Abstract
Linkage to HIV care and survival in sub-Saharan Africa is not well documented. In 2004 we conducted a randomized trial among medical inpatients in Mulago Hospital to assess the impact of HIV counseling and testing (HCT) on linkage to care and survival. Participants were randomized to inpatient HCT (intervention) or outpatient HCT 1 week post-discharge (control); inpatient HCT was not available at Mulago during the study. Among 590 eligible patients, 85% (500) agreed to participate; 98.8% (248) in the intervention arm received HCT compared to 68.7% (171) in the control arm. Within 6 months, 62.2% (92) of surviving HIV-infected participants received HIV care; 15.0% (20) received antiretroviral medications (ARVs). Overall mortality among HIV-infected participants was 34.6% (72). HCT had significant impact on linkage to care among surviving participants. Referral for HCT was a missed opportunity for diagnosis. There is need for earlier diagnosis and linkage to HIV care among inpatients.
Keywords: Provider Initiated HIV Testing and Counseling (PITC), Inpatient, Access to care, Survival, Africa
Introduction
HIV counseling and testing (HCT) reduces HIV transmission risk behavior especially among individuals diagnosed with HIV and within serodiscordant couples [1–4]. HCT is also a gateway to HIV care and treatment. However, access to HCT remains limited as over 90% of HIV infected individuals worldwide are unaware of their HIV status [5]. Available testing fails to reach many of those who develop life-threatening complications requiring hospitalization. Provision of confidential HIV testing among hospitalized patients in Africa has been demonstrated to increase the number of identified HIV infections [6–9]. Since the beginning of the HIV epidemic in Uganda, HIV/AIDS accounts for about 50% of medical ward beds [10, 11]. However, access to HIV testing in hospital settings remains limited [12].
To scale-up access to HIV testing, provider initiated HIV counseling and testing (PITC) has been adopted [13–15]. Because of the high HIV prevalence in the hospital population, hospital based HCT could be an efficient means of identifying and linking severely ill individuals to medical care including life saving antiretroviral drugs (ARVs). Studies have shown that many hospitalized HIV infected patients have advanced disease and are in urgent need of antiretroviral therapy [16]. It is however not known what proportion of patients diagnosed with HIV in the hospitals access care subsequent to discharge and how care may impact their survival. Several studies have documented the high early mortality among patients on ART in resource limited settings [17, 18]. However, these studies may underestimate mortality because they report on only those patients who enter care. If linkage to care is not accomplished, a major goal of the provision of inpatient HCT fails.
While inpatient HCT may be an efficient and effective way to diagnose HIV infected individuals and refer them to care, there have been concerns about the impact of expansion of provider-initiated testing within health facilities in terms of patient rights, disclosure of HIV status and the negative social outcomes of disclosure [19]. The frequency and severity of such outcomes, however, has not been documented. We conducted a randomized controlled trial of a sample of medical inpatients with unknown HIV status in Mulago hospital. Patients were randomized to receive either free inpatient HCT or referral for free HCT post-discharge. All participants were followed for 6 months to assess the impact of HIV testing on linkage to HIV care. We conducted the intervention between March 2004 to March 2005, before free ARVs and provider-initiated HCT were widely available in Uganda [20]. The primary study outcomes were uptake of inpatient HIV testing, linkage to care including ARVs, and survival. A secondary outcome was disclosure of HIV status and effects of such disclosure (positive and negative).
Methods
This was a randomized controlled trial of routine offer of HCT to medical inpatients compared to referral for HCT post-discharge which was the standard of care in Mulago hospital at the time. Until 2005, Mulago hospital did not have a dedicated HIV testing facility and minimal testing occurred during hospitalization [12]. Participants were identified in consultation with the medical teams responsible for their care. Potential participants were selected randomly from hospitalized patients and were approached to explain the study’s purpose and procedures. If the patient expressed interest in participating, the study protocol was fully explained as part of the informed consent process. The eligibility criteria included age over 18 years, unknown HIV status, and residence within 20 km of Mulago hospital (for ease of follow-up). Patients with altered mental status and those who were too ill to provide informed consent or be interviewed were excluded from the study.
When a subject was determined to be eligible for the study s/he was enrolled and then assigned to a treatment arm. The treatment assignment list was generated prior to enrollment. After enrollment and treatment assignment the interviewers administered the baseline interview. Follow-up interviews were conducted with participants in the intervention and control groups (both HIV negative and HIV positive) at three and 6 months post-discharge. We conducted home visits for individuals lost to follow-up, in order to inquire about survival status and health care prior to death. The study was reviewed and approved by two Institutional Review Boards (University of California, San Francisco and Makerere University Faculty of Medicine) and by the Uganda National Council for Science and Technology (UNCST).
