Abstract
Background
In asymptomatic smokers, coronary microcirculatory dysfunction, assessed by coronary flow reserve (CFR), is an early indicator of cardiovascular risk. Inflammation and oxidative stress may be the mechanisms through which smoking affects the microvasculature.
Objectives
The purpose of this study was to determine the relationship between smoking and CFR, taking into account potential shared genetic effects.
Methods
We examined 360 male middle aged twins (288 non-smokers and 72 smokers), including 46 twin pairs discordant for current smoking. Coronary flow reserve (CFR) in response to adenosine was measured with positron emission tomography [N13] ammonia and quantitation of coronary blood flow at rest and after adenosine stress. Inflammation was assessed by measuring interleukin-6 and C-reactive protein, and oxidative stress was determined by measuring plasma hydroperoxides, glutathione (GSH), the oxidized form of GSH, GSSG, and the ratio of GSH to GSSG.
Results
CFR was significantly lower in smokers compared to nonsmokers (2.25 vs. 2.75, p<0.01). This relationship persisted after accounting for known cardiovascular disease risk factors, and was marginally affected by adjusting for inflammatory and oxidative stress biomarkers. In addition, in smoking-discordant twin pairs, CFR in the smoking twin was significantly lower than in the non-smoking co-twin (2.25 vs. 2.67, p = 0.03) even after adjustment for cardiovascular risk factors.
Conclusions
Our results demonstrate the adverse effects of smoking in the early phases of cardiovascular disease. Mechanisms other than peripherally-measured inflammation and oxidative stress are involved.
Keywords: biomarkers, smoking, cardiovascular disease, oxidative stress, inflammation
Introduction
Epidemiological evidence supports a link between cigarette smoking and coronary heart disease (CHD) 1. Most of the adverse effects of cigarette smoking on CHD risk are attributed to its hemodynamic influences (increase in blood pressure and heart rate, and decrease in exercise tolerance) and pro-coagulant effects 2. However, cigarette smoking also releases free radicals and pro-oxidant factors that may result in inflammation and oxidative damage to the vascular endothelium and impair coronary circulatory function.3
In asymptomatic smokers with no evidence of CHD, coronary microcirculatory dysfunction is considered an early indicator of CHD risk 4. Although no technique is available that directly depicts the coronary microcirculation in vivo, dysfunction in the coronary microcirculation can be assessed by coronary flow reserve (CFR), a measure of whole coronary circulation and coronary vasodilatory capacity. To date, only a few studies have measured CFR using PET in relation to long-standing, habitual smoking 5–11. The results of these studies have been conflicting. Some studies have reported similar CFR comparing long-term smokers to nonsmokers 5, 7, 9, while other studies have found that CFR was attenuated in smokers 10. Therefore, whether chronic smoking affects the function of the coronary circulation and the early phases of atherosclerosis is unclear. The small sample size of many of these studies, and use of differing stressors (i.e., dipyridamole, adenosine, or cold pressor test) may explain differences in results. Furthermore, none of these studies sought to determine whether elevated levels of biomarkers for inflammation and oxidative stress, which are augmented in smokers, may be implicated in the coronary vascular effects of smoking 12.
The purpose of this study was to assess the relationship between smoking status and CFR, a measure of coronary microcirculatory function and vasodilator capacity, using a co-twin control design. Because CHD has a large genetic component 13, as does nicotine addiction 14, it is important to take into account any potential shared genetic influences. Co-twin control studies allow tight control for genetic and shared environmental influences. Another objective was to determine whether inflammation or oxidative stress were potential mechanisms underlying the relationship between smoking and coronary microvascular function.
Methods
Subjects
The Twins Heart Study (THS) is an investigation of psychological, behavioral and biological risk factors for subclinical cardiovascular disease using twins 15, 16. It includes 180 monozygotic (MZ) and dizogytic (DZ) twin pairs who are members of the Vietnam Era Twin (VET) Registry 17. The sample consists of smoking and non-smoking middle aged male twins born between 1946 and 1956. The VET Registry includes twins that previously served in the United States military during the time of the Vietnam War. For the THS two groups of twin pairs were randomly sampled from the Registry. The first group included twins discordant for a lifetime history for major depression, and the second group included twin pairs where neither had a history of major depression. Twins were examined together at the Emory University General Clinical Research Center (GCRC) between March 2002 and March 2006. Medical history and smoking status were obtained at the time of examination. The study protocol was approved by the Institutional review board at Emory University, and informed consent was obtained from all subjects.
Positron Emission Tomography (PET)
Twins underwent imaging of myocardial blood flow (MBF) with PET [13N] ammonia at rest and following pharmacologic (adenosine) induced hyperemia during a single imaging session. All twins were admitted to the GCRC in the late morning or early afternoon on the day prior to the PET scan, and resided overnight in the GCRC facility. They all received a similar dinner following the American Heart Association Step 1 diet, and remained fasting until the PET scan was completed. They were instructed to abstain from smoking, drinking alcoholic or caffeinated beverages, and from eating any foods other than what was served to them, for their entire GCRC stay, that is, at least 12 hours prior to the blood draw and PET scan. All medications were held the morning of the PET scan.
