Infection with varicella-zoster virus (VZV) is common, occurring as primary varicella, usually during childhood, and as zoster, following reactivation of latent virus. Although serious VZV infections are infrequent, they can be life-threatening when they occur in susceptible immunosuppressed patients (1, 2). These patients have a high incidence of visceral involvement, including pneumonitis, meningoencephalitis, and rarely, hepatitis. We report here 3 cases of VZV hepatitis in adult liver transplant recipients that occurred in our institution between 1984 and 1989. We describe their clinical presentations and use these cases to illustrate some aspects in diagnosis and prevention of VZV infection after solid organ Tx.
Case 1
A 19-year-old woman underwent orthotopic liver Tx for end-stage liver disease secondary to chronic active autoimmune hepatitis. She was receiving prednisone and azathioprine before the transplant operation. Her immunosuppression after Tx included cyclosporine (CSA) and steroids. At 28 days after her transplant operation, she started complaining of low back pain over the coccyx area, but the exam of this area did not reveal any skin lesions. Two days later she developed few skin vesicles on her face, abdomen, and hands. Laboratory examination showed elevation in LFTs with serum alanine aminotrahsferase (ALT) of 196 lU/L (normal value [nv] <40, lU/L), serum aspartate aminotransferase (AST) of 221 IU/L (nv <40 IU/L) and bilirubin of 3.5 mg% (nv <1.0 mg/dl), and gamma GTP of 137 (nv <40 U/L). On day 33 after OLTx, her AST increased to 1057 lU/L and bilirubin increased to 4.7 mg/dl. Liver biopsy showed foci of coagulative necrosis with minimal mixed inflammatory cellular infiltrate and multinucleated cells, with negative histochemical stains for HSV 1 and HSV 2. The patient was started the same day on intravenous acyclovir at 10 mg/kg every 8 hr, but the following day she developed DIC and ARDS and expired 37 days after Tx. A blood buffy coat specimen was positive subsequently for VZV by culture. IgG antibody for VZV was negative on day 33 after OLTx by indirect fluorescence assay (IFA). No history of exposure to VZV was obtained.
Case 2
A 33-year-old man underwent a liver and pancreas Tx for end-stage liver disease secondary to hepatitis B infection and hepatoma. Immunosuppression treatment included CSA and steroids. His spleen and pancreas and part of his colon, duodenum, and stomach were also removed. His immediate postoperative course was complicated by abdominal bleeding, renal failure, pancreatitis, and failure of the transplanted graft. He underwent a second liver Tx 7 days after the first transplant operation. Then 7 days later (day 14 after first Tx) the patient started having daily temperature elevations to 39.5°C, abdominal pain, and metabolic acidosis. The liver function tests showed ALT of 61 IU/L and AST of 68 lU/L, AP of 265 IU/L, and bilirubin of 21.1 mg/dl. Liver biopsy showed coagulative necrosis with mild neutrophilic exudate (Fig. 1) and nuclear inclusions were seen (Fig. 2), but histochemical stains were negative for HSV 1 and HSV 2. The patient’s skin examination revealed vesicular lesions on the palmar aspect of his hands and also on his neck. He was placed on i.v. acyclovir at 10 mg/kg after each dialysis, and was also given a dose of VZIG. Buffy coat and vesicular skin lesions samples yielded VZV.
Figure 1.
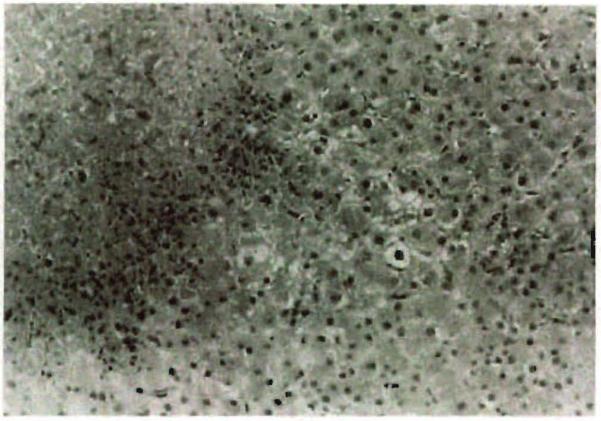
Focus of coagulative necrosis with surrounding mild neutrophilic exudate (H&E; original magnification: ×200).
Figure 2.

Intranuclear inclusion body in the periphery of the same necrotic area (H&E; original magnification: ×400).