Counseling, Testing, and Linkage to Care
The HCT protocol was adapted from the intervention proven effective at reducing HIV risk behavior in the randomized, controlled trial conducted in Trinidad, Tanzania, and Kenya, the VCT Efficacy Study [3]. This approach is based on the CDC guidelines for HIV counseling and testing [21] and employs an HIV counseling model that includes personalized risk assessment and the development of a personalized risk reduction plan for each client. In addition to counseling HIV infected individuals (in both the intervention and control groups) were given referrals to HIV/AIDS clinics for follow-up care; preparation for and initiation of HIV treatment only happened in the outpatient HIV clinics. Two full-time HIV counselors provided HCT for participants in the intervention and control groups, using the same model.
HCT in the Intervention Group
Participants who were randomized to the intervention group received HCT immediately after the baseline interview. HCT was conducted in a room within the medical ward. Patients underwent phlebotomy and serologic testing and results were disclosed the following day with post-test counseling.
HCT in the Control Group
Participants who were randomized to the control group were given a referral card and an appointment, by the interviewers, to return for HCT at Mulago hospital 1 week after discharge. The participants who returned for HCT received transport reimbursement. HCT for participants in the control arm was also delivered in a room within the medical ward.
Laboratory Methods
The sequential rapid testing algorithm using Determine HIV 1/2 assay (Abbott Laboratories, Abbott Park, IL) for screening, STAT-PAK HIV 1/2 DIPSTICK assay (Chembio Diagnostic Systems Inc) for confirmatory testing and Uni-Gold (Trinity Biotech, Wicklow, Ireland) as the tie-breaker was used to determine HIV status.
Measurements
The primary outcome measures were linkage to medical care including ARVs, and mortality. Assessment using structured interviews was conducted at baseline and follow-up. We elicited information regarding medical encounters with physicians, pharmacists, traditional healers, hospice, and other providers. Participants were asked about the use of antiretroviral therapy, treatment and prophylaxis for opportunistic infections since the last visit. When possible, reported medication use was verified by interviewer direct visualization of the medication during the structured interviews. Information on disclosure of HIV status to sexual partners, friends and relatives, and the positive as well as negative outcomes of disclosure (e.g. strengthening of relationships and emotional support from family, friends and others; friends, family and others feeling ashamed of them) was collected. The linkage to care and disclosure outcomes were calculated over the 6 months of follow-up (sum of those who disclosed or those who were linked to care at 3 and 6 months). We assumed that the study participants who did not receive HIV counseling and testing did not disclose their HIV status to anyone. In terms of disclosure to specific individuals, participants who indicated that they did not have certain categories of persons (e.g. spouses, parents) in their lives were also excluded.
Screening, Enrollment and Retention
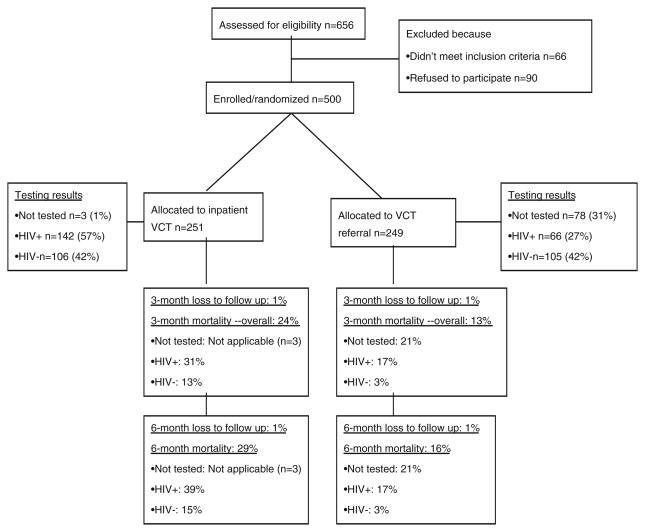
Out of 656 patients who were screened, 590 were found to be eligible and were approached for participation. Of these, 500 (85%) agreed and were enrolled (Fig. 1); 251 in the intervention and 249 in the control group. Five participants (1%) were lost to follow-up over the 6 month study period.
Fig. 1.
Study Profile
Analysis
We conducted t-tests and Chi-square tests to determine whether any socio-demographic and medical history variables at baseline differed by study arm. We examined the 6-month rate of disclosure of HIV status and positive and negative effects of such disclosure overall, by intervention arm, and by HIV status. We examined linkage to care by study arm among the HIV positives, and vital status by study arm and HIV status. The number of missing values is noted in tables when this value exceeds 2% of the sample size.