PET Dynamic Flow Measures
Myocardial blood flow (MBF) measurements were acquired at rest and during adenosine-induced hyperemia. The last frame of the dynamic sequence was used as a template for sectorial region-of-interest (ROI) analysis. The input function was generated by drawing an ROI in the left ventricle chamber on a mid-ventricular slice, and flow was calculated (expressed in ml/min per 100 gm of tissue) using established methodology 18. The left ventricle was sampled radially from 40 different angles and 40 samples of flow were obtained for each short axis slice. The resulting hundreds of samples were grouped into 20 segments.
CFR Calculations
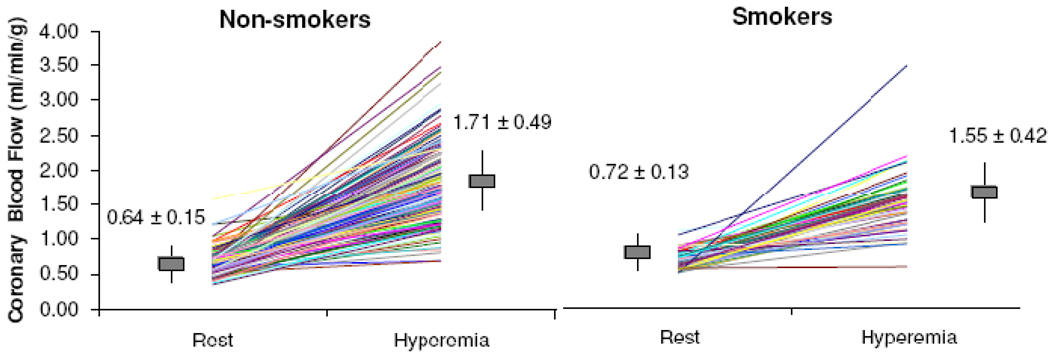
CFR was calculated using MBF measurements during rest (MBFrest) and hyperemia (MBFhyperemia) (Figure 1). CFR is defined as the ratio of MBFhyperemia to MBFrest. The measure of CFR was determined across all 20 segments. Coronary vascular resistance (CVR) was also calculated both at baseline and after hyperemia by dividing the mean arterial blood pressure by the respective flow value. To determine the amount of cardiac work conducted in smokers and non-smokers the rate pressure product (RPP) was calculated as the mean systolic blood pressure during adenosine infusion multiplied by the mean heart rate during adenosine infusion divided by 100.
Figure 1.
Myocardial blood flow, under resting (MBFrest) condition and after adenosine induced hyperemia (MBFhyperemia) in non-smokers and smokers. Vertical bars denote means ± standard deviations for each group.
Myocardial Perfusion Defect Score
In addition to CFR measures, we constructed a summation score describing the number and the severity of visible perfusion defects across the 20 segments of acquisition. In each segment, the defect severity was quantified on a 4-point scale and then was summed up across the 20 segments yielding a total score. Separate scores were obtained for the rest (summed rest score) and stress (summed stress score) scans. A reversible defect score (summed difference) was obtained by subtracting the rest score from the stress score. These scores, and a dichotomous indicator of perfusion abnormalities (defined as a summed stress score ≥ 4), represent secondary perfusion outcomes.
Smoking Status and Cardiovascular Risk Factor Assessment
Smoking status was determined using standardized questionnaires from population studies19. We classified participants: as never smoked - those who reported that they never smoked regularly, current smokers - those currently smoking cigarettes regularly, and past smokers - those who reported smoking more than 100 cigarettes in the past. For this study, we also classified participants into two groups: current smokers and non-smokers, the latter including never and past smokers. To determine the length an individual smoked, pack years were calculated by multiplying the number of packs of cigarettes smoked per day by the number of years the person had smoked. The time lapsed between the age at smoking cessation and the current age was also determined in past smokers.
Medical history and physical evaluation were obtained from all twins by a research nurse. Waist-to-hip ratio was calculated as the ratio of waist to hip circumference. Systolic and diastolic blood pressure was measured by a mercury sphygmomanometer on the right arm in sitting position after 10 minutes of rest. Two separate blood pressure measurements, taken 5 minutes apart, were averaged for analysis. Plasma fasting glucose, total, low, and high density cholesterol were determined from plasma samples collected after a 9 hour overnight fast. Total triglycerides were measured by enzymatic methods (Beckman Coulter Diagnostics, Fullerton, California). Direct high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were measured with homogeneous assays (Equal Diagnostics, Exton, Pennsylvania). Glucose was measured on the Beckman CX7 chemistry autoanalyzer. Current physical activity status was determined using the Baecke questionnaire, a 16-question instrument documenting level of physical activity at work, during sports and non-sports activities 20. The global physical activity score was used in the analysis. A history of coronary heart disease was defined as previously being diagnosed with myocardial infarction or angina pectoris or previous coronary revascularization procedures. Diabetes was defined as having a fasting plasma glucose >126 mg/dl or current treatment with antidiabetic medications. Demographic factors, such as education, marital and employment status were determined using a standardized questionnaire.
