Abstract
Nitrogen is a key regulator of primary productivity in many terrestrial ecosystems. Historically, only inorganic N (NH4 + and NO3 -) and L-amino acids have been considered to be important to the N nutrition of terrestrial plants. However, amino acids are also present in soil as small peptides and in D-enantiomeric form. We compared the uptake and assimilation of N as free amino acid and short homopeptide in both L- and D-enantiomeric forms. Sterile roots of wheat (Triticum aestivum L.) plants were exposed to solutions containing either 14C-labelled L-alanine, D-alanine, L-trialanine or D-trialanine at a concentration likely to be found in soil solution (10 µM). Over 5 h, plants took up L-alanine, D-alanine and L-trialanine at rates of 0.9±0.3, 0.3±0.06 and 0.3±0.04 µmol g−1 root DW h−1, respectively. The rate of N uptake as L-trialanine was the same as that as L-alanine. Plants lost ca.60% of amino acid C taken up in respiration, regardless of the enantiomeric form, but more (ca.80%) of the L-trialanine C than amino acid C was respired. When supplied in solutions of mixed N form, N uptake as D-alanine was ca.5-fold faster than as NO3 -, but slower than as L-alanine, L-trialanine and NH4 +. Plants showed a limited capacity to take up D-trialanine (0.04±0.03 µmol g−1 root DW h−1), but did not appear to be able to metabolise it. We conclude that wheat is able to utilise L-peptide and D-amino acid N at rates comparable to those of N forms of acknowledged importance, namely L-amino acids and inorganic N. This is true even when solutes are supplied at realistic soil concentrations and when other forms of N are available. We suggest that it may be necessary to reconsider which forms of soil N are important in the terrestrial N cycle.
Introduction
Nitrogen is a key factor in the control of carbon fixation by photosynthetic primary producers [1], [2]. Historically, higher plants were thought to be dependent on inorganic N (NH4 + and NO3 -) for all of their N requirements. However, in the absence of human inputs of synthetic inorganic N, most N enters soil as protein, and this remains the dominant form of soil organic N [3]–[5]. Consequently, plant productivity in N-limited ecosystems was thought to be controlled by the rate of microbial mineralization of organic N to inorganic N. In the 1990s our understanding of the regulation of plant productivity was revolutionised by the demonstration of a “short-circuit” in the N cycle. Plants were shown to take up L-enantiomers of amino acids [6], [7] with productivity being limited by the rate of microbial protein/peptide cleavage to amino acids. The importance of L-amino acids to the N cycle has subsequently received a great deal of interest [7]. However, soil soluble N is as abundant as small peptides (<1 kDa MW) as it is as free amino acids (Table 1) [8], [9]. Despite the identification of peptide transporters in various plant tissues including roots, there has been surprisingly little consideration of the nutritional and ecological significance of plants competing for N at an earlier stage of protein cleavage than free amino acids [9]–[13].
Table 1. Concentrations of inorganic, amino acid and peptide N in the soil solution of a UK agricultural soila.
| N concentration (µmol N l−1) | |
| Total dissolved N | 844±30 |
| Total dissolved N <1 kDa | 746±46 |
| Peptidic-N <1 kDa | 31±2 |
| Free amino acid N | 4±0.9 |
| NH4 + | 16±4 |
| NO3 - | 655±38 |
Values are mean ± SEM; n = 4.
Short peptides of D-amino acids are essential components of bacterial peptidoglycan and some D-amino acids exist in soil organic matter at 10 to 20% of the concentration of L-enantiomers [14], [15]. There is some existing evidence that plants are able to metabolise D-amino acids, and D-amino acids and amino acid racemases have been reported in plants [16]–[20]. Nevertheless, some reports of phytotoxic effects of certain D-amino acids (e.g. D-serine), when supplied at high concentrations relative to those in soil, have resulted in D-amino acids being discounted as important plant N resources [7], [16], [21], [22]. D-peptides have been reported in plant tissues [19], [20], but very little information exists on the capacity of plants to take up and assimilate them through their roots [23].
