Abstract
Background
While the preclinical development of type 2 diabetes is partly explained by obesity and central adiposity, psychosocial research has shown that chronic stressors such as discrimination have health consequences as well.
Purpose
We investigated the extent to which the well-established effects of obesity and central adiposity on nondiabetic glycemic control (indexed by HbA1c) were moderated by a targeted psychosocial stressor linked to weight: perceived weight discrimination.
Methods
Data came from the nondiabetic subsample (n=938) of the Midlife in the United States (MIDUS II) survey.
Results
Body mass index (BMI), waist-to-hip ratio, and waist circumference were linked to significantly higher HbA1c (p < .001). Multivariate-adjusted models showed that weight discrimination exacerbated the effects of waist-to-hip ratio on HbA1c ( p < .05), such that people who had higher WHR and reported weight discrimination had the highest HbA1c levels.
Conclusions
Understanding how biological and psychosocial factors interact at nondiabetic levels to increase vulnerability could have important implications for public health and education strategies. Effective strategies may include targeting sources of discrimination, rather than solely targeting health behaviors and practices of overweight and obese persons.
Keywords: diabetes, weight discrimination, obesity, individual differences
Introduction
The prevalence of type 2 diabetes has risen steadily over the last three decades (1) and this trend is expected to persist. In the United States, more than 23 million adults have diabetes, 57 million have pre-diabetes, and an estimated one-third of children born in the year 2000 will suffer from diabetes at some point in their lifetime (2, 3). The preclinical progression to type 2 diabetes is only partly explained by obesity and fat distribution, sedentary lifestyle, genetics, and aging. Even obesity and central adiposity, perhaps the most frequently documented and most well understood risk factors for type 2 diabetes, do not translate to an inevitable risk for diabetes: while more than 80% of people with type 2 diabetes are obese, most obese people never develop diabetes (4). Therefore, researchers have looked for additional factors at multiple levels that influence glycemic control (5). Emerging studies have documented that psychosocial vulnerabilities, such as various types of stress and depression, may dysregulate glycemic control even before type 2 diabetes is diagnosed (6–9). The interplay of these and other risk factors, however, is not well understood: biomedical research tends to overlook psychosocial influences, whereas psychosocial models frequently treat traditional biological risk factors as noise factors to be controlled for statistically.
The overarching goal of this study was to integrate these separate strands of previous biomedical and psychosocial research by investigating whether the impact of the established health risk factors on glycemic control was moderated by perceived discrimination. Stigma is described as a social construction influenced by cultural, historical, and situational factors (10), and the visibility and perceived controllability of the stigmatized condition are important determinants of who will be stigmatized. Characteristics perceived to be the responsibility of the person bearing the stigma are more likely to be denigrated (11). Biopsychosocial models of discrimination have a broader interest in the role of stress as a determinant of social disparities in health (12) and describe perceived race discrimination as a class of stressors with consequences for health outcomes (13). Previous research has documented the direct harmful physical health consequences of perceived discrimination for a range of outcomes including mortality, hypertension, poor self-rated health, and blood pressure reactivity (13–18), as well as mental health (19–21).
Weight discrimination is an important social stressor which has only recently garnered scholarly attention. Theoretical work on the origins of weight stigma has identified perceived controllability of the cause of obesity as particularly important (22, 23). The prevalence of weight discrimination is comparable to rates of race discrimination, particularly among women (24), and translates into unfair treatment for overweight and obese persons in domains of employment, healthcare, and education (25, 26). Obesity is considered one of the most enduring stigmas, due in part to the perception that extra weight is due to characterological flaws such as laziness, gluttony, or lack of self-discipline (25, 26).
Building on this prior work that has documented links between discrimination and health, the focus of our study was to investigate individual differences in the relationship between obesity, central adiposity, and glycemic control. Specifically, measures of obesity (body mass index=BMI) and central adiposity (waist-to-hip ratio and waist circumference) were the key biological risk factors, given their well-documented causal relationships with dysregulated glycemic control and type 2 diabetes (27, 28). Specific to obesity, perceived discrimination due to one’s body weight was the key psychosocial factor and was expected to amplify the effects of obesity and central adiposity on nondiabetic glycemic control. Treating weight discrimination as an individual difference variable draws explicit attention to the fact that some individuals, but not others, perceive themselves to experience such discrimination. Our focus on perceived discrimination as an individual difference variable is not about accuracy versus distortion in perceptions of how one is treated based on personal weight/body size— rather, it is about the fact that some obese people see themselves as treated unfairly by others, while other obese people do not. Thus, the central question was whether obese people who also perceived daily weight discrimination (whether warranted or not) were more likely to have dysregulated glycemic control than obese people who did not report discrimination due to weight.
We know of no studies that investigate whether perceived discrimination attributed specifically to one’s body weight exacerbates the harmful physical health effects of body weight. However, animal research provides initial evidence that stress and obesity have interactive effects on glycemic control. In a series of experiments, blood samples were drawn from obese and lean mice after exposure to stress. Both lean and obese animals evidenced increases in plasma glucose levels attributable to the stress: however, the effect was significantly larger in the obese mice (29).
