Abstract
To define a functional role for the endosomal/lysosomal cysteine protease cathepsin L (Ctsl) during squamous carcinogenesis, we generated mice harboring a constitutive Ctsl deficiency in addition to epithelial expression of the human papillomavirus type 16 oncogenes (human cytokeratin 14 (K14)–HPV16).We found enhanced tumor progression and metastasis in the absence of Ctsl. As tumor progression in K14–HPV16 mice is dependent on inflammation and angiogenesis, we examined immune cell infiltration and vascularization without finding any effect of the Ctsl genotype. In contrast, keratinocyte-specific transgenic expression of cathepsin V, the human orthologue of mouse Ctsl, in otherwise Ctsl-deficient K14–HPV16 mice restored the phenotype observed in the control HPV16 skin. To better understand this phenotype at the molecular level, we measured several oncogenic signal transduction pathways in primary keratinocytes on stimulation with keratinocyte-conditioned cell culture medium. We found increased activation of protein kinase B/Akt and mitogen-activated protein kinase pathways in protease-deficient cells, especially if treated with media conditioned by Ctsl-deficient keratinocytes. Similarly, the level of active GTP-Ras was increased in Ctsl-deficient epidermis. We conclude that Ctsl is critical for the termination of growth factor signaling in the endosomal/lysosomal compartment of keratinocytes and, therefore, functions as an anti-tumor protease.
Keywords: skin cancer, mouse model, protease, cathepsin
Introduction
Proteases have traditionally been thought to promote the invasive growth of carcinomas, contributing to the spread and homing of metastasizing cancer cells (Dano et al., 1999). Proteases that function outside tumor cells have been implicated in these processes because of their well-established release from tumors, causing the breakdown of basement membranes and extracellular matrix. Thus, extracellular proteases may in part facilitate tumor cell invasion into ectopic tissue by the generation of bioactive factors from extracellular matrix macromolecules (Kalluri, 2003; DeClerck et al., 2004). The groups of tumor-promoting proteolytic enzymes consist of: soluble and integral membrane matrix metalloproteases (MMP, MT-MMP, and ADAMTS), serine proteases and inhibitors of the plasminogen activator/plasminogen system, and cysteine- and aspartyl-type endosomal/lysosomal peptidases (Liotta and Kohn, 2001; Mohamed and Sloane, 2006). Alternatively, a number of proteases are considered to have tumor-suppressive functions (Lopez-Otin and Matrisian, 2007). This paradox is easily appreciated for caspases that regulate cell death programming in cells through initiation and execution of apoptotic programs. However, there is also evidence of anti-tumor functions for some members of the extracellular metalloprotease families, including MMPs and ADAMTS (Martin and Matrisian, 2007). The major mechanism(s) underlying anti-tumor functions is thought to result from the inhibition of tumor angiogenesis because of the generation of anti-angiogenic peptides (Nyberg et al., 2008).
Cathepsin L (Ctsl) is an endosomal/lysosomal papain-like cysteine endoprotease implicated in a variety of physiological and pathological processes (Vasiljeva et al., 2007). The mouse genome encodes for only one Ctsl while in humans there is not only the ‘classical’ cathepsin L (CTSL) but also the related cysteine protease cathepsin V (CTSV), which is frequently termed cathepsin L2. Human CTSV and murine Ctsl are true orthologues because they share 75% amino acid sequence identity, whereas the homology of murine Ctsl and human CTSL is less pronounced (Brömme, 2004). However, human CTSL is ubiquitously expressed, whereas CTSV expression is restricted to macrophages, thymus, testis, corneal epithelium and—importantly—the epidermis (Bernard et al., 2003; Tolosa et al., 2003; Cheng et al., 2006). In a variety of human cancers expression levels of CTSL have been positively correlated with poor prognosis (Jedeszko and Sloane, 2004), while CTSV is transcriptionally upregulated in skin squamous cell carcinomas (SCCs) as compared with the benign hyperproliferation in psoriatic skin (Haider et al., 2006). In addition to their intracellular localization, the various human and murine Ctsl enzymes have been detected in the extracellular portion of the plasma membrane and secreted into the extracellular space. Ctsl released by carcinoma cells has been shown to produce the angiogenesis inhibitor endostatin from collagen XVIII, the core protein of heparan sulfate proteoglycans in vascular and epithelial basement membranes (Felbor et al., 2000). In contrast, Urbich et al. showed that Ctsl is actually critical for the invasive capacity of endothelial progenitor cells, promoting neovascularization (Urbich et al., 2005). Intracellular Ctsl has been identified as a positive regulator of insulin-like growth factor 1 receptor signaling and of the experimental metastasis of tumor cells, which are dependent on insulin-like growth factor 1 receptor, and has also been found to have a role in promoting resistance toward chemotherapeutic drugs (Zheng et al., 2004; Navab et al., 2008). N-terminally truncated Ctsl has been found in the nucleus, in which it activates the CDP/Cux transcription factor, a positive regulator of the cell cycle involved in cellular transformation (Goulet et al., 2007).
As of the multiple potential mechanisms by which Ctsl might affect tumor progression, we decided to analyze this protease in a complex in vivo model of tumorigenesis that mimics the successive stages of human cancer development. Therefore, we used K14–HPV16 transgenic mice as a mouse model of epidermal carcinogenesis (Arbeit et al., 1994; Coussens et al., 1996). K14–HPV16 mice express the early region genes of human papillomavirus type 16 (HPV16) under the control of the human cytokeratin 14 (K14) promoter. HPV16 transgenic mice show a series of discrete neoplastic stages, including hyperplasia by 1 month of age and development of dysplasias between 3 and 6 month of age. These precursor lesions undergo malignant conversion into SCCs. In addition to epithelial alterations, neoplastic progression in HPV16 mice is characterized by chronic inflammation regulating microenvironmental remodeling and the activation of angiogenic programs in premalignant tissue (Coussens et al., 1999, 2000; de Visser et al., 2005).
To evaluate a functional role for Ctsl during squamous carcinogenesis, we modulated Ctsl expression systemically in keratinocytes and found that Ctsl is a keratinocyte-specific tumor suppressor that regulates autocrine signaling processes and, therefore, progression of epidermal SCCs.