He remained on a respirator, required hemodialysis, and developed bacterial and fungal abdominal sepsis secondary to leaks from the bowel. At 31 days after retransplantation he required distal pancreatectomy for pancreatic phlegmon. He was discharged 5 months after his original transplant operation. The patient’s blood sample 32 days before Tx tested positive for VZV antibodies by IFA, enzyme-linked immunoassay (ELISA), and fluorescent-antibody-to-membrane antigen (FAMA). He denied having had chickenpox in the past. Interview of his family members revealed that the patient’s young nephew was diagnosed with chickenpox 16 days before his first Tx, and later two of the patient’s children also contracted this illness. The first child was diagnosed 3 days before Tx and the second child was diagnosed on the same day the patient was electively admitted to the hospital for Tx.
Case 3
A 38-year-old man with chronic active hepatitis B, underwent OLTX and was maintained on CSA, prednisone, and azathioprine. Nineteen months after his Tx he visited his friend’s house, and two days later his friend’s son, whom he met, was diagnosed with chickenpox. The patient was given an injection of varicella-zoster immune globulin (VZIG) intramuscularly the day the exposure was reported. Thirty-two days after exposure he noticed for the first time malaise and the onset of a rash on his chest. He was admitted to the hospital with high temperature and vesicular lesions on his face, chest, and back. He was started on i.v. acyclovir at 10 mg/kg every 8 hr and was given an intramuscular injection of VZIG. His laboratory tests showed ALT of 824 IU/L, AST of 2091 lU/L, and bilirubin of 1.0 mg/dl. No liver biopsy was performed. A buffy coat culture of blood drawn on admission was positive for VZV and IgG varicella antibody titer was positive by IFA. A sample of blood prior to his transplant was positive with a low titer by IFA, and negative by ELISA and FAMA test. The patient was discharged home 12 days after admission.
Hepatic involvement has been described in childhood varicella, most often subclinically (3), and as hepatitis in immunosuppressed children who develop disseminated disease (1, 4). In adults, clinical hepatitis may appear in patients with varicella pneumonia (5) and sporadically as the only manifestation of visceral disease. In the former situation, the disease tends to be fatal (6–9), and to our knowledge, there has been only one report of successful treatment with acyclovir therapy (10). Thjs suggests that VZV hepatitis is a life-threatening disease, particularly in the immunocompromised adult host.
Varicella is reported to cause Reye’s syndrome (11, 12), making the distinction between this clinical entity and VZV hepatitis difficult without a liver biopsy. On the other hand, the foci of coagulative necrosis with little inflammatory response has been described in both herpes simplex and varicella zoster infections, with varicella often giving smaller and better-circumscribed lesions (13, 14). Since histology findings are similar, it is difficult to distinguish between herpes simplex and varicella hepatitis based on morphology alone, and evaluation by immunoperoxidase technique is also needed. It was not possible to perform specific immunostains for VZV because of some technical problems. However, the negative immunostains for HSV 1 and HSV 2 in two patients, along with the recovery of VZV from the buffY coats in the three patients, suggests that VZV was the cause of the hepatitis. Patient 3 did not have liver biopsy and the diagnosis was based on liver function test abnormalities and the recovery of VZV from a buffy coat culture. Although without histology we can not really be sure that this patient had hepatitis, VZV viremia has been considered as a marker of visceral disease in immunocompromised patients (15).
We have previously reported 12 patients who developed herpes simplex hepatitis after solid organ Tx (16). Five of these patients developed disseminated intravascular coagulation and expired. This fulminant disease was more common in primary infection compared with reactivation, similar to the clinical picture of patient 1 in this report. The main dissimilarity is that all three patients with varicella hepatitis had skin lesions. In our report of herpes simplex hepatitis, skin lesions were not common, even in cases with autopsy-proven disseminated disease to multiple organs (16). This is similar to bone marrow Tx, where higher rates of VZV visceral dissemination without skin manifestations have been reported (17).
In a surveillance performed among our adult candidates for liver Tx, only 5% were seronegative for VZV and therefore at risk for infection (unpublished data). Because seronegative patients are more susceptible to VZV infection, prophylaxis with varicella zoster immunoglobulin (VZIG) is recommended for them after virus exposure. However, there are conflicting reports regarding its beneficial effect (18). In fact some patients given VZIG after exposure to VZV nevertheless acquired severe infection (19, 20). On the other hand, passive immunity may morufy and “mitigate” the disease even if not giving an absolute protection. Patient 3 was most likely seronegative for VZV before Tx, since the ELISA and FAMA tests were negative. The low-positive antibody titer detected by IFA may have been related to passive acquisition of antibodies through blood transfusion. This patient received VZIG one day after exposure; he did contract varicella 32 days later, with visceral involvement, but survived. Patient 1 was seronegative for VZV. since serum from day 33 after Tx tested negative. The exposure in this case was not identified and the rash was seen for the first time 28 days after the transplant operation. The patient did not receive prophylactic treatment and developed a fulminant disease with DIC and expired.