Assessment of Inflammatory and Oxidative Stress Biomarkers
Inflammation
Plasma samples were collected and frozen at −70°C for later analysis of inflammatory biomarkers. IL-6 levels were measured using a commercially available enzyme linked immunosorbent assay kit (Quantikine hs human IL-6, R&D Systems, Minneapolis, Minnesota). Levels of C-reactive protein (CRP) were measured with the high-sensitivity Beckman Coulter assay. High values of IL-6 and CRP are indicative of inflammation.
Oxidative Stress
We examined two measures of oxidative stress. The first measure was the free oxygen radical test assay (FORT, Incomat). The FORT provides an indirect measure of hydroperoxides, the intermediate oxidative products of lipids, amino acids, and peptides. Briefly, a 10µL sample of plasma was mixed with acidic buffer containing transition metals. Chromagen was then added to the sample resulting in the formation of a colored radical cation. The amount of radical cation was determined by the intensity of its color spectrophotometrically. This color corresponds to the amount of peroxy and alkoxy radicals that were formed from alcohols and hydroperoxides upon addition of the transition metals. Results of the FORT are presented in FORT units, with higher values indicative of higher oxidative stress levels.
Our second measure was the plasma ratio of reduced to oxidized glutathione. Briefly, plasma samples were placed in a tube containing a preservative to decrease auto-oxidation and stored at −80°C until analysis. Samples were analyzied for GSH and GSSG levels using a high performance liquid chromatography assay 21. GSH and GSSG levels were used to determine the ratio of GSH/GSSG. Low values of GSH/GSSG are indicative of oxidative stress.
Statistical Analysis
Statistical analysis was performed using SAS software version 9.2 (SAS Institute). All analysis was conducted using mixed model linear regression analysis adapted for twin studies 22. Initial descriptive analysis treating the twins as individuals, while accounting for clustering by twin pair, compared smokers to non-smokers on cardiovascular disease risk factors and biomarkers for inflammation and oxidative stress. Inflammatory biomarkers were log-transformed to improve normality. Comparison of smokers and non-smokers (base model) was conducted on CFR. We then adjusted for cardiovascular risk factors that include age, body mass index (BMI), high, total, and low density lipoprotein cholesterol. In this model we also included use of aspirin and use of statins. Because blood pressure medications could be related to CFR, in a separate model we accounted for cardiovascular risk factors, aspirin, use of statins, as well as anti-hypertensive meds, including beta blockers, ace-inhibitors, diuretics, and angiotensin receptor blockers. We repeated the analysis further adjusting for history of major depression and posttraumatic stress disorder (PTSD). Each inflammatory and oxidative stress biomarker was then added to the model that included cardiovascular risk factors to assess if these factors confounded the relationship between smoking and CFR. In addition, we fit additional models that adjusted for the presence of perfusion abnormalities (summed stress score) to determine whether differences in CFR associated with smoking were due to microvascular disease as opposed to epicardial coronary stenoses. To further ensure the long-term effects of smoking on the coronary microcirculation was not due to perfusion defects, the same analysis was conducted in patients without perfusion abnormalities (summed stress score=0). In a separate model, we adjusted for RPP to account for any difference in cardiac work conducted in smokers compared to non-smokers. The Spearmen correlation coefficient was used to determine the relationship between smoking pack years and CFR.
Next, we focused our analysis on smoking discordant twin pairs where one member was a smoker and their twin brother was not a smoker. We conducted a within pair analysis of the relationship of smoking and the coronary flow measures that automatically takes into account shared familial and many early environmental factors. In this sample the twins were raised in the same home and environment. Within-pair analysis was further stratified by zygosity to determine whether the relationship between smoking and the coronary flow measures were different between monozygotic and dizygotic twins. Monozygotic twins share 100% of their genes, whereas dizygotic twins only share on average 50% of their genes. Our statistical analysis of the smoking discordant pairs uses mixed regression modeling to examine the association of smoking with the coronary flow measures both before and after adjustment for CVD risk factors and summed stress score.
Results
The study sample consisted of 288 non-smokers (164 monozygotic and 124 dizygotic) and 72 smokers (40 monozygotic and 32 dizygotic). The mean pack years ± SD for smokers was 41.1 ± 20.3 (Table 1). There were 20 MZ and 26 DZ twin pairs discordant for current cigarette smoking. Compared to non-smokers, smokers were less educated and physically active, and less likely to be employed and married. In addition, smokers had a lower diastolic BP and BMI, and were more likely to previously have been diagnosed with CHD.
Table 1.