We conducted a straightforward test of the effect of polymeric and enantiomeric form on the uptake and assimilation of amino acid N supplied to a higher plant in the absence of mycorrhizal symbionts. We directly compared D- and L-forms of the same amino acid, and the D- and L-forms of their corresponding tripeptides, to test the hypothesis that non-symbiotic higher plants are able to take up and assimilate amino acids and small peptides supplied at the low concentrations likely to be present in soil solution, irrespective of entantiomeric form. We further compared rates of uptake of these organic forms of N with those of inorganic forms of N. As a conservative test of organic N use, we chose an agricultural plant, wheat, which has been bred to grow with high inputs of synthetic inorganic N. As the amino acid monomer, we chose alanine which is common in all kingdoms of organisms as an individual amino acid and short homopeptides, and in soil as both L- and D-enantiomers [14], [15], [24].
Results and Discussion
Over 5 h, sterile roots of wheat took up 14C-labelled L-alanine, D-alanine and L-trialanine at rates of 0.9±0.3, 0.3±0.06, and 0.3±0.04 µmol g−1 DW root h−1, respectively (mean ± SEM; n = 3) from a 10 µM solution reflecting realistic soil solution concentrations. There was no difference in the rate of N uptake as L-trialanine and that as L-alanine (Fig. 1). Plants took up 80 to 90% less (P<0.05) D-peptide than other forms of organic N. D-trialanine was taken up at a rate of only 0.04±0.03 µmol g−1 DW root h−1. Recovery of plant 14C by combustion revealed that 14C was translocated and 66±5, 58±5 and 83±4% (L-alanine, D-alanine and L-trialanine, respectively) of substrate 14C removed from solution was lost in respiration (not recovered in plant tissues). The 14C recovered in plants exposed to D-trialanine was the same as that removed from solution and a much higher (P<0.001) proportion of D-trialanine 14C was recovered in the shoot than in the root in comparison to other substrates. Although possibly not accurately representing the partitioning of N, the ratio of 14C recovered in the root to 14C recovered in the shoot was 6.0±2, 4.6±0.4, 5.7±1.6 and 0.5±0.01 for D-alanine, L-alanine, L-trialanine and D-trialanine, respectively. This indicates that plants took up and assimilated L- and D-amino acids and L-peptide, but were unable to assimilate even the small quantity of D-peptide taken up. The ca.20-fold difference between L-alanine uptake and the uptake of D-trialanine is consistent with the previously reported 20-fold difference found in uptake of amino acids between control plants and those treated with protonophores e.g. CCCP [25]. Consequently, we suggest that uptake of D-trialanine was by passive uptake alone.
Figure 1. Uptake of peptide or amino acid N by sterile roots of wheat.
Uptake determined over 5 h from the depletion of 14C from 10 µM solutions of single N forms. Values are mean ± SEM; n = 3.
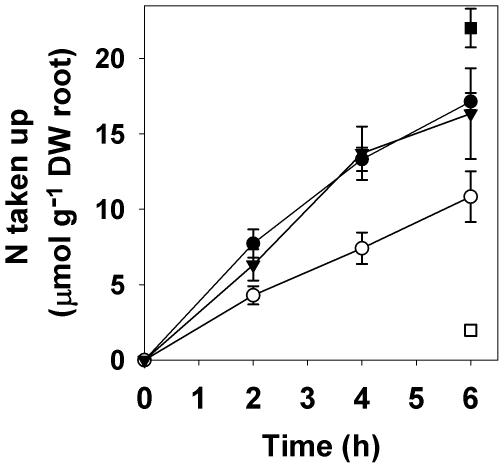
When other forms of N were available to plants in an equimolar solution containing five forms of N (L-alanine, D-alanine, L-trialanine, KNO3 and NH4Cl), N was taken up as the D-amino acid monomer at a five-fold higher (P = 0.004; Fig. 2) rate than NO3 -. Uptake of N as D-alanine was, however, 37% slower (P≤0.04) than as L-alanine, which was taken up at the same rate as L-trialanine N and NH4 +. Rates of metabolism of L-peptide and L- and D-amino acids, as determined from losses of 14C in respiration, were the same when acquired from the mixed solution as when N forms were supplied individually. In both cases, the proportion of the 14C taken up which was respired by plants was greatest (P≤0.03) when supplied as L-trialanine. This ca.25% increase in post-uptake metabolism between peptides and their amino acid monomers strongly suggests that there was no extracellular cleavage of peptides prior to uptake.
Figure 2. Uptake of N by sterile roots of wheat from a mixed N form solution.