It is assumed that seropositive individuals are not susceptible to varicella. However, there are some reports of immunosuppressed patients who developed varicella more than one time (20,21). Patients may be reinfected with a different strain of virus and develop a second episode of varicella (22). This is what probably occurred to patient 2, who was seropositive before Tx but was exposed to a new virus in the family from his two children and developed varicella with hepatitis. The patient might have had disseminated zoster secondary to VZV reactivation and not varicella. However, his vesicular rash rud not follow any dermatomal distribution at any time. Moreover, visceral dissemination is not a common event in dermatomal zoster infection, compared with varicella infection (23). The three cases that we reported here suggest that transplant recipients in close contact with VZV may benefit from VZV prophylaxis, even when the patients are seropositive. The question of whether this prophylaxis should be done with VZIG or with oral acyclovir, as recently reported in the pediatric population (24, 25) will require further investigation. Recently a second oral antiviral agent with activity against VZV, famciclovir, was introduced for clinical use, and it may have a role in prophylaxis (26). There are some data from leukemic children showing that varicella vaccine is both safe and immunogenic in immunocompromised patients (27). Seronegative transplant candidates would be good candidates for the use of this vaccine, which has recently been released for clinical use.
Since VZV infection may be life-threatening in solid organ transplant patients, and after our experience with these three cases, we suggest a tentative management plan for prevention of VZV infection in adult transplant recipients. These suggestions should be used as a working plan until more data are available and conclusive recommendations are made regarding the use of passive immunity, antivirals, and vaccination in this patient population:
A history of varicella (chickenpox) and zoster (shingles) is obtained from all patients during pre-Tx evaluation, and VZV IgG titer is checked. (Our laboratory uses ELISA.) Seronegative patients and possibly those with low titer may be candidates for varicella vaccine.
Transplant candidates and recipients are educated to avoid exposure to varicella (chickenpox) or zoster (shingles), and to immediately report to their nurse coordinators any accidental exposure.
After an accidental exposure to VZV (chickenpox or shingles), VZIG is offered to seronegative transplant recipients and those with low-positive titer. Varicella-zoster immune globulin (VZIG) is administered intramuscularly at a dose of 125 mg/l0 kg of weight (maximum dose 625 mg).
After any exposure to VZV, susceptible patients should be considered for placement on oral acyclovir at 800 mg five times a day (or possibly famcyc1ovir) for the duration of the incubation period (2–3 weeks).
Development of vesicular skin lesions after exposure should prompt viral cultures for herpesvirus (skin lesion and buffy coat), and immediate institution of high-dosage acyclovir therapy (10 mg/kg every 8 hr, i.v.) while awaiting confirmation of diagnosis.
Liver function tests abnormalities in a patient who has had a recent exposure to VZV should prompt liver biopsy.
Acknowledgment
We thank the staff of the Clinical Virology Laboratory for technical assistance.
REFERENCES
- 1.Feldhoff CM, Balfour HH, Simmons RL, Najarian JS, Mauer SM. Varicella in children with renal transplants. J Pediatr. 1981;98:25. doi: 10.1016/s0022-3476(81)80527-6. [DOI] [PubMed] [Google Scholar]
- 2.Bradley JR, Wreghitt TG, Evans DB. Chickenpox in adult renal transplant recipients. Nephrol Dial Transpl. 1987;1:242. [PubMed] [Google Scholar]
- 3.Pitel PA, McCormick KL, Fitzgerald E, Orson JM. Subclinical hepatic changes in varicella infection. Pediatrics. 1980;65:631. [PubMed] [Google Scholar]
- 4.Feldman S, Hughes WT, Daniel CB. Varicella in children with cancer: seventy-seven cases. Pediatrics. 1975;56:388. [PubMed] [Google Scholar]