Demographic, lifestyle, clinical, hemodynamic and biochemical characteristics in smokers and non-smokers
| Non-smokers | Smokers | p-value | |
|---|---|---|---|
| Age | 55 (3) | 53 (3) | 0.23 |
| Education | 14 (2) | 14 (0.06) | 0.001 |
| Body Mass Index, kg/m2 | 30 (5) | 28 (5) | 0.90 |
| Waist-to-hip ratio | 0.94 (0.06) | 0.96 (.06) | 0.15 |
| Physical Activity | 7.30 (2) | 6.74 (2) | 0.007 |
| Married, n (%) | 241 (84%) | 43 (60%) | <0.001 |
| Employed, n (%) | 235 (82%) | 51 (71%) | 0.07 |
| Plasma glucose, mg/dL | 101 (17) | 99 (21) | 0.29 |
| Systolic blood pressure, mmHg | 130 (16) | 128 (14) | 0.47 |
| Diastolic blood pressure, mmHg | 81 (11) | 79 (11) | 0.11 |
| Blood Lipids, mg/dL | |||
| Total Triglycerides | 178 (96) | 190 (115) | 0.50 |
| Total cholesterol | 186 (38) | 186 (38) | 0.64 |
| HDL cholesterol | 39 (10) | 39 (10) | 0.83 |
| LDL cholesterol | 122 (34) | 122 (34) | 0.54 |
| Previous coronary heart disease, n (%) | 20 (7%) | 15 (21%) | <0.001 |
| Diabetes mellitus, n (%) | 27 (9%) | 6 (8%) | 0.56 |
| Hypertension, n (%) | 97 (34%) | 26 (37%) | 0.75 |
| Use of statins, n (%) | 72 (25%) | 19 (26%) | 0.43 |
| Use of aspirin, n (%) | 32 (11%) | 10 (14%) | 0.65 |
| Inflammatory biomarkers | |||
| IL-6 | 2.62 (6.83) | 3.84 (3.58) | <0.001 |
| hs-CRP | 2.98 (13.7) | 3.38 (4.70) | 0.13 |
| Oxidative Stress biomarkers | |||
| FORT Test | 293.1 (59.1) | 321.1 (69.22) | <0.001 |
| GSH | 2.06 (2.60) | 1.71 (1.60) | 0.36 |
| GSSG | 0.11 (0.37) | 0.11 (0.29) | 0.38 |
| GSH/GSSG | 42.25 (43.66 | 50.66 (63.6) | 0.26 |
| Zygosity | |||
| Monozygotic | 164 (57%) | 40 (56%) | 0.74 |
| Dizygotic | 124 (43%) | 32 (44%) |
Unless otherwise started, values presented are means (SD). P-values are obtained from mixed models that included a random intercept for pair.
Smokers had significantly higher levels of IL-6 (p<0.01) and higher oxidative stress as measured by the FORT test (p<0.01). In addition, smokers had significantly higher summed stress scores, summed rest scores, maximal RPP, and lower resting CVR (Table 2).
Table 2.
Myocardial perfusion imaging data in smokers compared with non-smokers
| Myocardial perfusion | Non- smokers |
Smokers | p-value |
|---|---|---|---|
| Abnormal perfusion (summed stress score ≥ 4) No (%) | 63 (26.4) | 24 (36.9) | 0.09 |
| Summed stress score | 2.18 (4.32) | 4.23 (7.63) | 0.02 |
| Summed rest score | 0.59 (2.34) | 1.42 (3.37) | <0.001 |
| Summed difference score | 1.59 (3.54) | 2.82 (6.37) | 0.34 |
| Average rate-pressure product during adenosine | 92.0 (23.9) | 86.1 (22.9) | 0.11 |
| Maximal rate-pressure product during adenosine | 114.8 (31.1) | 105.2 (28.6) | 0.04 |
| Coronary vascular resistance during rest | 1.56 (0.31) | 1.32 (0.28) | <0.001 |
| Coronary vascular resistance during hyperemia | 0.59 (0.19) | 0.62 (0.19) | 0.50 |
Values presented are means (SD). P-values are obtained from mixed models for continuous variables that included a random intercept for pair.
Coronary Flow Reserve
Resting flow was significantly higher in smokers compared to nonsmokers (p=0.002) and MBFhyperemia was significantly lower in smokers compared to non-smokers (p=0.03) (Figure 1). In the base model, CFR was significantly lower in smokers (2.25) than in non-smokers (2.75) (p<0.01) (Table 3). After adjustment for CVD risk factors, CFR remained lower in smokers compared to non-smokers. Additionally, the relationship between smoking and CFR did not substantially change after adjusting for the summed stress score (Table 3), history of major depression, PTSD, and remained significant even when patients with perfusion defects were excluded from the analysis (p=0.02).
Table 3.