Uptake determined by solution 14C depletion (organic N) or 15N recovery in plants (inorganic N). L-alanine •, D-alanine ○, L-trialanine ▾, NO3 - □, NH4 + ▪. Values are mean ± SEM; n = 3.
Organic N uptake has been identified as important in natural habitats [6], [7], [9], [26], [27]. However, our results show that even plants such as wheat, bred to grow with high inorganic N additions, can take up and assimilate peptide N at a rate comparable to those of N forms of known importance for plant nutrition, namely L-amino acid and NH4 +, and greatly exceeding that of NO3 -. This is true even when peptides are supplied at low soil concentrations and when other forms of N are available to the plant. The concentration of solutes in soil is maintained by the balance between their input or production, and their consumption by soil microorganisms and plants. Consequently, successful root uptake and assimilation of peptides when supplied at the low concentrations maintained in soil, strongly suggests that plants are capable of competing with soil microorganisms for N at an early stage of protein decomposition. Thus, the rate-limiting step in N-limited plant productivity may be the rate of protein cleavage to short peptides rather than the rate of protein/peptide cleavage to free amino acids or the rate of microbial mineralisation of amino acids to inorganic N. There is some evidence that plants may be able to take up intact protein through their roots, but quantities appear to be very low [28]. Consequently, uptake of peptides very likely represents the uppermost level of plant competition with soil microbes for N resources.
Plants are apparently unable to utilise D-peptide N, assuming D-trialanine and wheat are representative. However, our data show that they are clearly able to take up and assimilate D-alanine when supplied at soil solution concentrations and do so in preference to NO3 -. As D-amino acids, such as D-alanine, are common in bacteria and in soil, we suggest that they may be more important as a source of N to plants than has previously been recognised. We further suggest that the often relatively high concentrations of NO3 - in soil solution [29] (Table 1) may not reflect its importance to plants as a large pool of available N, but rather the preference of plants for other forms of N, which leads to slower depletion of the soil NO3 - pool.
These findings indicate that plants can acquire and metabolise N in forms that are not currently considered to be of importance for plant nutrition, and at an earlier stage in the N cycle than previously thought. Further, such early uptake of more complex soil N by plants must necessarily affect the availability of substrate for downstream microbial N transformations and the flux of N through soil pools. There are many possible variations in peptide composition, and much further work is necessary to fully elucidate the relative importance of the various forms of soil N available to plants. Nevertheless, we suggest that it may be necessary to reconsider current assumptions concerning the fundamental pattern of N flow in the plant-microbe-soil continuum.
Materials and Methods
Soil solution characterisation
Agricultural soil was collected from a depth of 0–10 cm in four locations at Bangor University's Henfaes Research Station (53° 14′N, 4° 01′W). Background soil characteristics are given in [30]. Soil solution was extracted by centrifugal drainage [31], sterilised by filtration to 0.2 µm and passed through a 1 kDa ultrafiltration membrane (Millipore, Billerica, MA, USA). Amino acid N was measured fluorometrically according to [32] before and after hydrolysis in 6 M HCl at 105°C for 16 h under N2. Total dissolved N was measured in a TOC-V-TN analyzer (Shimadzu, Kyoto, Japan). Nitrate and ammonium were measured colorimetrically according to [33] and [34], respectively.
Uptake from solutions of single N forms
Seeds of wheat (Triticum aestivum L. cv. Claire) were surface sterilised in 10% NaClO followed by 80% ethanol, and grown in Phytatrays (Sigma Aldrich, Gillingham, UK) on 10% Murashige and Skoog agar in natural light. At the third leaf stage, roots of single plants (n = 3) were placed in 4 ml of sterile (0.2 µm-filtered) solutions of either 10 µM, ca.1.5 kBq U-14C-labelled, L-alanine (C3H7NO2), D-alanine, L-trialanine (C9H17N3O4), or D-trialanine (unlabelled from Bachem, Bubendorf, Switzerland; labelled from American Radiolabeled Chemicals, St Louis, MA, USA). All operations were carried out aseptically in a laminar flow cabinet at ca.25°C and a light intensity of 170 µmol photons m-2 s-1 PAR. After 5 h, plants were washed in deionised water for ca.1.5 min and the remaining 14C activity of solutions was measured by liquid scintillation counting in a Wallac 1404 scintillation counter (Perkin-Elmer, Boston, MA, USA). Plants were dried at 80°C, before combustion in an OX400 biological oxidizer (RJ Harvey, Hillsdale, NJ, USA). Liberated 14CO2 was captured in Oxosol scintillant (National Diagnostics, Atlanta, GA, USA) and measured by liquid scintillation counting.
