Abstract
In the current study, we have altered the surface oxide properties of a Ti6Al4V alloy using heat treatment or radiofrequency glow discharge (RFGD) in order to evaluate the relationship between the physico-chemical and biological properties of the alloy's surface oxide. The effects of surface pretreatments on the attachment of cells from two osteogenic cell lines (MG63 and MC3T3) and a mesenchymal stem cell line (C3H10T1/2) to fibronectin adsorbed to the alloy were measured. Both heat and RFGD pretreatments produced a several-fold increase in the number of cells that attached to fibronectin adsorbed to the alloy (0.001 and 10 nM FN) for each cell line tested. An antibody (HFN7.1) directed against the central integrin binding domain of fibronectin produced a 65-70% inhibition of cell attachment to fibronectin-coated disks, incdicating that cell attachment to the metal discs was dependent on fibronectin binding to cell integrin receptors. Both treatments also accelerated the cell spreading response manifested by extensive flattening and an increase in mean cellular area. The treatment-induced increases in the cell attachment activity of adsorbed fibronectin were correlated with previously demonstrated increases in Ti6Al4V oxide negative net surface charge at physiological pH produced by both heat and RFGD pretreatments. Since neither treatment increased the adsorption mass of fibronectin, these findings suggest that negatively charged surface oxide functional groups in Ti6Al4V can modulate fibronectin's integrin receptor activity by altering the adsorbed protein's conformation. Our results further suggest that negatively charged functional groups in the surface oxide can play a prominent role in the osseointegration of metallic implant materials.
Keywords: cell attachment, coatings, fibronnectin, metal oxides, surface charge
1. Introduction
A variety of strategies have been explored to reduce implant failure. These include the use of cell adhesive proteins to enhance osteoblast implant surface coverage and, thereby, accelerate osteoinduction and bone integration. Proteins of the extracellular matrix (ECM) can stimulate cell adhesion through interactions with a superfamily of cell-surface receptors called integrins via the integrin-binding arg-gly-asp (RGD) tripeptide motif [1-2]. These ECM proteins have the potential to bind to the titanium oxide surface when present in the surrounding biologic milieu [3-6]. Our laboratory and others have studied ECM proteins such as fibronectin or human bone sialoprotein (hBSP) or hBSP peptides following their non-covalent adsorption [7-8] or covalent grafting [9-12] to implant model surfaces to facilitate osteoprogenitor cell attachment. Since such attachment of cells is a priori a necessary step for osteogenesis and implant integration, enhancing osteoblast activity at the implant surface shortly after fixation is likely to extend implant longevity and reduce failures. In order to optimize the effects of cell attachment proteins such as BSP or fibronectin on implant integration, it is crucial to understand how these proteins interact with the implant surface.
Several recent approaches have emphasized the modification of the implant surface's physical and chemical properties in order to enhance protein binding, the attraction of appropriate cell types and implant integration [13-17]. Notably, a number of studies of non-metallic model surfaces have demonstrated that a substrate's surface charge can strongly influence the conformation of fibronectin and thus alter its ability to attach to cells. The adsorption of fibronectin on nonpolar surfaces results in drastic conformational changes due to severe unfolding of the protein compared to more polar substrates [18-20], confirming other studies suggesting that hydrophobic surfaces cause the unfolding of random coil protein structure including that of fibronectin [21-23]. Another study has suggested that the “hinge” domain bridging the RGD and another site that acts in synergy with RGD to bind integrin receptors [24] modulates their accessibility to these cell receptors. This hinge domain would hypothetically alter fibronectin's integrin binding affinity by modulating the distance between the RGD and synergy sites. The distance between these sites might be controlled by the selective unfolding of the hinge domain when it binds to a substrate with a particular surface chemistry [24]. Therefore, a model has emerged in which substrate surface charge can induce conformational changes that increase the functional presentation of fibronectin's integrin binding domain [25].
How the physico-chemical properties of the implant metal oxide may affect fibronectin's 3-D structure, osteoblast binding activity and capacity to promote osteogenesis is poorly understood. Heat treatment of the surface oxide layer has been shown to increase the biocompatibility of the metallic surface [26-29] and promote mineralization [30-31]. We have previously reported that heat treatment of a titanium alloy (Ti6Al4V) increased the ability of adsorbed fibronectin to bind to bone cells [16]. However, in our companion article we have shown that heat pretreatment increased a number of the Ti6Al4V oxide's physico-chemical properties, including its negative net charge at physiological pH, per cent composition of aluminum, thickness and nanotopographical structure [32]. Therefore, the particular oxide property that is altered by heat treatment to modulate the cell binding activity of fibronectin remains to be identified. In contrast to the effects of heat treatment on oxide properties, treatment with radiofrequency glow discharge (RFGD) was shown, in our companion article, to selectively increase the negative net charge of the oxide at physiological pH, without altering its topography, thickness or elemental composition [32] Therefore, the basic objective of this study was to compare the effects of heat treatment and RFGD treatment on the ability of adsorbed fibronectin to promote bone cell attachment and spreading. in order to better understand the relationship between the oxide's physico-chemical and biological properties. This study suggests that increases in the negative net charge of the alloy's surface oxide charge produced by heat or RFGD pretreatment mediates an increase in the ability of adsorbed fibronectin to bind to osteogenic cell receptors.
2. Materials and Methods
2.1 Materials
Fetal bovine serum (FBS) and cell culture media were from Invitrogen (Carlsbad, CA). Bovine serum albumin (BSA) (Fraction V; essentially fatty acid-free), p-nitrophenol-N-acetyl-B-D-glucoseaminide and human plasma fibronectin were purchased from Sigma-Aldrich. RBS 35® detergent was obtained from Fisher Scientific Inc. (Rockford, IL) All other chemicals were from Sigma-Aldrich (St. Louis, MO) and were of spectroscopic grade. Tissue culture flasks (75 cm2), 96-well tissue culture plates, and 96-well non-tissue culture treated plates were obtained from Laboratory Disposable products (North Haledon, NJ). Cells from a subclone of the MC3T3-E1 osteoblast-like cell line (subclone 4) that exhibit high levels of osteoblast differentiation [33], MG63 osteoblast-like cells and the C3H10T1/2 mesenchymal stem cell line were obtained from the American Type Culture Collection (ATCC; Manassas, VA).
2.2 Disk Preparation
2.2.1 Controls
Disks were prepared as described in the companion article [32].
2.2.2. Heat and RFGD Treatment
The alloy disks were heat or RFGD-treated as previously described [32].
2.3 Titanium-aluminum-vanadium – protein interaction
2.3.1. Effects of treatments on binding of fibronectin to titanium alloy disks
To determine the effect of surface treatments on the adsorption of human plasma fibronectin to titanium alloy disks, the concentration of adsorbed fibronectin following an overnight incubation of disks with a 0.1 – 10 nM solution of the protein in 1 X phosphate-buffered saline (PBS) was measured using the Assay Max human fibronectin ELISA kit (Assaypro, St Charles, MO). Fibronectin was assayed by binding to a biotinylated polyclonal antibody specific for human fibronectin that is recognized by a streptavidin-peroxidase conjugate. Following incubation with a peroxidase substrate for 10 min, the absorbances were measured at 450 nm in a 96-well plate reading Titerek Multiskan Plus spectrophotometer (LabSystems, Finland). A standard curve obtained with known concentrations of human fibronectin was used to quantify the unknown concentrations of fibronectin adsorbed to alloy disks.
2.4 Cell Culture
MG63 (ATCC) cells were cultured in Modified Eagles Medium + nonessential amino acids (MEM+ NEAA) with heat-inactivated 10% FBS as previously described [16]. MC3T3-E1 cells (subclone 4) were cultured in MEM-α with 10% FBS. C3H10T1/2 mesenchymal stem cells (ATCC) were cultured in DME with 10 % FBS.
