is abolished on hydrolysis of the C-1 acetate moiety (31) (Ma and Lee, 2008). More recently, C-12 furan-modified salvinicin A (17; Ki = 390 nM) and salvidivin A (19; Ki = 440 nM) were identified as KOP receptor ligands, 19 being identified as the first naturally occurring neoclerodane with KOP antagonist activity (Simpson et al., 2007).
TABLE 1.
Binding affinity, potency, and efficacy data for analogs of 1 with appreciable opioid receptor activity
| Cmpd |
Ki ± S.D. |
KOP Radiolabel | EC50 ± S.D. (Emax ± S.D.)a |
References | ||||
|---|---|---|---|---|---|---|---|---|
| MOP | DOP | KOP | MOP | DOP | KOP | |||
| nM | nM | |||||||
| 1 | >10,000 | >10,000 | 18.74 ± 3.38 | [3H]Bremazocine | >10,000 | >10,000 | 7 (104 ± 7) | Chavkin et al., 2004 |
| 1.9 ± 0.2 | [125I]IOXY | 45 ± 10 (108 ± 4)b | Simpson et al., 2007 | |||||
| 12-epi-1 | N.D. | N.D. | 41 ± 5 | [3H]Diprenorphine | N.D. | N.D. | 84 ± 8 (67 ± 5)b | Béguin et al., 2009 |
| 2 | >10,000 | >10,000 | >10,000 | [3H]Bremazocine | >10,000 | >10,000 | >10,000 | Chavkin et al., 2004 |
| 3 | >1000 | >1000 | 1022 ± 262 | [3H]U69,593 | >1000 | >1000 | N.D. | Munro et al., 2005b |
| 7 | >10,000 | >10,000 | 418 ± 117 | [3H]Diprenorphine | N.D. | N.D. | N.D.b | Lee et al., 2005b |
| 14 | >10,000 | 625 ± 42 | 230 ± 21 | [3H]Diprenorphine | N.D. | N.D. | 359 ± 17 (103)b | Lee et al., 2005b |
| 15 | >10,000 | >10,000 | >10,000 | [3H]Diprenorphine | N.D. | N.D. | N.D.b | Lee et al., 2005b |
| 17 | >10,000 | >10,000 | 390 ± 30 | [125I]IOXY | N.D. | N.D. | N.D. | Simpson et al., 2007 |
| 18 | >10,000 | >10,000 | 7020 ± 750 | [125I]IOXY | N.D. | N.D. | N.D. | Simpson et al., 2007 |
| 19 | N.D. | N.D. | N.D. | [125I]IOXY | 760 ± 320 (Ke)c | 2830 ± 320 (Ke) | 440 ± 140 (Ke) | Simpson et al., 2007 |
| 32 | >1000 | >1000 | 18 ± 2 | [3H]U69,593 | >1000 | >1000 | 315 ± 35 (108 ± 11) | Munro et al., 2005b |
| 33 | >10,000 | >10,000 | 32.63 ± 15.7 | [3H]Bremazocine | N.D. | N.D. | 4.7 (100) | Chavkin et al., 2004 |
| 34 | 520 ± 50 | 4030 ± 250 | 4 ± 1 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 35 | 310 ± 50 | 3970 ± 270 | 15 ± 2 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 36 | 520 ± 80 | 4240 ± 290 | 70 ± 4 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 37 | >10,000 | >10,000 | 3199 ± 961.2 | [3H]Bremazocine | N.D. | N.D. | N.D. | Chavkin et al., 2004 |
| 38 | 2980 ± 110 | >10,000 | 19 ± 2 | [125I]IOXY | N.D. | N.D. | 360 ± 50 (105 ± 3)b | Harding et al., 2005 |
| 41 | 260 ± 6 | 8880 ± 390 | 42 ± 1 | [125I]IOXY | N.D. | N.D. | N.D.b | Harding et al., 2005 |
| 42 | >10,000 | >10,000 | 430 ± 10 | [125I]IOXY | N.D. | N.D. | N.D.b | Harding et al., 2005 |
| 46 | 5660 ± 250 | >10,000 | 90 ± 10 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 47 | 12 ± 1 | 1170 ± 60 | 90 ± 2 | [125I]IOXY | 500 ± 140 (130 ± 4) | N.D. | 1320 ± 150 (140 ± 2)b | Harding et al., 2005 |
| 48 | 1030 ± 80 | >10,000 | 2010 ± 110 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2008 |
| 49 | 110 ± 10 | >10,000 | 90 ± 7 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 50 | 110 ± 10 | >10,000 | 70 ± 7 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 51 | >10,000 | >10,000 | >10,000 | [3H]Bremazocine | >10,000 | >10,000 | >10,000 | Chavkin et al., 2004 |
| 10 ± 1 | 1410 ± 80 | 740 ± 40 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 | |
| 52 | 1640 ± 90 | >10,000 | 230 ± 20 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2008 |
| 53 | 30 ± 2 | 1140 ± 60 | 550 ± 30 | [125I]IOXY | 1670 ± 250 (72 ± 3) | N.D. | 3590 ± 550 (97 ± 2)b | Tidgewell et al., 2008 |
| 54 | 70 ± 4 | 1860 ± 140 | 540 ± 40 | [125I]IOXY | 830 ± 100 (94 ± 3) | N.D. | 2610 ± 470 (106 ± 5)b | Tidgewell et al., 2008 |
| 56 | 7550 ± 970 | >10,000 | 900 ± 50 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2008 |
| 57 | >10,000 | >10,000 | 800 ± 50 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2008 |
| 58 | 260 ± 210 | >10,000 | 570 ± 40 | [125I]IOXY | 1370 ± 230 (46 ± 1) | N.D. | N.D.b | Tidgewell et al., 2008 |
| 59 | 73 ± 2 | 4820 ± 300 | 1930 ± 50 | [125I]IOXY | 2100 ± 300 (110 ± 5) | N.D. | 5740 ± 890 (76 ± 4)b | Harding et al., 2005 |
| 60 | 10 ± 2 | 1380 ± 130 | 260 ± 20 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 61 | 10 ± 1 | 690 ± 30 | 80 ± 3 | [125I]IOXY | 690 ± 60 (108 ± 3) | N.D. | 480 ± 210 (95 ± 8)b | Tidgewell et al., 2008 |
| 62 | >10,000 | >10,000 | >10,000 | [3H]Bremazocine | >10,000 | >10,000 | >10,000 | Chavkin et al., 2004 |
| >10,000 | >10,000 | 410 ± 40 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2008 | |
| 63 | 180 ± 20 | >10,000 | 5490 ± 640 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2008 |
| 64 | 10 ± 1 | 580 ± 30 | 70 ± 2 | [125I]IOXY | 1680 ± 250 (104 ± 5) | N.D. | 1120 ± 170 (109 ± 5)b | Tidgewell et al., 2008 |
| 65 | 1090 ± 250 | >10,000 | 290 ± 40 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 66 | 280 ± 40 | 9330 ± 1010 | 180 ± 10 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 67 | N.D. | N.D. | 149 ± 1 | [3H]Diprenorphine | N.D. | N.D. | 188 ± 2 (106)b | Béguin et al., 2006 |
| 4180 ± 310 | >10,000 | 30 ± 2 | [125I]IOXY | N.D. | N.D. | 120 ± 20 (108 ± 3)b | Tidgewell et al., 2008 | |
| 68 | N.D. | N.D. | 374 ± 19 | [3H]Diprenorphine | N.D. | N.D. | 444 ± 35 (109)b | Béguin et al., 2006 |
| 69 | N.D. | N.D. | 3.2 ± 0.1 | [3H]Diprenorphine | N.D. | N.D. | 2.4 ± 0.7 (103)b | Béguin et al., 2006 |
| 135 ± 4 | 1690 ± 285 | 0.37 ± 0.30 | [3H]U69,593 | Béguin et al., 2008 | ||||
| 70 | N.D. | N.D. | 1.6 ± 0.1 | [3H]Diprenorphine | N.D. | N.D. | 0.75 ± 0.08 (100)b | Béguin et al., 2006 |
| 15 ± 3 | 366 ± 38 | 0.11 ± 0.10 | [3H]U69,593 | Béguin et al., 2008 | ||||
| 71 | N.D. | N.D. | 27.6 ± 1.8 | [3H]Diprenorphine | N.D. | N.D. | 25.2 ± 0.2 (104)b | Béguin et al., 2006 |
| 72 | N.D. | N.D. | 38.1 ± 1.9 | [3H]Diprenorphine | N.D. | N.D. | 37.2 ± 0.2 (100)b | Béguin et al., 2006 |
| 73 | 3.1 ± 0.4 | 810 ± 30 | 7430 ± 880 | [125I]IOXY | 360 ± 60 (134 ± 5) | N.D. | N.D.b | Tidgewell et al., 2008 |
| 74 | N.D. | N.D. | 3.2 ± 0.2 | [3H]Diprenorphine | N.D. | N.D. | 6.2 ± 1.4 (99)b | Béguin et al., 2005 |
| nM | nM | |||||||
| 75 | N.D. | N.D. | 83.0 ± 8.5 | [3H]Diprenorphine | N.D. | N.D. | 201 ± 10 (81)b | Béguin et al., 2005 |
| 76 | N.D. | N.D. | 462 ± 20 | [3H]Diprenorphine | N.D. | N.D. | >1000b | Béguin et al., 2005 |
| 77 | 640 ± 30 | 6460 ± 390 | 120 ± 4 | [125I]IOXY | N.D. | N.D. | N.D.b | Harding et al., 2005 |
| 78 | 16 ± 1 | 230 ± 10 | 93 ± 3 | [125I]IOXY | 590 ± 50 (92 ± 2) | 2530 ± 380 (82 ± 3) | 480 ± 60 (100 ± 3)b | Harding et al., 2005 |
| 81 | 6820 ± 660 | >10,000 | 2.3 ± 0.1 | [125I]IOXY | N.D. | N.D. | 30 ± 5 (112 ± 4)b | Harding et al., 2005 |
| 82 | >10,000 | >10,000 | 60 ± 6 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 83 | 220 ± 20 | 3720 ± 400 | 50 ± 5 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2006 |
| 84 | N.D. | N.D. | 18.4 ± 7.9 | [3H]U69,593 | N.D. | N.D. | 4.77 ± 2.72 (107 ± 4) | Bikbulatov et al., 2007 |
| 4370 ± 310 | 3990 ± 290 | 5.7 ± 0.4 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2008 | |
| 85 | 290 ± 70 | 1930 ± 70 | 1410 ± 80 | [125I]IOXY | N.D. | N.D. | N.D.b | Tidgewell et al., 2008 |
| 86 | N.D. | N.D. | 54.5 ± 25.7 | [3H]U69,593 | N.D. | N.D. | 287 ± 85 (89 ± 14) | Bikbulatov et al., 2007 |
| 87 | N.D. | N.D. | 220 ± 12 | [3H]Diprenorphine | N.D. | N.D. | 389 ± 76 (98)b | Béguin et al., 2005 |
| 88 | N.D. | N.D. | 7.9 ± 0.3 | [3H]Diprenorphine | N.D. | N.D. | 18.6 ± 2.6 (103)b | Béguin et al., 2005 |
| 89 | N.D. | N.D. | 28.7 ± 3.0 | [3H]Diprenorphine | N.D. | N.D. | 67.4 ± 9.9 (100)b | Béguin et al., 2005 |
| 90 | N.D. | N.D. | 35.8 ± 5.1 | [3H]Diprenorphine | N.D. | N.D. | 104 ± 17 (105)b | Béguin et al., 2005 |
| 91 | N.D. | N.D. | 60.1 ± 9.1 | [3H]Diprenorphine | N.D. | N.D. | 145 ± 33 (106)b | Béguin et al., 2005 |
| 92 | N.D. | N.D. | 75.7 ± 5.9 | [3H]Diprenorphine | N.D. | N.D. | 161 ± 14 (102)b | Béguin et al., 2005 |
| 93 | >10,000 | >10,000 | 1610 ± 120 | [125I]IOXY | N.D. | N.D. | N.D.b | Harding et al., 2005 |
| 94 | N.D. | N.D. | 0.4 ± 0.02 | [3H]Diprenorphine | N.D. | N.D. | 0.6 ± 0.2 (98)b | Lee et al., 2005a |
| 95 | >1 μM | >1 μM | 0.32 ± 0.02 | [3H]Diprenorphine | N.D. | N.D. | 0.14 ± 0.01 (81–106)d | Munro et al., 2008 |
| 96 | >1 μM | >1 μM | 2.2 ± 0.6 | [3H]Diprenorphine | N.D. | N.D. | 5.2 ± 0.4 (81–106)d | Munro et al., 2008 |
| 97 | >1 μM | >1 μM | 5.3 ± 1.7 | [3H]Diprenorphine | N.D. | N.D. | 20 ± 3.5 (81–106)d | Munro et al., 2008 |
| 98 | >1 μM | >1 μM | 1.6 ± 0.5 | [3H]Diprenorphine | N.D. | N.D. | 4.2 ± 0.7 (81–106)d | Munro et al., 2008 |
| 99 | >1 μM | >1 μM | 35 ± 15 | [3H]Diprenorphine | N.D. | N.D. | 108 ± 18 (81–106)d | Munro et al., 2008 |
| 100 | >1 μM | >1 μM | 1.9 ± 0.5 | [3H]Diprenorphine | N.D. | N.D. | 3.8 ± 0.3 (81–106)d | Munro et al., 2008 |
| 101 | >1 μM | >1 μM | 31 ± 8 | [3H]Diprenorphine | N.D. | N.D. | 75 ± 7 (81–106)d | Munro et al., 2008 |
| 102 | >1 μM | >1 μM | 141 ± 29 | [3H]Diprenorphine | N.D. | N.D. | 320 ± 13 (81–106)d | Munro et al., 2008 |
| 103 | >1 μM | >1 μM | >1000 | [3H]Diprenorphine | N.D. | N.D. | 1660 ± 60 (81–106)d | Munro et al., 2008 |
| 104 | >1 μM | >1 μM | 147 ± 26 | [3H]Diprenorphine | N.D. | N.D. | 274 ± 16 (81–106)d | Munro et al., 2008 |
| 105 | >1 μM | >1 μM | 13 ± 3 | [3H]Diprenorphine | N.D. | N.D. | 31 ± 8 (81–106)d | Munro et al., 2008 |
| 106 | >1 μM | >1 μM | 50 ± 9 | [3H]Diprenorphine | N.D. | N.D. | 26 ± 6 (81–106)d | Munro et al., 2008 |
| 107 | >1 μM | >1 μM | 11 ± 1 | [3H]Diprenorphine | N.D. | N.D. | 10 ± 1 (81–106)d | Munro et al., 2008 |
| 108 | >1 μM | >1 μM | 6.6 ± 0.3 | [3H]Diprenorphine | N.D. | N.D. | 5.7 ± 0.7 (81–106)d | Munro et al., 2008 |
| 109 | >1 μM | >1 μM | 72 ± 13 | [3H]Diprenorphine | N.D. | N.D. | 72 ± 5 (81–106)d | Munro et al., 2008 |
| 110 | >1 μM | >1 μM | 4.0 ± 0.4 | [3H]Diprenorphine | N.D. | N.D. | 2.8 ± 0.3 (81–106)d | Munro et al., 2008 |
| 111 | N.D. | N.D. | N.D. | [125I]IOXY | 3650 ± 1970 (Ke) | 964 ± 2200 (Ke) | 385 ± 74 (104 ± 6)b | Harding et al., 2006b |
| 424 ± 16 | [3H]Diprenorphine | N.D. | N.D. | 306 ± 23 (102)b | Béguin et al., 2006 | |||
| 112 | N.D. | N.D. | 614 ± 122 | [3H]Diprenorphine | N.D. | N.D. | N.D. | Béguin et al., 2006 |
| 113 | N.D. | N.D. | 665 ± 100 | [3H]Diprenorphine | N.D. | N.D. | N.D. | Béguin et al., 2006 |
| 117 | N.D. | N.D. | 151 ± 53 | [3H]U69,593 | N.D. | N.D. | 123 ± 30 (106 ± 1) | Bikbulatov et al., 2007 |
| 118 | N.D. | N.D. | 546 ± 140 | [3H]U69,593 | N.D. | N.D. | >2000 (71 ± 12) | Bikbulatov et al., 2007 |
| 119 | N.D. | N.D. | 332 ± 41 | [3H]Diprenorphine | N.D. | N.D. | 339 ± 33 (103)b | Béguin et al., 2006 |
| 120 | N.D. | N.D. | 117 ± 63 | [3H]Diprenorphine | N.D. | N.D. | 718 ± 31 (102)b | Béguin et al., 2006 |
| 121 | N.D. | N.D. | 16.5 ± 1.1 | [3H]Diprenorphine | N.D. | N.D. | 21.0 ± 0.9 (106)b | Béguin et al., 2006 |
| 122 | N.D. | N.D. | 6.9 ± 1.1 | [3H]Diprenorphine | N.D. | N.D. | 12.6 ± 0.9 (103)b | Béguin et al., 2006 |
| 123 | N.D. | N.D. | 240 ± 17 | [3H]Diprenorphine | N.D. | N.D. | 614 ± 92 (95)b | Béguin et al., 2006 |
| 124 | N.D. | N.D. | 376 ± 36 | [3H]Diprenorphine | N.D. | N.D. | 857 ± 136 (96)b | Béguin et al., 2006 |
| 125 | N.D. | N.D. | 328 ± 40 | [3H]Diprenorphine | N.D. | N.D. | 825 ± 93 (82)b | Béguin et al., 2006 |
| 126 | N.D. | N.D. | 65.2 ± 24.6 | [3H]Diprenorphine | N.D. | N.D. | 72.8 ± 4.0 (104)b | Béguin et al., 2006 |
| 127 | N.D. | N.D. | 17.6 ± 3.1 | [3H]Diprenorphine | N.D. | N.D. | 18.9 ± 0.6 (99)b | Béguin et al., 2006 |
| 111 ± 49 | >10 μM | 4.5 ± 2.0 | [3H]U69,593 | Béguin et al., 2008 | ||||
| nM | nM | |||||||
| 128 | N.D. | N.D. | 168 ± 10 | [3H]Diprenorphine | N.D. | N.D. | 240 ± 23 (110)b | Béguin et al., 2006 |
| 129 | N.D. | N.D. | 223 ± 123 | [3H]Diprenorphine | N.D. | N.D. | 1373 ± 155 (84)b | Béguin et al., 2006 |
| 131 | N.D. | N.D. | 28.9 ± 1.0 | [3H]Diprenorphine | N.D. | N.D. | 68.9 ± 5.3 (111)b | Béguin et al., 2006 |
| 132 | N.D. | N.D. | 2.3 ± 0.6 | [3H]Diprenorphine | N.D. | N.D. | 7.2 ± 0.3 (107)b | Béguin et al., 2006 |
| 133 | N.D. | N.D. | 90.9 ± 2.5 | [3H]Diprenorphine | N.D. | N.D. | 343 ± 12 (105)b | Béguin et al., 2006 |
| 136 | >3 μM | >3 μM | 422 ± 44 | [3H]Diprenorphine | N.D. | N.D. | 1966 ± 94 (87) | Lee et al., 2010 |
| 137 | >3 μM | >3 μM | 198 ± 43 | [3H]Diprenorphine | N.D. | N.D. | 648 ± 152 (92) | Lee et al., 2010 |
| 138 | >3 μM | >3 μM | 197 ± 14 | [3H]Diprenorphine | N.D. | N.D. | 539 ± 50 (77) | Lee et al., 2010 |
| 139 | >3 μM | >3 μM | 167 ± 6.5 | [3H]Diprenorphine | N.D. | N.D. | 673 ± 111 (112) | Lee et al., 2010 |
| 140 | >3 μM | >3 μM | 42.3 ± 3.3 | [3H]Diprenorphine | N.D. | N.D. | 282 ± 8.5 (90) | Lee et al., 2010 |
| 141 | >3 μM | >3 μM | 245 ± 14 | [3H]Diprenorphine | N.D. | N.D. | 239 ± 23 (46) | Lee et al., 2010 |
| 142 | >10 μM | >10 μM | 2.9 ± 0.4 μM | [3H]U69,593 | N.D. | N.D. | N.D.b | Munro et al., 2005a |
| 143 | >1000 | >1000 | 1125 ± 365 | [3H]U69,593 | >1000 | >1000 | N.D.b | Munro et al., 2005b |
| 144 | >1000 | >1000 | 18 ± 2 | [3H]U69,593 | >1000 | >1000 | 141 ± 43 (122 ± 27) | Munro et al., 2005b |
| 170 ± 20 (Ke) | 100 ± 3 (Ke) | 280 ± 60 (49 ± 5) | Holden et al., 2007 | |||||
| 145 | N.D. | N.D. | N.D. | 4700 ± 910 (Ke) | 8780 ± 2130 (Ke) | 580 ± 30 (Ke) | Holden et al., 2007 | |
| 146 | N.D. | N.D. | N.D. | 1830 ± 930 (Ke) | 2900 ± 150 (Ke) | 1050 ± 600 (Ke) | Holden et al., 2007 | |
| 147 | N.D. | N.D. | N.D. | N.D. | N.D. | 2700 ± 400 (95 ± 12) | Holden et al., 2007 | |
| 148 | N.D. | N.D. | N.D. | 200 ± 30 (Ke) | 400 ± 90 (Ke) | 570 ± 140 (Ke) | Holden et al., 2007 | |
| 150 | N.D. | N.D. | N.D. | 700 ± 220 (Ke) | 3500 ± 1700 (Ke) | 460 ± 70 (Ke) | Holden et al., 2007 | |