In our study, glycemic control was indexed by glycosylated hemoglobin (HbA1c) and provided a time-integrated measure of blood glucose levels over the previous two to three months. Glycemic control is essential for the management of type 1 and type 2 diabetes, as high HbA1c is linked to diabetes-related complications and cardiovascular events (30). Recent research highlights the importance of nondiabetic HbA1c as a cardiovascular risk factor (31) and predictor of other diseases (32, 33), suggesting that some health correlates of glycemic control might emerge earlier (i.e., at nondiabetic levels) than normally recognized. Current guidelines by the American Diabetes Association recommend the use of HbA1c to diagnose diabetes, with a threshold of ≥6.5 (34).
Specific Hypotheses
Consistent with prior studies, BMI, waist-to-hip ratio, and waist circumference were expected to be linked to higher nondiabetic HbA1c, after control variables were adjusted. We predicted that perceived weight discrimination would exacerbate the effects of BMI, waist-to-hip ratio, and waist circumference, resulting in higher HbA1c. This inquiry builds upon studies documenting the direct effects of perceived discrimination on health outcomes; however, prior work on the moderating effects of discrimination are notably absent from existing research.
Methods and Procedures
Study Population and Design
This study used data from the Midlife in the US (MIDUS) II, a longitudinal follow-up of the original MIDUS I study (N = 7,108) conducted in 1995/96 to investigate the role of behavioral, psychological, and social factors in understanding differences in physical and mental health. All eligible participants were non-institutionalized, English-speaking adults in the coterminous United States, aged 25 to 74. Approximately 9–10 years after the baseline interview respondents were re-contacted (longitudinal retention rate was 75%, adjusted for mortality). MIDUS II added comprehensive biomarker assessments on a subsample of participants who had completed a phone interview and self-administered questionnaires. Forty-three percent of the MIDUS II participants who were eligible to participate in the biological data collection agreed to be in the study. This rate is somewhat lower than other epidemiological studies involving a visit to a health clinic (e.g., 57% response rate in the Cardiovascular Health Study) (35). However, the MIDUS biological protocol is more intensive than other studies, requiring significant travel for most respondents, and also an overnight stay at the clinic. There were no significant differences between the biological subsample and the main MIDUS sample in the proportion who are obese or who reported experiences of weight-related discrimination. Additionally, the biological subsample was not significantly different from the main MIDUS sample on age, sex, race, marital status, or income, although respondents in the biological protocol were significantly more likely to have a college degree and significantly less likely to have only high school or some college compared with the main sample (36).
All analyses in this study used data from the biological subsample of MIDUS II and included 1255 participants ages 35 to 86 (M=57.32, SD=11.55), more than half of whom (57%) were female. We excluded 317 participants from analyses because of a self-reported diabetes diagnosis, taking anti-diabetic medications, fasting glucose above 126 mg/dl, HbA1c level above 6.5%, or missing data on any variables used in the analyses, yielding a final sample of 938 nondiabetic participants with complete data. The nondiabetic subsample of the biomarker sample included 96 twin pairs, thus raising potential concerns about non-independence. We employed a resampling strategy: one family member from each family in the MIDUS data was selected and then analyses were re-estimated with data that had been purged of dependencies among sample respondents. These findings were then compared with the results obtained when MIDUS samples were combined (and thus included dependencies in the data). We found no evidence for bias: the patterns of all main effects and interactions remained the same and coefficient sizes varied only slightly.
More details on MIDUS participants and subsamples is available in another publication (36). Table 1 includes the descriptive information for all variables.
Table 1.
Means (and SDs) or Proportions for All Measures (N = 938)
| Mean (SD) or proportion | |
|---|---|
| Dependent Variable | |
| HbA1c (range: 3.6–6.5) | 5.76 (.38) |
| Independent Variables | |
| Body mass index (range: 14.99–64.06) | 29.02 (6.05) |
| Waist to hip ratio (range: .62–1.15 ) | 0.88 (0.09) |
| Waist circumference (range: 24.02–56.10) | 37.75 (6.4) |
| Perceived weight discrimination (1=Yes) | 0.09 |
| Control Variables | |
| Race (1=White) | 0.83 |
| Gender (1=Male) | 0.43 |
| Age (range: 35–86) | 56.87 (11.55) |
| Income (range: 0–300,000) | 73,801 (59,275) |
| Education (8–21) | 14 (3.1) |
| Taking cholesterol medications (1=Yes) | 0.24 |
| Number of Cholesterol Medications (range: 0–2) | 0.26 (.5) |
| Exercise 3 Times a Week (1=yes) | 0.79 |
| Fast Food Consumption Weekly (range: 0– 5) | 2.34 (.91) |
| Current Smoker (1=yes) | 0.13 |
| Global Sleep Score (range: 0–19) | 6.05 (3.6) |
| Time Lag (months between survey and bio assessments) (range: 0–62) | 26.39 (14.33) |
Measures
Dependent variable: HbA1c
The HbA1c assay was a colorimetric total-hemoglobin determination combined with an immunoturbidometric HbA1c assay, carried out using a Cobas Integra Systems instrument (Roche Diagnostics) (37, 38). The Roche Diagnostics protocol stated that an intra-assay coefficient of variation (CV) for this method ranged between 2.2%–2.3%, and the inter-assay CV was 2.4%. Duplicate samples submitted for quality control (QC) monitoring of our samples documented a 0.43% CV.