2.41. Effect of Fibronectin concentration on MG63, MC3T3 and C3H10T1/2 cell attachment to titanium alloy surfaces
To determine the effect of fibronectin coating concentration on MG63, MC3T3 and C3H10T1/2 cell attachment to Ti6Al4V, titanium alloy disks were first placed into 96-well nontissue culture plates. Control disks or disks modified by heat (600 ° C for 1 h) or RFGD (5 min.) pretreatment were then incubated with increasing concentrations of fibronectin (0.005 to 10 nM in PBS, pH 7.4) in covered 96-well non-tissue culture plates overnight at room temperature under a cell culture hood. The supernatant was then aspirated, 200 μl of 0.1% BSA/ 1 X PBS added to each well, and liquid aspirated 2 hours later. MG63 cells were then added to each well (150,000 cells) and incubated for 2 hours at 37 ° C. Samples were then washed 6X with serum-free media and cell attachment numbers quantitated using a hexosaminidase assay [22] as previously described [16].
2.4.2. Inhibitory effects of HFN7.1 antibody on MG63, MC3T3 and C3H10T1/2 cell attachment to fibronectin coiated titanium alloy surfaces
Cell attachment was also measured as described above in the presence of the anti-fibronectin HFN7.1 monoclonal antibody at dilutions between 1:100 and 1:10 and a constant fibronectin coating concentration of 10 nM. The HFN7.1 antibody is directed against the central integrin binding domain of fibronectin, is specific for human fibronectin and does not cross-react with mouse or bovine fibronectin [24].
2.4.3. Morphological changes in MG63 cells following plating on unmodified and modified titanium alloy disks measured by scanning electron microscopy (SEM)
Untreated and modified disks were coated with fibronectin (0.25 nM) and incubated with MG63 cells as described above in section 2.5.1. for a cell adhesion period of 1 hr Following the aspiration of the supernatant, disks were washed 1X with a fixative for electron microscopy containing 0.5 % glutaraldehyde / 2 % paraformaldehyde in 0.05 M cacodylate sodium buffer, pH 7.4 and incubated in a second aliquot of this fixative for 2-3 hours. The disks were washed 3X with distilled water and then stored in distilled water. The disks were air-dried, sputter-coated with a thin layer of palladium and analyzed by high vacuum scanning electron microscopy (FEI, Quanta 600, Peabody, MA) in the secondary electron mode and imaged at 5000 – 10,000X [34].
SEM micrographic images were captured digitally. The perimeter and area of MG63 cells obtained from three independent cultures that attached to control, heat or RFGD-modified disks were measured for at least 6 representative fields using a Bioquant II software program. The cell shape factor, which is inversely related to cell roundness, was calculated using the formula : 4π (area / perimeter2) [34]. The mean cellular area and shape factor were then calculated for each of the control and modified disk groups.
2.5 Statistical analysis
Data are presented as mean ± standard error of the mean (N= total number of independent cell cultures). Statistical comparisons were performed using an ANOVA with the alpha level set at 0.05. For cell culture experiments, the number of attached cells is expressed as % control. Control is defined as the number of attached cells measured at the 0 nM fibronectin coating concentration for the untreated group or measured at the 0 nM fibronectin concentration for each corresponding treatment condition.
3. Results
3.1. Adsorption of fibronectin to metallic surface
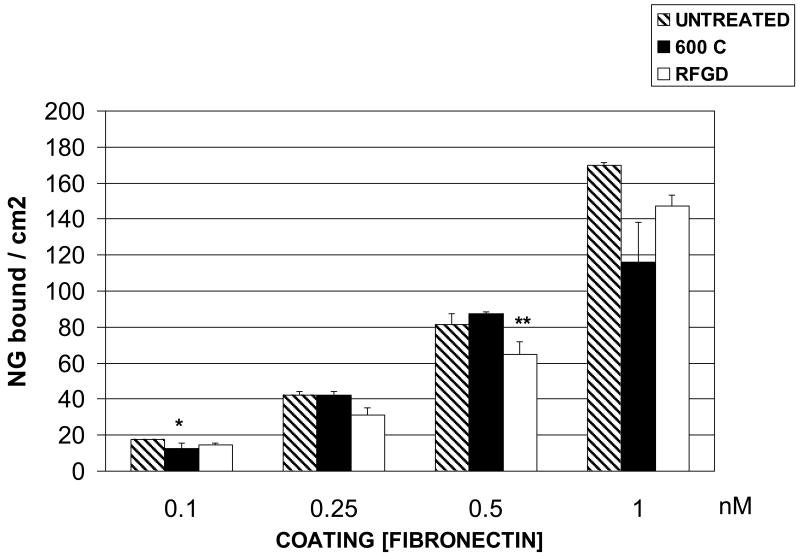
The adsorption of fibronectin to untreated and treated Ti6Al4V disks was measured over a range of coating concentrations from 0.1 – 1 nM as shown in Figure 1. There were no consistent differences found between control or heat -treated disks in the amount of fibronectin that adsorbed to the surface (following an overnight incubation) at any of the coating concentrations (Figure 1) or at a concentration of 10 nM (unpublished results). RFGD treatment slightly decreased fibronectin adsorption compared to polished controls, although statistically significant differences were not observed (Figure 1).
Fig. 1.
Effects of heat and RFGD treatment of Ti6Al4V disks on the adsorption of solution phase fibronectin. Disks were either untreated, heat-treated at 600 C for 1 h or exposed to RFGD for 5 min as described in methods and disks and then coated overnight with the indicated solution phase concentrations of fibronectin. The adsorbed mass of fibronectin was measured by ELISA and expressed as nanograms per unit of metallic disc surface area. Data are from 3-8 replicate disks per coating concentration. * < control (p < 0.05) ; ** < 600 C (p < 0.05) based on one-way ANOVA.
3.2 Cell attachment
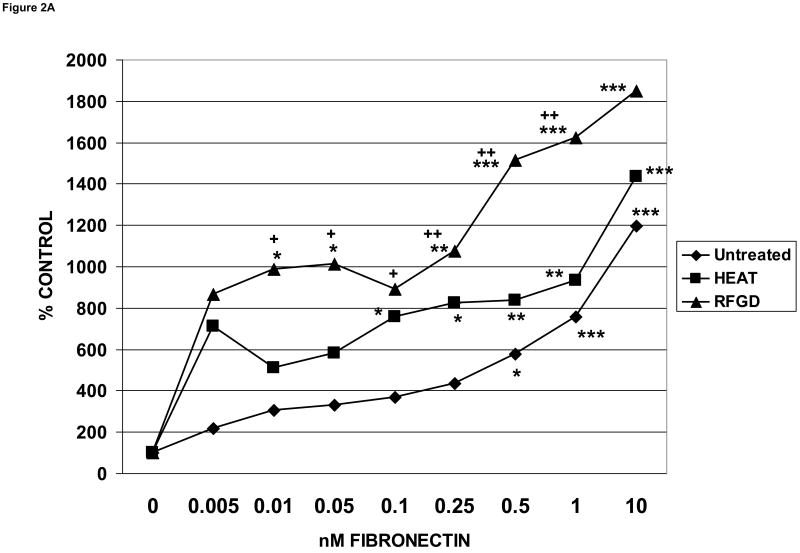
MG63 cells, which display many of the characteristics of differentiated osteoblasts, have been extensively studied following attachment to titanium implant materials [35]. The number of MG63 cells that attached to untreated and modified metallic surfaces over a period of 2 hours was measured after an overnight precoating of the disks with range of solution phase fibronectin concentrations between 0.005 and 10 nM (Figure 2A). Heat and RFGD treatments of polished alloy disks each substantially increased the levels of cell attachment compared to untreated disks at fibronectin coating concentrations of 1 nM or less, although there were no differences between these three groups at a [fibronectin] of 10 nM. Fibronectin preadsorbed to untreated disks promoted a significant increase in cell attachment only at a [fibronectin] of 0.5 nM or higher. In contrast, the protein significantly increased cell attachment at coating concentrations as low as 0.01 to 0.1 nM when precoated on heat or RFGD-treated surfaces (Figure 2A).