| 152 | N.D. | N.D. | 1000 ± 269 | [3H]Diprenorphine | N.D. | N.D. | N.D. (68% @ 10 μM)b,e | Béguin et al., 2006 |
| 8-epi-156 | N.D. | N.D. | 48.6 ± 4.4 | [3H]Diprenorphine | N.D. | N.D. | 74.1 ± 2.2 (94)b | Lee et al., 2005a |
| 157 | N.D. | N.D. | 28.5 ± 0.9 | [3H]Diprenorphine | N.D. | N.D. | 94.4 ± 4.1 (110)b | Lee et al., 2005a |
| 159 | N.D. | N.D. | 201 ± 26 | [3H]Diprenorphine | N.D. | N.D. | 223.5 ± 3.7 (104)b | Lee et al., 2005a |
| 160 | N.D. | N.D. | 99.6 ± 15.9 | [3H]Diprenorphine | N.D. | N.D. | 58.2 ± 5.7 (105)b | Lee et al., 2005a |
| 8-epi-160 | N.D. | N.D. | 110 ± 15 | [3H]Diprenorphine | N.D. | N.D. | 191 ± 5 (102)b | Lee et al., 2005a |
| 161 | N.D. | N.D. | 613 ± 54.1 | [3H]Diprenorphine | N.D. | N.D. | 210 ± 47 (98)b | Lee et al., 2006 |
| 162 | N.D. | N.D. | 1392 ± 218 | [3H]Diprenorphine | N.D. | N.D. | N.D. (71% @ 10 μM)b | Béguin et al., 2006 |
| 165 | N.D. | N.D. | 26.9 ± 1.8 | [3H]Diprenorphine | N.D. | N.D. | 46.7 ± 7.3 (95)b | Lee et al., 2005a |
| 166 | N.D. | N.D. | 470 ± 92 | [3H]Diprenorphine | N.D. | N.D. | 227 ± 15 (105)b | Lee et al., 2005a |
| 167 | N.D. | N.D. | 210 ± 32 | [3H]Diprenorphine | N.D. | N.D. | 348 ± 26 (100)b | Lee et al., 2005a |
| 168 | >1000 | >1000 | 59 ± 11 | [3H]U69,593 | >1000 | >1000 | 78 ± 21 (107 ± 5) | Munro et al., 2005b |
| 171 | >1000 | >1000 | 6 ± 1 | [3H]U69,593 | >1000 | >1000 | 223 ± 60 (103 ± 13) | Munro et al., 2005b |
| 172 | >1000 | >1000 | 6 ± 2 | [3H]U69,593 | >1000 | >1000 | 624 ± 200 (116 ± 10) | Munro et al., 2005b |
| 175 | 1926 ± 147 | N.D. | 219 ± 59 | [3H]U69,593 | N.D. | N.D. | N.D. | Bikbulatov et al., 2008 |
| 176 | 7487 ± 2141 | N.D. | 6003 ± 1242 | [3H]U69,593 | N.D. | N.D. | N.D. | Bikbulatov et al., 2008 |
| 177 | >3 μM | >3 μM | >1000 | [3H]Diprenorphine | N.D. | N.D. | N.D. | Lee et al., 2010 |
| 178 | >10,000 | N.D. | 1991 ± 708 | [3H]U69,593 | N.D. | N.D. | N.D. | Bikbulatov et al., 2008 |
| 179 | 7240 ± 480 | >10,000 | 14 ± 1 | [125I]IOXY | N.D. | N.D. | N.D. | Simpson et al., 2007 |
| R-179 | 9790 ± 1090 | >10,000 | 3.7 ± 0.2 | [125I]IOXY | N.D. | N.D. | 750 ± 60 (81 ± 7) | Simpson et al., 2007 |
| 180 | >10,000 | >10,000 | 25 ± 1 | [125I]IOXY | 1370 ± 400 (Ke) | N.D. | 2350 ± 870 (95 ± 5) | Simpson et al., 2007 |
| 181 | >10,000 | >10,000 | 125 ± 1 | [125I]IOXY | N.D. | N.D. | N.D. | Simpson et al., 2007 |
| 182 | 3190 ± 230 | >10,000 | 420 ± 20 | [125I]IOXY | 2320 ± 1650 (Ke) | 3280 ± 1640 (Ke) | 9700 ± 1000 (Ke) | Simpson et al., 2007 |
| 183 | >10,000 | >10,000 | 125 ± 4 | [125I]IOXY | 2930 ± 1000 (Ke) | 1280 ± 270 (Ke) | 2370 ± 1240 (Ke) | Simpson et al., 2007 |
| 184 | 1450 ± 60 | 7620 ± 180 | 3.0 ± 0.2 | [125I]IOXY | N.D. | N.D. | 50 ± 10 (104 ± 4) | Simpson et al., 2007 |
| 185 | N.D. | N.D. | 7.1 ± 0.1 | [3H]Diprenorphine | N.D. | N.D. | 4.6 ± 0.1 (120 ± 6)b | Béguin et al., 2009 |
| 186 | >10,000 | >10,000 | 300 ± 20 | [125I]IOXY | N.D. | N.D. | N.D. | Simpson et al., 2007 |
| 187 | >10,000 | >10,000 | 8530 ± 550 | [125I]IOXY | N.D. | N.D. | N.D.b | Harding et al., 2006a |
| 188 | >10,000 | >10,000 | 840 ± 90 | [125I]IOXY | N.D. | N.D. | 3600 ± 580 (70 ± 3)b | Harding et al., 2006a |
| 189 | >10,000 | >10,000 | 410 ± 30 | [125I]IOXY | N.D. | N.D. | 9160 ± 1900 (60 ± 3)b | Harding et al., 2006a |
| 190 | >10,000 | >10,000 | 1620 ± 110 | [125I]IOXY | N.D. | N.D. | 15190 ± 3590 (60 ± 5)b | Harding et al., 2006a |
| 191 | >10,000 | >10,000 | 56 ± 3 | [125I]IOXY | 430 ± 80 (Ke) | Inactive (Ke) | 360 ± 140 (Ke) | Simpson et al., 2007 |
| nM | nM | |||||||
| 204 | 1900 ± 90 | 3380 ± 240 | 2340 ± 120 | [3H]U69,593 | N.D. | N.D. | N.D. | Simpson et al., 2009 |
| 205 | 1510 ± 100 | 3650 ± 260 | >10,000 | [3H]U69,593 | N.D. | N.D. | N.D. | Simpson et al., 2009 |
| 206 | >10,000 | >10,000 | 3400 ± 150 | [125I]IOXY | N.D. | N.D. | N.D. | Simpson et al., 2007 |
| 207 | N.D. | N.D. | 55 ± 23 | [3H]Diprenorphine | N.D. | N.D. | 167 ± 35 (99 ± 1)b | Béguin et al., 2009 |
| 208 | N.D. | N.D. | 154 ± 27 | [3H]Diprenorphine | N.D. | N.D. | 361 ± 25 (99 ± 2)b | Béguin et al., 2009 |
| 209 | N.D. | N.D. | 196 ± 23 | [3H]Diprenorphine | N.D. | N.D. | 508 ± 8 (94 ± 2)b | Béguin et al., 2009 |
| 210 | N.D. | N.D. | 109 ± 12 | [3H]Diprenorphine | N.D. | N.D. | 337 ± 54 (94 ± 2)b | Béguin et al., 2009 |
| 215 | >10,000 | 3360 ± 340 | 610 ± 30 | [3H]U69,593 | N.D. | N.D. | N.D. | Simpson et al., 2009 |
| 217 | N.D. | N.D. | 38 ± 10 | [3H]Diprenorphine | N.D. | N.D. | 101 ± 6 (103 ± 1)b | Béguin et al., 2009 |
| 220 | N.D. | N.D. | 83 ± 28 | [3H]Diprenorphine | N.D. | N.D. | 195 ± 6 (103 ± 3)b | Béguin et al., 2009 |
| 226 | 1630 ± 100 | >10,000 | 7580 ± 720 | [3H]U69,593 | N.D. | N.D. | N.D. | Simpson et al., 2009 |
| 227 | 1610 ± 80 | >10,000 | >10,000 | [3H]U69,593 | N.D. | N.D. | N.D. | Simpson et al., 2009 |
| 228 | 1570 ± 90 | 2600 ± 220 | 7280 ± 400 | [3H]U69,593 | N.D. | N.D. | N.D. | Simpson et al., 2009 |
| 230 | N.D. | N.D. | 498 ± 71 | [3H]Diprenorphine | N.D. | N.D. | 330 ± 30 (98 ± 2)b | Béguin et al., 2009 |
| 231 | N.D. | N.D. | 497 ± 13 | [3H]Diprenorphine | N.D. | N.D. | >1000b | Béguin et al., 2009 |
| 232 | N.D. | N.D. | 555 ± 97 | [3H]Diprenorphine | N.D. | N.D. | 299 ± 13 (133 ± 3)b | Béguin et al., 2009 |
| 233 | N.D. | N.D. | 20 ± 2 | [3H]Diprenorphine | N.D. | N.D. | 36 ± 5 (111 ± 4)b | Béguin et al., 2009 |
| 234 | >10,000 | >10,000 | 39 ± 11 | [3H]Diprenorphine | N.D. | N.D. | 0.077 ± 0.016 (95 ± 2)b | Yan et al., 2009 |
N.D., not determined.
Biological activity compared with subtype-selective agonists [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (MOP), SNC-80 (DOP), and U50,488 (KOP) unless noted otherwise.
Biological activity compared with KOP-selective ligand U69,593.
Antagonism assays (data given as Ke ± S.D.).
Exact value not reported. Data reported as follows: “All compounds were full agonists (Emax = 81 to 106% relative to U50,488).”
Data given as percentage stimulation at the highest concentration tested.
Epimerization of the C-8 position is a potential result of pyrolysis of the leaves of S. divinorum and has a great effect on binding affinity for KOP receptors. The C-8 epimer of salvinorin A (23) was found to have 41-fold lower affinity for KOP receptors compared with salvinorin A (Ki = 163 versus 4 nM) (Chavkin et al., 2004; Munro et al., 2005b). Other C-8 epimeric derivatives (24–30) found in the smoke of salvinorin A were inactive (Ma et al., 2010a; Ma et al., 2010b). Constituents of S. splendens contain a trans-decalin ring system similar to that of salvinorin A; however, they differ significantly in that they are epimeric at both C-8 and C-12. Thorough synthetic modification of salvisplendens A to D failed to produce ligands with significant KOP, MOP, or DOP affinity, however, highlighting the structural requirements for KOP receptor binding that are contained in salvinorin A-based ligands (Li et al., 2007; Fontana et al., 2008).
The activities of naturally occurring neoclerodane derivatives of salvinorin A highlight several sites for synthetic derivatization toward developing SAR at opioid receptors (Lozama and Prisinzano, 2009). In particular, much attention has been given to structural modifications to several key regions: 1) the C-2 position acetoxy substituent; 2) the tricyclic trans-decalin core; and 3) the C-12 furan ring. All structural derivatization studies reported to date have involved systematic modification of salvinorin A extracted directly from the leaves of S. divinorum. Several total synthesis efforts have been reported (Lingham et al., 2006; Scheerer et al., 2007; Burns and Forsyth, 2008; Nozawa et al., 2008; Bergman et al., 2009; Hagiwara et al., 2009); however, their time and resource investment, compared with the relative ease in isolation from natural sources, render their application thus far suboptimal for use in SAR development. Binding affinities and functional activities of analogs at MOP, DOP, and KOP receptors are described in Table 1.
1. Structural Derivatization of the C-2 Position.
A plurality of SAR development studies have focused on sequential modifications to the acetoxy substituent of C-2. This can be rationalized in part by the ease of hydrolysis of this group to the nonacetylated derivative salvinorin B, as well as the early observation that this naturally occurring derivative possesses little activity at KOP receptors. Numerous derivatives were thus produced with the aim of probing the steric and physiochemical tolerance of this region, as well as minimizing metabolism to the inactive salvinorin B.
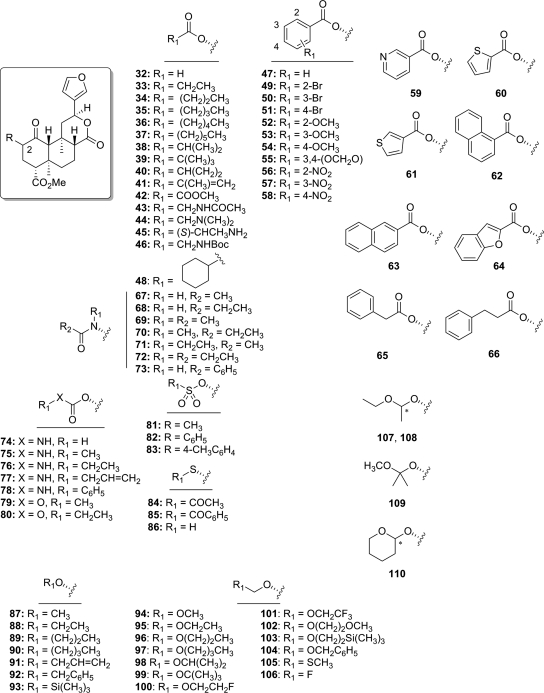
Early efforts aimed to elucidate the role of the C-2 carbonyl substituent through synthesis of various aliphatic chain length esters (33–37; Fig. 5) (Chavkin et al., 2004). Structural modification of this position produced ligands with various activity, from full agonism to partial agonism, for inhibition of forskolin-stimulated cAMP production. In particular, salvinorin A was found to be a full agonist, whereas propionate 33 and heptanoate 37 were found to be partial agonists in this assay (Chavkin et al., 2004). Salvinorin A was found to be more efficacious than the selective KOP receptor agonist U50,488 and similar in efficacy to dynorphin A, the naturally occurring peptide ligand for KOP receptors. Replacement of the C-2 acetyl group with a formate (32) decreased affinity and potency by approximately 5-fold at KOP receptors compared with salvinorin A (Munro et al., 2005b). Increasing the chain to a butyl ester (35) decreased affinity for KOP receptors approximately 2-fold, as well as introducing binding affinity for MOP receptors. Binding affinity for KOP receptors decreased with increasing the ester chain length (C3–C5; 35–37) but had no effect on MOP receptor affinity (Tidgewell et al., 2006). Branching was generally poorly tolerated. Insertion of a methyl group to 34 (iso-propoyl derivative 38) decreased affinity 10-fold at KOP receptors (Harding et al., 2005a). Furthermore, addition of a second methyl group (tert-butoyl derivative 39) or cyclization (cyclopropoyl derivative 40) abolished KOP receptor affinity (Ki > 10,000 nM). Introduction of an alkene (41) decreased affinity 3-fold for KOP receptors but increased affinity 11-fold at MOP receptors. Replacement of the 2-methylacroyl group with a methyl glyoxyl group (42) decreased affinity 11-fold at KOP receptors.
Fig. 5.
Analogs of salvinorin A derivatized at the C-2 position.
Introduction of nitrogen substituents into aliphatic esters generally had a deleterious effect on KOP receptor binding affinity (43–46). Binding affinity for KOP receptors was abolished (Ki > 10,000 nM) for acetamido derivative 43, as well as for derivatives 44 and 45, which contain basic amino substituents (Béguin et al., 2005). The introduction of a tert-butoxycarbonylamino group (46) reduced affinity 47-fold for KOP receptors compared with salvinorin A (Ki = 90 versus 1.9 nM) (Tidgewell et al., 2006).
Introduction of aromaticity to the C-2 acetate produced interesting results. A benzoyl substitution (47) resulted in 47-fold loss in affinity at KOP receptors and 25-fold increased in affinity for MOP receptors compared with salvinorin A. Functional studies showed 47 to be a full agonist at MOP (Emax = 130% ± 4) and KOP (Emax = 140% ± 2) receptors, signifiying the first example of a non-nitrogenous MOP receptor agonist (Harding et al., 2005a). Pharmacological examination of 47, termed herkinorin, produced important results that will be detailed later (section V.C) (Groer et al., 2007; Xu et al., 2007). The structural features responsible for the preference for MOP receptors over KOP receptors were then investigated (Tidgewell et al., 2006). Aromaticity plays a significant role in MOP and KOP receptor binding, in that saturation of the benzoyl ring (cyclohexoyl derivative 48) greatly reduced affinity for all opioid receptors approximately 100-fold compared with 47. Introduction of a bromo substituent to the 2-position (49) or 3-position (50) of the benzene ring had no effect on KOP affinity but decreased affinity for MOP receptors 9-fold compared with 47. Substitution of a 4-bromo substituent (51) decreased affinity for KOP receptors 8-fold compared with 47 (Ki = 740 versus 90 nM) while retaining high affinity for MOP receptors (Ki = 10 versus 12 nM) (Tidgewell et al., 2006). This was in contrast to a previous report (Chavkin et al., 2004) that indicated that 51 had no affinity for MOP. Electron-donating (52–55) and withdrawing (56–58) groups were examined similarly (Tidgewell et al., 2008). Substitutions to the 2-benzoyl position (52, 56) decreased binding affinity to MOP receptors compared with 47, suggesting that steric factors may impede binding of these ligands to MOP receptors; however, this hypothesis has yet to be experimentally investigated. Introduction of a 3-methoxy substituent to the benzoyl ring (53) reduced MOP receptor binding approximately 2-fold; however, KOP receptor binding was greatly diminished, resulting in enhanced selectivity of 53 for MOP over KOP receptors compared with 47. This effect was also seen with 4-methoxy-substituted derivative 54. Nitro substitution of position 3 (57) abolished MOP receptor binding and reduced KOP receptor binding approximately 10-fold compared with 47, and 4-nitro derivative 58 showed a 20-fold reduction in MOP receptor binding affinity with a 6-fold loss of KOP receptor affinity. These 4-position derivatives (54, 58) exhibit similarly selective binding affinities for MOP and KOP receptors, indicating that factors other than electronic effects are responsible for MOP receptor binding.