- 5.Gershon AA, LaRussa P. Varicella-zoster virus infections. In: Krugman S, Katz SL, Gershon AA, Wifert CM, editors. Infectious diseases of children. Mosby; St. Louis, MO: 1992. p. 587. [Google Scholar]
- 6.Ross JS, Fanning WL, Beautyman W, Craighead JE. Fatal massive hepatic necrosis from varicella-zoster hepatitis. Am J Gastroenterol. 1980;74:423. [PubMed] [Google Scholar]
- 7.Morishita K, Kodo H, Asano S, Fujii H, Miwa S. Fulminant varicella hepatitis following bone marrow transplantation. JAMA. 1985;253:511. [PubMed] [Google Scholar]
- 8.Patti ME, Selvaggi KJ, Kroboth FJ. Varicella hepatitis in the immunocompromised adult: a case report and review of the literature. Am J Med. 1990;88:77. doi: 10.1016/0002-9343(90)90133-x. [DOI] [PubMed] [Google Scholar]
- 9.Soriano V, Bru F, Gonzalez-Lahoz J. Fatal varicella hepatitis in a patient with AIDS. J Infection. 1992;25:107. doi: 10.1016/0163-4453(92)93681-f. [DOI] [PubMed] [Google Scholar]
- 10.Morales JM. Successful acyclovir therapy for severe varicella hepatitis in an adult renal transplant recipient. Am J Med. 1991;90:401. [PubMed] [Google Scholar]
- 11.Landay SE. Varicella hepatitis and Reye’s syndrome: an interrelationship. Pediatrics. 1977;60:746. [PubMed] [Google Scholar]
- 12.Lichtenstein PK, Heubi JE, Daugherty CC, et al. Grade 1 Reye’s syndrome: a frequent cause of vomiting and liver dysfunction after varicella and upper respiratory tract infection. N Engl J Med. 1983;309:133. doi: 10.1056/NEJM198307213090302. [DOI] [PubMed] [Google Scholar]
- 13.Johnson HN. Visceral lesions associated with varicella. Arch Pathol. 1940;30:292. [Google Scholar]
- 14.Demetris AJ, Jaffe R, Starzl TE. A review of adult and pediatric posttransplant liver pathology. Pathol Annu. 1987;2:347. [PubMed] [Google Scholar]
- 15.Myers MG. Viremia caused by varicella-zoster virus: association with malignant progressive varicella. J Infect Dis. 1979;140:229. doi: 10.1093/infdis/140.2.229. [DOI] [PubMed] [Google Scholar]
- 16.Kusne S, Schwartz M, Breinig MK, et al. Herpes simplex virus hepatitis after solid organ transplantation in adults. J Infect Dis. 1991;163:1001. doi: 10.1093/infdis/163.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locksley RM, Flournoy N, Sullivan KM, Meyers JD. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172. doi: 10.1093/infdis/152.6.1172. [DOI] [PubMed] [Google Scholar]
- 18.Zaia JA, Levin MJ, Preblud SR, et al. Evaluation of varicella-zoster immune globulin? protection of immunosuppressed children after household exposure to varicella. J Infect Dis. 1983;147:737. doi: 10.1093/infdis/147.4.737. [DOI] [PubMed] [Google Scholar]
- 19.Lynfield R, Herrin JT, Rubin RH. Varicella in pediatric renal transplant recipients. Pediatrics. 1992;90:216. [PubMed] [Google Scholar]
- 20.McGregor RS, Zitelli BJ, Urbach AH, Malatack JJ, Gartner JC. Varicella in pediatric orthotopic liver transplant recipients. Pediatrics. 1989;83:256. [PubMed] [Google Scholar]
- 21.Howarth CB. Recurrent varicella-like illness in a child with leukemia. Lancet. 1974:342. doi: 10.1016/s0140-6736(74)91710-3. [DOI] [PubMed] [Google Scholar]
- 22.Gershon AA, Steinberg SP, Gelb L. Clinical reinfection with varicella-zoster virus. J Infect Dis. 1984;149:137. doi: 10.1093/infdis/149.2.137. [DOI] [PubMed] [Google Scholar]
- 23.Mazur MH, Dolin R. Herpes zoster at the NIH: a 20 year experience. Am J Med. 1978;65:738. doi: 10.1016/0002-9343(78)90791-x. [DOI] [PubMed] [Google Scholar]
- 24.Dunkle LM, Arvin AM, Whitley RJ, et al. A controlled trial of acyclovir for chickenpox in normal children. N Engl J Med. 1991;325:1539. doi: 10.1056/NEJM199111283252203. [DOI] [PubMed] [Google Scholar]
- 25.Asano Y, Yoshikawa T, Suga S, et al. Postexposure prophylaxis of varicella in family contact by oral acyclovir. Pediatrics. 1993;92:219. [PubMed] [Google Scholar]
- 26.Saltzman R, Jurewicz R, Boon R. Safety of famciclovir in patients with herpes zoster and genital herpes. Antimicrob Agents Chermother. 1994;38:2454. doi: 10.1128/aac.38.10.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gershon AA. Human immune responses to live attenuated vaccine. Rev Infect Dis. 1991;13(suppl 13):S957. doi: 10.1093/clind/13.supplement_11.s957. [DOI] [PubMed] [Google Scholar]