Results
Socio-Demographic Characteristics and Prior Medical Care of Participants
The majority of the participants were female (59.0%), and had primary level education (50.5%), while 42% of participants were married (Table 1). Overall, 75.8% of the participants received prior treatment in a medical clinic within 3 months of hospitalization while 50.8% had received treatment from a traditional healer, and 25.1% were hospitalized in the prior year. The control group had a larger proportion of individuals who were not-married (χ2 = 10.14, P = 0.02). Otherwise, there were no other significant socio-demographic and prior health provider encounter differences between the intervention and control groups at baseline (Table 1).
Table 1.
Demographic characteristics and health seeking behavior of study participants by study group
| Characteristic | Intervention (Inpatient VCT) N = 251 | Control VCT post-discharge N = 249 | Total N = 500 | Test statistics (comparing intervention and control) |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age | ||||
| Mean, std dev | 34.4 ± 11.0 | 32.5 ± 11.1 | 33.5 ± 11.1 | t = 1.98, P = 0.049* |
| Sex | ||||
| Male | 109 (43.4%) | 96 (38.6%) | 205 (41.0%) | χ2 = 1.23, P = 0.268 |
| Education level | ||||
| None | 22 (8.8%) | 21 (8.4%) | 43 (8.6%) | χ2 = 1.42, P = 0.701 |
| Primary | 133 (53.0%) | 120 (48.2%) | 253 (50.5%) | |
| Secondary | 80 (31.9) | 89 (35.7) | 169 (33.8%) | |
| Tertiary | 16 (6.4%) | 19 (7.6%) | 35 (7.0%) | |
| Monthly income in Uganda shillings | ||||
| < 50,000 | 88 (35.1%) | 107 (43.0%) | 195 (39.0%) | χ2 = 3.35, P = 0.187 |
| 50–100,000 | 55 (21.9%) | 46 (18.5%) | 101 (20.2%) | |
| > 100,000 | 108 (43.0%) | 96 (38.6%) | 204 (40.8%) | |
| Marital status | ||||
| Married and/or living together | 137 (54.6%) | 118 (47.4%) | 255 (0.51) | χ2 = 10.14, P = 0.017* |
| Not married, not living with someone | 44 (17.5%) | 72 (29.3%) | 116 (23.3%) | |
| Separated/Divorced | 46 (18.3%) | 40 (16.2%) | 86 (17.3) | |
| Widowed | 24 (9.6%) | 16 (6.5%) | 40 (8.1%) | |
| Health care provider encounters (in the year prior to baseline) | ||||
| Received treatment in a medical clinic | ||||
| No | 17 (6.9%) | 21 (8.6%) | 38 (7.7%) | χ2 = 0.47, P = 0.491 |
| Yes | 229 (93.1%) | 224 (91.4%) | 453 (92.3%) | |
| Received treatment from a traditional healer (n = 480) | ||||
| No | 116 (48.3%) | 128 (53.3%) | 244 (50.8%) | χ2 = 1.20, P = 0.273 |
| Yes | 124 (51.7%) | 112 (46.7%) | 236 (49.2%) | |
| Admitted to the hospital | ||||
| No | 189 (75.3%) | 175 (70.6%) | 364 (73.0%) | χ2 = 1.43, P = 0.232 |
| Yes | 62 (24.7%) | 73 (29.4%) | 135 (27.1%) | |
| Diagnosed with tuberculosis in previous 5 years | ||||
| No | 199 (81.2%) | 207 (83.8%) | 406 (82.5%) | χ2 = 0.57, P = 0.451 |
| Yes | 46 (18.8%) | 40 (16.2%) | 86 (17.5%) | |
Completion of Treatment (i.e. Receipt of HCT) and HIV Status
All, except three of the participants (1.2%) who were randomized to the inpatient testing arm were tested and received their results before discharge; of these, 57.3% (142) were HIV infected. Among participants who were randomized to receiving HIV testing post-discharge 68.7% (171) were tested and received results; 38.6% (66) of these were HIV infected (Fig. 1).
Disclosure of HIV Status
We determined disclosure rates and the social outcomes of disclosure among participants who tested for HIV (248 in the intervention and 171 in the control arm). Overall 6-month disclosure of HIV status to at least one person was high, at 77.4%. The rate of disclosure to other family members was higher than to spouses; 68.1% (109) disclosed to spouses compared to 76.9% (249) who disclosed to brothers and sisters (Table 2).
Table 2.