Heteroaromatic esters and extended aromatic esters were also examined. The 3-pyridyl ester 59 showed a 6-fold loss of affinity for MOP receptors and a greater (20-fold) drop in affinity for KOP receptors. Replacement of the benzoyl group with 2-thiophene (60) produced a moderate (3-fold) reduction of affinity for KOP receptors with no change in MOP receptor affinity. Modification to a 3-thiophene (61) produced little difference in binding affinity and MOP/KOP selectivity compared with 47. The depth of the putative aromatic binding pocket was probed by extension with naphthyl (62, 63) and benzofuranyl (64) substituents. Replacement of the benzoyl group in 47 with a 1-naphthoyl group (62) (Chavkin et al., 2004) decreased affinity roughly 1000-fold at MOP receptors; substitution of a 2-naphthoyl group (63), however, reduced affinity at MOP receptors approximately 10-fold compared with 47. Similar to thiophenes 60 and 61, benzofuranoyl derivative 64 exhibited equivalent binding affinity and selectivity for MOP and KOP receptors. Extension of the aromatic ring through introduction of a single methylene spacer (65) greatly reduced MOP and KOP receptor binding affinity, resulting in an approximate 5-fold preference for KOP over MOP. In addition, introducing a second methylene group (66) increased affinity at all receptors compared with 65 and abolished receptor binding selectivity.
Bioisosteric replacement of the C-2 acetoxy group of salvinorin A with acetamido substituents has been investigated (67–73; Fig. 5). Substitution of an acetamido group (67) for the acetoxy group in salvinorin A decreases affinity and potency at KOP receptors, and extension of this chain (propionamido derivative 68) further diminishes binding affinity and potency. Addition of an N-methyl substituent to 67 (69) and 68 (70) increased affinity and potency at KOP receptors, with derivative 70 exhibiting greater potency in in vitro assays than salvinorin A (EC50 = 0.75 nM versus EC50 = 4.5 nM). A similar trend in binding affinity was seen with addition of N-ethyl substituents to 67 (71) and 68 (72); however, these analogs were less potent than 70 and 71. Similar to benzoyl derivative 47, the N-benzamide derivative 73 was found to increase affinity and selectivity for MOP receptors (Ki = 3.4 versus 12 nM) (Tidgewell et al., 2008), resulting in the most potent MOP receptor agonist derived from salvinorin A (EC50 = 360 nM) described to date.
Evaluation of carbamoyl derivatives 74–78 indicate that derivative 74 exhibits high affinity (Béguin et al., 2005), with decreasing KOP receptor binding affinity for N-methyl (75) and N-ethyl (76) substitutions, whereas modification to an allyl carbamoyl group (77) further decreased affinity 63-fold at KOP receptors (Harding et al., 2005a). In addition, this change resulted in moderate affinity at MOP receptors. Substitution of a phenylcarbamoyl group (78) for the allyl carbamoyl group in 77 had little effect at KOP receptors but increased affinity for MOP and DOP receptors (Harding et al., 2005a). Carbonates, on the other hand, are poorly tolerated at the C-2 position of salvinorin A. Conversion of 75 and 76 to their corresponding carbonates (79 and 80, respectively) caused a complete loss of affinity at KOP receptors (Ki > 1000 nM) (Lee et al., 2005c).
Sulfonate esters were targeted as an isosteric replacement for the C-2 acetate group (Harding et al., 2005a). Substitution of a mesylate group (81) was well tolerated because this change had little effect on binding and potency at KOP receptors (EC50 = 30 versus 40 nM) (Harding et al., 2005a). Consistent with SAR for ester modifications, conversion of the methyl group of 81 to a phenyl group (benzene sulfonate 82) diminished affinity for KOP receptors; however, 82 showed no affinity for MOP receptors (Ki > 10,000 nM). Introduction of a 4-methyl group to 82 (83) had no effect on KOP affinity (Ki = 50 versus 60 nM) and increased affinity for DOP (Ki = 3720 versus >10,000 nM) compared with 82. This change, however, also increased affinity for MOP receptors compared with 82 (Ki = 220 versus >10,000 nM). Combined, these results do not parallel those seen with the ester series, suggesting that the sulfonate esters are not binding in an identical manner at either MOP receptors or KOP receptors.
The SAR described earlier for esters are consistent for thioacetoxy substitution at C-2. Isosteric substitution of a thioacetoxy group (84) decreased affinity and activity at KOP receptors (EC50 = 4.77 versus 2.82 nM) (Bikbulatov et al., 2007; Tidgewell et al., 2008). In addition, as shown in the amide and ester series, introduction of a benzene ring to 84 (85) increased affinity for MOP receptors, although to a lesser extent than ester 47 and amide 73 (Tidgewell et al., 2008). Removal of the acetyl group in 84 (86) decreased affinity and potency at KOP receptors.
The conversion of salvinorin A to various ethers has been studied (Béguin et al., 2005, 2006; Lee et al., 2005c). The methyl ether derivative 87 has similar affinity and efficacy at KOP receptors as salvinorin A (Béguin et al., 2005). Extending the chain to ethyl (88) increases affinity and potency 20-fold compared with 87; however, further extension of the chain (89–90) diminishes affinity and potency compared with 88. Allyl ether 91 and benzyl ether 92 were found to have similar activity at KOP receptors but were less potent than 88 (Béguin et al., 2005). Trimethylsilyl ether 93 exhibited greatly reduced affinity compared salvinorin A (Harding et al., 2005a).
Introduction of a methoxymethyl group (94) to salvinorin B was found to increase affinity and potency at KOP receptors compared with salvinorin A (Lee et al., 2005c). It was hypothesized that the additional oxygen substituent could be involved in synergistic binding interactions with the KOP receptor (Munro et al., 2008). This prompted investigation into a series of oxygenated, halogenated, and silylated ether derivatives (95–110). Aliphatic straight-chain (95–97) and branched (98) derivatives exhibited similar affinity and potency as salvinorin A, ethoxy derivative 95 demonstrating the highest KOP affinity (Ki = 0.32 nM) and potency (EC50 = 0.14 nM) of all salvinorin A derivatives described to date. Introducing halogens (100 and 101) and oxygen (102) greatly reduced KOP receptor binding compared with 95. Other larger derivatives (102, 103) also showed a great loss of affinity and potency compared with 85. Conversion of 85 to methylthiomethyl analog 105 caused a 20-fold drop of affinity for KOP receptors and a 10-fold drop in potency. Fluoromethyl derivative 106 exhibited approximately 20-fold lower affinity and 15-fold lower potency than salvinorin A. Alkylation of the acetal carbon had a negative effect on binding affinity and potency at KOP receptors, as evidenced by the isolated epimers of monomethyl analog of 95 (107, 108) and dimethyl analog of 94 (109) exhibiting 20- and 100-fold losses in potency, respectively. The epimeric mixture of tetrahydropyran derivative 110 had similar binding affinity and potency as salvinorin A. The effects of each individual epimer of 110 remain to be elucidated, however.
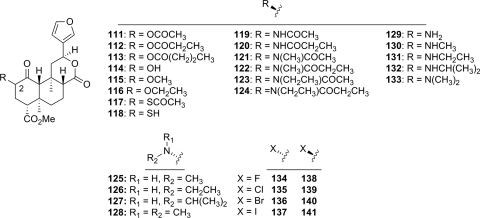
The stereochemical requirements at C-2 have also been examined (Fig. 6) (Béguin et al., 2006; Harding et al., 2006b). Inversion of the C-2 acetate of salvinorin A (111) resulted in a significant loss of affinity at KOP receptors and was found to be the first neoclerodane diterpene with DOP antagonist activity (Harding et al., 2006b). Epimerization of the C-2 position was detrimental for binding affinity and potency of C-2 esters (111–113), ethers (114–116), thiols (117, 118), and amides (119–124) (Béguin et al., 2006; Bikbulatov et al., 2007).
Fig. 6.
C-2 epimeric derivatives of salvinorin A.
The conversion of the methoxy group in 87 to a methylamino group (125) had little effect on affinity but decreased potency at KOP receptors (Béguin et al., 2006). Extension of the chain to an ethylamino group (126) increased affinity and potency compared with 125, and substitution of an isopropylamino group (127) increased potency at KOP receptors compared with 126. Activity at KOP receptors was also increased upon addition of an N-methyl group to 125 (128). In general, inversion of C-2 stereochemistry of these analogs was found to increase activity at KOP receptors (129–133) (Béguin et al., 2006). The most potent analog (132) was found to be roughly equipotent with salvinorin A (EC50 = 7.2 versus 4.5 nM) (Béguin et al., 2006). This is in contrast to SAR seen with C-2 esters and thioacetoxy esters, whose β-epimers exhibit decreased activity compared with their natural α-counterparts.
Halogenation of the C-2 position was first reported in 2006 (Stewart et al., 2006), and then again in 2008 (Tidgewell et al., 2008); however, a complete series of C-2 halogenated analogs was described only recently (Lee et al., 2010). Pharmacological evaluation of a series of eight analogs (134–141) showed that C-2-β analogs generally displayed higher binding affinity than their corresponding α-isomers, with the exception of iodo analogs 140 and 141 (Ki = 198 ± 43 versus 245 ± 14 nM, respectively). Both affinity and efficacy were generally diminished within this series, with 141 displaying an Emax of 46% (Emax = 106% for salvinorin A).
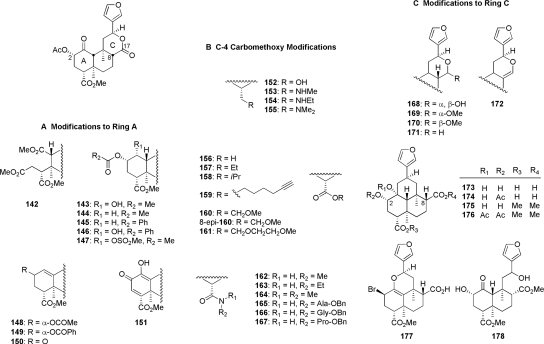
2. Structural Modifications of the trans-decalin Core of Salvinorin A.
In addition to structural modifications to the 2-position acetoxy substituent, other modifications to the A ring have been studied (Fig. 7A) (Lee et al., 2005c; Munro et al., 2005a; Holden et al., 2007). Basic autoxidation of salvinorin A produces the ring-opened analog 142, which was found to have weak affinity at KOP receptors (Ki = 2.9 μM) (Munro et al., 2005a). Reduction of the C-1 ketone to an α-alcohol (143) caused a reduction in affinity of more than 250-fold compared with salvinorin A (Ki = 1125 versus 4 nM) (Munro et al., 2005b). This modification also changed the efficacy at KOP receptors from a full agonist (salvinorin A, Emax = 108%) to an antagonist (143; Ke = 240 nM) (Holden et al., 2007). Complete removal of the C-1 ketone (144) resulted in a 5-fold loss of affinity compared with salvinorin A (Ki = 18 versus 4 nM) (Munro et al., 2005b). It was further found that 144 was 3-fold less potent than salvinorin A yet more efficacious as a KOP receptor agonist (Holden et al., 2007). A more recent study found that 144 was approximately as potent as salvinorin A but less efficacious and showed antagonist activity at MOP and DOP receptors. Replacement of the 2-acetyl substituent with a 2-benzoyl group (145) resulted in an antagonist across all subtypes (MOP, DOP, KOP). In light of the fact that 47 is a full agonist at MOP receptors (Harding et al., 2005a), this finding suggests that C-1 deoxo analogs may be interacting at MOP receptors differently than its C-1 keto counterparts. A similar 2-O-benzoyl replacement of 143 (146) resulted in a 2-fold loss of activity at KOP receptors (Ke = 450 versus 240 nM). Addition of a 1-O-mesylate group (147) resulted in a loss of antagonist activity at KOP receptors.
Fig. 7.
Derivatives of salvinorin A with modifications to the trans-decalin ring system.
Introduction of a 1,10-alkene to 144 (148) lowered efficacy across all opioid receptors, resulting in a switch of efficacy from KOP partial agonist to antagonist, reducing antagonist activity at DOP receptors and maintaining antagonist efficacy at MOP receptors (Holden et al., 2007). Replacement of the 2-α-acetyl substituent of 148 with a benzoyl moiety (149) reduced activity 9- and 6-fold at MOP and DOP receptors, respectively, but had little effect on actions at KOP receptors. Combined, these results also suggest a dissimilar binding mode of 1,10-dehydro analogs than their C-1 keto congeners. Oxidation of the C-2 position of 1,10-dehydro derivatives 148 and 149 produced α,β-unsaturated derivatives 150 and 151. The C-1 desoxy derivative 150 showed KOP receptor antagonist activity similar to that of 148, al though the enol 151 was shown to have no affinity for KOP receptors (Ki > 10 μM) (Lee et al., 2005c; Munro et al., 2005a; Holden et al., 2007).
The role of the 4-carbomethoxy group has also been explored (Lee et al., 2005a, 2006; Munro et al., 2005b; Béguin et al., 2006). Reduction of this group was generally poorly tolerated (Fig. 7B). The primary alcohol 152 showed approximately 90-fold reduced affinity for KOP receptors, and replacement of the alcohol with primary (153), secondary (154), and tertiary (155) amines produced abolished affinity for KOP receptors (Ki > 10,000 nM) (Béguin et al., 2006). As with other analogs described previously, C-8 epimerization of 153–155 did not lead to increased affinity for KOP receptors. Hydrolysis of salvinorin A to the corresponding carboxylic acid (156) was not tolerated, resulting in a loss of affinity for KOP receptors (Ki > 1000 nM); it is noteworthy that epimerization (8-epi-156) was reported to have largely restored affinity (Ki = 48.6 ± 4.4 nM) and potency (Ki = 74.1 ± 2.2 nM) (Lee et al., 2005a). Extension of the carbon chain of the methyl ester of salvinorin A to ethyl (157) and iso-propyl (158) resulted in a loss of affinity and potency at KOP receptors (Lee et al., 2005a). Further extension of the chain and incorporation of an acetylene function (159) did result in modest KOP receptor affinity (Ki = 201 nM); however, methoxymethyl (160) and methoxyethoxymethyl ester (161) modifications were poorly tolerated (Lee et al., 2006). It is noteworthy that the C-8 epimer of 160 (8-epi-160) had affinity for KOP receptors similar to that of salvinorin A but was 3-fold less potent as an agonist (Lee et al., 2005a).
Other modifications of the C-4 carbomethoxy substituent were also poorly tolerated; methyl amide 162 displayed a greater than 500-fold loss of affinity at KOP receptors compared with salvinorin A (Béguin et al., 2006), and extending the carbon chain to N-ethyl (163) and N,N-dimethylation (164) failed to rescue affinity (Ki > 10,000 nM) (Lee et al., 2005a). It is noteworthy that incorporation of several amino acids (165–167) led to modest affinity and activity at KOP receptors, the most potent analog being alanine derivative 165 (EC50 = 46.7 nM) (Lee et al., 2005a).
Derivatization of the C-ring has focused on modification of the C-17 carbonyl substituent (Fig. 7C). Reduction of the lactone carbonyl to the lactol 168 reduced affinity 14-fold and potency 2-fold at KOP receptors (Munro et al., 2005b). Methylation of 168 was well tolerated for both C-17 α-methoxy (170) and C-17 β-methoxy (169) analogs. There was little difference between epimers, as both exhibited similar binding affinities for all opioid receptors. Complete removal of the C-17 carbonyl resulted in the tetrahydropyran derivative 171 and dihydropyran derivative 172. These modifications had little effect on KOP receptor binding compared with salvinorin A; however, potency was reduced 5- and 14-fold, respectively (Munro et al., 2005b). Hydrolysis of the C-ring lactone has been reported (Fig. 7C), and hemiacetal derivatives 173 to 177 exhibit generally poor affinity for KOP receptors (Bikbulatov et al., 2008; Béguin et al., 2009; Lee et al., 2010). The diester derivative 175 displayed the highest binding affinity for KOP and MOP receptors (Ki = 219 ± 59 nM and K−i = 1926 ± 147 nM, respectively), while acetylation (176) greatly diminished affinity. Subsequent acidic hydrolysis of 176 resulted in the ring-opened salvidivin analog 178, which was also shown to be fairly inactive at KOP receptors.
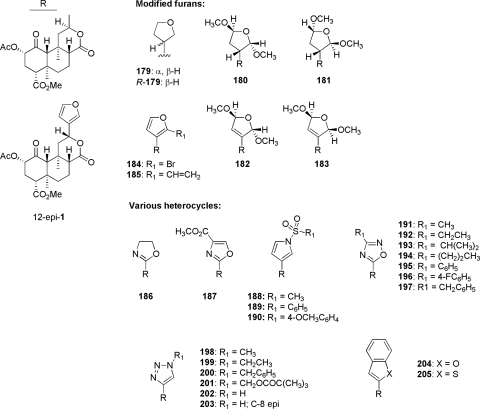
3. Modifications to the C-12 Furan Ring.
There has been much recent interest into evaluation of the SAR of the furan moiety on the C-12 position of ring C (Munro et al., 2005b; Harding et al., 2006a; Simpson et al., 2007; Yang et al., 2009). Epimerization of C-12 (12-epi-1) resulted in a modest drop in KOP receptor binding affinity compared with the natural isomer (Ki = 41 versus 2.5 nM; Fig. 8) (Béguin et al., 2009). Reduction of the furan ring resulted in a mixture of C-13 tetrahydrofuran epimers (179) that displayed reduced KOP receptor affinity compared with salvinorin A (Ki = 156 versus 4 nM) (Munro et al., 2005b). In addition, 179 was reported to possess high affinity at KOP receptors (Ki = 14 nM) (Simpson et al., 2007). These studies also found that the R-epimer [i.e., (R)-179] had binding affinity similar to that of salvinorin A but was 17-fold less potent than salvinorin A at KOP receptors. The addition of 2,5-dimethoxy groups to (R)-179 (180 and 181) was also examined and found to decrease affinity at KOP receptors. Incorporation of an alkene between C-13 and C-14 to 180 (182) and 181 (183) did not enhance affinity at KOP receptors. Bromination of the furan C-16 position (184) is well tolerated, as is addition of a vinyl group (185).
Fig. 8.
Furan ring modified analogs of salvinorin A.
Mixed results have been found regarding modification of the 3-furan to other heterocyclic derivatives (186–205). Replacement of the furan ring with either 2-oxazoline (186) or 4-carbomethoxyoxazole (187) was deleterious to KOP receptor binding (Harding et al., 2006a; Simpson et al., 2007). A series of N-sulfonylpyrroles (188–190) showed reduced affinity and efficacy at KOP receptors compared with salvinorin A (Harding et al., 2006a). Conflicting results have been seen with replacement of the furan ring with 1,3,5-oxadiazoles. An initial report (Simpson et al., 2007) described 4-methyl-1,3,5-oxadiazole 191 as a MOP/KOP receptor antagonist with a 29-fold loss in affinity at KOP receptors compared with salvinorin A. A subsequent study (Béguin et al., 2009) sought to elucidate the SAR of substitution of the 4-position of the 1,3,5-oxadiazole of 191 with various alkyl (192–194) and aryl (195–197) substituents. None of these derivatives was found to have affinity for KOP receptors, however. A more thorough investigation of 195 showed that whereas KOP receptor affinity was lost (Ki > 10,000 nM), 195 showed binding affinity for MOP receptors (Ki = 1610 nM). Replacement of the furan ring with variably substituted 1,2,3-triazole rings (198–203) abolished opioid receptor binding (Ki > 10,000 nM for MOP, DOP, and KOP) (Yang et al., 2009). Extension of the aromaticity of the furan resulted in benzofuran derivative 204, which was found to reduce affinity for KOP receptors by 300-fold yet had little effect on MOP receptor binding compared with salvinorin A (Ki = 1900 versus 1370 nM). It is noteworthy that replacement of 204 with a 2-benzothiophene (205) resulted in a loss of KOP receptor binding (Ki > 10,000 nM) but maintained similar affinity for MOP and DOP receptors (Simpson et al., 2009).