Results
Ctsl deficiency potentiates neoplastic progression in K14–HPV16 transgenic mice
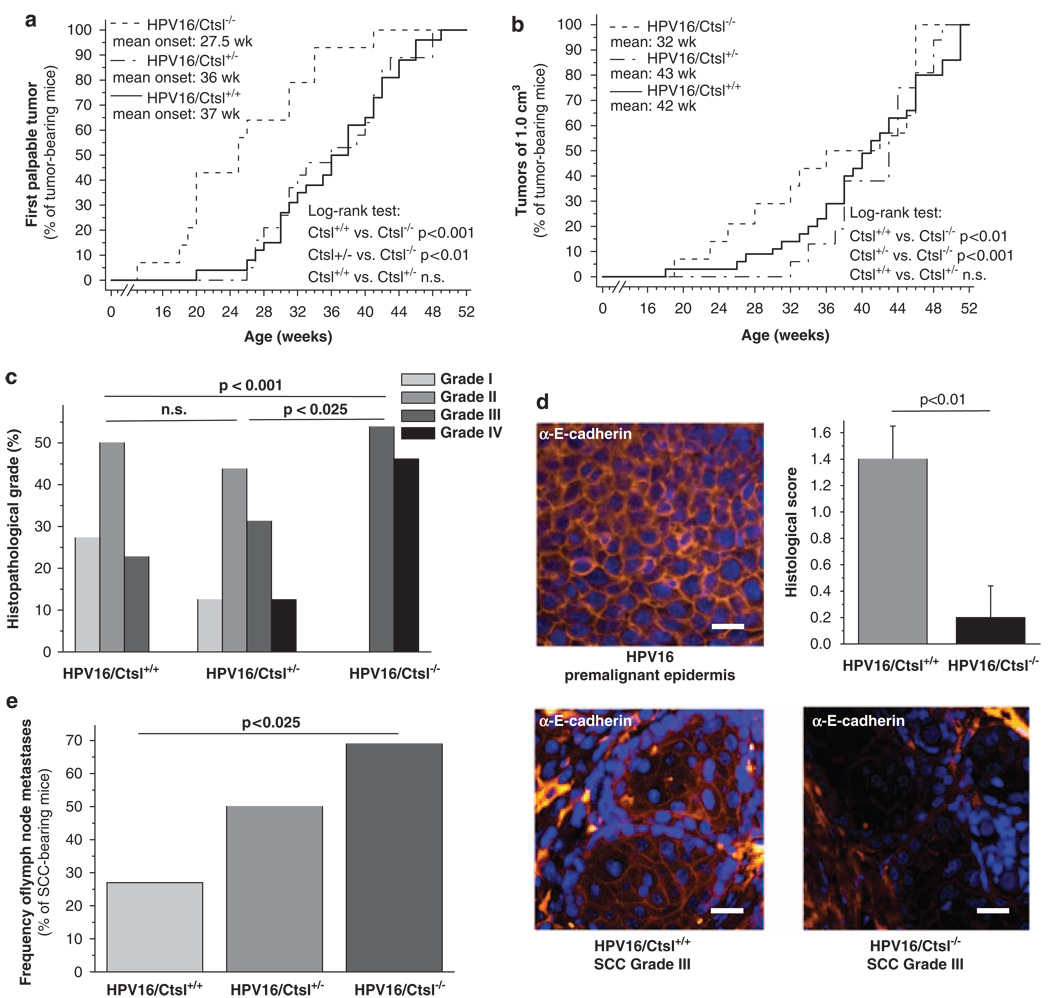
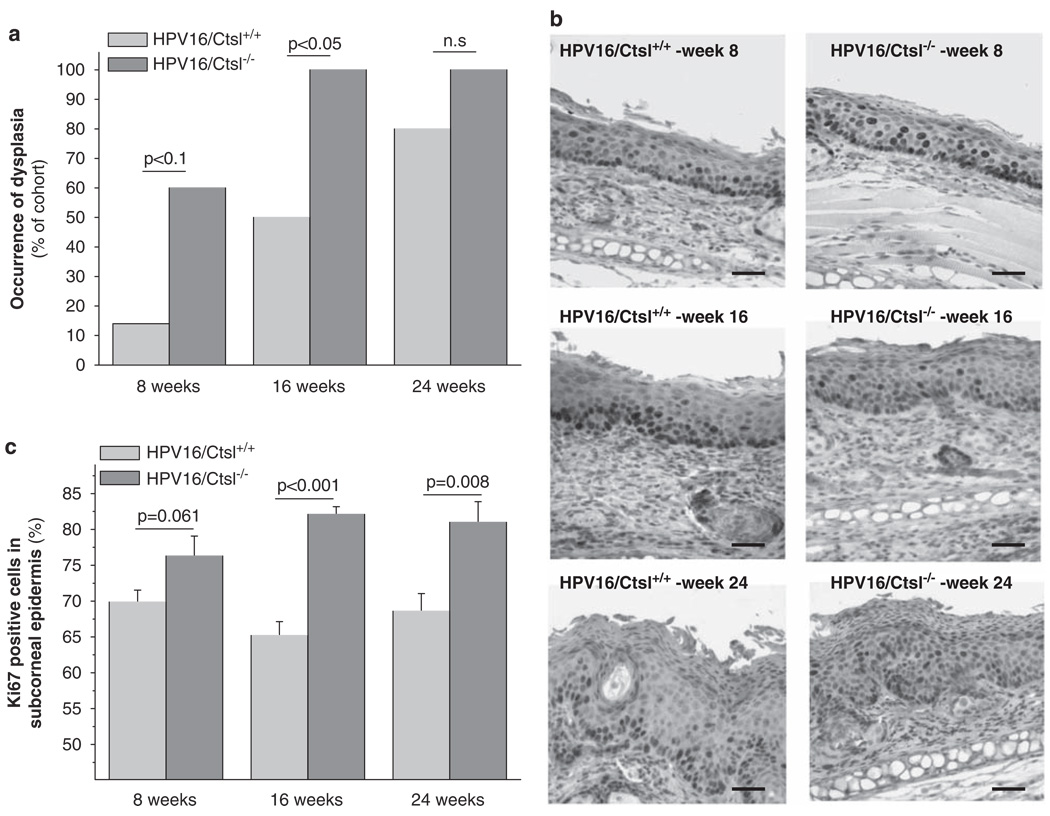
Cysteine cathepsins, such as Ctsl, are considered to be multifunctional proteases in cancer (Mohamed and Sloane, 2006). To evaluate which aspects of neoplastic progression Ctsl might regulate, we backcrossed Ctsl−/− mice (Roth et al., 2000) into the FVB/n genetic background and crossed them to congenic K14–HPV16 (HPV16) mice. We found that HPV16/Ctsl−/− mice developed the first palpable tumors significantly earlier than HPV16/Ctsl+/− and HPV16/Ctsl+/+ littermates (Figure 1a). The final tumor size of 1 cm3 appeared in the HPV16/Ctsl−/− mice approximately 10 weeks earlier than in the controls (Figure 1b). In addition, histopathological grading of carcinomas from HPV16/Ctsl−/− mice revealed the emergence of only poorly differentiated grade III and grade IV SCCs as opposed to a higher percentage of well- and moderately differentiated SCCs in littermate controls (Figure 1c). To further assess the histological characteristics of carcinomas, we quantified membrane-bound E-cadherin in grade III SCCs from HPV16/Ctsl+/+ and HPV16/Ctsl−/− mice (Figure 1d). We found that membrane-bound E-cadherin was reduced in the Ctsl-deficient carcinomas, substantiating their poor histological differentiation and suggesting a highly invasive and potentially metastatic phenotype. Accordingly, the frequency of metastases in axillary lymph nodes was found to be significantly higher in the Ctsl-deficient mice, 25% in the HPV16/Ctsl+/+ cohort and 70% in HPV16/Ctsl−/− mice (Figure 1e). The early onset and poor state of differentiation in HPV16/Ctsl−/− cancers was preceded by an earlier development of dysplasias (Figure 2a). Epidermal dysplasia was found at 16 weeks of age in each of the HPV16/Ctsl−/− mice, whereas only 50% of control mice had advanced to the dysplastic state by this time point. Quantification of the cell proliferation marker Ki67 revealed significantly increased percentages of proliferating keratinocytes in the premalignant epidermis of HPV16/Ctsl−/− mice at 8, 16 and 24 weeks of age (Figures 2b and c). Together, these data indicate a potential anti-tumor role for Ctsl during squamous carcinogenesis.
Figure 1.
Cathepsin L (Ctsl) deficiency promotes carcinogenesis and metastasis in K14–HPV16 mice. (a) First palpable tumors in HPV16/Ctsl+/+ (n = 26), HPV16/Ctsl+/− (n = 19) and HPV16/Ctsl−/− mice (n = 14). (b) The occurrence of end-stage tumors, 1 cm3 in volume. (c) Histopathological grading of end-stage squamous cell carcinomas (SCCs); grade I: well-differentiated SCC, grade II: moderately differentiated SCC; grade III and IV: poorly differentiated SCC. (d) E-cadherin (yellow) and nuclei (Hoechst; blue) staining in premalignant hyperplastic epidermis and SCC grade III from HPV16/Ctsl+/+ and HPV16/Ctsl−/− mice. Bars indicate 200 µm. The graph shows the semi-quantitative evaluation of E-cadherin staining at the plasma membrane of grade III SCC. The staining was classified as absent, weak, moderate or strong by two blinded observers and assigned a score ranging from 0 to 3 points, respectively (n = 6). (e) The frequency of lymph node metastases (cytokeratin-positive cell clones) in SCC-bearing HPV16 mice with different Ctsl genotypes.
Figure 2.
Cathepsin L (Ctsl) deficiency potentiates premalignant neoplastic progression in K14–HPV16 mice. (a) Ear skin from HPV16/Ctsl+/+ and HPV16/Ctsl−/− mice was histologically evaluated for the occurrence of dysplasia at 8, 16 and 24 weeks of age (each group n = 8–10). (b) Immunohistochemical staining of the proliferation marker Ki67 in ear skin from HPV16 mice. (c) Quantification of Ki67 staining in the subcorneal epidermal layers. Bars indicate 50 µm.