Obesity and central adiposity
Obesity and central adiposity were the biological independent variables expected to predict nondiabetic HbA1c. Obesity, indexed by BMI, was calculated using measurements obtained by MIDUS staff and was derived by dividing a respondent’s weight (in kilograms) by their height (in meters squared). Consistent with guidelines established by the National Heart, Lung, and Blood Institute (NHLBI), values below 18.5 indicated an individual was underweight, values from 18.5 to 24.9 were considered normal, values from 25 to 29.9 indicated an individual was overweight, and values of 30 or greater identified an individual as obese (39). Central adiposity was indexed by waist-to-hip ratio and waist circumference. Waist-to-hip ratio was calculated by dividing an individual’s waist in inches (measured around the abdomen just above the hip bone) by their hip (maximum hip extension measurement); a waist-to-hip ratio of 0.90 or higher (for men) and 0.80 or higher (for women) was considered high-risk (39). Current high-risk cut points for waist circumference were 40 inches for men and 35 inches for women (39).
Perceived weight discrimination
Perceived weight discrimination was the psychosocial independent variable in this study (19). Nine questions assessed the frequency of exposure to daily occurrences of perceived discrimination (40). We focus here on indicators of interpersonal or daily discrimination, rather than institutional discrimination because the latter represents only a small proportion of the actual instances of unfair treatment based on personal characteristics (41). Respondents were asked, “How often on a day-to-day basis do you experience each of the following types of discrimination?” (1) “you are treated with less courtesy than other people”; (2) “you are treated with less respect than other people”; (3) “you receive poorer service than other people at restaurants or stores”; (4) “people act as if they think you are not smart”; (5) “people act as if they are afraid of you”; (6) “people act as if they think you are dishonest”; (7) “people act as if they think you are not as good as they are”; (8) “you are called names or insulted”; and (9) “you are threatened or harassed.” The four response categories ranged from 1 (“never”) to 4 (“often”). Respondents who indicated that they had ever experienced any such mistreatment were then asked: “What was the main reason for the discrimination you experienced?” A dichotomous indicator was created based on responses to both sets of questions and revealed whether one had ever (at least once) experienced mistreatment and this mistreatment was due to weight or height. Approximately 9% of the sample reported experiencing such discrimination. While it was not possible to ascertain how many of the participants reported mistreatment due specifically to weight, further analyses showed that all the individuals reporting such discrimination had high-risk BMI, waist-to-hip ratio, or waist circumference as defined by established cut-points described in the previous paragraph (39).
Control variables
Selected sociodemographic, health and psychosocial variables that have been linked to higher HbA1c levels and risk for type 2 diabetes were included in the regression models as covariates. Since higher HbA1c levels have been documented among older adults and ethnic minorities (42, 43), sociodemographic covariates included age, race, and gender. BMI is a powerful predictor of type 2 diabetes that is independent of central adiposity (44) and was also included as a control variable. Statins influence glycemic control (45), thus we controlled for taking cholesterol medications and number of cholesterol medications. Health behaviors linked to risk for type 2 diabetes were controlled for and included exercise frequency (46), frequent consumption of fast food (47), and current smoking (48). Sleep patterns have been consistently linked to risk for type 2 diabetes (49) so we included a measure of global sleep, based on The Pittsburgh Sleep Quality Index (PSQI) questionnaire that summed sleep disturbances over a 1-month time interval (continuous, range 0–19). The final control variable pertained to the time lag (in months) between the time when psychosocial and biological data were collected.
Overview of Data Analytic Plan
Hierarchical multiple regression was used and all models were multivariate-adjusted. All continuous independent variables and control variables were mean-centered and all categorical variables were dichotomous. All interaction terms were computed such that they were the product of the main effects variables centered at their mean. Statistically significant interaction terms were interpreted by graphing predicted scores for respondents in theoretically meaningful groups (i.e., people who report weight discrimination vs. those who do not). Building main effect models involved two steps. At step 1 of the multivariate model all covariates were entered (age, race, gender, income, education, taking cholesterol medications, number of cholesterol medications, exercise, fast food consumption, smoking status, and global sleep score). At step 2, one of each of the three measures of obesity and central adiposity (BMI, waist-to-hip ratio, or waist circumference) was added to the covariates. Building interaction models included three steps: at step 1 all covariates were entered, at step 2 one measure of either obesity or central adiposity was included together with perceived weight discrimination (i.e., BMI and Perceived Weight Discrimination), and at step 3 the interactive effect of the two measures from step 2 was included (i.e., BMI × Perceived Weight Discrimination).