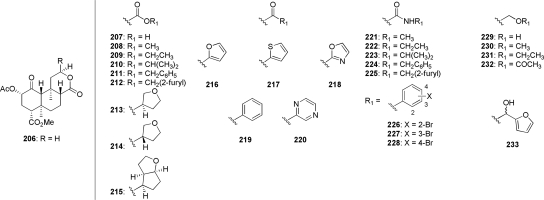
Combined, these results provide ample evidence that a furan ring at C-12 is not required for biological activity. It should be noted, however, that complete removal of the furan moiety (206) resulted in a reduction in affinity of more than 1700-fold for KOP receptors compared with salvinorin A (Ki = 3400 versus 1.9 nM) (Simpson et al., 2007). This has led to several studies aiming to investigate the effect of substitutions to C-12 of des-furyl analog 206 (Fig. 9) (Béguin et al., 2009; Simpson et al., 2009; Yang et al., 2009). The C-12 carboxylic acid derivative 207, which was shown to be a full agonist at KOP receptors with a modest reduction in affinity compared with salvinorin A (Ki = 55 versus 2.5 nM), was used to produce esters (208–215), ketones (216–220), and amides (221–228), and was reduced to produce a series of hydroxymethyl derivatives 229–233. In the ester series (208–215), short alkyl chains displayed KOP receptor binding affinity that was approximately 50- to 100-fold lower than salvinorin A, whereas larger chains (benzoyl and 2-methylfuroyl derivatives 211 and 212, respectively) were devoid of activity at all opioid receptor subtypes. It is noteworthy that although the bicycle 215 also showed low affinity for KOP receptors (Ki = 610 nM), marginal affinity was also seen at DOP receptors (Ki = 3360 nM). A series of aromatic ketones was also evaluated for KOP binding and activity. Keto-2-thiophene 217 and ketopyrazine 220 were the only derivatives to show detectable affinity (Ki = 38 and 83 nM, respectively) in this series. This is interesting, considering that the keto-2-furan 216 showed no activity in this assay (Ki > 10,000 nM). Another interesting finding from this study was that the reduced derivative of 216 (233) was found to be a high-affinity (Ki = 20 nM) KOP receptor agonist; however, the respective contribution of each individual epimer is currently unknown. Amide derivatives 221–228 generally exhibited low affinity for KOP receptors. It is noteworthy that bromophenylamide derivatives 226 to 228 all showed preferential binding affinity for MOP over KOP receptors, reinforcing the notion that opioid receptor subtype selectivity can be altered by modification of the C-12 furan ring. With the exception of the aforementioned 2-hydroxymethylfuryl derivative 233, removal of the carbonyl of acid 207 was generally poorly tolerated (229–232). A general summary of the known structure-activity relationship of 1 is provided in Fig. 10.
Fig. 9.
C-17 des-furyl homologated analogs of salvinorin A.
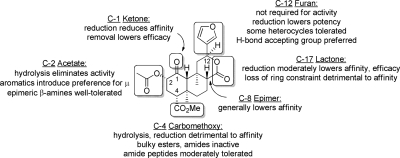
Fig. 10.
General SAR for salvinorin A activity at KOP receptors. [Adapted from Prisinzano TE and Rothman RB (2008) Salvinorin A analogs as probes in opioid pharmacology. Chem Rev 108:1732–1743. Copyright © 2008 The American Chemical Society. Used with permission.]
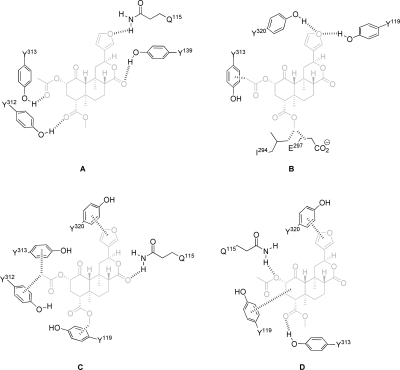
B. Proposed Binding Interactions with κ-Opioid Receptors
Salvinorin A was identified as the first non-nitrogenous opioid receptor-selective ligand, raising questions as to the binding epitope of this unique structural scaffold. It had previously been hypothesized that a positively charged amino substituent would form a salt bridge with a negatively charged aspartate residue, which is highly conserved within the third transmembrane domain of the opioid receptor (Surratt et al., 1994; Mansour et al., 1997; Lu et al., 2001). The lack of such functionality within the tricyclic skeleton makes it unlikely that these ionic interactions are stabilizing salvinorin A in a manner similar to that of other nitrogenous opioids. Numerous studies have been devoted to elucidating the interactions between salvinorin A and KOP receptors. These include biochemical approaches, such as development of chimeric opioid receptors and single-point modifications to KOP receptors, as well as the development of computational models that aim to relate the physiochemical properties of salvinorin A analogs with observed pharmacological effects.
1. Receptor-Based Binding and Activity Studies.
Several models have been proposed to explain the selectivity and binding mode of KOP receptor-selective ligands. One of the earliest studies aimed to identify the structural features of the bivalent selective antagonist nor-binaltorphimine (norBNI), responsible for KOP receptor selectivity (Hjorth et al., 1995). It was found, using a systematic series of chimeras between MOP and KOP receptors, that extracellular domain III and transmembrane domains (TM) VI and VII play prominent roles in the KOP-selective binding of norBNI, with a Glu297 residue located at the top of TM-VI identified as particularly important for KOP selectivity. In addition to this residue, a series of mutagenesis studies identified additional elements of KOP receptors recognized by antagonists norBNI and guanidino naltrexone, which impart their observed selectivity, namely 1) a highly conserved Asp138 in TM III, and (2) a lipophilic, aromatic pocket formed by TMs V, VI, and VII (Hjorth et al., 1995; Jones et al., 1998; Larson et al., 2000; Metzger et al., 2001) (Fig. 11).
Fig. 11.
Graphical representations of the human KOP receptor. A, amino acid sequence alignments for TM II and the EL II of human and rat KOP receptor (hKOR and rKOR, respectively), human MOP receptor (hMOR), and human and mouse DOP receptor (hDOR and mDOR, respectively). B, molecular models of KOP receptor before rotation (green) and after rotation (orange) with salvinorin A docked and energy minimized in both receptors. [Reprinted from Vortherms TA, Mosier PD, Westkaemper RB, and Roth BL (2007) Differential helical orientations among related G protein-coupled receptors provide a novel mechanism for selectivity. Studies with salvinorin A and the kappa-opioid receptor. J Biol Chem 282:3146–3156. Copyright © 2007 The American Society for Biochemistry and Molecular Biology. Used with permission.]
In contrast to reports proposing KOP receptor binding interactions for nitrogenous opioid ligands, the binding of salvinorin A to KOP receptors is enigmatic, particularly because salvinorin A lacks the basic amine substituents that are hypothesized to be vital for binding of the “address” to Glu297 and Asp138 residues in the KOP receptor. One of the first studies to describe the effect of single-point mutations on salvinorin A binding to KOP receptors highlighted a region of TM VII and TM II characterized by lipophilic tyrosine residues (Yan et al., 2005). Mutations of Tyr313, Tyr119, and Tyr320 caused a dramatic decrease in salvinorin A binding affinity to KOP receptors compared with radioligand [3H]diprenorphine. For mutations to Tyr313 (Y313A) and Tyr119 (Y119A), a diminished loss of affinity was seen with arylacetamide agonist (5α,7α,8β)-(+)-N-methyl-N-(7-(1-pyrrolidinyl)-1-oxaspiro(4,5)dec-8-yl)benzeneacetamide (U69,593) and endogenous peptide agonist dynorphin A(1–13), indicating that these residues preferentially engage in more favorable interactions with salvinorin A. It is notable that although Y313A mutation on TM 7 caused a 22-fold drop in affinity of salvinorin A for KOP receptors, a Y313F mutation, in which phenylalanine maintains hydrophobic interactions but removes the hydrogen-bonding potential of tyrosine, causes no loss of affinity of salvinorin A. Agonist potency experiments indicated a 6-fold loss of potency of salvinorin A as the result of a Y313A mutation, and Y313F had no effect on salvinorin A potency, consistent with the finding that this mutation had little effect on KOP receptor binding affinity. Mutations to Y139A, Y312A, and Y119A also negatively affected agonist potency, whereas the corresponding Tyr-to-Phe mutations had no discernible effect. The 2-thiosalvinorin B analog 86 was also compared with salvinorin A in KOP receptor double-mutant studies, wherein C315S mutations were combined with cysteine mutations to several residues located within the binding pocket. One significant finding was the fact that a C315S–Y313C double mutation was not tolerated by salvinorin A (14-fold drop in affinity compared with C315S) but had no effect on binding of 2-thio analog 86, further supporting the notion that the 2-position of salvinorin A engages in interactions with Tyr313.
An additional model proposed by Kane et al. (2006) uses KOP receptor chimeras and single-point mutations to describe salvinorin A binding. Using chimeric KOP receptors spliced with portions of MOP and DOP receptors, it was found that only a combination of KOP(1–227)/DOP(215–372) maintained similar binding affinity for salvinorin A as KOP receptors, in fact exhibiting an increase in affinity of approximately 10-fold. Single-point mutation studies then revealed that mutation of Y320A caused a 32-fold loss in affinity relative to salvinorin A, consistent with previous reports (Yan et al., 2005) that demonstrated the importance of Tyr320. Combined, this work proposes a unique binding epitope for salvinorin A that vertically spans TM II, TM VII, and EL II. Residues determined to be important for KOP receptor binding include Tyr119, Tyr320, Asn115, Tyr313, and Tyr312. A follow-up study (Kane et al., 2008) examined the effects of additional single-point mutations to TM VII, and found that a mutation of I316A abolished binding affinity of salvinorin A to KOP receptors by interrupting the lipophilic α-helical bundle of Tyr313 and Tyr320 in TM VII. Modifications to TM I had little effect on KOP receptor binding, and modifications to TM VI, which contains Glu297 required for KOP selectivity of nitrogenous opioid ligands, also had little effect, suggesting that this domain is not required for salvinorin A binding and selectivity.
Using chimeras, site-directed mutagenesis, and the substituted cysteine accessibility method, more key residues for KOP receptor binding of salvinorin A were identified (Vortherms et al., 2007). It was found that inclusion of TM II of DOP receptors to KOP receptors had a deleterious effect on salvinorin A binding, whereas binding affinity was retained in MOP and DOP receptors mutated with TM II and EL II of KOP receptors. Single-point mutations of nonconserved residues V108A and V118K proved detrimental to KOP receptor binding for salvinorin A but not for the nonselective antagonist naloxone. This suggests that these residues are responsible for the selectivity of salvinorin A for KOP receptors, which would indicate a unique binding mode that is not shared by nitrogenous ligands. This study further suggests that residues in the helix of TM II are rotated within the KOP receptor, which maximizes favorable interactions with salvinorin A. This would represent a unique mechanism of selectivity among G-protein-coupled receptor (GPCR) binding.
After the many receptor modification studies, structural analogs of salvinorin A have been developed that act as active-state probes of the native KOP receptor (Yan et al., 2009). As described in section IV.A, short-chain, lipophilic modifications to the C-2 acetate group are typically tolerated in KOP receptor binding. Furthermore, several models have indicated that the residue Cys315 is located in TM VII in a region near the salvinorin A recognition site of KOP receptors. Covalent KOP receptor labeling probes were developed that would covalently bind the KOP receptor through this site (Fig. 12). In addition to showing enhanced binding affinity and potency (Table 1), the 22-thiocyanatosalvinorin A derivative 234 exhibited wash-resistant KOP receptor binding. This irreversible binding probe is also active in vivo and provides a unique tool for elucidating the potential active site interactions of salvinorin A in vitro and the activity of KOP receptors in vivo.
Fig. 12.
Mechanism of covalent receptor modification by KOP receptor probe, 234.
2. Computational Models for KOP Receptor Binding.
Computational models have been used to describe KOP receptor binding and selectivity for KOP agonists (Metzger et al., 1996; Subramanian et al., 1998; Wan et al., 2000; Iadanza et al., 2002; Holzgrabe and Brandt, 2003). It has been proposed that the Asp138 carboxylate in TM III forms a salt bridge with the protonated amine of arylacetamides and benzomorphans, and a hydrophobic pocket consisting of Tyr312, Leu224, Leu295, and Ala298 side chains hosts the phenyl ring of arylacetamides. Benzomorphans are thought to form favorable hydrogen bonding interactions with His291 (Lavecchia et al., 2000). For KOP-selective peptide dynorphin A(1–8), a model identified residues in EL II, and TMs III, IV, and V as determinants of KOP selectivity for peptide agonists at KOP receptors (Wan et al., 2000).
Receptor- and ligand-based studies described in section IV.B.2 led to the hypothesis that the binding mode of salvinorin A differs from that of nitrogenous KOP ligands. Target-based computational models initially identified four interactions that were proposed to explain the binding of salvinorin A (Roth et al., 2002) (Fig. 13A): 1) hydrogen bonding interactions between Gln115 and the furan oxygen; 2) Tyr139 forming a hydrogen bond with the C-17 lactone carbonyl; 3) Tyr312 interacting with the C-4-carbomethoxy substituent; and 4) Tyr313 interacting with the C-2 acetyl group. After observations of salvinorin A binding interactions with KOP receptor mutants, a newer model was proposed that highlighted three functional group interactions required for KOP receptor activity (Yan et al., 2005) (Fig. 13B): 1) the furan oxygen interacting with Tyr119 and Tyr320; 2) the 4-carbomethoxy group interacting with Glu297 and Ile294; and 3) the 2-acetyl group interacting via a hydrophobic manner with Tyr313. A further refined model, developed after studies using receptor chimeras and site-directed mutagenesis (Kane et al., 2006), also proposed interactions between KOP receptors and the C-12 furan (Tyr320), C-17 lactone (Gln115), C-4-carbomethoxy group (Tyr119), and C-2 acetate (Tyr312, Tyr313) (Fig. 13C).
Fig. 13.
Proposed binding modes between salvinorin A and key residues within the KOP receptor. A, Roth et al. (2002). B, Yan et al. (2005). C, Kane et al. (2006). D, Singh et al. (2006).
In conjunction with target-based models, ligand-based pharmacophore models have also been effective in determining SAR for KOP receptor ligands. A model that described a pharmacophore based on KOP-selective arylacetamide agonists has been described (Singh et al., 2008). Salvinorin A was not identified as a KOP ligand in this study, reinforcing the hypothesis that salvinorin A binds the KOP receptor through a unique recognition site. Yamaotsu et al. (2010) has recently proposed a general 3D pharmacophore for KOP receptor agonists that incorporates salvinorin A in the model training set. Despite the wealth of information supporting the suggestion that salvinorin A binds in a manner unique from other nitrogenous opioids, this model was developed with the idea that the structural features required for salvinorin A to bind with the receptor would directly overlap with similar features of 4,5-epoxymorphinan and arylacetamide agonists.
Another computational model used a novel integrated approach to pharmacophore development, developing quantitative, ligand-based pharmacophores simultaneously refined with target-based methods (Singh et al., 2006). First, a training set of 15 salvinorin A-based derivatives was used to develop an initial, ligand-based pharmacophore that was validated against a test set. This model successfully distinguished among ligands with moderate to low affinity for KOP receptors. Several key features of this first model was the presence of hydrophobic regions separated by 8.38 Å, which correspond to the C-12 furan and C-4 carbomethoxy substituent, and hydrogen bond-accepting groups surrounding the C-2 oxygen and C-4 carbonyl moieties. A target-based model was then created from induced fit-docking of salvinorin A into a receptor binding site that was guided by site-directed mutagenesis studies described earlier (section IV.B.1) (Yan et al., 2005; Kane et al., 2006). In accordance with previous reports (Kane et al., 2006), the KOP receptor binding pocket was formed primarily between TMs II and VII and was largely lipophilic. Lipophilic interactions between the furan ring and Tyr320 were once again noted, as were hydrophobic interactions between the C-4 carbomethoxy methyl group and Tyr119. However, two key interactions were noted that further refined the ligand-receptor model (Fig. 13D): 1) Gln115, which had previously been suggested to form hydrogen bonds with the furan oxygen or C-17 lactone oxygen, instead preferentially identified the C-2 oxygen; and 2) Tyr313, which all other previous models suggested interacts with the C-2 acetate (Roth et al., 2002; Yan et al., 2005; Kane et al., 2006), was instead proposed to form hydrogen-bonding interactions with the C-4 carbomethoxy ester carbonyl. It was found that the combined hydrophobic and hydrogen bonding regions of the pharmacophore model coincide well with residues identified from the docked receptor model.
A more recent computational study used comparative molecular field analysis methodology to describe three-dimensional quantitative SARs of salvinorin A derivatives (McGovern et al., 2010). Here, a training set consisting of structural modifications confined to the C-2 acetate of salvinorin A was aligned and correlated with experimentally obtained affinity data. These data confirm that short (3-carbon length) alkyl chains are tolerated at the C-2 position and identify a sizable region of steric incompatibility immediately surrounding this group. Although previously observed qualitatively, these comparative molecular field analysis studies provided further evidence that regions exist within the KOP receptor that engage in favorable interactions with electronegative atoms of the C-2 position of salvinorin A. This model was also able to identify regions above the plane of ring A of salvinorin A where electropositive interactions are favorable. This would help to explain how C-2 epimeric amino derivatives 129 to 133 exhibited enhanced binding affinity over corresponding C-2-β ethers (115, 116) and thioethers (117, 118).
V. Effects of Salvinorin A In Vivo
As noted in the Introduction (section I), salvinorin A is unique among opioids, not only because it shares little structural similarity to other classes of nonpeptidic opioid receptor ligands but also because it does not have the positively charged nitrogen atom, which was thought to be an absolute requirement for the interaction of opioid ligands with opioid receptors (Rees and Hunter, 1990). The discovery of a novel structural class of opioid receptor ligands raised the possibility of developing new opioid receptor probes with novel properties. Moreover, the role of the endogenous dynorphin (KOP) system in stress (Bruchas et al., 2010) and reward (Bruijnzeel, 2009), as well as the possible role of KOP receptor agonists and antagonists for the treatment of psychiatric disorders, such as mood and thought disorders (Carlezon et al., 2009), emphasizes the need to explore the development of novel KOP agonists and antagonists. In addition, peripherally active KOP agonists, including salvinorin A, may have a role in treating chronic pain (Vanderah, 2010) and gastrointestinal disorders (Capasso et al., 2006; Fichna et al., 2009).
The discovery of the KOP agonist activity of salvinorin A (Roth et al., 2002) led to numerous pharmacological studies of this small molecule. Although many studies demonstrated that salvinorin A produced classic KOP agonist effects, others revealed different responses compared with standard KOP agonists, such as U69,593 or U50,488. This section will review these studies with an emphasis on studies that reveal such differences.