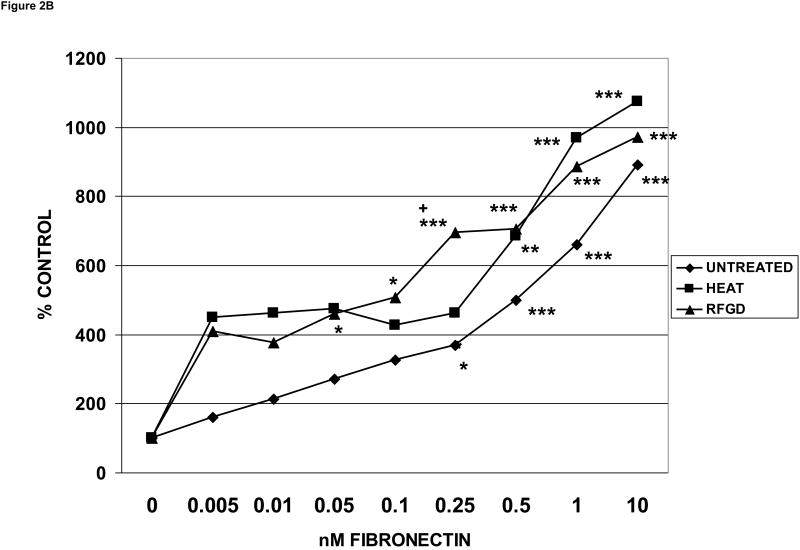
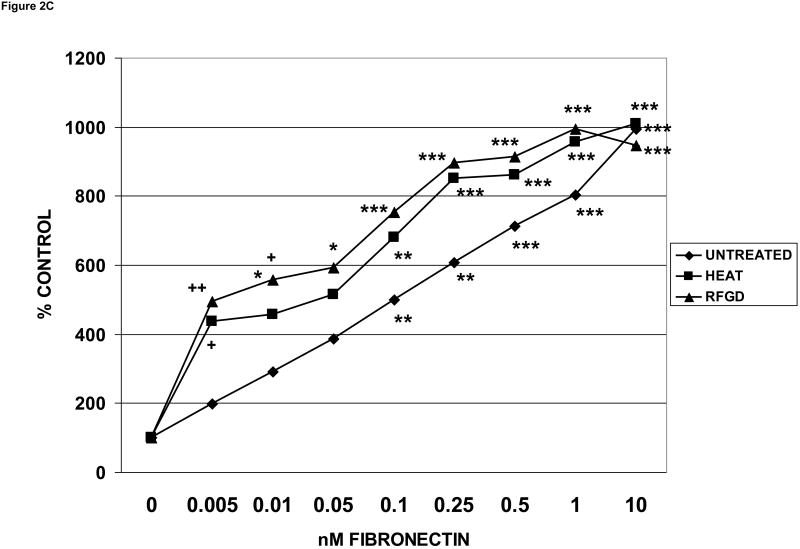
Fig. 2.
Effects of heat and RFGD treatment of Ti6Al4V disks on the attachment of osteogenic cells to adsorbed fibronectin. Disks were either untreated or pretreated with heat or RFGD, coated overnight with the indicated solution phase concentrations of fibronectin, incubated wih cells for 2 hours and and the number of cells that attached to coated disks was measured. Three disk metallic surface categories : untreated, HEAT, RFGD, were tested, The number of cells that attached to these disks measured at each [fibronectin] is expressed as a percentage of control attachment, or the number of cells that attached at 0 nM fibronectin. Data are from 4-11 independent cell cultures per fibronectin coating concentration for each surface category. A – MG63 cells ; B - MC3T3 cells ; C – C3H10T1/2 mesenchymal stem cells. * > 0 nM fibronectin for that surface category (p < 0.05) ; ** > 0 nM fibronectin for that surface category (p < 0.01) ; *** > 0 nM fibronectin for that surface category (p < 0.001) ; + > untreated disks at the corresponding [fibronectin] (p < 0.05) ; ++ > untreated disks at the corresponding [fibronectin] (p < 0.01) based on one-way ANOVA.
The MC3T3-E1 osteoblast-like cell line (subclone 4) exhibits high levels of osteoblast differentiation in cell culture [33]. When MC3T3 cells were tested, RFGD and heat treatments of polished disks again produced nearly identical per cent increases in the number of attached cells (Figure 2B). The increases in cell attachment promoted by each pretreatment (Figure 2B) were slightly less than that shown for MG63 cells (Figure 2A).
The C3H10T1/2 mesenchymal stem cell line consists of pluripotential cells that have the capacity to differentiate into cells of the osteoblastic lineage [36]. The same pattern of augmented levels of cell attachment to heat or RFGD-modified disks was repeated when C3H10T1/2 cells were tested (Figure 2C). Although the levels of attachment appeared to be higher for RFGD-treated compared to preheated samples, there were no statistically significant differences between the two treatments for any of the cell lines tested (Figures 2A, 2B and 2C). Notably, neither treatment increased the attachment of cells to the alloy disks in the absence of coated fibronectin for any of the cell lines tested.
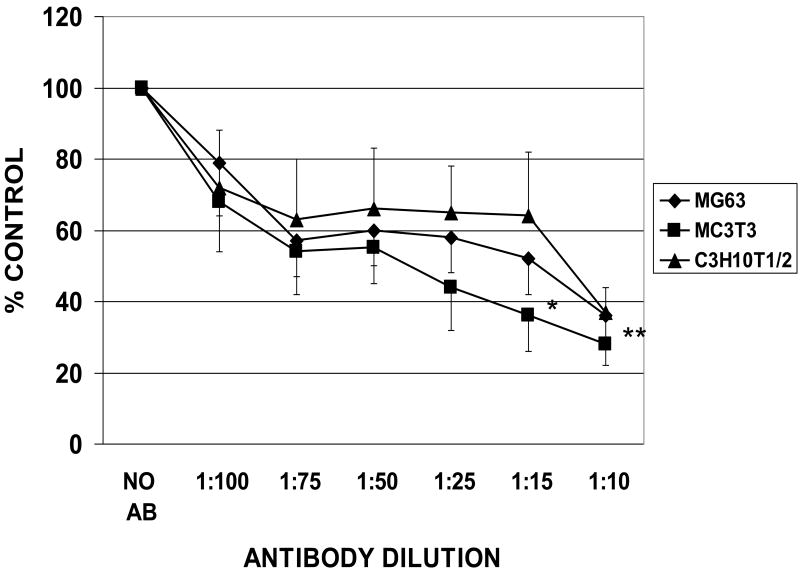
3.3. Inhibitory effects of HFN7.1 antibody on cell attachment
The anti-fibronectin antibody produced a concentration-dependent inhibition of the attachment of MG63, MC3t3 and C3H10T1/2 cells to fibronectin coated on untreated discs (Figure 3). Approximately 65 – 70 % of cell attachment was blocked in the presence of the antibody at a dilution of 1:10 (Figure 3), indicating that most if not all of the cell attachment to the metal discs was fibronectin-dependent.
Fig. 3.
Inhibitory effects of HFN7.1 antibody on osteogenic cell attachment to fibronectin-coated titanium alloy surfaces. Untreated disks were coated overnight with 10 nM fibronectin, incubated with cells for 2 hours and the number of cells that attached to coated disks was measured in the presence of various dilutions of HFN7.1 anti-fibronectin antibody. The number of cells that attached to these disks measured at each antibody dilution is expressed as a percentage of the attached cell number measured in the absence of antibody. Data are from 3-5 independent cell cultures for each antibody dilution and each surface category. * > the number of attached cells measured in the absence of antibody. (p < 0.05) ; ** > the number of attached cells measured in the absence of antibody. (p < 0.01) based on one-way ANOVA.