Ctsl deficiency does not alter immune cell infiltration or angiogenesis during neoplastic progression in HPV16 mice
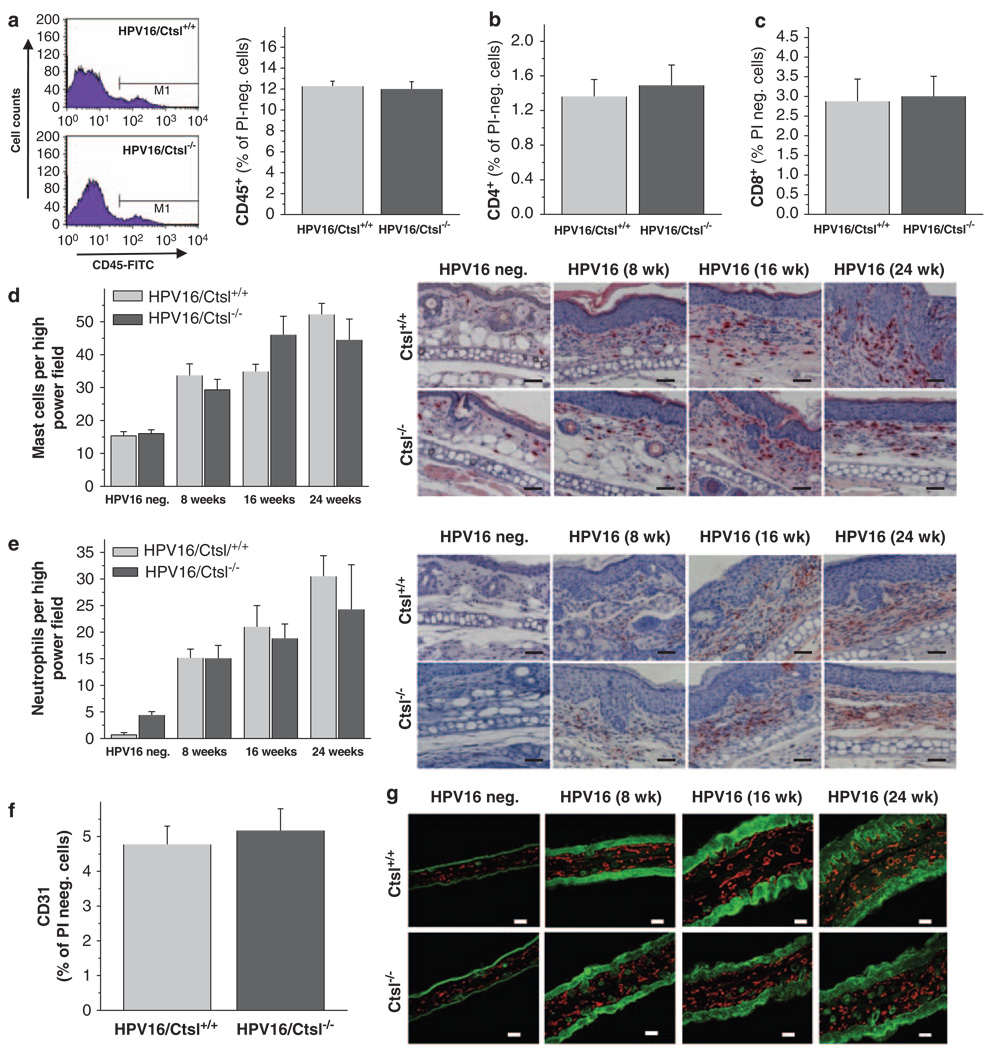
Neoplastic progression in HPV16 mice is characterized by extensive tissue remodeling. This occurs simultaneously with the activation of angiogenic programming in premalignant skin, which is largely regulated by cytokines, growth factors and proteases released by stroma-infiltrating immune cells (Coussens et al., 2000; de Visser et al., 2006). Thus, to determine if Ctsl regulates malignancy in HPV16 mice as a function of its altering the recruitment and/or activation of immune cells, we used flow cytometry and immunohistology (Figure 3). Our data revealed that the skin of both HPV16/Ctsl+/+ and HPV16/Ctsl−/− mice contained similar percentages of CD45-positive immune cells (Figure 3a) composed of cytotoxic T (CD8+) and T-helper (CD4+) cells (Figures 3b and c), mast cells (Figure 3d) and neutrophil granulocytes (Figure 3e). In addition, flow cytometry (Figure 3f) and immunofluorescence (Figure 3g) evaluation of the development of angiogenic vasculature revealed equal numbers of CD31+ endothelial cells, as well as similar vascular expansion in the skin of HPV16/Ctsl+/+ versus HPV16/Ctsl−/− mice.
Figure 3.
Immune cell infiltration and angiogenesis in K14–HPV16 mice. (a) Flow cytometric analysis of CD45+ leukocytes, (b) CD4+ T-helper cells, and (c) CD8+ cytotoxic T cells in the mechanical/enzymatical dissociated back skin of 16-week-old HPV16/Ctsl+/+ and HPV16/Ctsl−/− mice. (d) Histochemical staining and quantification of mast cells in the ear skin of 8-, 16- and 24-week-old HPV16/Ctsl+/+, HPV16/Ctsl−/− and wild-type (HPV16-negative) littermates. (e) Immunohistochemistry and quantification of neutrophil granulocytes in the ear skin of 8-, 16- and 24-week-old HPV16/Ctsl+/+, HPV16/Ctsl−/− and wild-type littermates. (f) Flow cytometry of CD31+ endothelial cells in 16-week-old HPV16/Ctsl+/+ and HPV16/Ctsl−/− mice. (g) Immunofluorescence detection of CD31+ endothelial cells (red: CD31+, green: pan-keratin) in HPV16/Ctsl+/+, HPV16/Ctsl−/−, and wild-type littermates. N = 6 in all groups; magnification bars: (d,e) = 50 µm, g = 100 µm.
Ctsl is known to be expressed in bone marrow-derived cells. Therefore, we decided to analyze the effect of selective Ctsl-deletion in bone marrow-derived cells on stromal remodeling and dysplasia formation in HPV16 mice (Supplementary Figure 1). Bone marrow from Ctsl+/+ or Ctsl−/− mice was transferred into 8-week old, lethally irradiated HPV16/Ctsl+/+ recipients. However, no differences were detected between the two experimental groups in dysplasia development, endothelial cell numbers or immune cell counts in the skin of the recipients 10 weeks after the bone marrow transfers (Supplementary Figure 1). These results show that a significant effect for Ctsl deficiency on chronic inflammation and stromal remodeling during premalignant progression in HPV16 skin can be excluded. This indicates that increased malignancy in HPV16/Ctsl−/− mice is not likely to be due to microenvironmental alterations regulating malignant conversion.