Relationship between cigarette smoking and CFR, treating twins as separate individuals.
| Coronary Flow Reserve |
Non-smokers | Smokers | p-value | ||
|---|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | ||
| Unadjusted (Base Model) | |||||
| 2.75 | (2.64, 2.86) | 2.25 | (2.04, 2.46) | <0.001 | |
| Adjusted for coronary risk factors and medications* | |||||
| 2.74 | (2.62, 2.86) | 2.28 | (2.04, 2.53) | <0.001 | |
| Further adjusted for presence and severity of perfusion defects† | |||||
| 2.77 | (2.61, 2.93) | 2.27 | (2.03, 2.50) | <0.001 | |
| Further adjusted depression and PTSD‡ | |||||
| 2.81 | (2.65, 2.97) | 2.22 | (1.98, 2.46) | <0.001 | |
Abbreviations: CFR: coronary flow reserve
All values presented are least square means (95% confidence interval) derived from mixed models that included a random intercept for pair.
Coronary flow reserve (CFR) is defined as the ratio of MBFhyperemia to MBFrest.
Adjusted for age, body mass index (BMI), previous history of coronary heart disease, plasma lipids (high, total, and low density lipoprotein cholesterol); use of aspirin and use of statins.
Adjusted for coronary risk factors, medications, and summed stress score.
Adjusted for coronary risk factors, medications, history of major depression, and posttraumatic stress disorder (PTSD).
The effect of smoking on CFR remained after adjusting for all inflammatory biomarkers and oxidative stress biomarkers (Table 4). In a separate model, RPP was added to the base model to account for the amount of cardiac activation during hyperemia. CFR in smokers (2.25, 95% CI: 2.04, 2.47) compared to non-smokers (2.72, 95% CI: 2.61, 2.83) remained significantly lower (p<.001). Differences in CFR between smokers and non-smokers remained unchanged after adding beta blockers (2.25 vs. 2.79, p<0.001), and antihypertensive medications (2.24 vs. 2.80, p<0.001) to the model.
Table 4.
Relationship between cigarette smoking and CFR, after adjustment for inflammatory and oxidative stress biomarkers.
| % difference in CFR in smokers vs. nonsmokers |
p-value | |
|---|---|---|
| Base model* | −16.8 | <0.001 |
| Further adjusted for biomarkers†: | ||
| IL-6 | −14.0 | <0.001 |
| hs-CRP | −15.5 | <0.001 |
| FORT | −17.6 | <0.001 |
| GSH | −17.8 | <0.001 |
| GSSG | −17.5 | <0.001 |
| GSH/GSSG | −18.6 | <0.001 |
Abbreviations: CFR: coronary flow reserve; IL-6: interleukin-6, hsCRP: high sensitivity C-reactive protein; FORT: free oxygen radical test assay; GSH: glutathione; GSSG: oxidized form of GSH; GSH/GSSG: defined as the ratio of GSH to GSSG
All values presented are % difference between smokers and non-smokers derived from mixed models that included a random intercept for pair.
Adjusted for coronary risk factors: age, body mass index (BMI), previous history of coronary heart disease, plasma lipids (high, total, and low density lipoprotein cholesterol); use of aspirin and use of statins.
Adjusted for coronary risk factors (same factors as in Table 3), inflammatory biomarkers IL-6 or hsCRP, and oxidative stress biomarkers: oxidized free radicals determined by the free oxygen radical test assay, GSH, GSSG, or GSG/GSSG
Table 5 illustrates the within-pair comparisons of CFR in twin pairs discordant for smoking. CFR was lower in the smoking twin compared to his non-smoking co-twin (p= 0.005). The association of smoking with CFR persisted even after accounting for known cardiovascular disease risk factors (p=0.02). Results were similar in monozygotic and dizygotic twin pairs and the interaction between smoking and zygosity was not significant (p=0.95).
Table 5.
Relationship between cigarette smoking and CFR within twin pairs discordant for smoking.
| Non-Smokers (N=46) Mean (95%CI) |
Smokers (N=46) Mean (95%CI) |
Within-Pair Difference | |||
|---|---|---|---|---|---|
| Mean ± SE |
% | p | |||
| Unadjusted | |||||
| 2.63 (2.42, 2.83) |
2.23 (2.02, 2.45) |
0.39± 0.13 | −14.8 | <0.001 | |
| Adjusted for Coronary Risk Factors and Medications* | |||||
| 2.66 (2.37, 2.96) |
2.24 (1.95, 2.53) |
0.42 ± 0.16 | −15.8 | 0.03 | |
| Further adjusting for IL-6 and oxidized free radicals† | |||||
| 2.59 (2.33, 2.84) |
2.23 (1.97, 2.44) |
0.36 ± 0.15 | −13.9 | 0.04 | |
Abbreviations: CFR: coronary flow reserve; IL-6: interleukin-6
All values presented are least square means (95% Confidence Interval) derived from mixed models that included a random intercept for pair.
Coronary flow reserve (CFR) is defined as the ratio of MBFhyperemia to MBFrest.
Adjusted for coronary risk factors: age, body mass index (BMI), plasma lipids (high, total, and low density lipoprotein cholesterol); use of aspirin and use of statins, and summed stress score.