3.4. Effects of disk modifications on cell morphology
Many of the cells attached to heat or RFGD-treated disks showed a dramatic transformation from a spherical shape to a more flattened fibroblast-like morphology (Figure 4). Although some of the cells on untreated disks appeared less rounded than others, none exhibited a flattened morphology like those attached to the modified disks. Cells attached to the modified surfaces showed a network of cytoplasmic processes extending to the metallic substrate and to neighboring cells (Figure 4). In contrast, such cytoplasmic extensions were substantially shorter for cells attached to untreated disks.
Fig. 4.




Effects of heat and RFGD treatment of Ti6Al4V disks on the morphology of MG63 cells following a 1 h. period of attachment. Disks were either untreated or pretreated with heat or RFGD, coated with fibronectin at a solution phase concentration of 0.25 nM, incubated with MG63 cells and analyzed by SEM. A-C. Cells attached to untreated (control), heat-treated or RFGD-treated disks imaged at a magnification of 5000. D. A cell attached to an RFGD-treated disk imaged at a magnification of 10,000.
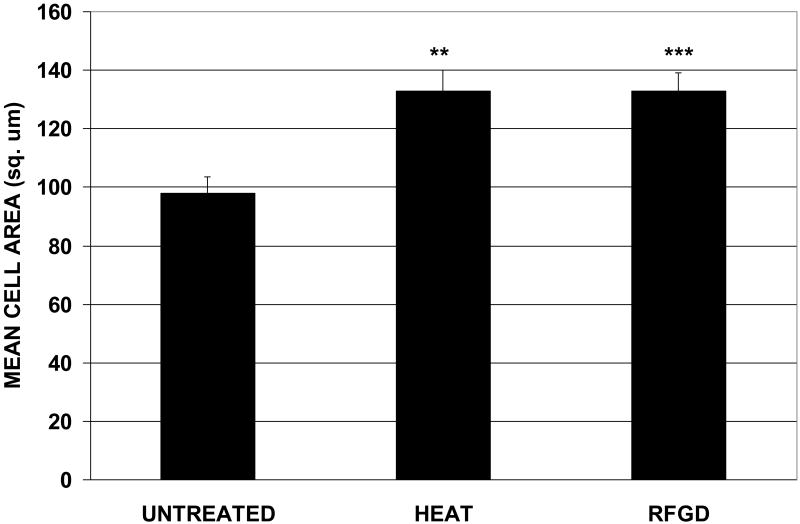
Both heat and RFGD pretreatments promoted a statistically significant 33 % increase in the mean cell area for individual MG63 cells attached to fibronectin-coated disks for 1 hour compared to fibronectin-coated untreated disks (Figure 5). The cells attached to untreated disks were found to have a mean shape factor of 0.473 +/- 0.01 compared to 0.494 +/- 0.01 and 0.465 +/- 0.01 for heat and RFGD-treated disks, respectively (mean +/- S.E ; no statistically significant differences between groups). The shape factor of a perfect circle is defined as 1 and that of a straight line as 0.00. Since the shape factors for cells attached to untreated, heat-treated or RFGD-treated discs were all <1.0, our results indicate that the cells underwent a change in morphology to a less round shape following attachment to all three surfaces. In summary, cells attached to both untreated and treated disks displayed similar decreases in roundness during cell adhesion. However, the increase in mean cell area, longer cytoplasmic projections and more flattened three-dimensional morphology of cells on treated disks indicate that cells spread more on pretreated surfaces compared to untreated disks, even though the cells on untreated and treated discs had the same general 2-D shape.
Fig. 5.
Effects of heat and RFGD treatment of Ti6Al4V disks on MG63 cell area following a 1 hr. period of attachment. Disks were prepared, coated with fibronectin, incubated wit MG63 cells, analyzed by SEM as described in Figure 4 and the mean area of cells attached to coated disks was measured. ** > untreated disks (p < 0.01) *** > untreated disks (p < 0.001) based on one-way ANOVA. N (number of cells analyzed) = 52, 69 and 87 for untreated, heat-treated or RFGD-treated disks, respectively. Cells from 3 independent cultures were analyzed for each treatment group.
4. Discussion
The objective of this investigation was to develop a deeper understanding of how the physical and chemical properties of a titanium alloy's (Ti6Al4V) surface oxide modulate the osteogenic cell attachment activity of adsorbed fibronectin. In a companion study [32], the material's surface oxide was first modified with RFGD or heat pretreatments and their physico-chemical effects on the oxide were then evaluated. We found that the effects of RFGD and heat treatment on surface oxide properties were qualitatively and quantitatively very different. Preheating substantially increased oxide roughness, topography and elemental composition compared to polished controls whereas RFGD failed to significantly alter any of these properties. In addition, although both treatments produced a more hydrophilic surface and increased the negative net charge of the oxide at physiological pH, the RFGD treatment increased the oxide's negative net charge to a greater degree that heat treatment [32]. In biological studies performed in parallel and reported here, the pretreated surfaces were coated with fibronectin and the effects of surface modification on the number of osteogenic cells that attached to this protein and the spreading response of attached cells were determined. Despite the differences in their topographical and chemical effects on the oxide [32], RFGD and heat pretreatments were equivalent in their stimulatory effects on the attachment of osteogenic cells to adsorbed fibronectin and the spreading of attached cells. Most importantly, our results with RFGD suggest that increases in oxide negative net charge per se enhance fibronectin's cell attachment activity without any accompanying changes in oxide topography or elemental composition. Notably, our results have shown that the biological effects of our surface pretreatments were not peculiar to one specific cell line but were reproduced with three different cell lines. Our findings support the notion, heretofore novel in the investigation of metallic implant materials, that negatively charged functional groups in the metal surface oxide play a determinant role in the conformational bioactivity of adsorbed proteins.
4.1 Effects of treatments on binding of adsorbed fibronectin to integrin receptors
Three particular findings reported here collectively suggest that heat or RFGD prertreatments of Ti6Al4V increased fibronectin's integrin receptor binding activity. First, we found that heat or RFGD treatment of Ti6Al4V increased the number of cells attached to fibronectin at a wide range of coating concentrations compared to polished controls. Second, neither treatment increased the amount of fibronectin adsorbed to the metal oxide suggesting that the treatments influence the intrinsic bioactivity of fibronectin rather than its propensity for surface binding. Third, our results also showed that a specific antibody directed against fibronectin's integrin-binding domain [24] blocked 65-70 % of the attachment of osteogenic cells to fibronectin adsorbed to Ti6Al4V discs. Preliminary results in our laboratory have shown a similar degree of inhibition of osteogenic cell attachment to heat or RFGD-pretreated discs subsequently coated with fibronectin using the same anti-fibronectin antibody (unpublished observations). Based on all of these findings, we propose that heat and RFGD pretreatments of the metal oxide enhanced the intrinsic ability of adsorbed fibronectin to bind to bone cell integrin receptors.
4.2 Mechanisms for the substrate-mediated control of fibronectin conformation
The mechanism by which changes in the surface chemistry of the metal oxide induced by our treatments alter fibronectin's integrin receptor activity is undoubtedly complex. The initial adsorption of proteins to implantable materials is certainly influenced by surface energy [37], which is a function of the surface density of chemical functional groups and their reactivities. Another important aspect of the behavior of proteins that may be affected by surface chemistry is their capacity to undergo structural rearrangements upon adsorption to a substrate. A number of environmental and surface variables, such as ionic strength, pH, temperature, surface charge and surface hydrophilicity have been shown to modulate the three-dimensional structure of fibronectin upon binding to a substrate [5,25,38-46]. The conformation of adsorbed proteins can be highly influenced by the hydrophobicity of the material surface, since protein binding to highly nonpolar surfaces results in drastic conformational changes and loss of activity due to severe unfolding of the protein [18-20]. In contrast, structural rearrangements in proteins that retain or even enhance biological activity can be promoted by Coulomb interactions (electrostatic forces) between the charged groups in the material and in the protein [37, 25]. A similar own demonstrated that a OH-functionalized surface [25], likely to be charged at physiological study to our pH, increased the conformational exposure and the binding of fibronectin to cell integrins compared to a nonpolar hydrophobic surface. Preliminary results in our laboratory have shown that pretreatment of the titanium alloy with heat or RFGD significantly increased the binding of the HFN7.1 antibody to the core integrin binding domain of adsorbed fibronectin (unpublished results). These findings suggest that the integrin binding domain was more accessible to the antibody due to conformational changes following adsorption to the treated surfaces. These studies suggest that some metal oxide property that is related to oxide net charge promotes changes in the 3-D structure of fibronectin's integrin binding domain which increases its functional presentation to integrins. Future studies in our laboratory will use Raman spectroscopy to confirm that RFGD and heat pretreatments alter the conformation of adsorbed fibronectin.