Uptake from solutions of mixed N-forms
Plant roots were placed in 4.5 ml of a mixed N form solution of L-alanine, D-alanine, L-trialanine, NH4Cl and KNO3. Each of 3 replicates had one N form labelled with either ca.4 kBq 14C (peptide and amino acids) or 98 atom % 15N (NH4 + and NO3 -; Sigma Aldrich, Gillingham, UK). In this case, substrates were all supplied at a concentration of 50 µM to ensure that sufficient 15N for accurate measurement could be recovered in plants. Aliquots of 50 µL were removed after 2, 4 and 6 h and 14C activity measured by liquid scintillation counting where appropriate. After 6 h plants were washed for ca.2 min in 0.1 M CaCl2. The 14C activity of washings was measured. Plants were dried and combusted in the biological oxidizer or ground and analyzed for 15N in a Eurovector EA-Isoprime IRMS (Eurovector SpA, Milan, Italy) as appropriate. All methods and conditions were as described for uptake from solutions of single N-form, except where stated.
Statistical analysis
All statistical analysis by one-way ANOVA with LSD post-hoc test (SPSS v14, SPSS Inc, Chicago, USA).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by UK Natural Environment Research Council grant AFI 8/08. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry. 1991;13:87–115. [Google Scholar]
- 2.Liu LL, Greaver TL. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett. 2010;13:819–828. doi: 10.1111/j.1461-0248.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson FJ. Agronomy no. 22. Madison, USA: Soil Science Society of America Inc; 1982. Nitrogen in Agricultural Soils. [Google Scholar]
- 4.Knicker H, Schmidt WI, Kögel-Knabner I. Nature of organic nitrogen in fine particle size separates of sandy soils of highly industrialised areas as revealed by NMR spectroscopy. Soil Biol Biochem. 2000;32:241–252. [Google Scholar]
- 5.Jan M T, Roberts P, Tonheim SK, Jones DL. Protein breakdown represents a major bottleneck in nitrogen cycling in grassland soils. Soil Biol Biochem. 2009;41:2272–2282. [Google Scholar]
- 6.Chapin FS, Moilanen L, Kielland K. Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature. 1993;361:150–153. [Google Scholar]
- 7.Näsholm T, Kielland K, Ganeteg U. Uptake of organic nitrogen by plants. New Phytol. 2009;182:31–48. doi: 10.1111/j.1469-8137.2008.02751.x. [DOI] [PubMed] [Google Scholar]
- 8.Farrell M, Hill PW, Farrar J, Bardgett RD, Jones DL. Seasonal variation in soluble soil carbon and nitrogen across a grassland productivity gradient. Soil Biol Biochem. 2011;43:835–844. [Google Scholar]
- 9.Hill PW, Farrar J, Roberts P, Farrell M, Grant H, et al. Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nature Clim Change. 2011;1:50–53. [Google Scholar]
- 10.Steiner H-Y, Song W, Zhang L, Naider F, Becker JM, et al. An Arabidopsis peptide transporter is a member of a new class of membrane transport proteins. Plant Cell. 1994;6:1289–1299. doi: 10.1105/tpc.6.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waterworth WM, Bray CM. Enigma variations for peptides and their transporters in higher plants. Ann Bot London. 2006;98:1–8. doi: 10.1093/aob/mcl099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komarova NY, Thor K, Gubler A, Meier S, Dietrich D, et al. AtPTR1 and AtPTR5 transport dipeptides in planta. Plant Physiol. 2008;148:856–869. doi: 10.1104/pp.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paungfoo-Lonhienne C, Schenk PM, Lonhienne TGA, Brackin R, Meier S, et al. Nitrogen affects cluster root formation and expression of putative peptide transporters. J Exp Bot. 2009;60:2665–2676. doi: 10.1093/jxb/erp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amelung W. Nitrogen biomarkers and their fate in soil. J Plant Nutr Soil Sc. 2003;166:677–686. [Google Scholar]