Adjusting for coronary risk factors, inflammatory biomarker interleukin-6 (IL-6), and oxidized free radicals determined by the free oxygen radical test assay (FORT).
Smoking History
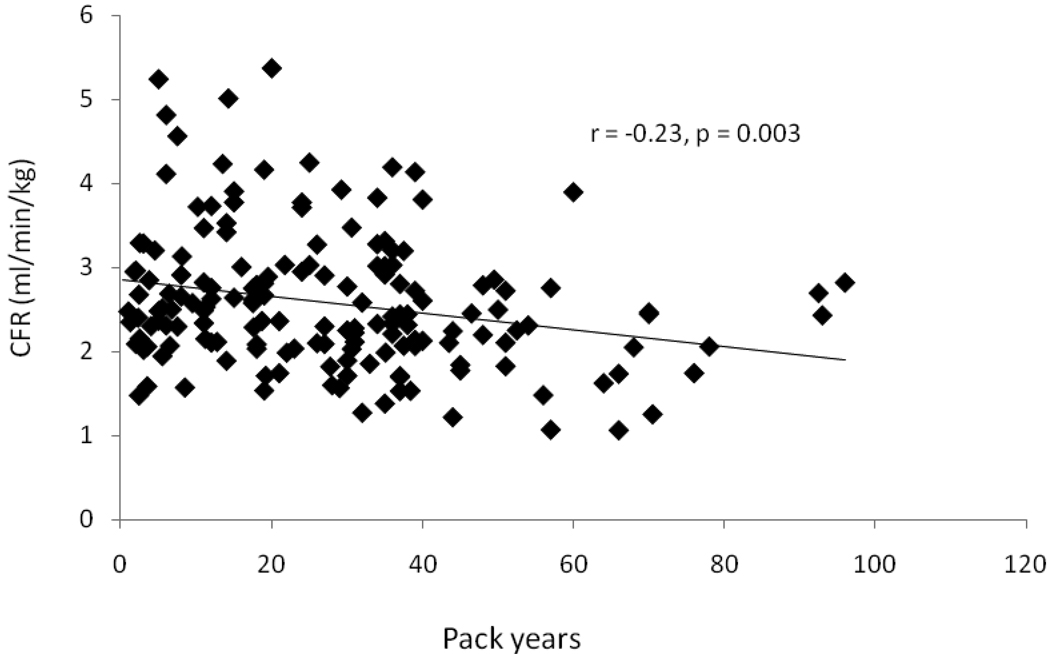
In current smokers and past smokers, pack years of smoking was negatively related to CFR (r= −0.23, p=0.003), (Figure 2). However, the time lapsed after past smokers quit smoking was not related to CFR (β= 0.002, p=0.79).
Figure 2.
Scatterplot and linear regression line between coronary flow reserve (CFR) and smoking pack-years in current and past smokers. Smoking pack-years was negatively related to CFR.
Discussion
Our study demonstrates that smoking is associated with microvascular dysfunction as measured by CFR. Both reduced hyperemic flow and increased resting flow were implicated in the lower CFR of smoking twins. The lower CFR in smokers compared to non-smokers remained even after adjusting for a comprehensive set of confounding factors. Since results were unchanged after adjusting for presence and severity of perfusion defects, and the relationship persisted after exclusion of individuals with perfusion defects, the lower CFR in smokers reflects lower microvascular function rather than epicardial stenoses. Importantly, we continued to observe the influence of smoking in a within pair analysis in both dizygotic and monozygotic smoking discordant twins. These results suggest a relationship between smoking and myocardial blood flow, while they rule out shared genetic or environmental confounding factors in this relationship.
Only a few studies have examined the relationship between smoking and CFR using PET 5–11. Some studies failed to find a difference in CFR between long-term smokers and non-smokers 5–9, while others found that the hyperemic response to the cold-pressor test was reduced in smokers compared with non-smokers 6, 11. The different results may be due to variations in the age of the study populations, length of smoking, stress agents used (i.e., dipyridamole, adenosine), and ability to control for confounding factors. Using a co-twin control design, we were able to demonstrate that long-term smoking affects coronary flow reserve; since results were materially unaffected after adjusting for perfusion defects, differences in flow are likely due to differences in microvascular function.
Our results are in agreement with the acute effects of smoking on the coronary circulation in experimental studies. Acute smoking increases baseline myocardial flow, reduces hyperemic myocardial flow, and markedly reduces CFR 5. Our findings show that similar vascular effects apply to chronic, habitual smoking, and that such effects are likely due to abnormalities of the coronary microcirculation, not to perfusion defects. A relationship between long-term smoking and CFR was also demonstrated by Kaufmann et al. (2000) in a study of the influence of vitamin C on CFR in habitual smokers. However, biomarkers for oxidative stress were not measured in the Kaufmann et al. study.