We have compared the effects of heat and RFGD pretreatments on the surface oxide of Ti6Al4V, as reported in our companion study [32], to help elucidate the physico-chemical mechanism for the treated oxide-induced changes in fibronectin bioactivity. Although the overall physico-chemical effects of heat and RFGD treatment on the oxide were quite different [32], the two treatments produced quantitatively similar increases in oxide surface wettability. The most general principle governing the relationship between material surface properties and cell–substrate interactions that has emerged from decades of research is that anchorage-dependent mammalian cells favor modestly wettable surfaces exhibiting a water contact angle < 60 degrees [47]. However, since even our control Ti6Al4V surfaces displayed a mean water contact angle of 43 degrees [32], it is not clear whether our treatment-induced increases in the hydration of an already hydrophilic oxide surface per se were sufficient enough to affect fibronectin conformation. Notably, fibronectin has been shown to assume a more unfolded, extended conformation when adsorbed to hydroxyapatite (HA), with the cell binding domain more accessible to a specific antibody, compared to a gold surface, even though both materials were hydrophilic. These findings suggest that another mechanism besides the hydrophilic nature of HA must have been responsible for the more activated conformation of fibronectin on HA [48]. Furthermore, cell attachment to fibronectin adsorbed to chitosan membranes and cell spreading have been demonstrated to vary with surface chemistry rather than with wettability [49]. Therefore, there may be a more basic oxide chemical property than hydrophilicity that directly modulates fibronectin's conformational activity.
4.3 The role of metal hydroxide chemistry in the modulation of fibronectin conformation
In discussing the mechanism of the oxide-mediated changes in fibronectin conformation, it should be noted that oxide net charge and hydrophilicity are a reflection of changes in surface chemistry, particularly that of oxide metal-hydroxide complexes. For example, surface chemistry and wettability appear to be inseparably linked in view of the observation that it is the hydrogen bonding of water to surface functional groups that exerts the greatest influence on wettability [50,51]. Therefore, since the surface oxide of Ti6Al4V in contact with water is believed to be highly hydroxylated with M-OH groups, it is likely that the surface oxide's wettability is largely a function of the combined concentrations of the neutral, cationic and anionic forms of M-OH in an aqueous environment. As discussed in our companion article [32], the net charge of the surface oxide is attributable to the surface concentration of uncompensated charges, which is likely to be a function of the relative proportions of [M — O]- and [M — OH2]+ groups and the total surface concentration of all M — OH species. Therefore, the chemistry of M-OH complexes in the surface oxide of Ti6Al4V may be critical to understanding how the oxide may affect the behavior of adsorbed fibronectin.
Our finding that heat and RFGD treatments increased the oxide's negative net charge [32] suggests that these treatments produced alterations in M-OH chemistry that may provide insight into the mechanism by which the oxide modulates fibronectin's bioactivity. As discussed in the companion paper [32], the engineered increases in the negative net charge of the oxide, measured at physiological pH, are likely to arise from an elevation in the surface concentration of uncompensated [M — O]- groups. Consequently, as the number of anionic [M — O]- species increases, bonds between this oxide functional group and basic amino acids residues (of opposite charge) will become more likely. Such interactions would potentially lead to a selective unfolding of the protein's integrin recognition domain that can occur upon binding to a substrate with a particular surface chemistry [24]. RFGD has also been shown to increase the hydration of the oxide theoretically by creating oxygen vacancies at the surface [52]. However, treatment-induced increases in charged M-OH groups in the oxide probably exert a greater effect on adsorbed protein conformation compared to modest increases in surface adsorption by uncharged water molecules. Therefore, the effects of both heat and RFGD treatments on the chemistry of charged M-OH species in the oxide may be central to their modulation of fibronectin's cell attachment activity. Future studies will analyze treatment –induced changes in surface oxide concentrations of cationic and anionic M-OH species by electron spectroscopy for chemical analysis (ESCA).
4.4 The role of oxide charged groups in the modulation of fibronectin conformation
The effects of RFGD and heat treatments of Ti6Al4V on metal oxide negative net charge and cell attachment, as described here and in our companion article [32], suggest that [M — O]- groups modulate fibronectin's conformational bioactivity. A number of studies have investigated the effects of non-metallic surface charge on fibronectin structure and bioactivity. Kowalczynska et al. have demonstrated that fibronectin assumes a more flattened, extended and presumably unfolded conformation on negatively charged sulfonated polystyrene compared to nonsulfonated hydrophobic polystyrene. The charged sulfonated substrate also supported a higher level of cell adhesion to adsorbed fibronectin compared to the nonsulfonated surface [53]. Two other studies also reported that cell adhesion to fibronectin was increased when the protein adsorbed to surfaces functionalized with COOH and OH groups that are likely to be negatively charged at physiological pH [54,55]. Other studies using fluorescence resonance energy transfer and AFM to analyze fibronectin conformation have found that the protein retains a compact configuration on hydrophobic surfaces but manifests a slightly more extended conformation on hydrophilic glass. It was suggested that charge–charge interactions between fibronectin and negatively charged hydrophilic glass can disrupt electrostatic interactions that stabilize the compact state of the protein. As a result, fibronectin's crossed-over dimeric arms separate, leading to a change in the protein's quaternary conformation [56,57]. In a similar manner, negatively charged functional groups in the Ti6Al4V surface oxide may disrupt intramolecular interactions in or close to fibronectin's cell-binding domain, resulting in conformational changes that increase its exposure to cell integrin receptors.
4.5 The role of oxide ultrastructure in the modulation of fibronectin conformation
Our current results emphasize the importance of surface oxide chemistry in the effects of RFGD on fibronectin's cell binding capacity. In comparison, the relative contributions of metal oxide surface chemistry and topography to the effects of heat treatment on fibronectin are less clear. Notably, Kilpadi and Lemons [58] showed that surface energy, which is thought to influence protein adsorption and cell attachment [37], was attributable to the overlying oxide rather than the nanostructure grain dimensions of titanium. Another study showed that the sputter-coating of a mechanically roughened titanium oxide with gold significantly reduced osteoblast attachment even though the surface topography was retained [59]. These findings would suggest that oxide topography per se may not directly modulate adsorbed protein behavior or the surface attachment of cell receptor proteins. However, as discussed in our companion paper [32], oxide roughness [60-62] and topography [63] can contribute to the wettability of the heat-treated samples. Therefore, perhaps oxide topography can influence protein bioactivity and cell attachment indirectly through interactions with oxide chemistry.
It is also possible that nanotopography can modify the conformation of adsorbed fibronectin indirectly through interactions with surface oxide charge. For example, it has been demonstrated that, as the number of nanometer surface features increases, the charge density at the surface also increases [64,65]. In addition, the surface potential effect of individual charged or polar surface functional groups inside a nanopore or similar non-smooth nanostructure on counter ions or charged species in proteins can be intensified (compared to a smooth surface) by linear superposition [66]. Based on the principle of linear superposition, the Coulomb (electrostatic) force on a single charged functional group in a protein would be the sum of all the individual Coulomb forces exerted on that functional group by each charged species of opposite polarity inside the pore. We have demonstrated that the heat treatment of Ti6Al4V had no effect on surface oxide net charge but created a porous oxide with nano-protrusions approximately 50-100 nm in diameter [32]. As demonstrated in our companion paper, heat treatment exerted a more modest effect on oxide negative net charge, when measured at physiological pH, compared to RFGD treatment [32], even though the two treatments promoted equivalent increases in cell attachment and spreading. However, the effective charge of uncompensated [M — O]- groups could be enhanced by the linear superposition of multiple charges of similar polarity inside the porous oxide nanotopography created by heat treatment. In this manner, oxide nanotopography could increase the interactions between available charged M-OH groups and fibronectin residues, thereby making conformational changes in the protein more likely.