A. κ-Opioid Receptor-Mediated Effects of Salvinorin A
Evaluation of the drug discrimination effects was an important step toward thoroughly understanding the KOP receptor-mediated effects of salvinorin A in vivo. Drug discrimination is a widely used behavioral paradigm where a subject, typically a rat, is first trained to respond to a drug, then subsequently other drugs are tested for their ability to generalize to, or “substitute” for, the training drug (for review, see Porter and Prus, 2009). In one of the earliest studies, Butelman et al. (2004) tested the ability of systemically administered (subcutaneous) salvinorin A to substitute for U69,593 in rhesus monkeys. Salvinorin A fully substituted for U69,593. The highest dose of salvinorin A (0.032 mg/kg) did not decrease the rate of responding and produced mild overt behavioral effects, such as sedation. The effects of salvinorin A were completely blocked by pretreatment with the opioid antagonist quadazocine, using a dose that blocks the behavioral and endocrine effects of KOP receptor agonists in the rhesus monkey. The selective KOP antagonist 5′-guanidinonaltrindole (Stevens et al., 2000) reduced the effects of salvinorin A in two of three monkeys. Butelman et al. (2004) further reported that ketamine, a dissociative anesthetic and NMDA receptor antagonist with hallucinogenic effects in humans, did not substitute for U69,593, thereby suggesting that the hallucinogenic effects of salvinorin A in humans are not mediated by the NMDA receptor. Willmore-Fordham et al. (2007) extended these studies to rats. Salvinorin A (1–3 mg/kg i.p.) fully substituted for U69,593 without altering response rates. Moreover, the KOP-selective antagonist norBNI completely blocked the effects of salvinorin A but had no effect by itself. The research published by Baker et al. (2009) extended these findings. In this study, conducted in rats, salvinorin A, and the metabolically stabilized salvinorin A analogs salvinorin B ethoxymethyl ether and salvinorin B methoxymethyl ether substituted completely for U69,593. In addition, this study showed that U69,593 and U50,488 substituted for salvinorin A in rats trained to discriminate salvinorin A from saline.
In light of the hallucinogenic effects of salvinorin A in humans, several studies examined the effects of salvinorin A compared with known hallucinogens. As noted above, Butelman et al. (2004) showed that ketamine did not substitute for U69,593. Li et al. (2008) significantly extended these findings. Rhesus monkeys were trained to discriminate between saline and 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane, a 5-HT2A receptor agonist that is hallucinogenic in humans. Neither U69,593 nor salvinorin A was shown to substitute for 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane. Butelman et al. (2010) further showed that structurally diverse KOP agonists (bremazocine, U69,593, U50,488), but not psilocybin, a 5-HT2A receptor agonist that is hallucinogenic in humans, substituted for salvinorin A in rhesus monkeys trained to discriminate salvinorin A from saline. Nemeth et al. (2010) compared the effects of ketamine and salvinorin A in the five-choice serial reaction time task (5CSRTT) in rats. This paradigm is a food-motivated attention test that is similar to the continuous performance test used to study attention in human research subjects. Patients with schizophrenia demonstrate impaired performance in this test (Holzman, 1992), suggesting that drugs that impair performance in the 5CSRTT may produce cognitive dysfunction similar to that observed in psychiatric disorders such as schizophrenia. In these experiments, salvinorin A and ketamine, a dissociative anesthetic similar in its action to phencyclidine, produced the same type of disruptive effects in the 5CSRTT, including signs often associated with reduced motivation and processing deficits. These findings collectively support the hypotheses that the hallucinogenic effects of salvinorin A arise from a different neural mechanism (KOP receptor agonism) than that associated with the classic hallucinogens that act as 5-HT2A receptor agonists (Nichols, 2004) and that KOP receptors might be involved the cognitive dysfunction present in psychiatric illnesses such as schizophrenia.
B. Behavioral Evaluation of Salvinorin A
The endogenous KOP receptor system has been implicated in various diverse disease states. Agonists of MOP, KOP, and DOP receptors have been shown to mediate pain response and are intriguing targets for the treatment of various other centrally mediated phenomena, including mood disorders, stress, and psychosis, and for mediating reward. The therapeutic potential of salvinorin A and analogs has thus been thoroughly examined as potential treatments of these various conditions.
1. Antinociception Studies.
Several studies examined the effects of salvinorin A in animal models of antinociception. Wang et al. (2005) reported that salvinorin A administered to mice (at doses up to 40–50 mg/kg s.c.) produced low and inconsistent effects in the acetic acid abdominal constriction test as well as against compound 48/80-induced scratching, assays in which classic KOP agonists demonstrate antinociceptive activity. However, a subsequent study by McCurdy et al. (2006) reported that salvinorin A administered intraperitoneally to mice produced transient and relatively weak antinociception as well as hypothermia. In a subsequent study, Wang et al. (2008) reported that the presumably metabolically stabilized salvinorin A analog 2-methoxymethyl-salvinorin B (94) produced antinociception and hypothermia in rats. The discrepant results reported by Wang et al. (2005) and McCurdy et al. (2006) are most likely explained by differences in route of administration and pharmacokinetics. In nonhuman primates, salvinorin A has a short half-life in plasma but a longer elimination half-life (Schmidt et al., 2005a). In the rat, salvinorin A (intraperitoneal administration) rapidly produces high plasma levels and is then eliminated with a half-life of 75 min (Teksin et al., 2009). Because lower doses of salvinorin A do have effects in other bioassay systems, such as drug discrimination (Willmore-Fordham et al., 2007), and other bioassays (for example, Carlezon et al., 2006), it is also possible that factors other than pharmacokinetics might explain the inactivity of salvinorin A in the acetic acid writhing test and the scratch test as reported by Wang et al. (2005).
Consistent with the experiments reported by Wang et al. (2005), Ansonoff et al. (2006) also observed no salvinorin A-induced antinociception in the radiant heat tail-flick assay when salvinorin A (5 mg/kg i.p.) was administered to mice. However, intracerebroventricular salvinorin A did produce antinociception and hypothermia, an effect that was also not observed in KOP receptor knockout mice. This observation provides evidence that these effects of salvinorin A require the KOP receptor. John et al. (2006) also provided a key role for the KOP receptor in mediating salvinorin A antinociception. These investigators reported that intrathecally administered salvinorin A produced dose-dependent antinociception in the mouse tail-flick test and that this effect was completely attenuated by pretreatment with the KOP receptor antagonist norBNI. Pretreatment with the MOP-selective antagonist β-funaltrexamine or the DOP-selective antagonist naltrindole did not reduce the peak antinociceptive effect of salvinorin A at 10 min.
2. Studies Examining Stress, Mood, and Reward.
A growing literature supports a role of the endogenous KOP receptor system with mood disorders, stress, psychosis, and brain reward mechanisms (for review, see Kreek, 1996; Rothman et al., 2000; Sheffler and Roth, 2003; Shippenberg et al., 2007; Carlezon et al., 2009; Mysels and Sullivan, 2009; Knoll and Carlezon, 2010). Not surprisingly, salvinorin A and related analogs have been tested in animal models related to these endpoints. It is well established by in vivo microdialysis studies that opioid agonists such as U69,593 and U50,488 decrease dopamine (DA) levels in the caudate and nucleus accumbens of mice and rats (Di Chiara and Imperato, 1988; Spanagel et al., 1992). Consistent with this action, KOP agonists are known to produce aversive effects in a variety of behavioral assays, such as conditioned place preference (CPP) (Shippenberg and Herz, 1987), intracranial self-stimulation (ICSS) (Todtenkopf et al., 2004), and immobility in the forced swim test (FST) (Mague et al., 2003). Agonists of KOP receptors are also reported to decrease cocaine-induced behavioral sensitization (Heidbreder et al., 1993), to reduce cocaine self-administration (Glick et al., 1995; Schenk et al., 1999), and to attenuate cocaine-induced reinstatement of cocaine self-administration (Schenk et al., 1999).
Consistent with the known pharmacology of KOP agonists, Morani et al. (2009) reported that salvinorin A and other KOP agonists (U50,488 and spiradoline) attenuated cocaine-induced reinstatement of cocaine self-administration, after enough time had elapsed such that responding for cocaine had extinguished. Moreover, Zhang et al. (2005) reported that salvinorin A significantly decreased extracellular DA in the caudate of C57BL/J6 mice at doses of 1.0 and 3.2 mg/kg i.p. and in the nucleus accumbens at 3.2 mg/kg i.p. Moreover, salvinorin A produced an aversive response in the CPP test that was reversed by the KOP antagonist norBNI. Similar results were reported by Carlezon et al. (2006) in the nucleus accumbens of rats. In that study, salvinorin A (1.0 mg/kg i.v.) decreased extracellular DA but not 5-HT. Gehrke et al., (2008) extended these findings. This study showed that administration of salvinorin A (1.0 and 3.2 mg/kg i.p.) decreased extracellular DA in the nucleus accumbens. It is noteworthy that repeated daily administration of salvinorin A for 5 days did not alter extracellular DA levels 48 h after the last injection, although long-term treatment with salvinorin A (3.2 mg/kg) enhanced cocaine-induced increases in extracellular DA but not cocaine-induced locomotor activity. These results differ from previous experiments conducted with U69,593, which showed that long-term administration of U69,593 reduced cocaine-induced locomotor activity (Heidbreder et al., 1998). The different results obtained in these two studies with salvinorin A and U69,593 suggest that salvinorin A may produce different results than synthetic KOP agonists, a finding that is consistent with differential effects observed in a cellular model system (Wang et al., 2005).
A study by Braida et al. (2008), conducted in rats, suggests that the actions of salvinorin A on extracellular DA and CPP may be more complex than previously appreciated. In this study, salvinorin A was administered to rats over a broad range of doses (0.05–160 μg/kg i.p.). Salvinorin A produced a dose-dependent positive CPP response (0.05–40 μg/kg), a neutral response at a dose of 80 μg/kg, and a negative CPP response a dose of 160 μg/kg. The maximum CPP response observed with the 40 μg/kg dose was reduced by the CB1 receptor inverse-agonist rimonabant as well as norBNI. In contrast to other studies (Zhang et al., 2005; Gehrke et al., 2008), 40 μg/kg salvinorin A increased extracellular DA by ∼150% of baseline for 140 min in the shell of the nucleus accumbens. Consistent with the salvinorin A-induced increase in extracellular DA observed in this study, intracerebroventricular salvinorin A supported self-administration behavior, an effect that was attenuated by either rimonabant or norBNI. Beerepoot et al. (2008) also reported a finding, similar to that of Braida et al. (2008), that low and high doses of salvinorin A have opposite effects in a behavioral assay. This study observed that cotreatment of rats with U69,503 and the D2/D3 agonist quinpirole produced a potentiated degree of locomotor sensitization. In contrast, salvinorin A potentiated (2.0 mg/kg), had no effect (0.4 mg/kg), or attenuated (0.04 mg/kg) quinpirole-induced locomotor sensitization.
ICSS is a behavioral assay in which electrodes are surgically implanted in a brain area associated with mediating reward, such as the medial forebrain bundle at the level of the lateral hypothalamus, the ventral tegmental area, or the prefrontal cortex. Rats are then trained on a schedule of reinforcement to self-administer pulses of electricity-induced brain stimulation. All addictive drugs, when acutely administered, lower the threshold required to maintain ICSS. This is interpreted as the ability of these drugs to enhance the stimulating effects of ICSS (Kornetsky et al., 1979). In contrast, aversive drugs, such as the KOP agonist U69,593, increase the threshold required to maintain ICSS, an effect similar to that which occurs after withdrawal from various substances of abuse (Todtenkopf et al., 2004).
Carlezon et al. (2006) extended these observations to salvinorin A. This study demonstrated that short-term administration of salvinorin A to rats (0.125–2.0 mg/kg i.p.) did not affect locomotor activity but dose-dependently elevated the threshold for ICSS. In addition, salvinorin A administration increased immobility and decreased swimming behavior in the FST, an action opposite that observed with a wide variety of different antidepressant treatments (Cryan et al., 2002). These findings, together with other evidence, have raised the possibility that selective KOP antagonists might have clinical utility as antidepressants (Pliakas et al., 2001; Mague et al., 2003) or treatments for drug addiction (Rothman et al., 2000; Gerra et al., 2006; McCann, 2008). Béguin et al. (2008) subsequently showed that the orally active N-methylacetamide analog of salvinorin A (N-acetyl-N-methyl-2-amido salvinorin B; 69) also increased the ICSS threshold.
As noted above, Carlezon et al. (2006) also reported that salvinorin A decreased extracellular DA, as measured with in vivo microdialysis. Ebner et al. (2010) extended these observations by reporting that salvinorin A administration increased the ICSS threshold, decreased phasic DA release in the nucleus accumbens core and shell, and significantly lowered the break point on a progressive ratio responding for sucrose, suggesting that salvinorin A can decrease motivated behavior. As noted for CPP studies, lower doses of salvinorin A (10–80 μg/kg i.p.) had antidepressant effects, as assessed by swimming time in the FST (Braida et al., 2009). Further research will be needed to reconcile the apparently contradictory findings reported by Braida et al. (2008, 2009) and Carlezon et al. (2006). One possible key difference in the experimental paradigm used by these two groups is that whereas Braida et al. (2008, 2009) administered salvinorin A once before the FST procedure, Carlezon et al. (2006) administered salvinorin A three times, at 1, 19, and 23 h after the first baseline exposure to forced swimming. It should be noted that Braida et al. (2009) also observed anxiolytic effects, rather than aversive effects, in the elevated plus maze test after salvinorin A administration. Both the KOP receptor antagonist norBNI and the cannabinoid CB1 receptor antagonist N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) blocked the anxiolytic and antidepressant effect. Given that salvinorin A has very low affinity for CB1 receptors and lacks activity in various assays for CB1 receptor activation (Braida et al., 2009; Walentiny et al., 2010), the involvement of the CB1 receptor in some of the actions of salvinorin A is likely to involve indirect mechanisms. The report by Walentiny et al. (2010) demonstrated that the CB-related effects of salvinorin A are mediated not by direct modulation of CB receptors or indirect modulation of the endocannabinoid system but by direct activation of KOP receptors.
A number of studies indicate that salvinorin A acts differently than synthetic KOP agonists. Some of these were described above and will not be elaborated upon here (Beerepoot et al., 2008; Braida et al., 2008, 2009; Gehrke et al., 2008). In vitro studies demonstrated differential effects of salvinorin A and standard KOP agonists. For example, Wang et al. (2005) reported that although salvinorin A and U50,488 had similar potency in stimulation of [35S]GTP-γ-S binding, salvinorin A was approximately 40-fold less potent in inducing KOP receptor internalization. Xu et al. (2008) examined the effect of long-term treatment of Chinese hamster ovary cells that express the human KOP receptor with various KOP agonists on the expression of Gα12, a G-protein that is up-regulated in the brain of rats rendered tolerant and dependent on morphine (Xu et al., 2005). Whereas (−)-U50,488 and (−)-ethylketocyclazocine up-regulated Gα12, salvinorin A, etorphine, and U69,593 did not. Examining the proliferation of immortalized astrocytes, McLennan et al. (2008) observed that U69,593 rapidly activated extracellular signal-regulated kinase 1/2, an effect that was sustained for more than 2 h, and induced a proliferative response. In contrast, C-2-methoxymethyl salvinorin B (94) produced the rapid, but not the sustained, activation of extracellular signal-regulated kinase 1/2 and did not induce proliferation. These observations are consistent with the occurrence of functional selectivity, which is well established for many GPCRs (Kenakin, 2003). Rothman et al. (2007) reported that salvinorin A, unlike (−)-U50,488, partially inhibited MOP receptor binding and allosterically modulated the MOP receptor. It should be noted that other researchers have not observed partial inhibition of MOP receptor binding by salvinorin A (Wang et al., 2005). The reasons for these discrepant results remain to be clarified. Finally, Grilli et al. (2009) and Phipps and Butterweck (2010) observed differences in the effects of U69,593 and salvinorin A in neurochemical and behavioral experiments, respectively.
C. Pharmacological Evaluation of Herkinorin
An interesting development in the assessment of salvinorin A analogs was the evaluation of herkinorin (47) (Harding et al., 2005a). This compound has high affinity for the MOP receptor (Ki = 12 ± 1 nM) and, in the [35S]GTP-γ-S functional binding assay, it has an EC50 value of 500 ± 140 nM compared with 1320 ± 150 nM at the KOP receptor (Table 1). Unlike morphine and [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin, herkinorin does not promote recruitment of β-arrestin-2 to the MOP receptor and does not produce receptor internalization, even in the presence of G protein-coupled receptor kinase overexpression (Groer et al., 2007). A subsequent study identified four herkinorin analogs, three of which also did not promote recruitment of β-arrestin-2 to the MOP receptor or receptor internalization (Tidgewell et al., 2008). Herkinorin therefore provides a striking example of functional selectivity in opioid receptor pharmacology (Urban et al., 2007).
Herkinorin, as a ligand that does not promote MOP receptor internalization, has been a useful tool for studying the role of MOP receptor internalization in the development of opioid tolerance and dependence in a cellular model system. Thus, Xu et al. (2007) studied the effect of long-term treatment of Chinese hamster ovary cells that express the cloned human MOP receptor with internalizing ([d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin) and noninternalizing (herkinorin) MOP agonists. The results indicated that although each agonist produced a different spectrum of effects on cellular markers of tolerance and dependence, both agonists do produce tolerance and dependence, indicating that the absence or presence of MOP receptor internalization is not a critical factor in the development of cellular tolerance and dependence. A particular difference between these two types of MOP receptor agonists was that long-term exposure of herkinorin induced the formation of constitutively active MOP receptors to a profound degree. The evidence supporting the assertion that long-term herkinorin administration produced constitutively active MOP receptors was 3-fold: 1) an increase of basal [35S]GTP-γ-S binding, an effect that was reversed by the addition of antagonists; 2) an increase in the basal Bmax of the high-affinity agonist-responsive [35S]GTP-γ-S binding site; and 3) a great (approximately 60%) reduction of forskolin-stimulated cAMP accumulation. This action proved useful in generating a cellular system with which to facilitate the identification of neutral MOP receptor antagonists (Sally et al., 2010). Studies in nonhuman primates provided neuroendocrine evidence for both MOP agonist and partial KOP agonist effects of herkinorin (Butelman et al., 2008). It is clear that more studies must be initiated to facilitate understanding of this unique ligand.
D. Effects of Salvinorin A in Humans
As reviewed elsewhere (Vortherms and Roth, 2006), when smoked, salvinorin A produces a rapid and intense hallucinatory effect that typically lasts between 10 and 15 min and includes a “highly modified perception of external reality” (González et al., 2006). In many countries, including the United States, it is legal to purchase S. divinorum from internet suppliers. Twenty-three U.S. states have passed legislation controlling its use and sale (Siebert, 2010), 15 of which have made possession or use of S. divinorum illegal, and the U.S. Drug Enforcement Administration regards S. divinorum as a “drug of concern” (Brown, 2009).
The first anecdotal reports of the effect of salvinorin A in humans were described in the mid-1990s (Siebert, 1994; Ott, 1995). Various methods of administration of salvinorin A were evaluated, including absorption through the oral mucosa and inhalation of vaporized material. Oral administration of encapsulated salvinorin A produced no effects. Vaporization of 200 to 500 μg of salvinorin A produced subjective effects similar to those of the smoked herb, and threshold effect was typically noted around 200 μg. When inhaled in this manner, salvinorin A produced effects with a fast onset of action of approximately 30 s, peak effects lasting approximately 5 to 10 min before a gradual decrease over 20 to 30 min. Salvinorin A (2 mg in 1 ml of ethanol) absorbed through oral mucosa had a slightly delayed onset of action compared with vaporized material (5–10 min versus 30 s). The peak effects of orally absorbed material were reported to gradually build and last for approximately 1 h, before gradually subsiding over another 1-h period. It was speculated that gradual release of salvinorin A from the oral mucosa was responsible for the observed duration of effects; however, the effect of solvation in ethanol was not addressed in this study. A report in 1995 also described pharmacological effects of salvinorin A in humans after multiple routes of administration (Ott, 1995). In contrast to the Siebert (1994) study, Ott (1995) reported that infusions of S. divinorum and sublingual administration of a 1% solution in acetone were able to produce visionary effects at doses as low as 100 μg, 250 μg to 1 mg producing definite psychoactivity. As with the Siebert (1994) study, it should be noted that this route of administration used acetone and DMSO solvation, both of which are irritants, with DMSO capable of potentiating or producing psychological effects.