Inflammation and free-radical mediated oxidative stress are two potential mechanisms whereby smoking may be related to vascular dysfunction 23, 24. Cigarette smoking can lead to vascular damage by impairing endothelial function 25, and exacerbating proinflammatory and prothrombotic effects on the vasculature 26. In addition, free radicals, found in the gas or tar phase of cigarette smoke, can result in the formation of highly reactive oxygen species and favor the formation of oxidized LDL 27. This, in turn, can decrease microcirculatory function by producing the free radical superoxide anion, which decreases NO production 27. In our study, consistent with previous reports 12, 25 plasma inflammatory and oxidative biomarkers tended to be higher in smokers than in non-smokers. However even after accounting for inflammatory and oxidative stress biomarkers, the negative relationship between smoking and CFR persisted. This suggests that although inflammation and oxidative stress may be involved in the microvascular effects of acute smoking, they do not fully account for the relationship of long-standing, chronic effects of smoking. In this study, the FORT test and the plasma level of reduced and oxidized glutathione were utilized as biomarkers of oxidative stress. The validity and reliability of these tests has been shown before 28–30,31, 32. Although many biomarkers of oxidative stress have been proposed, none has yet emerged as the ideal marker 33. We chose these tests because they provide an acceptable balance between validity, easiness of use and potential clinical utility.
Length of smoking behavior may moderate the effects of smoking on coronary vascular function. In our study, there was a negative relationship between CFR and cumulative smoking exposure, determined by pack years, in current and past smokers, but no relationship with time lapsed since quitting smoking in past smokers. Consistent with these findings, previous studies have found a lower CFR in smokers compared to non-smokers if subjects were middle-aged 10 but not young smokers with a briefer smoking history 5, 7, 9. As a whole, these data suggest that the effects of smoking on the coronary circulation are cumulative, and long-standing smoking may be particularly deleterious.
CFR was determined by inducing hyperemia with adenosine, a potent coronary vasodilator. Adenosine causes vasodilation partly by endothelium-dependent mechanisms, but largely by endothelium-independent mechanisms through direct stimulation of adenosine A1 receptors. Nevertheless, the results presented here suggest that cigarette smoking is related to microcirculatory dysfunction in asymptomatic subjects. These effects are independent of traditional coronary risk factors and medications, as well as of cardiovascular activation during pharmacological stress as determined by RPP.
Our study results were similar in monozygotic and dizygotic twin pairs discordant for current smoking. This suggests that the effects of smoking on coronary microvascular function are independent of shared familial, genetic and early environmental factors. These results are consistent with studies that found a significant relationship between smoking and coronary heart disease mortality in monozygotic twins 34–36.
Our study has limitations that should be acknowledged. This is a cross-sectional study, and thus unable to address the temporal relationship between smoking and coronary blood flow. In addition, the inclusion of only middle aged males limits the generalizability of the results to women or individuals in other age groups.
In conclusion, long-term smoking is associated with impaired coronary vascular function in asymptomatic individuals. Our results, using a quasi-experimental design of matched twin pairs, highlight the adverse effects of smoking in the early phases of cardiovascular disease. Since coronary microvascular dysfunction is, at least in part, reversible, our findings point to the importance of smoking cessation as an effective strategy to prevent cardiovascular disease.
Acknowledgements
The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; NIH; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. We gratefully acknowledge the continued cooperation and participation of the members of the Vietnam Era Twin Registry. Without their contribution this research would not have been possible.
Funding Sources: This study was supported by K24HL077506, R01 HL68630 and R01 AG026255 from the National Institutes of Health; by the Emory University General Clinical Research Center MO1-RR00039 and by grant 0245115N from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Tracy Faber is a consultant and shareholder, and receives royalties from Syntermed Inc, which licenses the Emory Cardiac Toolbox, used for some analyses in this study.