Results
Bivariate Analyses
Bivariate correlations showed that HbA1c levels were positively linked to BMI (r= .16, p < .001), waist-to-hip ratio (r= .10, p < .01), and waist circumference (r= .17, p < .001). Sleep problem scores were correlated with BMI (r= .10, p < .01) but not waist-to-hip ratio and waist circumference. T-tests showed that people who reported discrimination due to weight were significantly younger (t (936) = 5.44, p < .001) and had higher BMI (t (936) = −9.54, p < .001) and waist circumference (t (936) = −5.95, p < .001) but not waist-to-hip ratio. People who reported discrimination did not differ significantly from those who did not in their health behaviors (smoking, exercise, and fast food consumption).
Main Effects Models: Obesity, Central Adiposity, and HbA1c
We estimated main effect models to investigate the independent effects of obesity and central adiposity on nondiabetic HbA1c. We hypothesized that individuals with high BMI, waist-to-hip ratio, and waist circumference would have higher HbA1c, net of control variables. Results of the three rounds of step additions confirmed that after adjusting for control variables, BMI (R2=0.156, b=0.008, p<.001), waist-to-hip ratio (R2=0.157, b=0.749, p<.001), and waist circumference (R2=0.164, b=0.011, p<.001) were linked to higher HbA1c levels.
Interaction Models
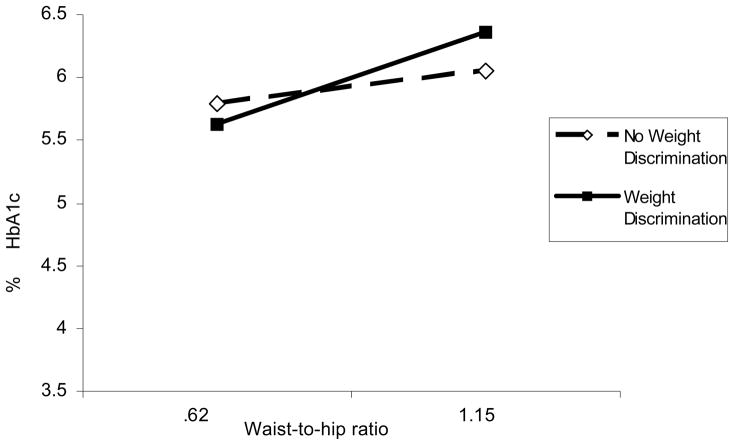
We estimated two-way interaction models to evaluate the combined effects of obesity and central adiposity and perceived weight discrimination on HbA1c. We hypothesized that perceived weight discrimination would exacerbate the effects of BMI, waist-to-hip ratio, and waist circumference, resulting in higher HbA1c. Results showed that, net of all control variables, there was a significant two-way interaction effect only between waist-to-hip ratio and weight discrimination on HbA1c (R2=0.171, b=0.851, p<.05). A graph of the analysis showed that weight discrimination exacerbated the effects of waist-to-hip ratio on HbA1c (see Table 2 and Figure 1). Specifically, weight discrimination did not affect HbA1c levels among people with lower waist-to-hip ratio: however, for people with high waist-to-hip ratio, weight discrimination emerged as a vulnerability factor. Perceived weight discrimination did not moderate the effects of BMI or waist circumference on HbA1c.
Table 2.
Multivariate Linear Regression Results (unstandardized coefficients) for Waist-to-hip Ratio, Weight Discrimination, and HbA1c (N=938)
| Variables | Step 1 | Step 2 | Step 3 | |||
|---|---|---|---|---|---|---|
| b (SE) | R2 | b (SE) | R2 | b (SE) | R2 | |
| Age | .009*** (.001) | .009*** (.001) | .009*** (.001) | |||
| Race (1=White) | −.083* (.033) | −.088** (.033) | −.086** (.033) | |||
| Gender (1=Male) | −.088*** (.024) | −.156*** (.033) | −.152*** (.033) | |||
| Global Sleep Score | .001 (.003) | .001 (.003) | .001 (.003) | |||
| Income | .001 (.001) | .001 (.001) | .001 (.001) | |||
| Education | −.004 (.005) | −.003 (.005) | −.004 (.005) | |||
| Taking Cholesterol Meds (1=Yes) | −.159 (.101) | −.189† (.101) | −.197* (.101) | |||
| # Cholesterol Meds | .217* (.091) | .232** (.090) | .238** (.090) | |||
| Regular Exercise (1=yes) | −.055† (.029) | −.048* (.029) | −.047 (.029) | |||
| Fast Food Consumption (1=yes) | .012 (.013) | .012 (.013) | .012 (.013) | |||
| Current Smoker (1=yes) | .077* (.036) | .067† (.036) | .068† (.036) | |||
| Body Mass Index | .008*** (.002) | .005* (.002) | .005* (.002) | |||
| Time Lag Variable | −.002* (.001) | −.002** (.001) | −.002** (.001) | |||
| Waist-to-hip Ratio | __________ | .580** (.180) | .488** (.186) | |||
| Weight Discrimination (1=Yes) | .156*** | .077† (.042) | .075† (.041) | |||
| Waist-to-hip Ratio × Weight Discrimination | __________ | .168** | .851* (.430) |
.17 1* |
||
p < .05.
p < .01.
p < .001.
p <.10.