Since these studies, two controlled trials using human volunteers have been reported. Mendelson et al. (2010) aimed to thoroughly investigate the effect of salvinorin A when administered sublingually. This placebo-controlled study examined the subjective effects (Subjective Drug Effects Questionnaire, Altered States of Consciousness Questionnaire, and Positive and Negative Affect Schedule) and physiological effects (heart rate, blood pressure, O2 saturation, and body temperature) of an ascending dose (0–4000 μg sublingual) of salvinorin A solvated in 25% DMSO/75% polyethylene glycol. The effects of salvinorin A administered in this way were not significantly different from those of placebo at even the highest doses (4000 μg), because there was no effect of dose on responses to the Subjective Drug Effects Questionnaire, Altered States of Consciousness Questionnaire, and Positive and Negative Affect Schedule. Heart rate, blood pressure, O2 saturation, and core temperature remained normal in this study, suggesting a favorable safety profile or lack of bioavailability of salvinorin A administered in this way. In addition, blood and urine samples were collected after administration of the highest dose of salvinorin A; most concentrations were found to be below the limit of quantitation.
Another double-blind, placebo-controlled study to report controlled use of salvinorin A in human trials has only recently been accepted for publication (Johnson et al., 2011). Here, a group of four volunteers was given increasing doses (0.375–21 μg/kg) of vaporized salvinorin A, and subjective effects (drug strength) and physiological effects (safety and tolerability, heart rate, blood pressure) were measured over a period of 60 min. After each session, volunteers were then asked to complete questionnaires designed to measure dysphoric effects (drug “liking” versus “disliking”), hallucinogenic effects (Hallucinogen Rating Scale) (Strassman et al., 1994), and mystical effects (Mysticism Scale) (Hood et al., 2001) experienced during the session. Time- and dose-related effects were observed: subject-rated drug strength peaked at the first time point (2 min) and gradually diminished over a 20-min period. The hallucinogenic and mystical effects of salvinorin A appeared similar to those produced by intravenously administered DMT and oral psilocybin. It is noteworthy that there was no significant effect of dose on blood pressure and heart rate, and no resting or kinetic tremors were observed during any session, suggesting a safe physiological profile of salvinorin A at the given doses under the controlled conditions of the study.
Survey-based studies describing the pattern of use and usage behavior have also been reported (González et al., 2006; Baggott et al., 2010; Sumnall et al., 2010). These reports are in general agreement that salvinorin A produces intense psychological effects, and Baggott et al. (2010) described that users reported positive after-effects (increased insight, improved mood) lasting for more than 24 h after use of S. divinorum. The survey reported by Sumnall et al. (2010) found that respondent use of S. divinorum was not categorized as a disorder according to the Severity of Dependence Scale (Gossop et al., 1995). This is in agreement with preclinical data suggesting that KOP receptor agonists are less reinforcing than MOP and DOP receptor agonists (Young et al., 1984; Shippenberg et al., 1987; Woods and Winger, 1987; Sumnall et al., 2010). It should be stressed, however, that, like other entheogens, S. divinorum, and salvinorin A in particular, produces very potent visionary effects that contribute to its recreational use, which is gaining in popularity. Because the concept of “drug abuse” considers factors such as “use for nontherapeutic effects,” in this context, salvinorin A may be considered an agent with recreational abuse liability similar to that of LSD. However, the results of the first randomized human trials have shown a safe physiological profile (heart rate, blood pressure, etc.) associated with exposure to S. divinorum and seem to suggest that there is low potential for drug-induced rewarding behavior. It should be noted that KOP receptor agonists generally produce dysphoria, as opposed to MOP receptor agonists, which produce euphoria, and have not been classified as controlled substances in the United States. Taken together, there is a great need for more studies to further evaluate the recreational abuse liabilities of S. divinorum and salvinorin A.
VI. Summary and Future Directions
Natural product lead optimization has represented a great resource for the development of novel therapeutics (Koehn and Carter, 2005). As a CNS receptor probe, salvinorin A has thus far already challenged many preconceived notions of opioid receptors and opioid ligands as therapeutic tools. Data regarding salvinorin A have called into question the idea that a basic amino substituent is required for opioid receptor binding. It was then shown that salvinorin A does not produce hallucinations mediated by the classic target of hallucinogenic natural products, the 5-HT2A receptor, but rather produces hallucinogenic effects through KOP receptor activation that are unique from other KOP receptor agonists. This raises even more questions about the intricate neurochemical pathways involved in the psychoactive effects of hallucinogenic agents. As a secondary metabolite possessing unique structural and pharmacological profiles, salvinorin A has produced a wealth of information about non-nitrogenous opioid receptor ligands and has rejuvenated interest in KOP receptor ligands as therapeutic targets (for a recent review of the therapeutic potential of KOP receptor agonists, please see Aldrich and McLaughlin, 2009).
Modulation of KOP receptor activation represents a unique approach toward treating schizophrenia. Typical antipsychotics act as modulators of DA receptors in the CNS and are prone to produce invasive side effects, which may vary from involuntary Parkinson-like tremors to akathisia and tardive dyskinesia. Although second-generation, atypical antipsychotics have a reduced instance of extrapyramidal effects, it remains to be seen whether the development of tardive dyskinesia remains a concern with prolonged administration (i.e., decades). A growing body of evidence (for a comprehensive review, see Schwarzer, 2009) suggests that the perceptual disturbances associated with schizophrenia may be mediated in part by dysregulation of the release of the endogenous KOP agonist dynorphin (Heikkilä et al., 1990; Hurd, 2002; Bortolato and Solbrig, 2007). In studies monitoring prepulse inhibition in Sprague-Dawley rats as a measure of sensorimotor gating in patients with schizophrenia, KOP agonists such as U50,488 have been found to produce dose-dependent reduction in prepulse inhibition that is reversed upon administration of the KOP receptor antagonist norBNI (Bortolato et al., 2005). A second group (Tejeda et al., 2010) failed to confirm these findings, however, indicating that more studies are required to understand the utility of KOP receptor activity in psychotic disorders. Salvinorin A-based antagonists may thus be beneficial in treating the sensorimotor deficits associated with this psychological disorder.
The suggestion that salvinorin A is an allosteric modulator of MOP receptors introduces the therapeutic potential of allosteric modulators of opioid receptors. Allosteric modulators of GPCR have the potential to potentiate the effects of endogenously expressed CNS receptor ligands, with the ceiling effect of the allosteric modulator potentially reducing the severity of narcotic effects currently associated with opioid overdose (Christopoulos and Kenakin, 2002). Before salvinorin A, cannabidiol was reported as an allosteric modulator of MOP and DOP receptors in two independent studies (Vaysse et al., 1987; Kathmann et al., 2006). The SAR highlighted here demonstrate that the salvinorin A scaffold can be derivatized to modify affinity and efficacy at all opioid receptor subtypes (MOP, DOP, KOP), and salvinorin A-based probes could direct investigation into a new class of neuroactive agents.
The C-2-benzoyl derivative herkinorin (47) is an illustration of how natural product-based optimization can lead to novel probes of GPCR signaling mechanisms. In addition to greatly increased MOP receptor binding affinity and selectivity compared with salvinorin A, herkinorin exhibits a profile of MOP receptor activation unique among opioid ligands. In particular, activation of MOP receptors by herkinorin does not promote recruitment of β-arrestin-2 to receptor surfaces and also does not promote receptor internalization. Conversely, the C-2-benzamide derivative 73 displays potent MOP agonism yet also produces β-arrestin-2 recruitment and receptor internalization. That such a subtle structural modification can produce vastly different cellular responses may lead to insights that uncover the ligand-receptor interactions responsible for functional selectivity in GPCRs. The concept of functional selectivity has been well characterized among GPCR (Urban et al., 2007). Functionally selective 5-HT2A receptor ligands, for example, have therapeutic potential for treatment of cognitive and mental deficits without hallucinogenic side effects commonly associated with 5-HT2A receptor activation. Few functionally selective 5-HT receptor agonists exist, however, and natural sources may provide new scaffolds for probing interactions between 5-HTR ligands with various G-protein signaling pathways. Likewise, salvinorin A analogs herkinorin and 73 are already useful tools for continuing to develop the concept of functionally selective opioid ligands as therapeutic targets (Keith et al., 1996; Whistler et al., 1999).
The future of salvinorin A will revolve around further elucidation of the mechanisms responsible for its observed behavioral and psychological effects and optimization of this skeleton to improve its therapeutic profile. In particular, although its short duration of action has been well established, the exact mechanisms behind the metabolism and elimination of salvinorin A largely remain a mystery, as do any mechanisms of tolerance that may arise from long-term administration. Elucidating these biological processes of inactivation will be crucial for increasing the duration of action to therapeutically relevant levels. Furthermore, separating the hallucinogenic actions of KOP receptor agonists would be crucial for developing clinically relevant pharmacotherapies, which is a contributor to the lack of success of previously developed KOP receptor agonists (for reviews, please see Barber and Gottschlich, 1997; DeHaven-Hudkins and Dolle, 2004). Despite the wealth of knowledge gained thus far regarding the SAR of salvinorin A at KOP receptors, much work remains to be done. In particular, the structural requirements of the B ring of salvinorin A have been largely uncharacterized, as have the contributions of the α methyl groups at C-5 and C-9. This is largely due to the lack of established methods that would produce selective transformation of these regions. The future of SAR development of salvinorin A therefore rests on the completion of a scalable total synthesis that would allow for selective modifications of these skeletal regions with relative ease.
Natural products are a fertile source of inspiration for medicinal chemists, and salvinorin A is a fine example of the power and scope of medicinal chemistry. Ethnopharmacological observation of the psychotropic effects of S. divinorum led to the arduous task of the initial isolation of salvinorin A; chemical methods were developed to modify the sensitive tricyclic scaffold, producing hundreds of analogs; and these analogs have, in turn, been tested and analyzed in cells, in rodents, and in primates, with these results collectively used to develop computational models designed to describe and predict biological activity. In summation, the results reviewed here are evidence supporting an assertion made by Prof. Samuel Danishefsky in 2002 regarding the role of natural products in modern drug development (Borman, 2002; Hudlicky and Reed, 2007; Danishefsky, 2010): “a small collection of smart compounds may be more valuable than a much larger hodgepodge collection mindlessly assembled.”
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse [Grant DA-018151] (to T.E.P.) and, in part, by the Intramural Research Program of the National Institute on Drug Abuse (to R.B.R.).
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Cunningham, Rothman, and Prisinzano.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.110.003244.
- CNS
- central nervous system
- CB
- cannabinoid
- KOP
- κ-opioid
- 5-HT
- 5-hydroxytryptamine (serotonin)
- LSD
- lysergic acid diethylamide
- SAR
- structure-activity relationship
- DMT
- N,N-dimethyltryptamine
- U50,488
- (−)-(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidiny)cyclohexyl]benzeneacetamide
- MOP
- μ-opioid
- HPLC
- high-performance liquid chromatography
- MS
- mass spectrometry
- LC
- liquid chromatography
- UGT
- UDP glucuronosyltransferase
- DA
- dopamine
- P-gp
- P-glycoprotein
- DOP
- δ-opioid
- norBNI
- nor-binaltorphimine
- TM
- transmembrane domain
- U69,593
- (5α,7α,8β)-(+)-N-methyl-N-(7-(1-pyrrolidinyl)-1-oxaspiro(4,5)dec-8-yl)benzeneacetamide
- EL
- extracellular loop
- GPCR
- G-protein-coupled receptor
- NMDA
- N-methyl-d-aspartate
- 5CSRTT
- five-choice serial reaction time task
- CPP
- conditioned place preference
- ICSS
- intracranial self-stimulation
- FST
- forced swim test
- AM251
- N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- GTP-γ-S
- guanosine 5′-O-(3-thio)triphosphate
- DMSO
- dimethyl sulfoxide
- SNC-80
- 4-[(R)-[(2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide.
References
- Aldrich JV, McLaughlin JP. (2009) Peptide kappa opioid receptor ligands: potential for drug development. AAPS J 11:312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansonoff MA, Zhang J, Czyzyk T, Rothman RB, Stewart J, Xu H, Zjwiony J, Siebert DJ, Yang F, Roth BL, et al. (2006) Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of kappa-opioid receptor-1 knockout mice. J Pharmacol Exp Ther 318:641–648 [DOI] [PubMed] [Google Scholar]
- Babu KM, McCurdy CR, Boyer EW. (2008) Opioid receptors and legal highs: Salvia divinorum and Kratom. Clin Toxicol (Phila) 46:146–152 [DOI] [PubMed] [Google Scholar]
- Baggott MJ, Erowid E, Erowid F, Galloway GP, Mendelson J. (2010) Use patterns and self-reported effects of Salvia divinorum: An internet-based survey. Drug Alcohol Depend 111:250–256 [DOI] [PubMed] [Google Scholar]
- Baker LE, Panos JJ, Killinger BA, Peet MM, Bell LM, Haliw LA, Walker SL. (2009) Comparison of the discriminative stimulus effects of salvinorin A and its derivatives to U69,593 and U50,488 in rats. Psychopharmacology (Berl) 203:203–211 [DOI] [PubMed] [Google Scholar]
- Barber A, Gottschlich R. (1997) Novel developments with selective, non-peptidic kappa-opioid receptor agonists. Expert Opin Investig Drugs 6:1351–1368 [DOI] [PubMed] [Google Scholar]
- Barnes S, Prasain JK, Wang CC, Moore DR., 2nd (2006) Applications of LC-MS in the study of the uptake, distribution, metabolism and excretion of bioactive polyphenols from dietary supplements. Life Sci 78:2054–2059 [DOI] [PubMed] [Google Scholar]
- Beerepoot P, Lam V, Luu A, Tsoi B, Siebert D, Szechtman H. (2008) Effects of salvinorin A on locomotor sensitization to D2/D3 dopamine agonist quinpirole. Neurosci Lett 446:101–104 [DOI] [PubMed] [Google Scholar]
- Béguin C, Duncan KK, Munro TA, Ho DM, Xu W, Liu-Chen LY, Carlezon WA, Jr, Cohen BM. (2009) Modification of the furan ring of salvinorin A: identification of a selective partial agonist at the kappa opioid receptor. Bioorg Med Chem 17:1370–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin C, Potter DN, Dinieri JA, Munro TA, Richards MR, Paine TA, Berry L, Zhao Z, Roth BL, Xu W, et al. (2008) N-methylacetamide analog of salvinorin A: a highly potent and selective kappa-opioid receptor agonist with oral efficacy. J Pharmacol Exp Ther 324:188–195 [DOI] [PubMed] [Google Scholar]
- Béguin C, Richards MR, Li JG, Wang Y, Xu W, Liu-Chen LY, Carlezon WA, Jr, Cohen BM. (2006) Synthesis and in vitro evaluation of salvinorin A analogues: effect of configuration at C(2) and substitution at C(18). Bioorg Med Chem Lett 16:4679–4685 [DOI] [PubMed] [Google Scholar]
- Béguin C, Richards MR, Wang Y, Chen Y, Liu-Chen LY, Ma Z, Lee DY, Carlezon WA, Jr, Cohen BM. (2005) Synthesis and in vitro pharmacological evaluation of salvinorin A analogues modified at C(2). Bioorg Med Chem Lett 15:2761–2765 [DOI] [PubMed] [Google Scholar]
- Bergman YE, Mulder R, Perlmutter P. (2009) Total synthesis of 20-norsalvinorin A. 1. Preparation of a key intermediate. J Org Chem 74:2589–2591 [DOI] [PubMed] [Google Scholar]
- Bertea CM, Luciano P, Bossi S, Leoni F, Baiocchi C, Medana C, Azzolin CM, Temporale G, Lombardozzi MA, Maffei ME. (2006) PCR and PCR-RFLP of the 5S-rRNA-NTS region and salvinorin A analyses for the rapid and unequivocal determination of Salvia divinorum. Phytochemistry 67:371–378 [DOI] [PubMed] [Google Scholar]
- Bigham AK, Munro TA, Rizzacasa MA, Robins-Browne RM. (2003) Divinatorins A-C, new neoclerodane diterpenoids from the controlled sage Salvia divinorum. J Nat Prod 66:1242–1244 [DOI] [PubMed] [Google Scholar]
- Bikbulatov RV, Stewart J, Jin W, Yan F, Roth BL, Ferreira D, Zjawiony JK. (2008) Short synthesis of a novel class of salvinorin A analogs with hemiacetalic structure. Tetrahedron Lett 49:937–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikbulatov RV, Yan F, Roth BL, Zjawiony JK. (2007) Convenient synthesis and in vitro pharmacological activity of 2-thioanalogs of salvinorins A and B. Bioorg Med Chem Lett 17:2229–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman S. (2002) Organic lab sparks drug discovery. Chem Eng News 80:23–24 [Google Scholar]
- Bortolato M, Aru GN, Frau R, Orrù M, Fà M, Manunta M, Puddu M, Mereu G, Gessa GL. (2005) Kappa opioid receptor activation disrupts prepulse inhibition of the acoustic startle in rats. Biol Psychiatry 57:1550–1558 [DOI] [PubMed] [Google Scholar]
- Bortolato M, Solbrig MV. (2007) The price of seizure control: dynorphins in interictal and postictal psychosis. Psychiatry Res 151:139–143 [DOI] [PubMed] [Google Scholar]
- Braida D, Capurro V, Zani A, Rubino T, Viganò D, Parolaro D, Sala M. (2009) Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br J Pharmacol 157:844–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Limonta V, Capurro V, Fadda P, Rubino T, Mascia P, Zani A, Gori E, Fratta W, Parolaro D, et al. (2008) Involvement of kappa-opioid and endocannabinoid system on Salvinorin A-induced reward. Biol Psychiatry 63:286–292 [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Pegorini S, Zani A, Guerini-Rocco C, Gori E, Sala M. (2007) Hallucinatory and rewarding effect of salvinorin A in zebrafish: kappa-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology (Berl) 190:441–448 [DOI] [PubMed] [Google Scholar]
- Brown DJ. (2009) Neuroscience. Salvia on schedule. Scientific American 301:20–21 [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. (2009) kappa-Opioid receptor signaling and brain reward function. Brain Res Rev 62:127–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AC, Forsyth CJ. (2008) Intramolecular Diels-Alder/Tsuji allylation assembly of the functionalized trans-decalin of salvinorin A. Org Lett 10:97–100 [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek MJ. (2004) The plant-derived hallucinogen, salvinorin A, produces kappa-opioid agonist-like discriminative effects in rhesus monkeys. Psychopharmacology (Berl) 172:220–224 [DOI] [PubMed] [Google Scholar]