Disclosure of HIV status within 6 months by study arm (excluding those who did not test for HIV)
| Intervention (Inpatient VCT) N = 248 | Control (VCT post-discharge) N = 171 | Total N = 419a | Test statistics (comparing intervention and control | |
|---|---|---|---|---|
| Disclosure in 6 months after baseline | ||||
| Disclosure to at least one person | ||||
| No | 6 (3.2%) | 89 (37.9%) | 95 | χ2 = 71.32, P <0.001* |
| Yes | 180 (96.8%) | 146 (62.1%) | 326 | |
| Disclosure to spouse | ||||
| No | 24 (26.7%) | 27 (38.6%) | 51 (31.9%) | χ2 = 2.57, P = 0.109 |
| Yes | 66 (73.3%) | 43 (61.4%) | 109 (68.1%) | |
| Disclosure to other sexual partners | ||||
| No | 35 (38.9%) | 44 (63.8%) | 79 (49.7%) | χ2 = 9.67, P = 0.002* |
| Yes | 55 (61.1%) | 25 (36.2%) | 80 (50.3%) | |
| Disclosure to brothers and sisters | ||||
| No | 47 (26.1%) | 28 (19.4%) | 75 (23.2%) | χ2 = 2.00, P = 0.157 |
| Yes | 133 (73.9%) | 116 (80.6%) | 249 (76.9%) | |
| Disclosure to mother/father | ||||
| No | 62 (46.6%) | 52 (46.4%) | 114 (46.5%) | χ2 = 0.001, P = 0.977 |
| Yes | 71 (53.4%) | 60 (53.6%) | 131 (53.5%) | |
| Disclosure to physician | ||||
| No | 47 (26.1%) | 44 (30.1%) | 91 (27.9%) | χ2 = 0.65, P = 0.420 |
| Yes | 133 (73.9%) | 102 (69.9%) | 235 (72.1%) | |
| Disclosure to employer | ||||
| No | 48 (84.2%) | 29 (87.9%) | 77 (85.6%) | χ2 = 0.23, P = 0.761 |
| Yes | 9 (15.8%) | 4 (12.1%) | 13 (14.4%) | |
| Outcomes of disclosure | ||||
| Spouse/sexual partners feels ashamed of you | ||||
| No | 95 (97.9%) | 61 (98.4%) | 156 (98.1%) | χ2 = 0.04, P = 1.000 |
| Yes | 2 (2.1%) | 8 (1.6%) | 3 (1.9%) | |
| Brothers/sisters feel ashamed of you | ||||
| No | 129 (97.7%) | 108 (96.4%) | 237 (97.1%) | χ2 = 0.37, P = 0.706 |
| Yes | 3 (2.3%) | 4 (3.6%) | 7 (2.9%) | |
| Mother/Father feel ashamed of you | ||||
| No | 66 (97.1%) | 58 (98.3%) | 124 (97.6%) | χ2 = 0.21, P = 1.000 |
| Yes | 2 (2.9%) | 1 (1.7%) | 3 (2.4%) | |
| Physician feels ashamed of you | ||||
| No | 128 (98.5%) | 95 (96.0%) | 223 (97.4%) | χ2 = 1.38, P = 0.407 |
| Yes | 2 (1.5%) | 4 (4.0%) | 6 (2.6%) | |
| Increased emotional support from employers | ||||
| No | 26 (14.9%) | 12 (9.0%) | 38 (12.3%) | χ2 = 2.51, P = 0.113 |
| Yes | 148 (85.1%) | 122 (91.0%) | 270 (87.7%) | |
| Strengthening relationship with spouse/sexual partners | ||||
| No | 35 (19.6%) | 21 (14.4%) | 56 (17.2%) | χ2 = 1.51, P = 240 |
| Yes | 144 (80.5%) | 125 (85.6%) | 269 (82.8%) | |
| Increased emotional support from peers | ||||
| No | 108 (60.3%) | 82 (56.6%) | 190 (58.6%) | χ2 = 0.47, P = 0.492 |
| Yes | 71 (39.7%) | 63 (43.5%) | 134 (41.4%) | |
| Increased emotional support from family and relatives | ||||
| No | 55 (30.7%) | 38 (26.2%) | 93 (28.7%) | χ2 = 0.80, P = 0.371 |
| Yes | 124 (69.3%) | 107 (73.8%) | 231 (71.3%) | |
| Increased support from health professionals | ||||
| No | 68 (38.0%) | 44 (30.3%) | 112 (34.6%) | χ2 = 2.07, P = 0.150 |
| Yes | 111 (62.0%) | 101 (69.7%) | 212 (65.4%) | |
Participants who did not test for HIV and those who did not have certain categories of persons (e.g. spouses, parents) in their lives were excluded
Disclosure by Study Arm
Overall disclosure to at least one person among individuals who tested for HIV, 6 months after enrollment was significantly higher in the intervention arm as compared to the control arm; 96.8% (180) in the intervention disclosed to at least one person compered to 62.1% (146) in the control arm (χ2 = 71.32, P<0.001). Disclosure to spouses and family members was not significantly different but disclosure to other sexual partners was significantly higher in the intervention group; 61.1% (55) in the intervention disclosed to sexual partners compered to 36.2% (25) in the control arm (χ2 = 9.67, P = 0.002).
Among those tested, there were no significant differences in the negative and positive outcomes of disclosure in the intervention and control groups (Table 2).