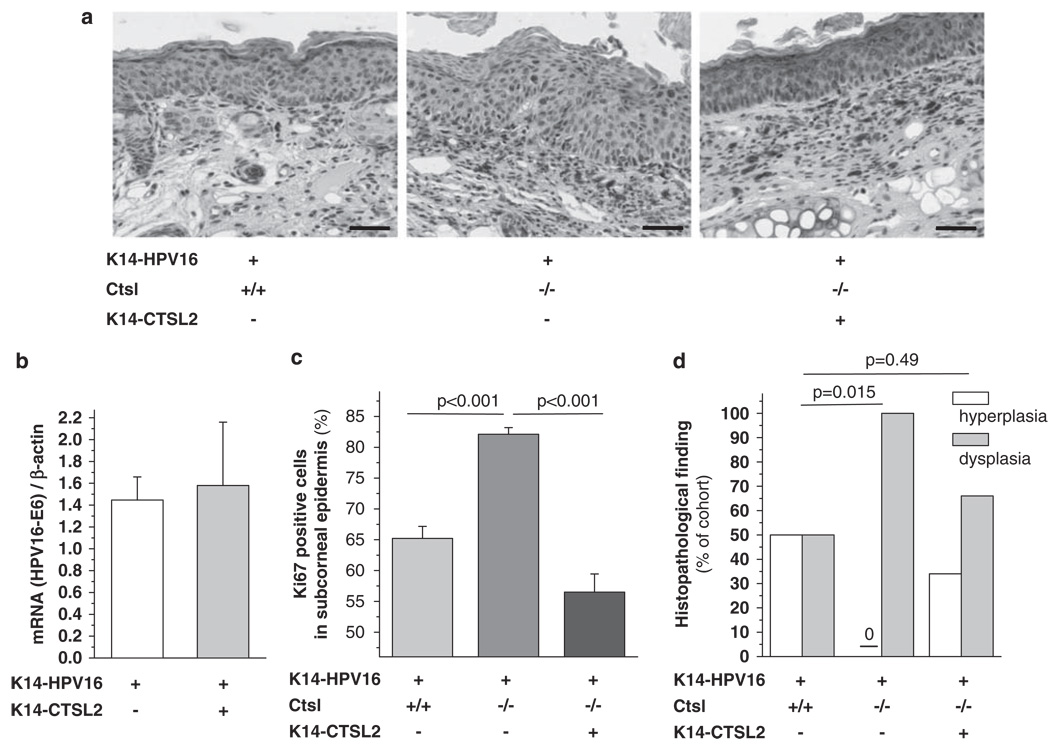
Reversal of aggravated neoplastic progression in HPV16/Ctsl−/− mice by selective restoration of epidermal Ctsl function
As immune cell infiltration and development of angiogenic vasculature were not altered by Ctsl deficiency, we hypothesized that selective re-expression of Ctsl activity in epidermal keratinocytes from HPV16/Ctsl−/− mice might reverse their enhanced malignant phenotype. To address this, we used a transgenic mouse line in which expression of CTSV, the human orthologue of murine Ctsl, is regulated by the human keratin 14 promoter, that is, K14–CTSV (Hagemann et al., 2004), and developed triple mutant HPV16/CTSV/Ctsl−/− mice that show prominent CTSV expression in the epidermis (Figure 4a). There was no difference between HPV16 and HPV16/CTSV mice in the expression of the E6 oncogene from the K14–HPV16 early region (Figure 4b). Thus, the addition of the second K14 promoter construct, that is, K14–CTSV, did not compromise the expression of the K14-controlled HPV16 oncogenes. Nevertheless, keratinocyte proliferation and penetrance of dysplasias in triple mutant HPV16/CTSV/Ctsl−/− mice were reinstated, reaching the characteristic levels observed in control HPV16 mice (Figures 4c and d). Thus, we conclude that epithelial expression of CTSV results in a significant delay of neoplastic progression that is induced by HPV16 oncogenes.
Figure 4.
The reversal of aggravated neoplastic progression in HPV16/Ctsl−/− mice by restoration of epidermal cathepsin L (Ctsl) function. The approach was to restore Ctsl function in Ctsl−/− epidermis by transgenic epithelial expression of human cathepsin V (CTSV; orthologue of mouse Ctsl) under the control of the human keratin 14 (K14) promoter. (a) Immunohistochemical detection of CTSV in a 16-week-old transgenic triple mutant (brown staining; right panel). Bars indicate 50 µm. (b) HPV16 E6 oncogene mRNA expression is not altered by expression of the second K14-promotor transgene (n = 3). (c) Proliferation rates and (d) the frequency of hyperplasia and dysplasia in the epidermis of 16-week-old HPV16/CTSV/Ctsl−/− mice compared with HPV16/Ctsl+/+ and HPV16/Ctsl−/− littermates (n = 10–12). The color reproduction of this figure is available on the html full text version of the manuscript. A full colour version of this figure is available at the Oncogene journal online.
Enhanced activation of oncogenic signal transduction pathways in Ctsl-deficient keratinocytes
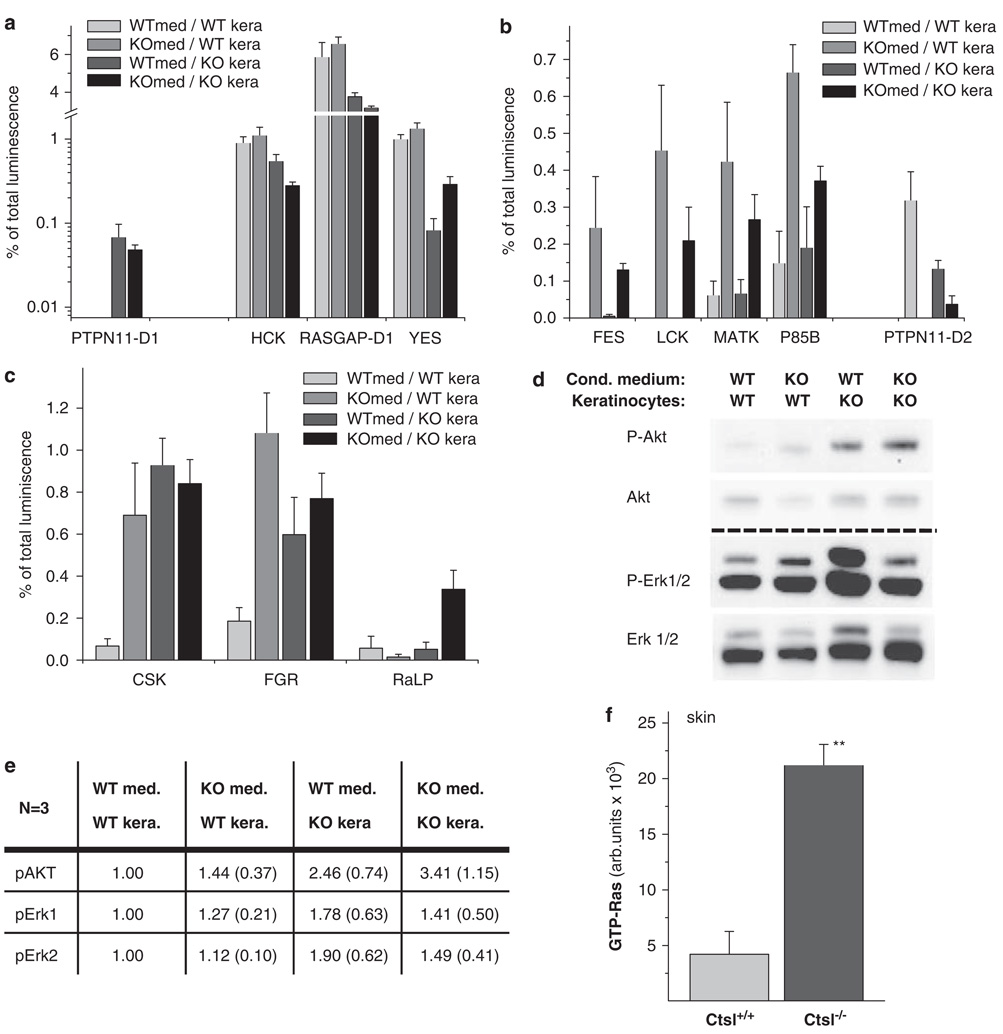
We previously reported increased recycling of growth factors in Ctsl−/− keratinocytes, and showed that proliferation of Ctsl−/− keratinocytes is hyper-responsive to stimulation by epidermal growth factor and keratinocyte-conditioned medium (Reinheckel et al., 2005). In this study, we investigated the effects of conditioned media on relevant oncogenic signal transduction pathways in Ctsl-deficient keratinocytes by analyzing the activation of tyrosine kinase pathways through the interactions of phosphotyrosine-containing proteins with their downstream Src-homology-2 (SH2) domain-containing signal transducers using a protein array containing 38 SH2 domains (Figure 5). Treatment of Ctsl+/+ and Ctsl−/− keratinocytes with cell culture medium conditioned by Ctsl+/+ and Ctsl−/− keratinocytes revealed significantly altered interaction of phosphoproteins with 12 SH2 domains belonging to 11 signaling proteins (interactions of PTPN11 (Shp2) are changed via both SH2 domains). For four of these interactions the Ctsl genotype of the analyzed keratinocytes was the critical variable (Figure 5a); for five SH2 domains the type of conditioned medium (that is, conditioned by Ctsl+/+ or Ctsl−/− keratinocytes) was critical (Figure 5b), whereas three SH2 domains evidenced variable results for the genotype of the keratinocytes and/or conditioned cell culture medium (Figure 5c). We hypothesized that these changes were indicative of tumorigenic tyrosine kinase signaling in Ctsl-deficient keratinocytes, which was enhanced by autocrine effects from the medium conditioned by Ctsl−/− keratinocytes (Figure 5). This conclusion was further supported by the activation of downstream targets of tyrosine kinase signals, including mitogen-activated protein (MAP) kinase Erk and protein kinase B (Akt) (Figure 5d). Quantification of western blots revealed a positive effect for the Ctsl−/−-conditioned cell culture medium as well as the Ctsl−/− genotype of the keratinocytes on Akt activation (Figures 5d and e). Phosphorylated Akt reached its highest levels when Ctsl-deficient keratinocytes were incubated with Ctsl−/−-conditioned cell culture medium, indicating an autocrine amplification loop. The activation of Erk was less pronounced in these experiments. The highest levels of phosphorylated Erk1/2 were detected in Ctsl−/− keratinocytes treated with medium conditioned by Ctsl+/+ keratinocytes. These results were consistent with the activation of MAP kinase signaling in Ctsl−/− keratinocytes. These findings using primary keratinocytes were further tested in vivo by comparing active Ras levels in the epidermis of Ctsl+/+ and Ctsl−/− mice (Figure 5f). The latter group of mice had significantly increased levels of active Ras, providing additional evidence for sustained oncogenic signaling in Ctsl-deficient epidermis (Figure 6).