- Butelman ER, Rus S, Prisinzano TE, Kreek MJ. (2010) The discriminative effects of the kappa-opioid hallucinogen salvinorin A in nonhuman primates: dissociation from classic hallucinogen effects. Psychopharmacology (Berl) 210:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Rus S, Simpson DS, Wolf A, Prisinzano TE, Kreek MJ. (2008) The effects of herkinorin, the first mu-selective ligand from a salvinorin A-derived scaffold, in a neuroendocrine biomarker assay in nonhuman primates. J Pharmacol Exp Ther 327:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Capasso F, Siebert DJ, Stewart DJ, Zjawiony JK, Izzo AA. (2006) The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A inhibit enteric cholinergic transmission in the guinea-pig ileum. Neurogastroenterol Motil 18:69–75 [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. (2006) Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316:440–447 [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Béguin C, Knoll AT, Cohen BM. (2009) Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther 123:334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EE. (2010) Natural products as chemical probes. ACS Chem Biol 5:639–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casy AF, Parfitt RT. (1986) Opioid Analgesics, Plenum Press, New York [Google Scholar]
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. (2004) Salvinorin A, an active component of the hallucinogenic sage Salvia divinorum is a highly efficacious kappa-opioid receptor agonist: structural and functional considerations. J Pharmacol Exp Ther 308:1197–1203 [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. (2002) G protein-coupled receptor allosterism and complexing. Pharmacol Rev 54:323–374 [DOI] [PubMed] [Google Scholar]
- Coffman BL, King CD, Rios GR, Tephly TR. (1998) The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268). Drug Metab Dispos 26:73–77 [PubMed] [Google Scholar]
- Coffman BL, Rios GR, King CD, Tephly TR. (1997) Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab Dispos 25:1–4 [PubMed] [Google Scholar]
- Craft RM. (2003) Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain 19:175–186 [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–245 [DOI] [PubMed] [Google Scholar]
- Cunningham CW, Mercer SL, Hassan HE, Traynor JR, Eddington ND, Coop A. (2008) Opioids and efflux transporters. Part 2: P-glycoprotein substrate activity of 3- and 6-substituted morphine analogs. J Med Chem 51:2316–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais C, Graff CL, Pollack GM. (2004) Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem Pharmacol 67:269–276 [DOI] [PubMed] [Google Scholar]
- Danishefsky S. (2010) On the potential of natural products in the discovery of pharma leads: a case for reassessment. Nat Prod Rep 27:1114–1116 [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins DL, Dolle RE. (2004) Peripherally restricted opioid agonists as novel analgesic agents. Curr Pharm Des 10:743–757 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. (2010) Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 210:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichna J, Schicho R, Janecka A, Zjawiony JK, Storr M. (2009) Selective natural kappa opioid and cannabinoid receptor agonists with a potential role in the treatment of gastrointestinal dysfunction. Drug News Perspect 22:383–392 [DOI] [PubMed] [Google Scholar]
- Fontana G, Savona G, Rodríguez B. (2006) Clerodane diterpenoids from Salvia splendens. J Nat Prod 69:1734–1738 [DOI] [PubMed] [Google Scholar]
- Fontana G, Savona G, Rodríguez B, Dersch CM, Rothman RB, Prisinzano TE. (2008) Synthetic studies of neoclerodane diterpenoids from Salvia splendens and evaluation of Opioid Receptor affinity. Tetrahedron 64:10041–10048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana G, Savona G, Rodriguez B, Dersch CM, Rothman RB, Prisinzano TE. (2009) Synthetic studies on neoclerodane diterpenes from Salvia splendens: oxidative modifications of ring A. Tetrahedron 65:1708–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedcke F. (1855) Über das Erythroxylin, dargestellt aus den Blättern des in Südamerika cultivirten Strauches Erythroxylon. Arch Pharm (Weinheim) 132:141–150 [Google Scholar]
- Gaoni Y, Mechoulam R. (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–1647 [Google Scholar]
- Gehrke BJ, Chefer VI, Shippenberg TS. (2008) Effects of acute and repeated administration of salvinorin A on dopamine function in the rat dorsal striatum. Psychopharmacology (Berl) 197:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Fantoma A, Zaimovic A. (2006) Naltrexone and buprenorphine combination in the treatment of opioid dependence. J Psychopharmacol 20:806–814 [DOI] [PubMed] [Google Scholar]
- Giroud C, Felber F, Augsburger M, Horisberger B, Rivier L, Mangin P. (2000) Salvia divinorum: an hallucinogenic mint which might become a new recreational drug in Switzerland. Forensic Sci Int 112:143–150 [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S. (1995) Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res 681:147–152 [DOI] [PubMed] [Google Scholar]
- González D, Riba J, Bouso JC, Gómez-Jarabo G, Barbanoj MJ. (2006) Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend 85:157–162 [DOI] [PubMed] [Google Scholar]
- Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W, Strang J. (1995) The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 90:607–614 [DOI] [PubMed] [Google Scholar]
- Griffin OH, Miller BL, Khey DN. (2008) Legally high? Legal considerations of Salvia divinorum. J Psychoactive Drugs 40:183–191 [DOI] [PubMed] [Google Scholar]
- Grilli M, Neri E, Zappettini S, Massa F, Bisio A, Romussi G, Marchi M, Pittaluga A. (2009) Salvinorin A exerts opposite presynaptic controls on neurotransmitter exocytosis from mouse brain nerve terminals. Neuropharmacology 57:523–530 [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. (2007) An opioid agonist that does not induce mu-opioid receptor beta-arrestin interactions or receptor internalization. Mol Pharmacol 71:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber JW, Siebert DJ, der Marderosian AH, Hock RS. (1999) High performance liquid chromatographic quantification of salvinorin A from tissues of Salvia divinorum Epling & Jativa-M. Phytochem Anal 10:22–25 [Google Scholar]
- Hagiwara H, Suka Y, Nojima T, Hoshi T, Suzuki T. (2009) Second-generation synthesis of salvinorin A. Tetrahedron 65:4820–4825 [Google Scholar]
- Hanson JR. (1992) The microbiological transformation of diterpenoids. Nat Prod Rep 9:139–151 [DOI] [PubMed] [Google Scholar]
- Harding WW, Schmidt M, Tidgewell K, Kannan P, Holden KG, Dersch CM, Rothman RB, Prisinzano TE. (2006a) Synthetic studies of neoclerodane diterpenes from Salvia divinorum: selective modification of the furan ring. Bioorg Med Chem Lett 16:3170–3174 [DOI] [PubMed] [Google Scholar]
- Harding WW, Schmidt M, Tidgewell K, Kannan P, Holden KG, Gilmour B, Navarro H, Rothman RB, Prisinzano TE. (2006b) Synthetic studies of neoclerodane diterpenes from Salvia divinorum: semisynthesis of salvinicins A and B and other chemical transformations of Salvinorin A. J Nat Prod 69:107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. (2005a) Neoclerodane diterpenes as a novel scaffold for mu opioid receptor ligands. J Med Chem 48:4765–4771 [DOI] [PubMed] [Google Scholar]
- Harding WW, Tidgewell K, Schmidt M, Shah K, Dersch CM, Snyder J, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE. (2005b) Salvinicins A and B, new neoclerodane diterpenes from Salvia divinorum. Org Lett 7:3017–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Goldberg SR, Shippenberg TS. (1993) The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res 616:335–338 [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Schenk S, Partridge B, Shippenberg TS. (1998) Increased responsiveness of mesolimbic and mesostriatal dopamine neurons to cocaine following repeated administration of a selective kappa-opioid receptor agonist. Synapse 30:255–262 [DOI] [PubMed] [Google Scholar]
- Heikkilä L, Rimón R, Terenius L. (1990) Dynorphin A and substance P in the cerebrospinal fluid of schizophrenic patients. Psychiatry Res 34:229–236 [DOI] [PubMed] [Google Scholar]
- Hjorth SA, Thirstrup K, Grandy DK, Schwartz TW. (1995) Analysis of selective binding epitopes for the kappa-opioid receptor antagonist nor-binaltorphimine. Mol Pharmacol 47:1089–1094 [PubMed] [Google Scholar]
- Hofmann A. (1980) LSD, My Problem Child, McGraw-Hill, New York [Google Scholar]
- Holden KG, Tidgewell K, Marquam A, Rothman RB, Navarro H, Prisinzano TE. (2007) Synthetic studies of neoclerodane diterpenes from Salvia divinorum: exploration of the 1-position. Bioorg Med Chem Lett 17:6111–6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzgrabe U, Brandt W. (2003) Mechanism of action of the diazabicyclononanone-type kappa-agonists. J Med Chem 46:1383–1389 [DOI] [PubMed] [Google Scholar]
- Holzman PS. (1992) Behavioral markers of schizophrenia useful for genetic studies. Journal of Psychiatric Research 26:427–445 [DOI] [PubMed] [Google Scholar]
- Hood RW, Jr, Ghorbani N, Watson PJ, Ghramaleki AF, Bing MN, Davison HK, Morris RJ, Williamson WP. (2001) Dimensions of the mysticism scale: confirming the three-factor structure in the United States and Iran. J Sci Study Relig 40:691–705 [Google Scholar]
- Hooker JM, Munro TA, Béguin C, Alexoff D, Shea C, Xu Y, Cohen BM. (2009) Salvinorin A and derivatives: protection from metabolism does not prolong short-term, whole-brain residence. Neuropharmacology 57:386–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker JM, Xu Y, Schiffer W, Shea C, Carter P, Fowler JS. (2008) Pharmacokinetics of the potent hallucinogen, salvinorin A in primates parallels the rapid onset and short duration of effects in humans. Neuroimage 41:1044–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudlicky T, Reed JW. (2007) The Way of Synthesis: Evolution of Design and Methods for Natural Products, Wiley-VCH Verlag GmbH, Weinheim, Germany [Google Scholar]
- Hurd YL. (2002) Subjects with major depression or bipolar disorder show reduction of prodynorphin mRNA expression in discrete nuclei of the amygdaloid complex. Mol Psychiatry 7:75–81 [DOI] [PubMed] [Google Scholar]
- Iadanza M, Höltje M, Ronsisvalle G, Höltje HD. (2002) Kappa-opioid receptor model in a phospholipid bilayer: molecular dynamics simulation. J Med Chem 45:4838–4846 [DOI] [PubMed] [Google Scholar]
- Jermain JD, Evans HK. (2009) Analyzing salvia divinorum and its active ingredient salvinorin A utilizing thin layer chromatography and gas chromatography/mass spectrometry. J Forensic Sci 54:612–616 [DOI] [PubMed] [Google Scholar]
- John TF, French LG, Erlichman JS. (2006) The antinociceptive effect of Salvinorin A in mice. Eur J Pharmacol 545:129–133 [DOI] [PubMed] [Google Scholar]
- Johnson MW, MacLean KA, Reissig CR, Prisinzano TE, Griffiths RR. (2011) Human psychopharmacology and dose-effects of salvinorin A, a kappa-opioid agonist hallucinogen present in the plant Salvia divinorum. Drug Alcohol Depend doi:10.1016/j.drugalcdep.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Hjorth SA, Schwartz TW, Portoghese PS. (1998) Mutational evidence for a common kappa antagonist binding pocket in the wild-type kappa and mutant mu[K303E] opioid receptors. J Med Chem 41:4911–4914 [DOI] [PubMed] [Google Scholar]
- Kalant H. (2010) Drug classification: science, politics, both or neither? Addiction 105:1146–1149 [DOI] [PubMed] [Google Scholar]
- Kane BE, McCurdy CR, Ferguson DM. (2008) Toward a structure-based model of salvinorin A recognition of the kappa-opioid receptor. J Med Chem 51:1824–1830 [DOI] [PubMed] [Google Scholar]
- Kane BE, Nieto MJ, McCurdy CR, Ferguson DM. (2006) A unique binding epitope for salvinorin A, a non-nitrogenous kappa opioid receptor agonist. FEBS J 273:1966–1974 [DOI] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. (2006) Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol 372:354–361 [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. (1996) Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem 271:19021–19024 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2003) Ligand-selective receptor conformations revisited: the promise and the problem. Trends Pharmacol Sci 24:346–354 [DOI] [PubMed] [Google Scholar]
- Kennedy JH, Wiseman JM. (2010) Direct analysis of Salvia divinorum leaves for salvinorin A by thin layer chromatography and desorption electrospray ionization multi-stage tandem mass spectrometry. Rapid Commun Mass Spectrom 24:1305–1311 [DOI] [PubMed] [Google Scholar]
- King CD, Green MD, Rios GR, Coffman BL, Owens IS, Bishop WP, Tephly TR. (1996) The glucuronidation of exogenous and endogenous compounds by stably expressed rat and human UDP-glucuronosyltransferase 1.1. Arch Biochem Biophys 332:92–100 [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr (2010) Dynorphin, stress, and depression. Brain Res 1314:56–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehn FE, Carter GT. (2005) The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4:206–220 [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. (1979) Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry 36:289–292 [DOI] [PubMed] [Google Scholar]
- Kreek MJ. (1996) Cocaine, dopamine and the endogenous opioid system. J Addict Dis 15:73–96 [DOI] [PubMed] [Google Scholar]
- Kutrzeba L, Dayan FE, Howell J, Feng J, Giner JL, Zjawiony JK. (2007) Biosynthesis of salvinorin A proceeds via the deoxyxylulose phosphate pathway. Phytochemistry 68:1872–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutrzeba LM, Ferreira D, Zjawiony JK. (2009a) Salvinorins J from Salvia divinorum: mutarotation in the neoclerodane system. J Nat Prod 72:1361–1363 [DOI] [PubMed] [Google Scholar]
- Kutrzeba LM, Karamyan VT, Speth RC, Williamson JS, Zjawiony JK. (2009b) In vitro studies on metabolism of salvinorin A. Pharm Biol 47:1078–1084 [Google Scholar]
- Kutrzeba LM, Li XC, Ding Y, Ferreira D, Zjawiony JK. (2010) Intramolecular transacetylation in salvinorins D and E. J Nat Prod 73:707–708 [DOI] [PubMed] [Google Scholar]
- Lange JE, Daniel J, Homer K, Reed MB, Clapp JD. (2010) Salvia divinorum: effects and use among YouTube users. Drug Alcohol Depend 108:138–140 [DOI] [PubMed] [Google Scholar]
- Lange JE, Reed MB, Croff JM, Clapp JD. (2008) College student use of Salvia divinorum. Drug Alcohol Depend 94:263–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson DL, Jones RM, Hjorth SA, Schwartz TW, Portoghese PS. (2000) Binding of norbinaltorphimine (norBNI) congeners to wild-type and mutant mu and kappa opioid receptors: molecular recognition loci for the pharmacophore and address components of kappa antagonists. J Med Chem 43:1573–1576 [DOI] [PubMed] [Google Scholar]
- Lavecchia A, Greco G, Novellino E, Vittorio F, Ronsisvalle G. (2000) Modeling of kappa-opioid receptor/agonists interactions using pharmacophore-based and docking simulations. J Med Chem 43:2124–2134 [DOI] [PubMed] [Google Scholar]
- Lee DY, He M, Kondaveti L, Liu-Chen LY, Ma Z, Wang Y, Chen Y, Li JG, Beguin C, Carlezon WA, Jr, et al. (2005a) Synthesis and in vitro pharmacological studies of C(4) modified salvinorin A analogues. Bioorg Med Chem Lett 15:4169–4173 [DOI] [PubMed] [Google Scholar]
- Lee DY, He M, Liu-Chen LY, Wang Y, Li JG, Xu W, Ma Z, Carlezon WA, Jr, Cohen B. (2006) Synthesis and in vitro pharmacological studies of new C(4)-modified salvinorin A analogues. Bioorg Med Chem Lett 16:5498–5502 [DOI] [PubMed] [Google Scholar]
- Lee DY, Ma Z, Liu-Chen LY, Wang Y, Chen Y, Carlezon WA, Jr, Cohen B. (2005b) New neoclerodane diterpenoids isolated from the leaves of Salvia divinorum and their binding affinities for human kappa opioid receptors. Bioorg Med Chem 13:5635–5639 [DOI] [PubMed] [Google Scholar]
- Lee DY, Karnati VV, He M, Liu-Chen LY, Kondaveti L, Ma Z, Wang Y, Chen Y, Beguin C, Carlezon WA, Jr, et al. (2005c) Synthesis and in vitro pharmacological studies of new C(2) modified salvinorin A analogues. Bioorg Med Chem Lett 15:3744–3747 [DOI] [PubMed] [Google Scholar]
- Lee DY, Yang L, Xu W, Deng G, Guo L, Liu-Chen LY. (2010) Synthesis and biological evaluation of C-2 halogenated analogs of salvinorin A. Bioorg Med Chem Lett 20:5749–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. (2008) Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rhesus monkeys. J Pharmacol Exp Ther 324:827–833 [DOI] [PubMed] [Google Scholar]
- Li Y, Husbands SM, Mahon MF, Traynor JR, Rowan MG. (2007) Isolation and chemical modification of clerodane diterpenoids from Salvia species as potential agonists at the kappa-opioid receptor. Chem Biodivers 4:1586–1593 [DOI] [PubMed] [Google Scholar]
- Lingham AR, Hügel HM, Rook TJ. (2006) Studies towards the synthesis of salvinorin A. Aust J Chem 59:340–348 [Google Scholar]
- Lozama A, Prisinzano TE. (2009) Chemical methods for the synthesis and modification of neoclerodane diterpenes. Bioorg Med Chem Lett 19:5490–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Weltrowska G, Lemieux C, Chung NN, Schiller PW. (2001) Stereospecific synthesis of (2S)-2-methyl-3-(2′,6′-dimethyl-4′-hydroxyphenyl)-propionic acid (Mdp) and its incorporation into an opioid peptide. Bioorg Med Chem Lett 11:323–325 [DOI] [PubMed] [Google Scholar]
- Ma Z, Deng G, Dai R, Xu W, Liu-Chen LY, Lee DWY. (2010a) Thermal degradation products derived from the smoke of Salvia divinorum leaves. Tetrahedron Lett 51:5480–5482 [Google Scholar]
- Ma Z, Deng G, Lee DY. (2010b) Novel neoclerodane diterpene derivatives from the smoke of salvinorin A. Tetrahedron Lett 51:5207–5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Lee DY. (2007) Revised structure of deacetyl-1,10-didehydrosalvinorin G. Tetrahedron Lett 48:5461–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Lee DY. (2008) Synthesis of deacetyl-1,10-didehydrosalvinorin G. Tetrahedron Lett 49:1782–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr (2003) Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305:323–330 [DOI] [PubMed] [Google Scholar]
- Mansour A, Taylor LP, Fine JL, Thompson RC, Hoversten MT, Mosberg HI, Watson SJ, Akil H. (1997) Key residues defining the mu-opioid receptor binding pocket: a site-directed mutagenesis study. J Neurochem 68:344–353 [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564 [DOI] [PubMed] [Google Scholar]
- McCann DJ. (2008) Potential of buprenorphine/naltrexone in treating polydrug addiction and co-occurring psychiatric disorders. Clin Pharmacol Ther 83:627–630 [DOI] [PubMed] [Google Scholar]
- McCurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. (2006) Antinociceptive profile of salvinorin A, a structurally unique kappa opioid receptor agonist. Pharmacol Biochem Behav 83:109–113 [DOI] [PubMed] [Google Scholar]
- McDonough PC, Holler JM, Vorce SP, Bosy TZ, Magluilo J, Jr, Past MR. (2008) The detection and quantitative analysis of the psychoactive component of Salvia divinorum, salvinorin A, in human biological fluids using liquid chromatography-mass spectrometry. J Anal Toxicol 32:417–421 [DOI] [PubMed] [Google Scholar]