References
- 1.Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46:11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 2.Czernin J, Waldherr C. Cigarette smoking and coronary blood flow. Prog Cardiovasc Dis. 2003;45:395–404. doi: 10.1053/pcad.2003.00104. [DOI] [PubMed] [Google Scholar]
- 3.Barua RS, Ambrose JA, Srivastava S, et al. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003;107:2342–2347. doi: 10.1161/01.CIR.0000066691.52789.BE. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med. 2005;46:75–88. [PubMed] [Google Scholar]
- 5.Czernin J, Sun K, Brunken R, et al. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891–2897. doi: 10.1161/01.cir.91.12.2891. [DOI] [PubMed] [Google Scholar]
- 6.Hwang KH, Lee BI, Kim SJ, et al. Evaluation of coronary endothelial dysfunction in healthy young smokers: Cold pressor test using [15O]H2O PET. Appl Radiat Isot. 2009;67:1199–1203. doi: 10.1016/j.apradiso.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Morita K, Tsukamoto T, Naya M, et al. Smoking cessation normalizes coronary endothelial vasomotor response assessed with 15O-water and PET in healthy young smokers. J Nucl Med. 2006;47:1914–1920. [PubMed] [Google Scholar]
- 8.Campisi R, Czernin J, Schoder H, et al. Effects of long-term smoking on myocardial blood flow, coronary vasomotion, and vasodilator capacity. Circulation. 1998;98:119–125. doi: 10.1161/01.cir.98.2.119. [DOI] [PubMed] [Google Scholar]
- 9.Iwado Y, Yoshinaga K, Furuyama H, et al. Decreased endothelium-dependent coronary vasomotion in healthy young smokers. Eur J Nucl Med Mol Imaging. 2002;29:984–990. doi: 10.1007/s00259-002-0818-1. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, et al. Coronary heart disease in smokers: vitamin C restores coronary microcirculatory function. Circulation. 2000;102:1233–1238. doi: 10.1161/01.cir.102.11.1233. [DOI] [PubMed] [Google Scholar]
- 11.Meeder JG, Blanksma PK, van der Wall EE, et al. Long-term cigarette smoking is associated with increased myocardial perfusion heterogeneity assessed by positron emission tomography. Eur J Nucl Med. 1996;23:1442–1447. doi: 10.1007/BF01254465. [DOI] [PubMed] [Google Scholar]
- 12.Tappia P, Troughton K, Langley-Evans S, et al. Cigarette smoking influences cytokine production and antioxidant defences. Clinical Science. 1995;88:485–489. doi: 10.1042/cs0880485. [DOI] [PubMed] [Google Scholar]
- 13.Scheuner MT. Genetic predisposition to coronary artery disease. Curr Opin Cardiol. 2001;16:251–260. doi: 10.1097/00001573-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1 Suppl 2:S51–S57. doi: 10.1080/14622299050011811. discussion S69–70. [DOI] [PubMed] [Google Scholar]
- 15.Vaccarino V, Lampert R, Bremner JD, et al. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med. 2008;70:628–636. doi: 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaccarino V, Brennan ML, Miller AH, et al. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry. 2008;64:476–483. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg J, Curran B, Vitek ME, et al. The Vietnam Era Twin Registry. Twin Res. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 18.Hutchins GD, Schwaiger M, Rosenspire KC, et al. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032–1042. doi: 10.1016/0735-1097(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 19.Howard G, Wagenknecht LE, Burke GL, et al. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279:119–124. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 20.Richardson MT, Ainsworth BE, Wu HC, et al. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–693. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 21.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 22.Carlin JB, Gurrin LC, Sterne JA, et al. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34:1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- 23.Heitzer T, Yla-Herttuala S, Luoma J, et al. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia. Role of oxidized LDL. Circulation. 1996;93:1346–1353. doi: 10.1161/01.cir.93.7.1346. [DOI] [PubMed] [Google Scholar]
- 24.Yokode M, Kita T, Arai H, et al. Cholesteryl ester accumulation in macrophages incubated with low density lipoprotein pretreated with cigarette smoke extract. Proc Natl Acad Sci U S A. 1988;85:2344–2348. doi: 10.1073/pnas.85.7.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell JT. Vascular damage from smoking: disease mechanisms at the arterial wall. Vasc Med. 1998;3:21–28. doi: 10.1177/1358836X9800300105. [DOI] [PubMed] [Google Scholar]
- 26.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 27.Hein TW, Kuo L. LDLs impair vasomotor function of the coronary microcirculation: role of superoxide anions. Circ Res. 1998;83:404–414. doi: 10.1161/01.res.83.4.404. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani G, Madeddu C, Gramignano G, et al. Subcutaneous interleukin-2 in combination with medroxyprogesterone acetate and antioxidants in advanced cancer responders to previous chemotherapy: phase II study evaluating clinical, quality of life, and laboratory parameters. J Exp Ther Oncol. 2003;3:205–219. doi: 10.1046/j.1359-4117.2003.01096.x. [DOI] [PubMed] [Google Scholar]
- 29.Garelnabi MO, Brown WV, Le NA. Evaluation of a novel colorimetric assay for free oxygen radicals as marker of oxidative stress. Clin Biochem. 2008;41:1250–1254. doi: 10.1016/j.clinbiochem.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Abramson JL, Hooper WC, Jones DP, et al. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005;178:115–121. doi: 10.1016/j.atherosclerosis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- 32.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Brown NJ, Vaughan DE, et al. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:IV6–IV19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 34.Carmelli D, Page WF. Twenty-four year mortality in World War II US male veteran twins discordant for cigarette smoking. Int J Epidemiol. 1996;25:554–559. doi: 10.1093/ije/25.3.554. [DOI] [PubMed] [Google Scholar]
- 35.Floderus B, Cederlof R, Friberg L. Smoking and mortality: a 21-year follow-up based on the Swedish Twin Registry. Int J Epidemiol. 1988;17:332–340. doi: 10.1093/ije/17.2.332. [DOI] [PubMed] [Google Scholar]
- 36.Kaprio J, Koskenvuo M. Twins, smoking and mortality: a 12-year prospective study of smoking-discordant twin pairs. Soc Sci Med. 1989;29:1083–1089. doi: 10.1016/0277-9536(89)90020-8. [DOI] [PubMed] [Google Scholar]