Disclosure and Social Outcomes of Disclosure by HIV Status
HIV infected individuals were much less likely than HIV negative individuals to disclose their HIV status to their spouses (χ2 = 19.20, P<0.001) and other sexual partners (χ2 = 20.66, P<0.001), but more likely to disclose their status to their physicians (χ2 = 16.55, P<0.001). In terms of the social outcomes, most individuals both HIV infected and HIV uninfected individuals reported positive outcomes including increased emotional support from family and strengthening of relationships with spouses and sexual partners. Seventy-five percent (107) of the HIV infected participants reported strengthening of relationships with sexual partners, 86.3% (119) increased emotional support from employers, 75.2% (106) increased support from family members, and 76.6% (108) increased support from health professionals. Similarly, there were no significant differences in the negative and positive outcomes of disclosure among HIV infected and HIV uninfected individuals, except that the HIV infected individuals were more likely to report increased support from health professionals (χ2 = 13.76, P<0.001).
Study Outcome: Linkage to HIV Care and Treatment
In the 6 month follow up period, 63.6% (84) of the HIV infected participants reported that they received HIV counseling services; 91.0% (121) received care at a general medical clinic; and 62.2% (92) attended a HIV clinic. Almost two-thirds of the HIV infected participants (64.7%, 88) reported that they could not afford medications for HIV/AIDS and 15.0% (20) reported that they received ARVs. The HIV infected participants also reported that they received other care including cotrimoxazole for prophylaxis (53.7%, 72) while 38.8% (50) sought treatment from a traditional healer. Overall, 32.8% (44) reported that they were diagnosed with tuberculosis (Table 3).
Table 3.
Linkage to care within 6 months by study arm among the HIV infected participants (excluding those who died before the 3 months follow-up)
| Intervention (Inpatient VCT) N = 98 | Control (VCT post-discharge) N = 55 | Total N = 153 | Test statistics (comparing intervention and control | |
|---|---|---|---|---|
| Health provider encounters in the 6 months after baseline | ||||
| Attended HIV/AIDS clinic | ||||
| No | 42 (44.2%) | 14 (26.4%) | 56 (37.8%) | χ2 = 4.58, P = 0.032* |
| Yes | 53 (55.8%) | 39 (73.6%) | 92 (62.2%) | |
| Attended any medical clinic | ||||
| No | 9 (10.7%) | 3 (6.1%) | 12 (9.0%) | χ2 = 0.80, P = 0.534 |
| Yes | 75 (89.3%) | 46 (93.9%) | 121 (91.0%) | |
| Treatment from a traditional healer | ||||
| No | 49 (59.8%) | 30 (63.8%) | 79 (61.2%) | χ2 = 0.21, P = 0.648 |
| Yes | 33 (40.2%) | 17 (36.2%) | 50 (38.8%) | |
| Meeting with a counselor | ||||
| No | 32 (38.1%) | 16 (33.3%) | 48 (36.4%) | χ2 = 0.30, P = 0.584 |
| Yes | 52 (61.9%) | 32 (66.7%) | 84 (63.6%) | |
| Admissions to hospital | ||||
| No | 65 (77.4%) | 36 (75.0%) | 101 (76.5%) | χ2 = 0.10, P = 0.756 |
| Yes | 19 (22.6%) | 12 (25.0%) | 31 (23.5%) | |
| Diagnosed with TB | ||||
| No | 51 (60.0%) | 39 (79.6%) | 90 (67.2%) | χ2 = 5.41, P = 0.020* |
| Yes | 34 (40.0%) | 10 (20.4%) | 44 (32.8%) | |
| Medications prescribed in the 6 months after baseline | ||||
| ARVs | ||||
| No | 73 (86.9%) | 40 (81.6%) | 113 (85.0%) | χ2 = 0.67, P = 0.456 |
| Yes | 11 (13.1%) | 9 (18.4%) | 20 (15.0%) | |
| Cotrimoxazole | ||||
| No | 43 (50.6%) | 19 (38.8%) | 62 (46.3%) | χ2 = 1.75, P = 0.187 |
| Yes | 42 (49.4%) | 30 (61.2%) | 72 (53.7%) | |
| Fluconazole | ||||
| No | 75 (88.2%) | 44 (89.8%) | 119 (88.8%) | χ2 = 0.08, P = 1.000 |
| Yes | 10 (11.8%) | 5 (10.2%) | 15 (11.2%) | |
Linkage to Care by Study Group
Although fewer people in the control arm were diagnosed with HIV, a higher proportion reported attending a HIV clinic than those in the intervention arm; 73.6% (39) in the control attended a HIV clinic compared to 55.8% (53) in the intervention arm (χ2 = 4.58, P = 0.032). A larger proportion of participants in the intervention arm reported a diagnosis of TB compared to those in the control arm (χ2 = 5.41, P = 0.020). There were no other significant differences between the intervention and control arms in terms of linkage to HIV care (Table 3).