4.6 Fibronectin adsorption
Another important aspect of protein behavior that can be influenced by the chemistry of the oxide and the M-OH groups therein is the ability of the protein to adsorb to the oxide surface. It has been reported that a DC current glow discharge plasma (GDP) treatment increased the adsorption of fibronectin to titanium [37,38]. However, differences between the GDP treatment and our RFGD procedure in their effects on fibronectin adsorption may be due to inherent differences between the two plasma generation systems, as discussed in our companion article [32]. In contrast to GDP treatment, Kern and coworkers obtained results similar to those reported here showing that the heat-treatment of cp-titanium (grade 2) surfaces did not significantly affect fibronectin and albumin adsorption although the treatment increased surface wettability [67]. Therefore, our results together with those of Kern and coworkers suggest that treatment-induced increases in the surface hydration of an already hydrophilic surface may not be great enough to significantly affect fibronectin adsorption to metal oxides.
Protein adsorption is complex in its own right, involving molecular-scale interactions that occur nearly instantaneously relative to the timeframe of cell adhesion [68, 69]. Polar amino acid residues that can participate in hydrogen bonding are likely to be sites of binding to other molecules including those in the substratum [70]. Therefore, it is possible that charged and uncharged M-OH species, both of which are capable of hydrogen bonding, can alter the degree of protein adsorption. However, our findings that neither heat nor RFGD treatments increased fibronectin adsorption suggest that the treatments did not increase the hydrogen bonding of fibronectin to the oxide substrate. Our findings suggest instead that the treatments used here either increased the density of uncompensated charged M — O– groups (at physiological pH ; see ref. 32) that interact selectively with amino acids of specific (and opposite) charge or else enhanced these interactions to modulate protein conformation. These latter interactions do not involve hydrogen bonding. Nevetheless, a role for hydrogen bonding in the effects of the pretreated oxide on fibronectin's conformational bioactivity cannot be ruled out. Future studies will analyze treatment –induced changes in surface oxide concentrations of cationic and anionic M-OH species by electron spectroscopy for chemical analysis (ESCA).
4.7 The effects of treatment on adsorbed fibronectin-promoted cell spreading
We also observed that the heat and RFGD treatment of Ti6Al4V samples precoated with fibronectin increased cellular spreading. Following the initial passive phases of cell contact and attachment, adherent cells then slowly spread over the surface within a period of a few hours via an active cellular mechanism that involves the recruitment and clustering of cell integrin receptors at anchoring sites called focal adhesions [71-73] Focal adhesions convey information across the cell membrane through integrins and related signaling proteins, such as focal adhesion kinase, to regulate a number of cell functions including differentiation.[74]. Properties of the surface titanium oxide, such as roughness, can enhance this process of osteogenic cell spreading / focal adhesion formation [75-76] to perhaps modulate the development of the osteoblast phenotype through intracellular signaling mechanisms. It is possible that heat treatment's effects on cell spreading were partially mediated by an increased in the oxide's nanoroughness (see ref. 32), which as discussed previously, may enhance the effects of surface charge on fibronectin's conformational bioactivity. Our observations that surface treatments augmented the cell spreading response indicate that integrin-dependent intracellular signaling may also have been amplified as a result of an increase in the number of fibronectin-integrin receptor complexes per cell. Our results suggest that implant surfaces engineered to stabilize adsorbed fibronectin's biologically active conformation are likely to increase fibronectin's binding to integrins as well as its activation of integrin receptor-dependent signaling mechanisms. Therefore, this surface engineering approach may accelerate osteoblast differentiation to a greater degree than strategies that only increase the adsorption of fibronectin without any conformational changes in the protein's receptor binding affinity.
In this study, we have presented evidence supporting the hypothesis that heat and RFGD treatments of Ti6Al4V enhance adsorbed fibronectin's intrinsic capacity to bind to mesenchymal and bone cell receptors. by modifying the electrostatic properties of the alloy's surface oxide. By elucidating the properties of metal oxides that control protein conformation and the accompanying cellular responses, treatment strategies can be developed to augment fibronectin's ability to promote bone cell attachment, spreading, osteogenesis and osseointegration in vivo.
Conclusions
In summary, in this study together with a companion study [32], we have examined the effects of heat or RFGD treatment on Ti6Al4V oxide surface properties, including topography, wettability, atomic composition and net surface charge in relationship to fibronectin binding to osteogenic cells and the cell spreading response. Our results showed that the stimulatory effects of heat and RFGD treatments on adsorbed fibronectin's cell attachment activity and capacity to promote cell spreading are more highly correlated with an increase in the oxide's negative net charge than with other oxide characteristics. Based on these findings, we conclude that negatively charged M-OH complexes modulate fibronectin's integrin binding activity probably through a change in conformation. Future studies will investigate the influence of specific oxide properties on the phenotypic expression of osteoblastic cells and osteogenesis.
Acknowledgments
The project described was supported by Grant Number NIH RO1 DE017695 (Awarded to DEM). The sponsor did not have any role in the study design; the collection, analysis and interpretation of data; the writing of the report; or the decision to submit the paper for publication. This material is also the result of work supported with resources and the use of facilities at the James J. Peters VA Medical Center, Bronx, New York. We would like to acknowledge Dr. Stephen Doty and Tony Labisserie for help with scanning electron microscopy. Special thanks goes to Christine Marsh and Kyle Hackshaw for their assistance with the cell culture experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 2.Stuiver I, O'Toole TE. Regulation of integrin function and cellular adhesion. Stem Cells. 1995;13:250–62. doi: 10.1002/stem.5530130306. [DOI] [PubMed] [Google Scholar]
- 3.Ivarsson B, Lundström I. Physical characterization of protein adsorption on metal and metal oxide surfaces. CRC Crit Rev Biocompat. 1986;2:1–96. [Google Scholar]
- 4.Kasemo B, Lausmaa J. Surface science aspects of inorganic biomaterials. CRC Crit Rev Biocompat. 1986;2:335–80. [Google Scholar]
- 5.MacDonald DE, Markovic B, Allen M, Somasundaran P, Boskey AL. Surface analysis of human plasma fibronectin adsorbed to commercially pure titanium materials. J Biomed Mater Res. 1998;41:120–30. doi: 10.1002/(sici)1097-4636(199807)41:1<120::aid-jbm15>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Wälivaara B, Lundström I, Tengvall P. An in vivo study of H2O2-treated titanium surfaces in contact with blood plasma and a simulated body fluid. Clin Mater. 1993;12:141–48. doi: 10.1016/0267-6605(93)90065-f. [DOI] [PubMed] [Google Scholar]