Note: R2 values at each step include the R2 value of the current and all previous steps (i.e. R2 for Step 3 is the cumulative R2 value for Steps 1, 2, and 3).
Figure 1.
Weight Discrimination Exacerbates the Effects of Waist-to-Hip Ratio on HbA1c (p<0.05)
Note: Waist-to-hip ratios in this figure include all waist-to-hip values used in the analyses. Unstandardized regression coefficients were used to plot the interaction.
Discussion
Our study capitalized on the strengths of a large national sample survey of Americans to investigate risk factors and psychosocial influences as complementary in their relationship to nondiabetic glycemic control. Results supported the predicted hypotheses: the negative influence of all indices of obesity and central adiposity on HbA1c was confirmed at nondiabetic levels, and importantly, the exacerbating power of perceived weight discrimination was documented.
Including weight discrimination as a targeted measure of stress linked with the key biological risk factors helped document that the physical burden of carrying excessive weight was significantly exacerbated by perceptions of discriminatory treatment due to the high body weight. Specifically, the highest HbA1c levels were observed among people who had high waist-to-hip ratio and who reported weight discrimination.
The prevalence of perceived weight discrimination has increased by 66% over the last decade (50) and obese individuals face multiple forms of prejudice and weight stigma, including both interpersonal slights, insults, and work-related discrimination (25). This study contributed to the growing literature on discrimination and health by documenting that the negative influence of self-reported weight discrimination was not limited to psychosocial aspects of daily life or self-reported health outcomes, but also extended to a powerful biomarker of physical health. This finding has potentially important implications for public health policy and education. Recent studies on race and health reveal that the well-documented health disadvantage of blacks relative to whites may be exacerbated even further due to blacks’ elevated risk of discriminatory treatment – which may discourage them from engaging in healthy behaviors or from seeking timely medical care (13). We suspect that a similar pattern may occur among overweight and obese Americans. While our study documented that the negative effects of weight discrimination were independent of certain health behaviors, such as smoking, exercise, and fast food consumption, previous studies (25, 51) have documented that obese individuals might not seek timely health care or comply with proper health care regimens due to fear of mistreatment, teasing, and the demoralization that results from this mistreatment. Thus, perceptions of persistent mistreatment may exacerbate the already harmful consequences of central adiposity for a range of physical outcomes, including glycemic control.
Possible Mechanisms
While the specific pathways that link obesity, central adiposity, discrimination, and glycemic control are unclear, the psychosocial moderation of waist-to-hip ratio but not other body weight measures provides initial clues into possible mechanisms. BMI, for example, is a surrogate measure of body fat that may provide misleading information about body fat content, especially among older adults for whom it may not detect the “conversion” of lean to fat tissue that accompanies normal aging, or among non-Caucasian people, for whom the relationship between BMI and body fat varies widely for different race/ethnic groups (52). Waist circumference and waist-to-hip ratio, in contrast, reflect central adiposity, which has been described as a superior measure in predicting risk in the Diabetes Prevention Program (53). Hip circumference is potentially important because it indexes muscle and/or fat mass at thehips (54, 55), and larger sizes of leg muscle and leg fat have been linked to metabolic protection against higher glucose levels and risk for diabetes (55). One mechanism that links lower leg fat to metabolic problems is its relative insensitivity to lipolytic stimuli and high sensitivity to antilipolytic stimuli: in effect, leg fat might act as a “sink” for circulating free fatty acids generated by central adiposity (52, 56). This uptake of free fatty acids prevents fat storage in liver, sketetal muscle, and pancreas, where high levels would cause insulin resistance and beta-cell dysfunction.
Since free fatty acids are a major mediator of the stress response (57) and widely accepted as important contributors to the development of diabetes (58), they represent a potential mechanism that might explain our results. Relevant to the present findings, chronic psychosocial stress such as perceived discrimination might introduce the major stress hormones (norepinephrine, epinephrine, and cortisol), as well as more free fatty acids that modify the relationship between the free fatty acids (produced by visceral fat and taken up by leg fat depots) and glycemic control, and this effect is more pronounced in people with an existing vulnerability (such as high waist-to-hip ratio). Future studies on psychosocial moderation of obesity and central adiposity are essential for documenting intervening mechanisms: for example, the testable hypothesis that free fatty acids are the main pathway that links stress to obesity, central adiposity, and glycemic control is an important next investigation.