Study Outcome: Mortality
Overall, 116 (23.2%) of the study participants had died by 6 months of follow-up. The majority of the deaths (79.3%) occurred in the first 3 months (Table 4).
Table 4.
Survival/Mortality by group and HIV status
| Vital status | Intervention N = 251 |
Control N = 249 |
Test statistics (comparing intervention and control) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV status unknown (col. %) | HIV negative (col. %) | HIV positive (col. %) | All (col. %) | HIV status unknown (col. %) | HIV negative (col. %) | HIV positive (col. %) | All (col. %) | ||
| Alive at 6 months | 0 (0.0%) | 89 (84.0%) | 87 (61.3%) | 176 (70.1%) | 60 (76.9%) | 97 (92.4%) | 49 (74.2%) | 206 (82.7%) | χ2 = 11.03, P = 0.0009 |
| Died 0–3 months | 2 (66.7%) | 15 (14.2%) | 44 (40.0%) | 61 (24.3%) | 17 (21.8 %) | 5 (4.8%) | 11 (16.7%) | 33 (13.3%) | χ2 = 10.00, P = 0.0016 |
| Died 3–6 months | 1 (33.3%) | 2 (1.9%) | 11 (7.7%) | 14 (5.6%) | 1 (1.2%) | 3 (2.9%) | 6 (9.1%) | 10 (4.0%) | χ2 = 1.36, P = 0.2430 |
Mortality by Treatment Arm
Mortality in the intervention group was 29.5% compared to 16.9% in the control group (χ2 = 11.03, P = 0.001). This varied by the period of follow-up. In the first 3 months mortality in the intervention group was 23.9% compared to 12.9% in the control group (χ2 = 11.03, P = 0.001) while between 3 and 6 months the mortality was not significantly different in the two groups; 7.3% in the intervention compared to 4.6% in the control group (χ2 = 1.36, P = 0.244).
Mortality by HIV Status
Overall mortality at the end of 6 months among HIV infected participants was 34.6% (72) compared to 11.9% (25) among the HIV negative, and 27.2% (22) among those with unknown HIV status. The highest mortality among HIV infected participants occurred in the first 3 months: 76.4% (55) of the deaths among HIV infected participants happened within the first 3 months, while 23.6% (17) died within 3–6 months (Table 4).
Discussion
These findings suggest that inpatient HCT identifies a high proportion of HIV infected individuals among hospitalized patients in an urban referral hospital in Uganda. Inpatient HCT is highly acceptable and is a more effective strategy to determine HIV status than referral for post-discharge HIV testing as a significant proportion of individuals referred for testing post-discharge will not be tested. This reinforces the need for provider-initiated HIV testing and counselling (PITC). Our findings also confirm prior reports of a high HIV sero-prevalence in similar inpatient settings, and reinforce the need for routine HIV testing among hospitalized patients in sub-Saharan Africa [6–8, 22].
More than one-half of surviving individuals were able to access some level of HIV related care, including counselling and cotrimoxazole. We were surprised by the greater proportion of deaths in the 3 months following discharge among patients receiving inpatient testing than patients referred for out-patient testing. This study did not assess the stage of HIV infection or other co-existing disease conditions. However, other studies show that hospitalized HIV-infected patients in this setting generally have advanced disease [16, 23] and high mortality [24]. A potential reason for the higher mortality rate in the inpatient testing group could include depression and potential withdrawal of care among patients diagnosed with what was then considered a terminal illness. While we cannot fully explain the higher mortality in the intervention group, it is clear that failure to test for HIV accounts for a heavy burden of preventable mortality in settings where HIV antiretroviral therapy is available. The very high early mortality we observed emphasizes the need for earlier diagnosis and immediate linkage to HIV care. A larger proportion of HIV infected individuals in the control group reported attending HIV clinics than those in the intervention group. This may be related to higher uptake of HIV testing by healthier individuals within the control group, as opposed to the intervention group where almost all participants tested. Although HIV diagnosis among the intervention group was made during hospitalization, they too were referred for HIV treatment at outpatient clinics, which was the practice at the time. Also, there was a high mortality among the HIV infected participants in the inpatient testing arm within the first 3 months of diagnosis, suggesting that some individuals died before the linkage to care assessment was completed. These delays and failure to enroll into HIV care emphasize the need for HIV testing programs to include a strong linkage to care component.