- McGovern DL, Mosier PD, Roth BL, Westkaemper RB. (2010) CoMFA analyses of C-2 position salvinorin A analogs at the kappa-opioid receptor provides insights into epimer selectivity. J Mol Graph Model 28:612–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, Mohabbat Y, Moyer RA, Bohn LM, Coscia CJ. (2008) Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways. J Neurochem 107:1753–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JE, Coyle JR, Lopez JC, Baggott MJ, Flower K, Everhart ET, Munro TA, Galloway GP, Cohen BM. (2010) Lack of effect of sublingual salvinorin A, a naturally occurring kappa opioid, in humans: a placebo-controlled trial. Psychopharmacology (Berl). doi:10.1007/s00213-010-2103-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer SL, Cunningham CW, Eddington ND, Coop A. (2008) Opioids and efflux transporters. Part 3: P-glycoprotein substrate activity of 3-hydroxyl addition to meperidine analogs. Bioorg Med Chem Lett 18:3638–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer SL, Hassan HE, Cunningham CW, Eddington ND, Coop A. (2007) Opioids and efflux transporters. Part 1: P-glycoprotein substrate activity of N-substituted analogs of meperidine. Bioorg Med Chem Lett 17:1160–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger TG, Paterlini MG, Ferguson DM, Portoghese PS. (2001) Investigation of the selectivity of oxymorphone- and naltrexone-derived ligands via site-directed mutagenesis of opioid receptors: exploring the “address” recognition locus. J Med Chem 44:857–862 [DOI] [PubMed] [Google Scholar]
- Metzger TG, Paterlini MG, Portoghese PS, Ferguson DM. (1996) Application of the message-address concept to the docking of naltrexone and selective naltrexone-derived opioid antagonists into opioid receptor models. Neurochem Res 21:1287–1294 [DOI] [PubMed] [Google Scholar]
- Morani AS, Kivell B, Prisinzano TE, Schenk S. (2009) Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol Biochem Behav 94:244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65 [DOI] [PubMed] [Google Scholar]
- Munro TA, Duncan KK, Xu W, Wang Y, Liu-Chen LY, Carlezon WA, Jr, Cohen BM, Béguin C. (2008) Standard protecting groups create potent and selective kappa opioids: salvinorin B alkoxymethyl ethers. Bioorg Med Chem 16:1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro TA, Goetchius GW, Roth BL, Vortherms TA, Rizzacasa MA. (2005a) Autoxidation of Salvinorin A under Basic Conditions. J Org Chem 70:10057–10061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro TA, Rizzacasa MA. (2003) Salvinorins D-F, new neoclerodane diterpenoids from Salvia divinorum, and an improved method for the isolation of salvinorin A. J Nat Prod 66:703–705 [DOI] [PubMed] [Google Scholar]
- Munro TA, Rizzacasa MA, Roth BL, Toth BA, Yan F. (2005b) Studies toward the pharmacophore of salvinorin A, a potent kappa opioid receptor agonist. J Med Chem 48:345–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysels D, Sullivan MA. (2009) The kappa-opiate receptor impacts the pathophysiology and behavior of substance use. Am J Addict 18:272–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. (1999) Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther 290:1132–1140 [PubMed] [Google Scholar]
- Negus SS, Wurrey BA, Mello NK. (2004) Sex differences in thermal nociception and prostaglandin-induced thermal hypersensitivity in rhesus monkeys. J Pain 5:92–103 [DOI] [PubMed] [Google Scholar]
- Negus SS, Zuzga DS, Mello NK. (2002) Sex differences in opioid antinociception in rhesus monkeys: antagonism of fentanyl and U50,488 by quadazocine. J Pain 3:218–226 [DOI] [PubMed] [Google Scholar]
- Nemeth CL, Paine TA, Rittiner JE, Béguin C, Carroll FI, Roth BL, Cohen BM, Carlezon WA., Jr (2010) Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology (Berl) 210:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477 [DOI] [PubMed] [Google Scholar]
- Nichols DE. (2004) Hallucinogens. Pharmacol Ther 101:131–181 [DOI] [PubMed] [Google Scholar]
- Nozawa M, Suka Y, Hoshi T, Suzuki T, Hagiwara H. (2008) Total synthesis of the hallucinogenic neoclerodane diterpenoid salvinorin A. Org Lett 10:1365–1368 [DOI] [PubMed] [Google Scholar]
- Ortega A, Blount JF, Manchand PS. (1982) Salvinorin, a new trans-neoclerodane diterpene from Salvia divinorum. J Chem Soc Perkin Trans I 10:2505–2508 [Google Scholar]
- Ott J. (1995) Ethnopharmacognosy and human pharmacology of Salvia divinorum and salvinorin A. Curare 18:103–129 [Google Scholar]
- Phipps SM, Butterweck V. (2010) A new digitized method of the compulsive gnawing test revealed dopaminergic activity of salvinorin A in vivo. Planta Med 76:1405–1410 [DOI] [PubMed] [Google Scholar]
- Pichini S, Abanades S, Farré M, Pellegrini M, Marchei E, Pacifici R, Torre Rde L, Zuccaro P. (2005) Quantification of the plant-derived hallucinogen Salvinorin A in conventional and non-conventional biological fluids by gas chromatography/mass spectrometry after Salvia divinorum smoking. Rapid Commun Mass Spectrom 19:1649–1656 [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr (2001) Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21:7397–7403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. (2001) Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther 299:620–628 [PubMed] [Google Scholar]
- Porter JH, Prus AJ. (2009) Discriminative stimulus properties of atypical and typical antipsychotic drugs: a review of preclinical studies. Psychopharmacology (Berl) 203:279–294 [DOI] [PubMed] [Google Scholar]
- Prisinzano TE. (2005) Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci 78:527–531 [DOI] [PubMed] [Google Scholar]
- Prisinzano TE, Rothman RB. (2008) Salvinorin A analogs as probes in opioid pharmacology. Chem Rev 108:1732–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DC, Hunter JC. (1990) Opioid receptors, in Comprehensive Medicinal Chemistry (Emmet JC. ed) pp 805–846, Pergamon Press, New York [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. (2002) Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci USA 99:11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Gorelick DA, Heishman SJ, Eichmiller PR, Hill BH, Norbeck J, Liberto JG. (2000) An open-label study of a functional opioid kappa antagonist in the treatment of opioid dependence. J Subst Abuse Treat 18:277–281 [DOI] [PubMed] [Google Scholar]
- Rothman RB, Murphy DL, Xu H, Godin JA, Dersch CM, Partilla JS, Tidgewell K, Schmidt M, Prisinzano TE. (2007) Salvinorin A: allosteric interactions at the mu-opioid receptor. J Pharmacol Exp Ther 320:801–810 [DOI] [PubMed] [Google Scholar]
- Runge FF. (1820) Neueste phytochemische Entdeckungen zur Begründung einer wissenschaftlichen Phytochemie, G. Reimer, Berlin [Google Scholar]
- Sally EJ, Xu H, Dersch CM, Hsin LW, Chang LT, Prisinzano TE, Simpson DS, Giuvelis D, Rice KC, Jacobson AE, et al. (2010) Identification of a novel “almost neutral” micro-opioid receptor antagonist in CHO cells expressing the cloned human mu-opioid receptor. Synapse 64:280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer JR, Lawrence JF, Wang GC, Evans DA. (2007) Asymmetric synthesis of salvinorin A, a potent kappa opioid receptor agonist. J Am Chem Soc 129:8968–8969 [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. (1999) U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 144:339–346 [DOI] [PubMed] [Google Scholar]
- Schmidt MD, Schmidt MS, Butelman ER, Harding WW, Tidgewell K, Murry DJ, Kreek MJ, Prisinzano TE. (2005a) Pharmacokinetics of the plant-derived kappa-opioid hallucinogen salvinorin A in nonhuman primates. Synapse 58:208–210 [DOI] [PubMed] [Google Scholar]
- Schmidt MS, Prisinzano TE, Tidgewell K, Harding W, Butelman ER, Kreek MJ, Murry DJ. (2005b) Determination of Salvinorin A in body fluids by high performance liquid chromatography-atmospheric pressure chemical ionization. J Chromatogr B Analyt Technol Biomed Life Sci 818:221–225 [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes J, O'Connor P, Chawarski M, Oliveto A, Kosten TR. (2000) Thrice-weekly versus daily buprenorphine maintenance. Biol Psychiatry 47:1072–1079 [DOI] [PubMed] [Google Scholar]
- Schwarzer C. (2009) 30 years of dynorphins—new insights on their functions in neuropsychiatric diseases. Pharmacol Ther 123:353–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Guan HC, Hirbec H. (2009) Dopamine D2High receptors stimulated by phencyclidines, lysergic acid diethylamide, salvinorin A, and modafinil. Synapse 63:698–704 [DOI] [PubMed] [Google Scholar]
- Sertürner FW. (1817) Über das Morphium, eine neue salzfähige Grundlage, und die Mekonsäure, als Hauptbestandtheile des Opiums. Ann Phys 55:56–89 [Google Scholar]
- Sheffler DJ, Roth BL. (2003) Salvinorin A: the “magic mint” hallucinogen finds a molecular target in the kappa opioid receptor. Trends Pharmacol Sci 24:107–109 [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. (1987) Motivational properties of opioids: evidence that an activation of delta-receptors mediates reinforcement processes. Brain Res 436:234–239 [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. (1987) Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res 436:169–172 [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. (2007) Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther 116:306–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota O, Nagamatsu K, Sekita S. (2006) Neo-clerodane diterpenes from the hallucinogenic sage Salvia divinorum. J Nat Prod 69:1782–1786 [DOI] [PubMed] [Google Scholar]
- Siebert D. (2010) The legal status of Salvia divinorum. The Salvia divinorum Research and Information Center, http://www.sagewisdom.org/legalstatus.html
- Siebert DJ. (1994) Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol 43:53–56 [DOI] [PubMed] [Google Scholar]
- Simpson DS, Katavic PL, Lozama A, Harding WW, Parrish D, Deschamps JR, Dersch CM, Partilla JS, Rothman RB, Navarro H, et al. (2007) Synthetic studies of neoclerodane diterpenes from Salvia divinorum: preparation and opioid receptor activity of salvinicin analogues. J Med Chem 50:3596–3603 [DOI] [PubMed] [Google Scholar]
- Simpson DS, Lovell KM, Lozama A, Han N, Day VW, Dersch CM, Rothman RB, Prisinzano TE. (2009) Synthetic studies of neoclerodane diterpenes from Salvia divinorum: role of the furan in affinity for opioid receptors. Org Biomol Chem 7:3748–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Chevé G, Ferguson DM, McCurdy CR. (2006) A combined ligand-based and target-based drug design approach for G-protein coupled receptors: application to salvinorin A, a selective kappa opioid receptor agonist. J Comput Aided Mol Des 20:471–493 [DOI] [PubMed] [Google Scholar]
- Singh N, Nolan TL, McCurdy CR. (2008) Chemical function-based pharmacophore development for novel, selective kappa opioid receptor agonists. J Mol Graph Model 27:131–139 [DOI] [PubMed] [Google Scholar]
- Snider NT, Walker VJ, Hollenberg PF. (2010) Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: physiological and pharmacological implications. Pharmacol Rev 62:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA 89:2046–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WC, Jr, Jones RM, Subramanian G, Metzger TG, Ferguson DM, Portoghese PS. (2000) Potent and selective indolomorphinan antagonists of the kappa-opioid receptor. J Med Chem 43:2759–2769 [DOI] [PubMed] [Google Scholar]
- Stewart DJ, Fahmy H, Roth BL, Yan F, Zjawiony JK. (2006) Bioisosteric modification of salvinorin A, a potent and selective kappa-opioid receptor agonist. Arzneimittelforschung 56:269–275 [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. (1994) Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry 51:98–108 [DOI] [PubMed] [Google Scholar]
- Subramanian G, Paterlini MG, Larson DL, Portoghese PS, Ferguson DM. (1998) Conformational analysis and automated receptor docking of selective arylacetamide-based kappa-opioid agonists. J Med Chem 41:4777–4789 [DOI] [PubMed] [Google Scholar]
- Sumnall HR, Measham F, Brandt SD, Cole JC. (2010) Salvia divinorum use and phenomenology: results from an online survey. J Psychopharmacol doi:10.1177/0269881110385596 [DOI] [PubMed] [Google Scholar]
- Surratt CK, Johnson PS, Moriwaki A, Seidleck BK, Blaschak CJ, Wang JB, Uhl GR. (1994) -mu opiate receptor. Charged transmembrane domain amino acids are critical for agonist recognition and intrinsic activity. J Biol Chem 269:20548–20553 [PubMed] [Google Scholar]
- Titeler M, Lyon RA, Glennon RA. (1988) Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology (Berl) 94:213–216 [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Chefer VI, Zapata A, Shippenberg TS. (2010) The effects of kappa-opioid receptor ligands on prepulse inhibition and CRF-induced prepulse inhibition deficits in the rat. Psychopharmacology (Berl) 210:231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teksin ZS, Lee IJ, Nemieboka NN, Othman AA, Upreti VV, Hassan HE, Syed SS, Prisinzano TE, Eddington ND. (2009) Evaluation of the transport, in vitro metabolism and pharmacokinetics of Salvinorin A, a potent hallucinogen. Eur J Pharm Biopharm 72:471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidgewell K, Groer CE, Harding WW, Lozama A, Schmidt M, Marquam A, Hiemstra J, Partilla JS, Dersch CM, Rothman RB, et al. (2008) Herkinorin analogues with differential beta-arrestin-2 interactions. J Med Chem 51:2421–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidgewell K, Harding WW, Lozama A, Cobb H, Shah K, Kannan P, Dersch CM, Parrish D, Deschamps JR, Rothman RB, et al. (2006) Synthesis of salvinorin a analogues as opioid receptor probes. J Nat Prod 69:914–918 [DOI] [PubMed] [Google Scholar]
- Tidgewell K, Harding WW, Schmidt M, Holden KG, Murry DJ, Prisinzano TE. (2004) A facile method for the preparation of deuterium labeled salvinorin A: synthesis of [2,2,2-2H3]-salvinorin A. Bioorg Med Chem Lett 14:5099–5102 [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172:463–470 [DOI] [PubMed] [Google Scholar]
- Tsujikawa K, Kuwayama K, Miyaguchi H, Kanamori T, Iwata YT, Inoue H. (2009) In vitro stability and metabolism of salvinorin A in rat plasma. Xenobiotica 39:391–398 [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Valdés LJ., 3rd (1994) Salvia divinorum and the unique diterpene hallucinogen, Salvinorin (divinorin) A. J Psychoactive Drugs 26:277–283 [DOI] [PubMed] [Google Scholar]
- Valdés LJ, 3rd, Butler WM, Hatfield GM, Paul AG, Koreeda M. (1984) Divinorin A, a psychotropic terpenoid, and divinorin B from the hallucinogenic Mexican mint Salvia divinorum. J Org Chem 49:4716–4720 [Google Scholar]
- Valdés LJ, 3rd, Chang HM, Visger DC, Koreeda M. (2001) Salvinorin C, a new neoclerodane diterpene from a bioactive fraction of the hallucinogenic Mexican mint Salvia divinorum. Org Lett 3:3935–3937 [DOI] [PubMed] [Google Scholar]
- Valdés LJ, 3rd, Díaz JL, Paul AG. (1983) Ethnopharmacology of ska María Pastora (Salvia divinorum, Epling and Játiva-M.). J Ethnopharmacol 7:287–312 [DOI] [PubMed] [Google Scholar]
- Vanderah TW. (2010) Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain 26 (Suppl 10):S10–S15 [DOI] [PubMed] [Google Scholar]
- Vaysse PJ, Gardner EL, Zukin RS. (1987) Modulation of rat brain opioid receptors by cannabinoids. J Pharmacol Exp Ther 241:534–539 [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, et al. (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386:827–830 [DOI] [PubMed] [Google Scholar]
- Vortherms TA, Mosier PD, Westkaemper RB, Roth BL. (2007) Differential helical orientations among related G protein-coupled receptors provide a novel mechanism for selectivity. Studies with salvinorin A and the kappa-opioid receptor. J Biol Chem 282:3146–3156 [DOI] [PubMed] [Google Scholar]
- Vortherms TA, Roth BL. (2006) Salvinorin A: from natural product to human therapeutics. Mol Interv 6:257–265 [DOI] [PubMed] [Google Scholar]
- Walentiny DM, Vann RE, Warner JA, King LS, Seltzman HH, Navarro HA, Twine CE, Jr, Thomas BF, Gilliam AF, Gilmour BP, et al. (2010) Kappa opioid mediation of cannabinoid effects of the potent hallucinogen, salvinorin A, in rodents. Psychopharmacology (Berl) 210:275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan XH, Huang XQ, Zhou DH, Jiang HL, Chen KX, Chi ZQ. (2000) Building 3D-structural model of kappa opioid receptor and studying its interaction mechanism with dynorphin A(1–8). Acta Pharmacol Sin 21:701–708 [PubMed] [Google Scholar]
- Wang Y, Chen Y, Xu W, Lee DY, Ma Z, Rawls SM, Cowan A, Liu-Chen LY. (2008) 2-Methoxymethyl-salvinorin B is a potent kappa opioid receptor agonist with longer lasting action in vivo than salvinorin A. J Pharmacol Exp Ther 324:1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY. (2005) Comparison of pharmacological activities of three distinct kappa ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther 312:220–230 [DOI] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. (1999) Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron 23:737–746 [DOI] [PubMed] [Google Scholar]
- Willmore-Fordham CB, Krall DM, McCurdy CR, Kinder DH. (2007) The hallucinogen derived from Salvia divinorum, salvinorin A, has kappa-opioid agonist discriminative stimulus effects in rats. Neuropharmacology 53:481–486 [DOI] [PubMed] [Google Scholar]
- Wolowich WR, Perkins AM, Cienki JJ. (2006) Analysis of the psychoactive terpenoid salvinorin A content in five Salvia divinorum herbal products. Pharmacotherapy 26:1268–1272 [DOI] [PubMed] [Google Scholar]
- Woods JH, Winger G. (1987) Opioids, receptors, and abuse liability, in Psychopharmacology: The Third Generation of Progress (Meltzer HY. ed) pp 1555–1564, Raven Press, New York [Google Scholar]
- Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. (2007) A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) mu-opioid agonists on cellular markers related to opioid tolerance and dependence. Synapse 61:166–175 [DOI] [PubMed] [Google Scholar]
- Xu H, Wang X, Partilla JS, Bishop-Mathis K, Benaderet TS, Dersch CM, Simpson DS, Prisinzano TE, Rothman RB. (2008) Differential effects of opioid agonists on G protein expression in CHO cells expressing cloned human opioid receptors. Brain Res Bull 77:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wang X, Zimmerman D, Boja ES, Wang J, Bilsky EJ, Rothman RB. (2005) Chronic morphine up-regulates G{alpha}12 and cytoskeletal proteins in chinese hamster ovary cells expressing the cloned mu opioid receptor. J Pharmacol Exp Ther 315:248–255 [DOI] [PubMed] [Google Scholar]
- Yamaotsu N, Fujii H, Nagase H, Hirono S. (2010) Identification of the three-dimensional pharmacophore of kappa-opioid receptor agonists. Bioorg Med Chem 18:4446–4452 [DOI] [PubMed] [Google Scholar]
- Yan F, Bikbulatov RV, Mocanu V, Dicheva N, Parker CE, Wetsel WC, Mosier PD, Westkaemper RB, Allen JA, Zjawiony JK, et al. (2009) Structure-based design, synthesis, and biochemical and pharmacological characterization of novel salvinorin A analogues as active state probes of the kappa-opioid receptor. Biochemistry 48:6898–6908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Mosier PD, Westkaemper RB, Stewart J, Zjawiony JK, Vortherms TA, Sheffler DJ, Roth BL. (2005) Identification of the molecular mechanisms by which the diterpenoid salvinorin A binds to kappa-opioid receptors. Biochemistry 44:8643–8651 [DOI] [PubMed] [Google Scholar]
- Yang L, Xu W, Chen F, Liu-Chen LY, Ma Z, Lee DY. (2009) Synthesis and biological evaluation of C-12 triazole and oxadiazole analogs of salvinorin A. Bioorg Med Chem Lett 19:1301–1304 [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Alheid GF, Heimer L. (1984) The ventral pallidal projection to the mediodorsal thalamus: a study with fluorescent retrograde tracers and immunohistofluorescence. J Neurosci 4:1626–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. (2005) Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 179:551–558 [DOI] [PubMed] [Google Scholar]