- 7.Rapuano BE, Wu C, MacDonald DE. Osteoblast-like cell adhesion to bone sialoprotein peptides. J Orthop Res. 2004;22:353–361. doi: 10.1016/S0736-0266(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 8.Sauberlich S, Klee D, Richter EJ, Hocker H, Spiekermann H. Cell culture tests for assessing the tolerance of soft tissue to variously modified titanium surfaces. Clin Oral Implants Res. 1999;10:379–393. doi: 10.1034/j.1600-0501.1999.100505.x. [DOI] [PubMed] [Google Scholar]
- 9.Harbers GM, Healy KE. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. J Biomed Mater Res. 2005;75:855–69. doi: 10.1002/jbm.a.30482. [DOI] [PubMed] [Google Scholar]
- 10.Barber TA, Golledge SL, Castner DG, Healy KE. Peptide-modified p(AAm-co-EG/AAc) IPN's grafted to bulk titanium modulate osteoblast behavior in vitro. J Biomed Mater Res. 2003;64:38–47. doi: 10.1002/jbm.a.10321. [DOI] [PubMed] [Google Scholar]
- 11.Rezania A, Thomas CH, Branger AB, Waters CM, Healy KE. The detachment strength and morphology of bone cells contacting materials modified with a peptide sequence found within bone sialoprotein. J Biomed Mater Res. 1997;37:9–19. doi: 10.1002/(sici)1097-4636(199710)37:1<9::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Hynes RO. Integrins : bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 13.Anselme K, Linez P, Bigerelle M, Le Maguer D, Le Maguer A, Hardouin P, et al. The relative influence of the topography and chemistry of TiAl6V4 surfaces on osteoblastic cell behavior. Biomaterials. 2002;21:1567–77. doi: 10.1016/s0142-9612(00)00042-9. [DOI] [PubMed] [Google Scholar]
- 14.Kieswetter K, Schwartz Z, Hummert TW, Cochran DL, Simpson J, Dean DD, et al. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996;32:55–63. doi: 10.1002/(SICI)1097-4636(199609)32:1<55::AID-JBM7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald DE, Deo N, Markovic B, Stranick M, Somasundaran P. Adsorption and dissolution behavior of human plasma fibronectin on heatly and chemically modified titanium dioxide particles. Biomaterials. 2002;23:1269–1279. doi: 10.1016/s0142-9612(01)00317-9. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald DE, Rapuano BE, Deo N, Stranick M, Boskey AL, Somasundaran P. Heat and chemical modification of titanium-aluminum-vanadium implant materials : effects on surface properties, glycoprotein adsorption, and MG63 cell attachment. Biomaterials. 2004;25:3135–3146. doi: 10.1016/j.biomaterials.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J, et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) J Biomed Mater Res. 1995;29:389–401. doi: 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson U, Lundstrom I, Ronnberg I. IgG and secretory fibronectin adsorption to Silica. J Colloid Interface Sci. 1987;117:127–138. [Google Scholar]
- 19.Pitt WG, Spiegelberg SH, Cooper SL. Adsorption of fibronectin to polyurethane Fourier transform infrared spectroscopy studies. In: Horbett TA, Brash JL, editors. Proteins at Interfaces. American Chemical Society; Washington, D.C.: 1987. pp. 324–338. [Google Scholar]
- 20.Narasimhan C, Lai CS. Conformational changes of plasma fibronectin detected upon adsorption to solid substrates : a spin-label study. Biochemistry. 1989;28:5041–5046. doi: 10.1021/bi00438a021. [DOI] [PubMed] [Google Scholar]
- 21.Grinnell F, Feld MK. Adsorption characteristics of plasma fibronectin in relationship to biological activity. J Biomed Mater Res. 1981;15:363–381. doi: 10.1002/jbm.820150308. [DOI] [PubMed] [Google Scholar]
- 22.Pettit DK, Hoffman AS, Horbett TA. Correlation between corneal epithelial cell outgrowth and monoclonal antibody binding to the cell binding domain of adsorbed fibronectin. J Biomed Mater Res. 1994;28:685–691. doi: 10.1002/jbm.820280605. [DOI] [PubMed] [Google Scholar]
- 23.Sigal GB, Mrksich M, Whitesides GM. Effect of surface wettability on the adsorption of proteins and detergents. J Am Chem Soc. 1998;120:3464–3473. [Google Scholar]
- 24.Bowditch RD, Halloran CE, Aota S, Obara M, Plow EF, Yamada KM, Ginsberg MH. Integrin alpha IIb beta 3 (platelet GPIIb-IIIa) recognizes multiple sites in Fibronectin. J Biol Chem. 1991;266:23323–23328. [PubMed] [Google Scholar]
- 25.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res. 2003;66A:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 26.Gotman I. Characteristics of metals used in implants. J Endourol. 1997;11:383–9. doi: 10.1089/end.1997.11.383. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Cheng XR. Characterizations of three surface-modified titanium oxide Films. Chin J Dent Res. 2000;3:49–52. [PubMed] [Google Scholar]
- 28.Szabo G, Kovacs L, Vargha K, Barabas J, Nemeth Z. A new advanced surface modification technique--titanium oxide ceramic surface implants: the background and long-term results. J Long Term Eff Med Implants. 1999;9:247–59. [PubMed] [Google Scholar]
- 29.Eisenbarth E, Velten D, Schenk-Meuser K, Linez P, Bieh V, Duschner H, Breme J, Hildebrand H. Interactions between cells and titanium surfaces. Biomol Eng. 2002;19:243–9. doi: 10.1016/s1389-0344(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 30.Hanawa T. Titanium and its oxide film: A substrate for formation of apatite. In: Davies JE, editor. The Bone-Biomaterial Interface. University of Toronto Press; Toronto: 1991. pp. 49–61. [Google Scholar]
- 31.Nishiguchi S, Kato H, Fujita H, Oka M, Kim HM, Kokubo T, Nakamura T. Titanium metals form direct bonding to bone after alkali and heat treatments. Biomater. 2001;22:2523–2533. doi: 10.1016/s0142-9612(00)00443-9. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald DE, Rapuano BE, Schniepp HC. Surface oxide charge and hydrophilicity of a titanium alloy : modification by treatment with heat or radiofrequency plasma glow discharge. Colloids and Surfaces B : Biointerfaces. 2010 doi: 10.1016/j.colsurfb.2010.08.031. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Christensen R, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast sub-clones with distinct in vitro differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 34.Nayab SN, Jones FH, Olsen I. Effects of calcium ion implantation on human bone cell interaction with titanium. Biomaterials. 2005;26:4717–4727. doi: 10.1016/j.biomaterials.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv Dent Res. 1999;13:38–48. doi: 10.1177/08959374990130011301. [DOI] [PubMed] [Google Scholar]
- 36.Bam G, Muller T, Wang X, Papkoff J. Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun. 2003;301:84–91. doi: 10.1016/s0006-291x(02)02951-0. [DOI] [PubMed] [Google Scholar]
- 37.Kieswetter K, Schwartz Z, Dean DD, Boyan BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996;7:329–345. doi: 10.1177/10454411960070040301. [DOI] [PubMed] [Google Scholar]
- 38.Altankov G, Groth T. Reorganization of substratum-bound fibronectin on hydrophilic and hydrophobic materials is related to biocompatibility. Journal of Material Science: Materials in Medicine. 1994;5:732–37. [Google Scholar]
- 39.Grinnell F, Feld MK. Adsorption characteristics of plasma fibronectin in relationship to biological activity. J Biomed Mater Res. 1981;15:363–81. doi: 10.1002/jbm.820150308. [DOI] [PubMed] [Google Scholar]
- 40.Sjöberg B, Eriksson M, Österlund E, Pap S, Österlund K. Solution structure of human plasma fibronectin as a function of NaCl concentration determined by small-angle neutron diffraction. European Journal of Biophysics. 1989;17:5–11. doi: 10.1007/BF00257140. [DOI] [PubMed] [Google Scholar]