Limitations
Finally, despite this study’s conceptual and methodological strengths, the main limitations pertained to its cross-sectional design which does not allow claims of causality. Furthermore, an important next step is identifying factors that differentiate between obese people who report discrimination and those who do not. Including competing measures of different stressors should be the first part of this inquiry: does perceived general stress make people more susceptible to perceive discrimination, and importantly, are coping skills a potential buffer in this relationship?
Implications for Prevention and Intervention
Public policies and educational interventions designed to lessen risk for type 2 diabetes and regulate glycemic levels typically target the behaviors of overweight and obese persons, including their diets, caloric intake levels, and physical fitness levels. The value of such work is undeniable. However, our study suggests that perceived weight discrimination among people at risk for developing type 2 diabetes is another potentially useful target for interventions. Fostering effective strategies for managing health behaviors as well as for coping with weight-related discrimination may be useful combined objectives for clinicians. For instance, research on coping with weight stigma has documented that a range of coping responses used to deal with stigma and effective strategies (e.g., positive self-talk, obtaining social support) are linked with higher psychological well-being among women who report weight stigma (59). Promoting both effective coping skills for dealing with weight stigma while also diminishing negative responses such as overeating or using food for comfort could have important consequences for glycemic control.
Our study did not adjudicate whether perceptions of weight discrimination were objective or perceived only, although previous research has documented both perceived and objective weight-based stigmatization and unfair treatment for overweight and obese persons in domains of employment, healthcare, and education (25, 26). In fact, even health professionals specializing in obesity show strong weight bias, indicating pervasive and powerful stigma (60, 61). Therefore, interventions also need to address “those who do the discriminating” against overweight and obese persons (62). Public education about the challenges facing obese persons and about the pervasiveness of prejudicial attitudes towards them may help to reduce discriminatory treatment. Legislative changes also may be effective. To date, Michigan is the only state that prohibits employment discrimination on the basis of weight: the Elliot Larsen Civil Rights Act bans discrimination in employment on the basis of height and weight. In the remaining 49 states, obesity is not a protected category. The Civil Rights Act of 1964 does not identify weight as a protected characteristic, and only in rare instances can severely obese people seek legal protection under Americans with Disabilities Act (ADA) legislation. Expanding protected categories to include obese persons may be effective for reducing the extent to which prejudicial beliefs against stigmatized individuals are translated into discriminatory treatment.
This study provides initial evidence that viewing biological and psychosocial factors as complementary influences is important for understanding variations in glycemic control. Ultimately, the goal is to design targeted interventions for individuals who are considered at risk for developing diabetes due to the combined presence of various psychosocial (i.e., perceived weight discrimination) and biological risk factors (e.g. obesity). Strategies that tackle both the discriminatory social environment faced by obese persons and their adaptations to these environments may be consequential for minimizing diabetes risk at the population level.
Footnotes
Conflict of Interest Statement
The authors have no financial disclosures related to products or corporate holdings mentioned in this manuscript.
Contributor Information
Vera K. Tsenkova, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, USA
Deborah Carr, Department of Sociology and Institute for Health, Health Care Policy and Aging Research, Rutgers University, New Brunswick, NJ, USA
Dale A. Schoeller, Department of Nutritional Sciences, University of Wisconsin— Madison, Madison, WI, USA
Carol D. Ryff, Department of Psychology, Institute on Aging, University of Wisconsin— Madison, Madison, WI, USA
References
- 1.National Diabetes Surveillance System. Center for Disease Control and Prevention; [Google Scholar]
- 2.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 3.N. I. o. D. a. D. a. K. Diseases. National Diabetes Statistics, 2007 fact sheet. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health; 2008. [Google Scholar]
- 4.Attie AD. Genetic and genomic studies of type 2 diabetes in mice. International Congress Series. 2004;1262:220–223. [Google Scholar]
- 5.Glasgow RE, Wagner EH, Kaplan RM, et al. If diabetes is a public health problem, why not treat it as one? A population-based approach to chronic illness. Ann Behav Med. 1999;21:159–170. doi: 10.1007/BF02908297. [DOI] [PubMed] [Google Scholar]