- 15.Amelung W, Zhang X, Flach KW. Amino acids in grassland soils: climatic effects on concentrations and chirality. Geoderma. 2006;130:207–217. [Google Scholar]
- 16.Manabe H, Ohira K. Effects of D- and L-alanine on the growth of suspension-cultured rice, soybean and tobacco cells. Soil Sci Plant Nutr. 1981;27:383–386. [Google Scholar]
- 17.Gördes D, Kolukisaoglu Ü, Thurow K. Uptake and conversion of D-amino acids in Arabidopsis thaliana. Amino Acids. 2011;40:553–563. doi: 10.1007/s00726-010-0674-4. [DOI] [PubMed] [Google Scholar]
- 18.Ono K, Yanagida K, Oikawa T, Ogawa T, Soda K. Alanine racemase of alfalfa seedlings (Medicago sativa L.): first evidence for the presence of an amino acid racemase in plants. Phytochemistry. 2006;67:856–860. doi: 10.1016/j.phytochem.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Frahn JL, Illman RJ. The occurrence of D-alanine and D-alanyl-D-alanine in Phalaris tuberosa. Phytochemistry. 1975;14:1464–1465. [Google Scholar]
- 20.Manabe H. Occurrence of D-alanyl-D-alanine in Oryza australiensis. Agr Biol Chem Tokyo. 1985;49:1203–1204. [Google Scholar]
- 21.Erikson O, Hertzberg M, Näsholm T. The dsdA gene from Escherichia coli provides a novel selectable marker for plant transformation. Plant Mol Biol. 2005;57:425–433. doi: 10.1007/s11103-004-7902-9. [DOI] [PubMed] [Google Scholar]
- 22.Forsum O, Svennerstam H, Ganateg U, Näsholm T. Capacities and constraints of amino acid utilization in Arabidopsis. New Phytol. 2008;179:1058–1069. doi: 10.1111/j.1469-8137.2008.02546.x. [DOI] [PubMed] [Google Scholar]
- 23.Bollard EG. A comparative study of the ability of organic nitrogenous compounds to serve as sole sources of nitrogen for the growth of plants. Plant Soil. 1966;25:153–166. [Google Scholar]
- 24.O'Dowd RW, Parsons R, Hopkins DW. Soil respiration induced by the D- and L-isomers of a range of amino acids. Soil Biol Biochem. 1997;29:1665–1671. [Google Scholar]
- 25.Jones DL, Darrah PR. Amino-acid influx at the soil-root interface of Zea mays L. and its implications in the rhizosphere. Plant Soil. 1994;163:1–12. [Google Scholar]
- 26.Schimel JP, Bennett J. Nitrogen mineralization: challenges of a changing paradigm. Ecology. 2004;85:591–602. [Google Scholar]
- 27.Weigelt A, Bol R, Bardgett RD. Preferential uptake of soil nitrogen forms by grassland plant species. Oecologia. 2005;142:627–635. doi: 10.1007/s00442-004-1765-2. [DOI] [PubMed] [Google Scholar]
- 28.Paungfoo-Lonhienne C, Lonhienne TGA, Rentsch D, Robinson N, Christie M, et al. Plants can use protein as a nitrogen source without assistance from other organisms. Proc Natl Acad Sci USA. 2008;105:4524–4529. doi: 10.1073/pnas.0712078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones DL, Willett VB. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem. 2006;38:991–999. [Google Scholar]
- 30.Hill PW, Farrar JF, Jones DL. Decoupling of microbial glucose uptake and mineralization in soil. Soil Biol Biochem. 2008;40:616–624. [Google Scholar]
- 31.Giesler R, Lundström US. Soil solution chemistry – the effects of bulking soil samples and spatial variation. Soil Sci Soc Am J. 1993;57:1283–1288. [Google Scholar]
- 32.Jones DL, Owen AG, Farrar JF. Simple method to enable the high resolution determination of total free amino acids in soil solutions and soil extracts. Soil Biol Biochem. 2002;34:1893–1902. [Google Scholar]
- 33.Miranda KM, Espey MG, Wink DA. A rapid, simple, spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 34.Mulvaney RL. Nitrogen—Inorganic forms. In: Sparks DL, editor. Methods of Soil Analysis. Part 3. Madison, USA: Soil Science Society of America Inc; 1996. pp. 1123–1184. [Google Scholar]