- 41.Benecky MJ, Kolvenbach CG, Wine RW, DiOrio JP, Mosesson MW. Human plasma fibronectin structure probed by steady-state fluorescence polarization. Evidence for a rigid oblate structure. Biochemistry. 1990;29:3082–91. doi: 10.1021/bi00464a027. [DOI] [PubMed] [Google Scholar]
- 42.Khan MY, Medow MS, Newman SA. Unfolding transitions of fibronectin and its domains, stabilization and structural alteration of the n-terminal domain by heparin. Biochem J. 1990;270:33–38. doi: 10.1042/bj2700033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erickson HP, Carrell NA. Fibronectin in extended and compact conformations. J Biol Chem. 1983;258:14539–44. [PubMed] [Google Scholar]
- 44.Iwamoto GK, Winterton LC, Stoker RS, van Wagenen RA, Andrade JD, Mosher DF. Fibronectin adsorption detected by interfacial fluorescence. J Colloid Interface Sci. 1985;105:459–64. [Google Scholar]
- 45.Emch R, Clivaz X, Taylor-Denes C, Vaudaux P, Descouts P. Scanning tunneling microscopy for studying the biomaterial-biological tissue interface. J Vac Sci Technol. 1990;A8:655–58. [Google Scholar]
- 46.Emch R, Zenhausern F, Jobin M, Taborelli M, Descouts P. Morphological difference between fibronectin sprayed on mica and on pmma. Ultramicroscopy. 1992;42-44:1155–60. doi: 10.1016/0304-3991(92)90417-i. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Lim JY, Donahue HJ, Dhurjau R, Mastro AM, Vogler EA. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and genotypic responses observed in vitro. Biomaterials. 2007;28:4535–4550. doi: 10.1016/j.biomaterials.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolatshahi-Pirouz A, Jensen T, Foss M, Chevallier J, Besenbacher F. Enhanced surface activation of fibronectin upon adsorption on hydroxyapatite. Langmuir. 2009;25:2971–2978. doi: 10.1021/la803142u. [DOI] [PubMed] [Google Scholar]
- 49.Amaral IF, Lamghari M, Sousa SR, Sampaio P, Barbosa MA. Rat bone marrow stromal cell osteogenic differentiation and fibronectin adsorption on chitosan membranes: The effect of the degree of acetylation. J Biomed Mater Res. 2005;A 75:387–397. doi: 10.1002/jbm.a.30436. [DOI] [PubMed] [Google Scholar]
- 50.Vogler EA. Interfacial chemistry in biomaterials science. In: Berg J, editor. Wettability. Marcel Dekker; New York: 1993k. pp. 184–250. [Google Scholar]
- 51.Vogler EA. Structure and reactivity of water at biomaterial surfaces. Adv Colloid Interf Sci. 1998;74:69–117. doi: 10.1016/s0001-8686(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 52.Han JB, Wang X, Wang N, Wei ZH, Yu GP, Zhou ZG, Wang QQ. Effect of plasma treatment on hydrophilic properties of TiO2 thin films. Surface & Coatings Technology. 2006;200:4876–4878. [Google Scholar]
- 53.Kowalczynska HM, Kołos R, Nowak-Wyrzykowska M, Dobkowski J, Elbaum D, Szczepankiewicz A, Kaminski J. Atomic force microscopy evidence for conformational changes of fibronectin adsorbed on unmodified and sulfonated polystyrene surfaces. J Biomed Mater Res. 2009;91A:1239–1251. doi: 10.1002/jbm.a.32473. [DOI] [PubMed] [Google Scholar]
- 54.Miller T, Boettiger D. Control of intracellular signaling by modulation of fibronectin conformation at the cell-materials interface. Langmuir. 2003;19:1723–1729. [Google Scholar]
- 55.Lee MH, Ducheyne P, Lynch L, Boettiger D, Composto RJ. Effect of biomaterial surface properties on fibronectin-α5β1 integrin interaction and cellular attachment. Biomaterials. 2006;27:1907–1916. doi: 10.1016/j.biomaterials.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Bergkvist M, Carlsson J, Oscarsson S. Surface-dependent conformations of human plasma fibronectin adsorbed to silica, mica, and hydrophobic surfaces, studied with use of atomic force microscopy. J Biomed Mater Res. 2003;64A:349–356. doi: 10.1002/jbm.a.10423. [DOI] [PubMed] [Google Scholar]
- 57.Baugh L, Vogel V. Structural changes of fibronectin adsorbed to model surfaces probed by fluorescence resonance energy transfer. J Biomed Mater Res. 2004;69A:525–534. doi: 10.1002/jbm.a.30026. [DOI] [PubMed] [Google Scholar]
- 58.Kilpadi DV, Lemons JE. Surface energy characterization of unalloyed titanium implants. Biomed Mater Res. 1994;28:1419–1425. doi: 10.1002/jbm.820281206. [DOI] [PubMed] [Google Scholar]
- 59.Anselme K, Bigerelle M. Statistical demonstration of the relative effect of surface chemistry and roughness on human osteoblast short-term adhesion. J Mater Sci Mater Med. 2006;17:471–479. doi: 10.1007/s10856-006-8475-8. [DOI] [PubMed] [Google Scholar]
- 60.Jennissen HP. Ultra-hydrophile metallische biomaterialien. Biomaterialien. 2001;2:45–53. [Google Scholar]
- 61.Adamson AW. Physical Chemistry of Surfaces. John Wiley & Sons Inc.; New York: 1990. p. 399. [Google Scholar]
- 62.Lim YJ, Oshida Y. Initial contact angle measurements on variously treated dental/medical titanium materials. Biomedical Materials and Engineering. 2001;11:325–341. [PubMed] [Google Scholar]
- 63.Zwilling V, Darque-Ceretti E, Boutry-Forveille A, David D, Perrin MY, Aucouturier M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surface and Interface Analysis. 1999;27:629–637. [Google Scholar]
- 64.Klabunde KJ, Strak J, Koper O, Mohs C, Park D, Decker S, Jiang Y, Lagadic L, Zhang D. Nanocrystals as stoichiometric reagents with unique surface chemistry. J Phys Chem. 1996;100:12141. [Google Scholar]
- 65.Baraton ML, Chen X, Gonsalves E. FTIR study of a nanostructured aluminum nitride powder surface: Determination of the acidic/basic sites by CO, CO2 and acetic acid adsorptions. Nanostruct Mater. 1997;8:435–444. [Google Scholar]
- 66.Bretag AH, editor. Membrane permeability: Experiments and models. Adelaide: Techsearch Inc.; 1983. [Google Scholar]
- 67.Kern T, Yang Y, Glover R, Ong JL. Effect of heat-treated titanium surfaces on protein adsorption and osteoblast precursor cell initial attachment. Implant Dent. 2005;14:70–76. doi: 10.1097/01.id.0000154795.93155.ee. [DOI] [PubMed] [Google Scholar]
- 68.Andrade JD. Principles of protein adsorption. In: Andrade JD, editor. Surf Interf Aspects Biomed Polym: Protein Adsorption. New York: Plenum Press; 1985. pp. 1–80. [Google Scholar]
- 69.Ramsden JJ. Puzzles and paradoxes in protein adsorption. Chem Soc Rev. 1995;24:73–8. [Google Scholar]
- 70.Turner NW, Jeans CW, Brain KR, Allender CJ, Hlady V, Britt DW. From 3D to 2D: A Review of the Molecular Imprinting of Proteins. Biotechnol Prog. 2006;22(6):1474–1489. doi: 10.1021/bp060122g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weiss L. The adhesion of cells. In: Bourne GH, Danielli JF, editors. International review of cytology. Academic Press Inc.; New York: 1960. pp. 187–225. [DOI] [PubMed] [Google Scholar]
- 72.Vogler EA. Interfacial chemistry in biomaterials science. In: Berg J, editor. Wettability. Marcel Dekker; New York: 1993. pp. 184–250. [Google Scholar]
- 73.Grinnell F. Cellular Adhesiveness and extracellular substrata. In: Bourne GH, Danielli JF, Jeon RW, editors. International review of cytology. Academic Press; New York: 1978. pp. 67–145. [DOI] [PubMed] [Google Scholar]
- 74.Docheva D, Popov C, Mutschier W, Schieker M. Human mesenchymal stem cells in contact with their environment surface characteristrics and the integrin system. J Cell Mol Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen J, Zhu XL, Scheideier L, Geis-Gerstorfer J. Effects of voltage during anodization of titanium surface on cell attachment and spreading of osteoblasts. Shanghai Kou Qiang Yi Xue. 2006;15:308–312. [PubMed] [Google Scholar]
- 76.Kommireddy DS, Sriram SM, Lvov YM, Mills DK. Stem cell attachment to layer-by-layerTiO2 nanoparticle thin films. Biomaterials. 2006;27:4296–4303. doi: 10.1016/j.biomaterials.2006.03.042. [DOI] [PubMed] [Google Scholar]