- 6.Netterstrom B, Danborg L, Olesen H. Glycated hemoglobin as a measure of physiological stress. Behav Med. 1988;14:13–16. doi: 10.1080/08964289.1988.9935118. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami N, Araki S, Hayashi T, Masumoto T. Relationship between perceived job-stress and glycosylated hemoglobin in white-collar workers. Ind Health. 1989;27:149–154. doi: 10.2486/indhealth.27.149. [DOI] [PubMed] [Google Scholar]
- 8.Norberg M, Stenlund H, Lindahl B, et al. Work stress and low emotional support is associated with increased risk of future type 2 diabetes in women. Diabetes Res Clin Pract. 2007;76:368–377. doi: 10.1016/j.diabres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Dedert EA, Calhoun PS, Watkins LL, Sherwood A, Beckham JC. Posttraumatic Stress Disorder, Cardiovascular, and Metabolic Disease: A Review of the Evidence. Ann Behav Med. doi: 10.1007/s12160-010-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dovidio JF, Major B, Crocker J. Stigma: Introduction and Overview. In: Heatherton TF, Neck RE, Hebl MR, Hill JG, editors. The Social Psychology of Stigma. Vol. 200. Guilford Press; pp. 1–28. [Google Scholar]
- 11.Crandall CS. Ideology and lay theories of stigma: the justification of stigmatization. In: Heatherton TF, Neck RE, Hebl MR, Hill JG, editors. The Social Psychology of Stigma. Guilford Press; 2000. [Google Scholar]
- 12.Pearlin LI, Schieman S, Fazio EM, Meersman SC. Stress, health, and the life course: some conceptual perspectives. J Health Soc Behav. 2005;46:205–219. doi: 10.1177/002214650504600206. [DOI] [PubMed] [Google Scholar]
- 13.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychol Bull. 2009;135:531–554. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes LL, de Leon CF, Lewis TT, et al. Perceived discrimination and mortality in a population-based study of older adults. Am J Public Health. 2008;98:1241–1247. doi: 10.2105/AJPH.2007.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brondolo E, Rieppi R, Kelly KP, Gerin W. Perceived racism and blood pressure: a review of the literature and conceptual and methodological critique. Ann Behav Med. 2003;25:55–65. doi: 10.1207/S15324796ABM2501_08. [DOI] [PubMed] [Google Scholar]
- 17.Clark R. Self-reported racism and social support predict blood pressure reactivity in Blacks. Ann Behav Med. 2003;25:127–136. doi: 10.1207/S15324796ABM2502_09. [DOI] [PubMed] [Google Scholar]
- 18.Matthews KA, Salomon K, Kenyon K, Zhou F. Unfair treatment, discrimination, and ambulatory blood pressure in black and white adolescents. Health Psychol. 2005;24:258–265. doi: 10.1037/0278-6133.24.3.258. [DOI] [PubMed] [Google Scholar]
- 19.Kessler RC, Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J Health Soc Behav. 1999;40:208–230. [PubMed] [Google Scholar]
- 20.Hatzenbuehler ML, Keyes KM, Hasin DS. Associations Between Perceived Weight Discrimination and the Prevalence of Psychiatric Disorders in the General Population. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. Am J Public Health. 2008;98:S29–37. doi: 10.2105/ajph.98.supplement_1.s29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodin M, Price J, Sanchez F, McElliot S. Derogation, exclusion, and unfair treatment of persons with social flaws: controllability of stigma and the attribution of prejudice. Personality and Social Psychology Bulletin. 1989;15:439–451. [Google Scholar]
- 23.Crandall CS, Martinez R. Culture, ideology, and antifat attitudes. Personality and Social Psychology Bulletin. 1997;22:1165–1176. [Google Scholar]
- 24.Puhl RM, Andreyeva T, Brownell KD. Perceptions of weight discrimination: prevalence and comparison to race and gender discrimination in America. Int J Obes (Lond) 2008;32:992–1000. doi: 10.1038/ijo.2008.22. [DOI] [PubMed] [Google Scholar]
- 25.Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17:941–964. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- 26.Puhl R, Brownell KD. Bias, discrimination, and obesity. Obes Res. 2001;9:788–805. doi: 10.1038/oby.2001.108. [DOI] [PubMed] [Google Scholar]
- 27.Bray GA, Jablonski KA, Fujimoto WY, et al. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr. 2008;87:1212–1218. doi: 10.1093/ajcn/87.5.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 29.Surwit RS, Feinglos MN, Livingston EG, Kuhn CM, McCubbin JA. Behavioral manipulation of the diabetic phenotype in ob/ob mice. Diabetes. 1984;33:616–618. doi: 10.2337/diab.33.7.616. [DOI] [PubMed] [Google Scholar]
- 30.Alam T, Weintraub N, Weinreb J. What is the proper use of hemoglobin A1c monitoring in the elderly? J Am Med Dir Assoc. 2005;6:200–204. doi: 10.1016/j.jamda.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Gerstein HC. Glycosylated hemoglobin: finally ready for prime time as a cardiovascular risk factor. Ann Intern Med. 2004;141:475–476. doi: 10.7326/0003-4819-141-6-200409210-00014. [DOI] [PubMed] [Google Scholar]
- 32.Muntner P, Wildman RP, Reynolds K, et al. Relationship between HbA1c level and peripheral arterial disease. Diabetes Care. 2005;28:1981–1987. doi: 10.2337/diacare.28.8.1981. [DOI] [PubMed] [Google Scholar]
- 33.Vitelli LL, Shahar E, Heiss G, et al. Glycosylated hemoglobin level and carotid intimal-medial thickening in nondiabetic individuals. The Atherosclerosis Risk in Communities Study. Diabetes Care. 1997;20:1454–1458. doi: 10.2337/diacare.20.9.1454. [DOI] [PubMed] [Google Scholar]