Figure 5.
Oncogenic signal transduction in cathepsin L (Ctsl) deficient keratinocytes. (a–c) Phosphotyrosine array experiments for the binding of phosphoproteins to 36 src-homology (SH2) domain-containing signal transducers. Epidermal growth factor (EGF)/fetal calf serum (FCS) starved wild-type primary keratinocytes (WTkera) and Ctsl−/− keratinocytes (KOkera) were incubated with cell culture medium conditioned by wild-type keratinocytes (WTmed) or Ctsl−/− keratinocytes (KOmed). Shown are results with a significance level of P < 0.05 for a multiple group comparison test (Kruskal–Wallis, n = 3). (a) The Ctsl genotype of keratinocytes was the critical variable. (b) The type of conditioned medium, whether by Ctsl+/+ or Ctsl−/− keratinocytes, was critical. (c) Mixed results were obtained for the genotypes of keratinocytes and/or conditioned cell culture media. (d) Western blot for phosphorylated Akt (P-Akt), unphosphorylated Akt (Akt), phosphorylated Erk (P-Erk) and unphosphorylated Erk (Erk) in wild-type (WT) and Ctsl−/− keratinocytes (KO) incubated with media conditioned with wild-type or Ctsl−/− keratinocytes. (e) Quantitative measurements of the chemiluminescence from the western blots in (d). Presented are the means (standard error of the mean) calculated from three independent experiments. Data are normalized to the wild-type. (f) Relative levels of active GTP-Ras in the skin of Ctsl−/− mice compared with wild-type littermates (n = 4; **P < 0.01). AKT (PKB, proteinkinase B); CSK (C-SRC tyrosine kinase); ERK (extracellular-signal regulated kinase); FES (proto-oncogene tyrosine-protein kinase FES/FPS (cFES)); FGR (Gardner-Rasheed feline sarcoma viral (v-fgr) oncogene homolog); HCK (hemopoietic cell kinase isoform p61HCK); P85B (phosphoinositide-3-kinase, regulatory subunit, polypeptide 2 (p85 beta)); PTPN11-D1/D2 (protein tyrosine phosphatase, non-receptor type 11, SH2domain #1/#2 (also termed Shp2)); RaLP (Rai-like protein RaLP); RASGAP (RAS p21 protein activator (GTPase activating protein) 1); YES (Yamaguchi sarcoma viral (v-yes) oncogene homolog 1).
Figure 6.
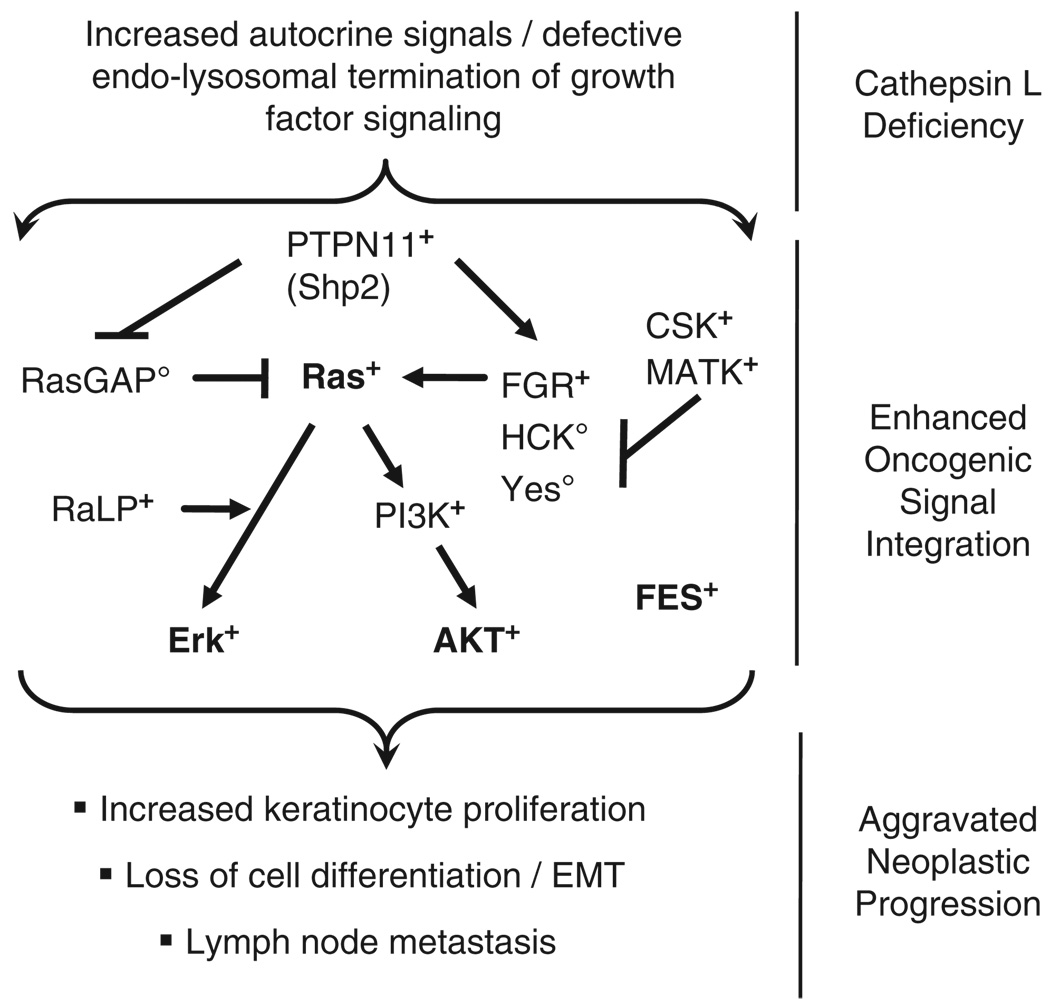
Oncogenic signaling in cathepsin L (Ctsl)-deficient keratinocytes. A Ctsl deficiency in keratinocytes causes the release of as yet unidentified growth factors from keratinocytes, resulting in oncogenic signaling within the keratinocytes (see Figure 5). This appears to be a keratinocyte-specific autocrine process (see Figures 3 and 4), explaining the aggravated neoplastic progression in Ctsl-deficient HPV16 mice (see Figures 1 and 2). +denotes proteins with increased function/activation in Ctsl−/− keratinocytes. ○denotes proteins with reduced activation state in Ctsl−/− keratinocytes. Activation ( ) and inhibition (
) and inhibition ( ) of the signaling pathways. AKT (PKB, proteinkinase B); CSK (C-SRC tyrosine kinase); ERK (extracellular-signal regulated kinase); FES (proto-oncogene tyrosine-protein kinase FES/FPS (cFES)); FGR (Gardner-Rasheed feline sarcoma viral (v-fgr) oncogene homolog); HCK (hemopoietic cell kinase isoform p61HCK); P85B (phosphoinositide-3-kinase, regulatory subunit, polypeptide 2 (p85 beta)); PTPN11-D1/D2 (protein tyrosine phosphatase, non-receptor type 11, SH2domain #1/#2 (also termed Shp2)); RaLP (Rai-like protein RaLP); RASGAP (RAS p21 protein activator (GTPase activating protein) 1); YES (Yamaguchi sarcoma viral (v-yes) oncogene homolog 1).