A very high proportion of participants both HIV infected and uninfected disclosed their HIV status to family members and health care providers, which is an important step in access to HIV services. The very high rates of disclosure to at least one person in the intervention group may be related to the fact that hospitalised patients in Uganda are usually nursed by a family member (to supplement the limited number of health providers) and may be more likely to disclose their results if they receive them in the presence of the caretaker. Disclosure of HIV status to sexual partners on the other hand was much less, especially among HIV infected participants and highlights challenges that still exist in terms of disclosure and testing of sexual partners. Studies have documented fears of breakup of relationships, physical violence and feelings of shame as reasons for non-disclosure to sexual partners but these studies also report more positive than negative social outcomes following disclosure [25, 26]. Notably, there was no significant difference in reported social harms between the two groups.
The high mortality in this study may have been related to late diagnosis of HIV, a situation that still persists in Uganda and several other countries in sub-Saharan Africa [16, 17, 23, 24]. Although, access to ART has improved over time, this alone, without earlier diagnosis and linkage to HIV care is unlikely to reverse the mortality trends among HIV infected individuals in Africa. Wide spread PITC may address the challenge of late diagnosis especially if it targets outpatients who are less ill and possibly with earlier stage HIV infection [27]. However, PITC alone might not eliminate this problem since PITC inherently targets ill individuals. It may be useful to combine PITC with other HCT approaches (e.g. home based HIV counseling and testing) which may reach individuals at an earlier stage of HIV infection [28].
In summary, this study reinforces the need for routine inpatient testing with immediate linkage to antiretroviral therapy for hospitalized patients. Inpatient testing is an acceptable and effective method to determine HIV status and begin discussions of antiretroviral therapy among those infected. The current practice of referring HIV infected inpatients to outpatient HIV clinics to initiate HIV care and treatment may cause unavoidable delays and mortality when HIV care and treatment is readily available.
Acknowledgments
This study was funded by the Bill and Melinda Gates Foundation. Dr. Bangsberg received support from K24-MH087227. We acknowledge the contribution of the management of Mulago hospital and Infectious Diseases Institute, the study team, and Prof. Fred Wabwire-Mangen from Makerere University School of Public Health.
Contributor Information
Rhoda K. Wanyenze, Email: rwanyenze@hotmail.com, Makerere University School of Public Health, P.O. Box 7072, Kampala, Uganda
Judith A. Hahn, University of California, San Francisco, CA, USA
Cheryl A. Liechty, Glen Cove Internal Medicine, Rockport, ME, USA
Kathie Ragland, University of California, San Francisco, CA, USA.
Allan Ronald, Infectious Diseases Institute, Kampala, Uganda.
Harriet Mayanja-Kizza, Makerere University School of Medicine, Kampala, Uganda.
Thomas Coates, University of California, Los Angeles, CA, USA.
Moses R. Kamya, Makerere University School of Medicine, Kampala, Uganda
David R. Bangsberg, Massachusetts General Hospital Center for Global Health, Harvard Medical School, Boston, MA, USA
References
- 1.Allen S, Tice J, Van de Perre P, Serufilira A, Hudes E, Nsengumuremyi F, et al. Effect of serotesting with counselling on condom use and seroconversion among HIV discordant couples in Africa. BMJ. 1992;304(6842):1605–9. doi: 10.1136/bmj.304.6842.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamenga M, Ryder RW, Jingu M, Mbuyi N, Mbu L, Behets F, et al. Evidence of marked sexual behavior change associated with low HIV-1 seroconversion in 149 married couples with discordant HIV-1 serostatus: experience at an HIV counselling center in Zaire. AIDS. 1991;5(1):61–7. doi: 10.1097/00002030-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. The Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Lancet. 2000 Jul 8;356(9224):103–12. [PubMed] [Google Scholar]
- 4.Gresenguet G, Sehonou J, Bassirou B, Longo Jde D, Malkin JE, Brogan T, et al. Voluntary HIV counseling and testing: experience among the sexually active population in Bangui, Central African Republic. J Acquir Immune Defic Syndr. 2002;31(1):106–14. doi: 10.1097/00126334-200209010-00014. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. Report on the global AIDS epidemic. Geneva, Switzerland: Joint United Nations Program on HIV/AIDS (UNAIDS); 2008. [Google Scholar]