- 34.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 36.Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS National Study: Protocol, Measures, Sample, and Comparative Context. JOURNAL OF AGING AND HEALTH. doi: 10.1177/0898264310374355. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zander R, Lang W, Wolf HU. Alkaline haematin D-575, a new tool for the determination of haemoglobin as an alternative to the cyanhaemiglobin method. I. Description of the method. Clin Chim Acta. 1984;136:83–93. doi: 10.1016/0009-8981(84)90250-x. [DOI] [PubMed] [Google Scholar]
- 38.Wolf HU, Lang W, Zander R. Alkaline haematin D-575, a new tool for the determination of haemoglobin as an alternative to the cyanhaemiglobin method. II. Standardisation of the method using pure chlorohaemin. Clin Chim Acta. 1984;136:95–104. doi: 10.1016/0009-8981(84)90251-1. [DOI] [PubMed] [Google Scholar]
- 39.National Heart LaBI. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in adults: The Evidence Report. Bethesda (MD): NIH Publication: National Institute of Health; 1998. [Google Scholar]
- 40.Williams DR, YU Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: Socioeconomic status, stress and discrimination. Journal of Health Psychology. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 41.Ridgeway C. Interaction and the Conservation of Gender Inequality: Considering Employment. American Sociological Review. 1997:62. [Google Scholar]
- 42.Boltri JM, Okosun IS, Davis-Smith M, Vogel RL. Hemoglobin A1c levels in diagnosed and undiagnosed black, Hispanic, and white persons with diabetes: results from NHANES 1999–2000. Ethn Dis. 2005;15:562–567. [PubMed] [Google Scholar]
- 43.Nuttall FQ. Effect of age on the percentage of hemoglobin A1c and the percentage of total glycohemoglobin in non-diabetic persons. J Lab Clin Med. 1999;134:451–453. doi: 10.1016/s0022-2143(99)90165-8. [DOI] [PubMed] [Google Scholar]
- 44.Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol. 1997;145:614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 45.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 46.Ivy JL. Role of exercise training in the prevention and treatment of insulin resistance and non-insulin-dependent diabetes mellitus. Sports Med. 1997;24:321–336. doi: 10.2165/00007256-199724050-00004. [DOI] [PubMed] [Google Scholar]
- 47.Pereira MA, Kartashov AI, Ebbeling CB, et al. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet. 2005;365:36–42. doi: 10.1016/S0140-6736(04)17663-0. [DOI] [PubMed] [Google Scholar]
- 48.Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care. 1999;22:1887–1898. doi: 10.2337/diacare.22.11.1887. [DOI] [PubMed] [Google Scholar]
- 49.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009 doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andreyeva T, Puhl RM, Brownell KD. Changes in perceived weight discrimination among Americans, 1995–1996 through 2004–2006. Obesity (Silver Spring) 2008;16:1129–1134. doi: 10.1038/oby.2008.35. [DOI] [PubMed] [Google Scholar]
- 51.Amy NK, Aalborg A, Lyons P, Keranen L. Barriers to routine gynecological cancer screening for White and African-American obese women. Int J Obes (Lond) 2006;30:147–155. doi: 10.1038/sj.ijo.0803105. [DOI] [PubMed] [Google Scholar]
- 52.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 53.Relationship of body size and shape to the development of diabetes in the diabetes prevention program. Obesity (Silver Spring) 2006;14:2107–2117. doi: 10.1038/oby.2006.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snijder MB, Visser M, Dekker JM, Seidell JC. Re: “Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men”. Am J Epidemiol. 2004;160:133–1134. doi: 10.1093/aje/kwh322. author reply 1134–1135. [DOI] [PubMed] [Google Scholar]
- 55.Seidell JC, Han TS, Feskens EJ, Lean ME. Narrow hips and broad waist circumferences independently contribute to increased risk of non-insulin-dependent diabetes mellitus. J Intern Med. 1997;242:401–406. doi: 10.1046/j.1365-2796.1997.00235.x. [DOI] [PubMed] [Google Scholar]
- 56.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–1210. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 57.Black PH. The inflammatory consequences of psychologic stress: Relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Medical Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 58.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 59.Puhl RM, Brownell KD. Confronting and coping with weight stigma: an investigation of overweight and obese adults. Obesity (Silver Spring) 2006;14:1802–1815. doi: 10.1038/oby.2006.208. [DOI] [PubMed] [Google Scholar]
- 60.Schwartz MB, Chambliss HO, Brownell KD, Blair SN, Billington C. Weight bias among health professionals specializing in obesity. Obes Res. 2003;11:1033–1039. doi: 10.1038/oby.2003.142. [DOI] [PubMed] [Google Scholar]
- 61.Foster GD, Wadden TA, Makris AP, et al. Primary care physicians’ attitudes about obesity and its treatment. Obes Res. 2003;11:1168–1177. doi: 10.1038/oby.2003.161. [DOI] [PubMed] [Google Scholar]
- 62.Link B, Phelan J. Conceptualizing Stigma. Annual Review of Sociology. 2001:27. [Google Scholar]