) of the signaling pathways. AKT (PKB, proteinkinase B); CSK (C-SRC tyrosine kinase); ERK (extracellular-signal regulated kinase); FES (proto-oncogene tyrosine-protein kinase FES/FPS (cFES)); FGR (Gardner-Rasheed feline sarcoma viral (v-fgr) oncogene homolog); HCK (hemopoietic cell kinase isoform p61HCK); P85B (phosphoinositide-3-kinase, regulatory subunit, polypeptide 2 (p85 beta)); PTPN11-D1/D2 (protein tyrosine phosphatase, non-receptor type 11, SH2domain #1/#2 (also termed Shp2)); RaLP (Rai-like protein RaLP); RASGAP (RAS p21 protein activator (GTPase activating protein) 1); YES (Yamaguchi sarcoma viral (v-yes) oncogene homolog 1).
Discussion
Several cysteine cathepsins, such as cathepsin B, have been shown to be pro-tumor proteases (Gondi et al., 2004; Vasiljeva et al., 2006, 2008). Therefore, the inhibition of cathepsins might represent a potential therapeutic option to slow the progression of cancer and metastatic disease (Mohamed and Sloane, 2006). In stark contrast, we found that Ctsl deficiency in a mouse model of de novo squamous carcinogenesis results in the increased proliferation of keratinocytes, accelerated development of dysplasias and the formation of more anaplastic and metastatic SCCs. This result is important when one bears in mind the failure of clinical trials using broad spectrum inhibitors for MMPs (Coussens et al., 2002). In this case, it was only afterward discovered that some of the MMPs, such as MMP8 and MMP9, are in fact anti-tumor proteases when tested in appropriate in vivo models (Coussens et al., 2000; Balbin et al., 2003; Gutierrez-Fernandez et al., 2008). Hence, one major conclusion of the present investigation is that inhibition of the human Ctsl enzymes for therapeutic purposes might be counterproductive. However, a recently developed potent CTSL inhibitor shows 80-fold selectivity over CTSV and other cysteine cathepsins (Bethel et al., 2009). Therefore, differential inhibition of CTSL and CTSV in vivo and a reasonable therapeutic index might be achievable using this new generation CTSL inhibitor.
Despite recent studies suggesting various mechanisms by which Ctsl could affect the inflammatory and angiogenic microenvironment of tumors, no such effect was revealed in the present experiments (Felbor et al., 2000; Abboud-Jarrous et al., 2008). Rather, keratinocyte-specific expression of CTSV in Ctsl−/− mice restored keratinocyte proliferation and the onset of dysplasia development similar to the control K14–HPV16 skin. Thus, the orthologous murine Ctsl and human CTSV keep a check on tumorigenesis in keratinocytes expressing dominant oncogenes. Interestingly, CTSV is induced sixfold transcriptionally in human cutaneous SCC as compared with benign psoriatic hyperplasia (Haider et al., 2006). In light of the present mouse study, it remains to be observed whether this expression signature is indeed correlated with well-differentiated/less invasive SCC.
The in vivo function of Ctsl during cancer development also appears to be determined by organ- and cell-specific conditions. This is because Ctsl deficiency in primary pancreatic islet cell cancers in mice has been shown to reduce primary tumor burden and cancer invasiveness (Gocheva et al., 2006). However, our data on squamous carcinogenesis indicate that a tumor-controlling function is present in mouse epidermis. This is supported by a recent report on the transgenic overexpression of the protease inhibitor hurpin in mouse skin (Walz et al., 2007). Hurpin, a known endogenous inhibitor of Ctsl activity, significantly increased the incidence of benign skin papillomas and adenomas in the course of a two-step 7,12-dimethylbenzanthracene/12-O-tetradecanoylphorbol-13-acetate chemical tumorigenesis experiment. Furthermore, crossing furless mice that harbor an inactivating point mutation in Ctsl to APCmin mice resulted in a significantly increased frequency of non-malignant adenomas in the colons of the double-mutant mice (Boudreau et al., 2007). This finding is consistent with the present findings in the K14–HPV16 model, and suggests an anti-tumor role for Ctsl in epithelial cells other than keratinocytes.
What is the molecular basis of Ctsl in the regulation of keratinocyte proliferation? Ctsl−/− mice present with periodic hair loss and epidermal hyperproliferation (Roth et al., 2000). It is noteworthy that the aberrant occurrence of proliferating cells during hair follicle regression largely contributes to the disturbed hair cycle of Ctsl−/− mice (Tobin et al., 2002). Normal epidermal proliferation and differentiation, as well as squamous carcinoma development, depends on the actions of multiple endocrine growth hormones, and para- and autocrine growth factors (Mueller and Fusenig, 2004; Schneider et al., 2008). A main function of the endosomal/lysosomal compartment is the modulation and termination of signal transduction pathways by dissociating growth factors from their receptors and then subsequently degrading them and/or recycling them to the plasma membrane (von Zastrow and Sorkin, 2007). We reported earlier that the proliferation of Ctsl−/− keratinocytes is hyper-responsive to stimulation by epidermal growth factor (Reinheckel et al., 2005). Tracing the fate of radioactively labeled epidermal growth factor in keratinocytes revealed that Ctsl-deficient cells show enhanced recycling of intact growth factor to the medium and to the plasma membrane. It has been shown that medium conditioned by Ctsl−/− keratinocytes results in higher keratinocyte proliferation than Ctsl+/+-conditioned medium (Reinheckel et al., 2005). Thus, we suggested a model in which enhanced recycling of plasma membrane receptors and their ligands results in the increased proliferation of basal keratinocytes, which in turn leads to epidermal hyperproliferation in Ctsl-deficient mice. This hypothesis was further investigated in this study by analyses of important signal transduction pathways in Ctsl+/+ and Ctsl−/− keratinocytes after exposure to keratinocyte-conditioned media. Profiling the interaction of signal transducers containing SH2 domains with their phosphotyrosine-containing partners revealed activation of a number of positive regulators and downregulation of inhibitors. For example, the cytoplasmic tyrosine phosphatase PTPN11, also known as Shp2, is a proto-oncogene activated in Ctsl−/− keratinocytes. PTPN11 is known to dephosphorylate activators of the RasGTPase activating protein (RasGAP) (Neel et al., 2003). Indeed, our data indicate reduced interaction of the RasGAP-SH2 domain with its phosphotyrosine-bearing activators in Ctsl−/− keratinocytes, resulting in diminished GTP hydrolysis of activated Ras and, thus, sustained Ras activity. On the other hand, PTPN11 activates the Src family of tyrosine kinases, such as FRG, which in turn activate Ras (Chan and Feng, 2007). Therefore, the present investigation of keratinocytes shows Ras activation on Ctsl deficiency. This proposition is supported in vivo by significantly elevated levels of active Ras in the skin of Ctsl−/− mice. Hyperactive Ras is central in many cancer-promoting signaling processes, such as MAP kinase and Akt pathways (Schubbert et al., 2007). Our studies revealed the activation of Akt and MAP-kinase Erk in Ctsl-deficient keratinocytes. Interestingly, Akt was further enhanced by the incubation of the Ctsl−/− cells in medium conditioned by Ctsl-deficient keratinocytes, supporting autocrine Akt stimulation caused by the absence of the protease.
In summary, the complex integration of extracellular signals by intracellular signal transduction cascades, that is, MAP-kinases, Akt and small non-receptor kinases of the CSK-, Fes- and Src-families, indicates a shift toward pronounced oncogenic signaling in Ctsl-deficient keratinocytes. Thus, it provides an explanation for our phenotypic findings in the Ctsl-deficient K14–HPV16 model: increased keratinocyte proliferation, early onset of dysplastic lesions and cancers, poorly differentiated carcinomas with loss of E-cadherin, and an increased frequency of lymph node metastases. As cancer progression caused by the bi-allelic inactivation of a gene product defines a tumor suppressor, we conclude that Ctsl acts as an epidermal tumor suppressor in mice. It is important to note that, whatever specific explanations for the contradicting roles of Ctsl in tumorigenesis and metastasis may be provided by further work in different models and experimental systems, Ctsl is a multifunctional protease whose inhibition has the potential to promote cancer rather than block it. A careful search of possible therapeutic windows for Ctsl inhibition in specific types of cancer using de novo mouse models and human studies is required.
