Abstract
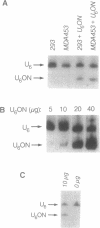
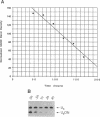
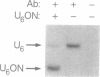
Antisense and triplex oligonucleotides continue to demonstrate potential as mediators of gene-specific repression of protein synthesis. However, inefficient and heterogeneous cellular uptake, intracellular sequestration, and rapid intracellular and extracellular degradation represent obstacles to their eventual clinical utility. Efficient cellular delivery of targeted ribozymes can present similar problems. In this report we describe a system for circumventing these obstacles and producing large quantities of short, sequence-specific RNA oligonucleotides for use in these gene regulation strategies. The oligonucleotides are generated from a vector containing promoter, capping, and termination sequences from the human small nuclear U6 gene, surrounding a synthetic sequence incorporating the oligonucleotide of interest. In vivo, these oligonucleotides are produced constitutively and without cell type specificity in levels up to 5 x 10(6) copies per cell, reach steady-state levels of expression within 9 hours post-transfection, and are still readily detectable 7 days post-transfection. In addition, these oligonucleotides are retained in the nucleus, obtain a 5' gamma-monomethyl phosphate cap, and have an intracellular half-life of approximately one hour. This expression vector provides a novel and efficient method of intracellular delivery of antisense or triplex RNA oligonucleotides (and/or ribozymes) for gene regulation, as well as a cost-effective means of comparing the biological activity arising from a variety of different potential oligonucleotide sequences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen J. S. Antisense oligodeoxynucleotides as antiviral agents. Antiviral Res. 1991 Sep;16(2):121–133. doi: 10.1016/0166-3542(91)90019-n. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gupta S., Busch R. K., Singh R., Reddy R. Characterization of U6 small nuclear RNA cap-specific antibodies. Identification of gamma-monomethyl-GTP cap structure in 7SK and several other human small RNAs. J Biol Chem. 1990 Nov 5;265(31):19137–19142. [PubMed] [Google Scholar]
- Krieg A. M., Gmelig-Meyling F., Gourley M. F., Kisch W. J., Chrisey L. A., Steinberg A. D. Uptake of oligodeoxyribonucleotides by lymphoid cells is heterogeneous and inducible. Antisense Res Dev. 1991 Summer;1(2):161–171. doi: 10.1089/ard.1991.1.161. [DOI] [PubMed] [Google Scholar]
- Kunkel G. R., Maser R. L., Calvet J. P., Pederson T. U6 small nuclear RNA is transcribed by RNA polymerase III. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8575–8579. doi: 10.1073/pnas.83.22.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel G. R., Pederson T. Transcription of a human U6 small nuclear RNA gene in vivo withstands deletion of intragenic sequences but not of an upstream TATATA box. Nucleic Acids Res. 1989 Sep 25;17(18):7371–7379. doi: 10.1093/nar/17.18.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Martinez H. M. Detecting pseudoknots and other local base-pairing structures in RNA sequences. Methods Enzymol. 1990;183:306–317. doi: 10.1016/0076-6879(90)83020-a. [DOI] [PubMed] [Google Scholar]
- McShan W. M., Rossen R. D., Laughter A. H., Trial J., Kessler D. J., Zendegui J. G., Hogan M. E., Orson F. M. Inhibition of transcription of HIV-1 in infected human cells by oligodeoxynucleotides designed to form DNA triple helices. J Biol Chem. 1992 Mar 15;267(8):5712–5721. [PubMed] [Google Scholar]
- Nishikura K. Modulation of double-stranded RNAs in vivo by RNA duplex unwindase. Ann N Y Acad Sci. 1992 Oct 28;660:240–250. doi: 10.1111/j.1749-6632.1992.tb21076.x. [DOI] [PubMed] [Google Scholar]
- Noonberg S. B., Garovoy M. R., Hunt C. A. Characteristics of oligonucleotide uptake in human keratinocyte cultures. J Invest Dermatol. 1993 Nov;101(5):727–731. doi: 10.1111/1523-1747.ep12371683. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Flint S. J., Kessler D. J., Hogan M. E. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8227–8231. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Henning D., Das G., Harless M., Wright D. The capped U6 small nuclear RNA is transcribed by RNA polymerase III. J Biol Chem. 1987 Jan 5;262(1):75–81. [PubMed] [Google Scholar]
- Roberts R. W., Crothers D. M. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science. 1992 Nov 27;258(5087):1463–1466. doi: 10.1126/science.1279808. [DOI] [PubMed] [Google Scholar]
- Sauterer R. A., Feeney R. J., Zieve G. W. Cytoplasmic assembly of snRNP particles from stored proteins and newly transcribed snRNA's in L929 mouse fibroblasts. Exp Cell Res. 1988 Jun;176(2):344–359. doi: 10.1016/0014-4827(88)90336-9. [DOI] [PubMed] [Google Scholar]
- Shumyatsky G., Wright D., Reddy R. Methylphosphate cap structure increases the stability of 7SK, B2 and U6 small RNAs in Xenopus oocytes. Nucleic Acids Res. 1993 Oct 11;21(20):4756–4761. doi: 10.1093/nar/21.20.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Singh R., Gupta S., Reddy R. Capping of mammalian U6 small nuclear RNA in vitro is directed by a conserved stem-loop and AUAUAC sequence: conversion of a noncapped RNA into a capped RNA. Mol Cell Biol. 1990 Mar;10(3):939–946. doi: 10.1128/mcb.10.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terns M. P., Dahlberg J. E., Lund E. Multiple cis-acting signals for export of pre-U1 snRNA from the nucleus. Genes Dev. 1993 Oct;7(10):1898–1908. doi: 10.1101/gad.7.10.1898. [DOI] [PubMed] [Google Scholar]
- Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986 Sep;13(2):97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]