- 6.Lie GT, Biswalo PM, Klepp KI. Counseling and HIV-testing among hospital patients in Arusha and Kilimanjaro. Tidsskr Nor Laegeforen. 1995;115(26):3286–8. [PubMed] [Google Scholar]
- 7.Kwesigabo G, Killewo JZ, Sandstrom A, Winani S, Mhalu FS, Biberfeld G, et al. Prevalence of HIV infection among hospital patients in north west Tanzania. AIDS Care. 1999;11(1):87–93. doi: 10.1080/09540129948225. [DOI] [PubMed] [Google Scholar]
- 8.Palmer DL, Mason PR, Pasi C, Tobiwa O. Value of mandatory testing for human immunodeficiency virus in a sub-Saharan hospital population. Clin Infect Dis. 2000;31(5):1258–65. doi: 10.1086/317453. [DOI] [PubMed] [Google Scholar]
- 9.Wanyenze RK, Nawavvu C, Namale AS, Mayanja B, Bunnell R, Abang B, et al. Acceptability of routine HIV counselling and testing, and HIV seroprevalence in Ugandan hospitals. Bull World Health Organ. 2008;86(4):302–9. doi: 10.2471/BLT.07.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tembo G, Friesan H, Asiimwe-Okiror G, Moser R, Naamara W, Bakyaita N, et al. Bed occupancy due to HIV/AIDS in an urban hospital medical ward in Uganda. AIDS. 1994;8(8):1169–71. doi: 10.1097/00002030-199408000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Fabiani M, Accorsi S, Aleni R, Rizzardini G, Nattabi B, Gabrielli A, et al. Estimating HIV prevalence and the impact of HIV/AIDS on a Ugandan hospital by combining serosurvey data and hospital discharge records. J Acquir Immune Defic Syndr. 2003;34(1):62–6. doi: 10.1097/00126334-200309010-00009. [DOI] [PubMed] [Google Scholar]
- 12.Wanyenze R, Kamya M, Liechty CA, Ronald A, Guzman DJ, Wabwire-Mangen F, et al. HIV counseling and testing practices at an urban hospital in Kampala, Uganda. AIDS Behav. 2006;10(4):361–7. doi: 10.1007/s10461-005-9035-9. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) Revised Recommendations for HIV Testing of Adults Adolescents and Pregnant Women in Health-Care Settings. MMWR Recomm Rep. 2006;55(RR-14):1–14. [PubMed] [Google Scholar]
- 14.World Health Organisation (WHO) Guidance on provider-initiated HIV testing and counseling in health facilities. Geneva: 2007. [Google Scholar]
- 15.Uganda MOH. Uganda National Policy on HIV Counseling and Testing. Kampala, Uganda: Ministry of Health; Sep, [Google Scholar]
- 16.Nakanjako D, Kyabayinze DJ, Mayanja-Kizza H, Katabira E, Kamya MR. Eligibility for HIV/AIDS treatment among adults in a medical emergency setting at an urban hospital in Uganda. Afr Health Sci. 2007;7(3):124–8. doi: 10.5555/afhs.2007.7.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu JK, Chen SC, Wang KY, Chang CS, Makombe SD, Schouten EJ, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85(7):550–4. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86(7):559–67. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennie S, Behets F. Desperately seeking targets: the ethics of routine HIV testing in low-income countries. Bull World Health Organ. 2006;84(1):52–7. doi: 10.2471/blt.05.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uganda AIDS Commission (UAC) Report on Implementation of National HIV and AIDS Strategic Plan—FY 2007/2008. Kampala, Uganda: Uganda AIDS Commission; Oct, 2008. [Google Scholar]
- 21.Centers for Disease C, Prevention. MMWR Recomm Rep. RR-19. Vol. 50. 2001. Nov 9, Revised guidelines for HIV counseling, testing, and referral; pp. 1–57. quiz CE1-19a1-CE6-a1. [PubMed] [Google Scholar]
- 22.Nakanjako D, Kamya M, Daniel K, Mayanja-Kizza H, Freers J, Whalen C, et al. Acceptance of routine testing for HIV among adult patients at the medical emergency unit at a national referral hospital in Kampala, Uganda. AIDS Behav. 2007;11(5):753–8. doi: 10.1007/s10461-006-9180-9. [DOI] [PubMed] [Google Scholar]
- 23.Kigozi IM, Dobkin LM, Martin JN, Geng EH, Muyindike W, Emenyonu NI, et al. Late-Disease Stage at Presentation to an HIV Clinic in the Era of Free Antiretroviral Therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009 Jun 10; doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82(4):299–307. [PMC free article] [PubMed] [Google Scholar]
- 26.Grinstead OA, Gregorich SE, Choi KH, Coates T. Positive and negative life events after counselling and testing: the Voluntary HIV-1 Counselling and Testing Efficacy Study. AIDS. 2001;15(8):1045–52. doi: 10.1097/00002030-200105250-00013. [DOI] [PubMed] [Google Scholar]
- 27.Kiene SM, Bateganya M, Wanyenze R, Lule H, Nantaba H, Stein MD. Initial outcomes of provider-initiated routine HIV testing and counseling during outpatient care at a rural Ugandan hospital: risky sexual behavior, partner HIV testing, disclosure, and HIV care seeking. AIDS Patient Care STDS. Feb;24(2):117–26. doi: 10.1089/apc.2009.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menzies N, Abang B, Wanyenze R, Nuwaha F, Mugisha B, Coutinho A, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23(3):395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]