Materials and methods
Animals
The generation of K14–HPV16 (HPV16) mice, Ctsl-deficient (Ctsl−/−) mice and K14–CTSV (CTSV) transgenic mice was as described earlier (Arbeit et al., 1994; Roth et al., 2000; Hagemann et al., 2004). The CTSV mice were generated in the FVB/N genetic background, whereas HPV16 and Ctsl−/− mice were backcrossed to FVB/N for at least 10 generations before intercrossing the mouse lines. The HPV16/Ctsl+/+, HPV16/Ctsl+/− and HPV16/Ctsl−/− mice were obtained by breeding HPV16/Ctsl+/− males with Ctsl+/− females. Further breeding resulted in the triple mutant HPV16/CTSV/Ctsl−/−. All tumor-prone mice were closely inspected from 8 to 52 weeks of age and palpated three times a week to identify the occurrence of tumors. The endpoints of the study were defined by a tumor volume of 1 cm3, an age of 52 weeks, or when the general health of the animal became severely impaired. The animals were kept under specific pathogen-free conditions, fed a standard diet and given free access to water. All animal studies were approved by the government commission for animal protection of the Regierungspräsidium Freiburg (AZ 35-9185.81 G-03/80).
Histology and immunohistology
Paraffin sections of ear skin, back skin, lymph nodes and cancers were stained with hematoxylin/eosin and evaluated for morphology in a blinded setting. Goat anti-human CTSV (0.2 µg/ml; R&D Systems, Minneapolis, MN, USA), anti-Ki67 (1:200 dilution; Dako-Cytomation, Glostrup, Denmark) and anti-mouse neutrophil (clone 7/4, 1:80; Cedarlane, Burlington, ON, Canada) antibodies were used for the detection of CTSV, the proliferation marker Ki67, and neutrophil granulocytes, respectively. Mast cells were visualized by chloroacetate-esterase histochemistry (Leder stain). The detection of E-cadherin by immunofluorescence on paraffin sections was performed as described earlier (Mayerle et al., 2005). The E-cadherin staining pattern was evaluated by two independent observers in a blinded setting, followed by the resolution of any differences by joint review and consultation with a third observer. Antibody staining at the cell membrane or in the cytosol was classified as absent, weak, moderate or strong and assigned a score ranging from 0 to 3 points, respectively. For immunoflourescence analyses of the endothelial marker CD31 and pan-keratin for epithelial cells were visualized by an anti-mouse CD31 (1:100; BD Bioscience, Franklin Lakes, NJ, USA)/anti-rat Cy3 (1:800; Dianova, Hamburg, Germany) and anti-pan-keratin (1:100; Progen, Heidelberg, Germany)/anti-guinea pig-fluorescein isothiocyanate (1:100; Dianova, Hamburg, Germany) double staining procedure.
Flow cytometry
Fresh mouse skin was dissociated in 20 ml Dulbecco’s phosphate-buffered saline containing 1% bovine serum albumin, 0.01 g. DNase I (Sigma, Munich, Germany), 0.1 g collagenase II (Worthington Biochemical Corporation, Lake-wood, NJ, USA) and collagenase IV (Invitrogen, Karlsruhe, Germany) by stirring for 30 min at 37 °C. The solution was passed through a 70 µm cell strainer, and centrifuged at 1200 × g for 5 min at 4 °C. The erythrocytes were lysed, the remaining cells were suspended in DMEM/5% fetal calf serum, and the cell number was adjusted to 0.5 × 106 cells/50 µl. After blocking with anti-mouse monoclonal CD16/CD32 antibody (clone 2.4G2; BD Pharmingen, San Diego, CA, USA) for 10 min, the antibody incubation was performed in a 96-well round bottom plate for 20 min with anti-mouse monoclonal CD31–APC (clone MEC 13.3; BD Pharmingen), anti-mouse monoclonal CD45– fluorescein isothiocyanate (clone 30-F11, RDI, Concord, MA, USA), anti-mouse monoclonal CD4–fluorescein isothiocyanate (clone RM4-5, BD Pharmingen) or anti-mouse monoclonal CD8a–PE (clone 53-6.7, BD Pharmingen). During the flow cytometric analyses, 40 000 living cells were counted. The exclusion of dead cells was achieved by staining with propidium iodide. Appropriate isotype controls were performed for each measurement.
Keratinocyte cell culture and cell lysis
Primary keratinocytes from neonatal mouse epidermis and keratinocyte-conditioned cell culture medium were prepared as described earlier (Reinheckel et al., 2005). Primary keratinocytes were grown to sub-confluency and starved in fetal calf serum-free medium for 6 h. Subsequently, the cells were incubated with conditioned cell culture medium for 10 min. Cells were harvested by scraping in a cell lysis buffer consisting of 20 mm Tris (pH 7.5), 150 mm NaCl, 1 mm ethylene-diaminetetraacetic acid, 1 mm EGTA, 1% Triton X 100, 2.5 mm sodium pyrophosphate, 1 mm β glycerophosphate, 1 mm Na3VO4, protease inhibitors (1 mm Pefabloc SC; 0.1 mm Leupeptin; 1 µm Pepstatin) (Roche, Basel, Switzerland) and phosphatase inhibitor cocktail (Sigma).
Assessment of signal transduction
Total keratinocyte lysates (50 µg protein per lane) were separated on 11% sodium dodecyl sulfate gels and subsequently semi-dry blotted onto PVDF membranes. Incubation with primary antibodies against phospho-Akt and phospho-Erk (1:500, Cell Signaling, Danvers, MA, USA) was performed at room temperature overnight and the incubation with peroxidase goat anti-rabbit secondary antibody (Jackson Immuno Research, West Grove, PA, USA) was at room temperature for 1 h. The membranes were ultraviolet-inactivated for 20 min, washed and incubated with the respective antibodies against the unphosphorylated protein (anti Akt: Cell Signaling; anti-Erk: Santa Cruz Biotechnology, Santa Cruz, CA, USA) before detection with the secondary antibody. A phosphotyrosine profiling array (TranSignal, Panomics, Fremont, CA, USA) was performed with total keratinocyte lysates (protein 1.0 mg/ml) as recommended. GTP-Ras was quantified in skin extracts (70 µg protein) via its binding to the Raf-SH2 domain using the Ras-GTPase Chemi-ELISA (Active Motif, Rixensart, Belgium) following the manufacturer’s instructions. The quantification of luminescence was always achieved with the Lumi-Imager System/Lumi Analyst Software (Roche).
Quantitative real-time PCR measurement of HPV16 E6 oncogene expression
Total RNA was isolated from skin and the mRNA was reverse transcribed into complementary DNA as described earlier (Reinheckel et al., 2005). The primers for quantitative real-time PCR (by MyiQ, BioRad, Hercules, CA, USA) were as follows: E6-1: 5′-AGA ACT GCA ATG TTT CAG GAC CCA CAG-3′, E6-2: 5′-TCT GCA ACA AGA CAT ACA TCG ACC GG-3′, β-actin-1: 5′-ACC CAG GCA TTG CTG ACA GG-3′, β-actin-2: 5′-GGA CAG TGA GGC CAG GAT GG-3′. Cycling conditions: one cycle of 72 °C for 1 min; 50 cycles of 94 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s; and one cycle of 72 °C for 7 min.
Data presentation and statistical analysis
The quantitative data are presented as means with their respective standard errors and analyzed by the Student’s t-test. Proportions were analyzed by χ2 statistics, and tumor incidences were compared by the log-rank test. Multiple group comparisons were carried out by analysis of variance (n > 10) or Kruskal–Wallis (n ≤ 10) testing. P-values ≤0.05 were considered significant.
Acknowledgements
We thank Ulrike Reif and Susanne Dollwet-Mack for excellent technical assistance and Dr Marie Follo for comments on the paper. The work was supported by a grant from the Deutsche Krebshilfe (Re106977) and in part by the Excellence Initiative of the German Federal and State Governments (EXC 294) and the European Union Framework Program (FP7 ‘MICROENVIMET’ No 201279).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Abboud-Jarrous G, Atzmon R, Peretz T, Palermo C, Gadea BB, Joyce JA, et al. Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J Biol Chem. 2008;283:18167–18176. doi: 10.1074/jbc.M801327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeit JM, Munger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol. 1994;68:4358–4368. doi: 10.1128/jvi.68.7.4358-4368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, et al. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- Bernard D, Mehul B, Thomas-Collignon A, Simonetti L, Remy V, Bernard MA, et al. Analysis of proteins with caseinolytic activity in a human stratum corneum extract revealed a yet unidentified cysteine protease and identified the so-called ‘stratum corneum thiol protease’ as cathepsin L2. J Invest Dermatol. 2003;120:592–600. doi: 10.1046/j.1523-1747.2003.12086.x. [DOI] [PubMed] [Google Scholar]
- Bethel PA, Gerhardt S, Jones EV, Kenny PW, Karoutchi GI, Morley AD, et al. Design of selective cathepsin inhibitors. Bioorg Med Chem Lett. 2009;19:4622–4625. doi: 10.1016/j.bmcl.2009.06.090. [DOI] [PubMed] [Google Scholar]
- Boudreau F, Lussier CR, Mongrain S, Darsigny M, Drouin JL, Doyon G, et al. Loss of cathepsin L activity promotes claudin-1 overexpression and intestinal neoplasia. FASEB J. 2007;21:3853–3865. doi: 10.1096/fj.07-8113com. [DOI] [PubMed] [Google Scholar]
- Brömme D. Cathepsin V. In: Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. London: Elsevier; 2004. pp. 1107–1110. [Google Scholar]
- Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109:862–867. doi: 10.1182/blood-2006-07-028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Hitomi K, van Vlijmen-Willems IM, de Jongh GJ, Yamamoto K, Nishi K, et al. Cystatin M/E is a high affinity inhibitor of cathepsin V and cathepsin L by a reactive site that is distinct from the legumain-binding site. A novel clue for the role of cystatin M/E in epidermal cornification. J Biol Chem. 2006;281:15893–15899. doi: 10.1074/jbc.M600694200. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Hanahan D, Arbeit JM. Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am J Pathol. 1996;149:1899–1917. [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, et al. Inflammatory mast cells upregulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Dano K, Romer J, Nielsen BS, Bjorn S, Pyke C, Rygaard J, et al. Cancer invasion and tissue remodeling—cooperation of protease systems and cell types. Apmis. 1999;107:120–127. doi: 10.1111/j.1699-0463.1999.tb01534.x. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- DeClerck YA, Mercurio AM, Stack MS, Chapman HA, Zutter MM, Muschel RJ, et al. Proteases, extracellular matrix, and cancer: a workshop of the path B study section. Am J Pathol. 2004;164:1131–1139. doi: 10.1016/S0002-9440(10)63200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felbor U, Dreier L, Bryant RA, Ploegh HL, Olsen BR, Mothes W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000;19:1187–1194. doi: 10.1093/emboj/19.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, et al. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–556. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondi CS, Lakka SS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, et al. Adenovirus-mediated expression of antisense urokinase plasminogen activator receptor and antisense cathepsin B inhibits tumor growth, invasion, and angiogenesis in gliomas. Cancer Res. 2004;64:4069–4077. doi: 10.1158/0008-5472.CAN-04-1243. [DOI] [PubMed] [Google Scholar]
- Goulet B, Sansregret L, Leduy L, Bogyo M, Weber E, Chauhan SS, et al. Increased expression and activity of nuclear cathepsin L in cancer cells suggests a novel mechanism of cell transformation. Mol Cancer Res. 2007;5:899–907. doi: 10.1158/1541-7786.MCR-07-0160. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Fernandez A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008;68:2755–2763. doi: 10.1158/0008-5472.CAN-07-5154. [DOI] [PubMed] [Google Scholar]
- Hagemann S, Gunther T, Dennemarker J, Lohmuller T, Bromme D, Schule R, et al. The human cysteine protease cathepsin V can compensate for murine cathepsin L in mouse epidermis and hair follicles. Eur J Cell Biol. 2004;83:775–780. doi: 10.1078/0171-9335-00404. [DOI] [PubMed] [Google Scholar]
- Haider AS, Peters SB, Kaporis H, Cardinale I, Fei J, Ott J, et al. Genomic analysis defines a cancer-specific gene expression signature for human squamous cell carcinoma and distinguishes malignant hyperproliferation from benign hyperplasia. J Invest Dermatol. 2006;126:869–881. doi: 10.1038/sj.jid.5700157. [DOI] [PubMed] [Google Scholar]
- Jedeszko C, Sloane BF. Cysteine cathepsins in human cancer. Biol Chem. 2004;385:1017–1027. doi: 10.1515/BC.2004.132. [DOI] [PubMed] [Google Scholar]
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–724. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- Mayerle J, Schnekenburger J, Kruger B, Kellermann J, Ruthenburger M, Weiss FU, et al. Extracellular cleavage of E-cadherin by leukocyte elastase during acute experimental pancreatitis in rats. Gastroenterology. 2005;129:1251–1267. doi: 10.1053/j.gastro.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Navab R, Pedraza C, Fallavollita L, Wang N, Chevet E, Auguste P, et al. Loss of responsiveness to IGF-I in cells with reduced cathepsin L expression levels. Oncogene. 2008;27:4973–4985. doi: 10.1038/onc.2008.144. [DOI] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci. 2008;13:6537–6553. doi: 10.2741/3173. [DOI] [PubMed] [Google Scholar]
- Reinheckel T, Hagemann S, Dollwet-Mack S, Martinez E, Lohmuller T, Zlatkovic G, et al. The lysosomal cysteine protease cathepsin L regulates keratinocyte proliferation by control of growth factor recycling. J Cell Sci. 2005;118:3387–3395. doi: 10.1242/jcs.02469. [DOI] [PubMed] [Google Scholar]
- Roth W, Deussing J, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A, et al. Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J. 2000;14:2075–2086. doi: 10.1096/fj.99-0970com. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Werner S, Paus R, Wolf E. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol. 2008;173:14–24. doi: 10.2353/ajpath.2008.070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G. Hyperactive ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, Foitzik K, Reinheckel T, Mecklenburg L, Botchkarev VA, Peters C, et al. The lysosomal protease cathepsin L is an important regulator of keratinocyte and melanocyte differentiation during hair follicle morphogenesis and cycling. Am J Pathol. 2002;160:1807–1821. doi: 10.1016/S0002-9440(10)61127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa E, Li W, Yasuda Y, Wienhold W, Denzin LK, Lautwein A, et al. Cathepsin V is involved in the degradation of invariant chain in human thymus and is overexpressed in myasthenia gravis. J Clin Invest. 2003;112:517–526. doi: 10.1172/JCI18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR, et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;11:206–213. doi: 10.1038/nm1182. [DOI] [PubMed] [Google Scholar]
- Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, et al. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66:5242–5250. doi: 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- Vasiljeva O, Reinheckel T, Peters C, Turk D, Turk V, Turk B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr Pharm Des. 2007;13:387–403. doi: 10.2174/138161207780162962. [DOI] [PubMed] [Google Scholar]
- Vasiljeva O, Korovin M, Gajda M, Brodoefel H, Bojic L, Kruger A, et al. Reduced tumour cell proliferation and delayed development of high-grade mammary carcinomas in cathepsin B-deficient mice. Oncogene. 2008;27:4191–4199. doi: 10.1038/onc.2008.59. [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz M, Kellermann S, Bylaite M, Andree B, Ruther U, Paus R, et al. Expression of the human cathepsin L inhibitor hurpin in mice: skin alterations and increased carcinogenesis. Exp Dermatol. 2007;16:715–723. doi: 10.1111/j.1600-0625.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- Zheng X, Chou PM, Mirkin BL, Rebbaa A. Senescenceinitiated reversal of drug resistance: specific role of cathepsin L. Cancer Res. 2004;64:1773–1780. doi: 10.1158/0008-5472.can-03-0820. [DOI] [PubMed] [Google Scholar]