Abstract
Background
Cyp1a1-Ren2 transgenic rats [strain name: TGR(Cyp1a1Ren2)], administered indole-3-carbinol (I3C) develop angiotensin (ANG) II-dependent hypertension due to hepatic expression of the Ren2 renin gene. Although AT1 receptor blockade prevents the development of hypertension and normalizes the elevated arterial blood pressure of Cyp1-Ren2 rats, little information is available regarding the blood pressure and renal functional responses to direct inhibition of renin in this high circulating renin model of ANG II-dependent hypertension. The present study was performed to determine the effects of acute direct renin inhibition with aliskiren on blood pressure and renal hemodynamics in Cyp1a1-Ren2 rats with ANG II-dependent malignant hypertension.
Methods
Mean arterial pressure (MAP) and renal hemodynamics were measured in pentobarbital-anesthetized male Cyp1a1-Ren2 rats during control conditions and following administration of the renin inhibitor, aliskiren (10 mg/kg, iv).
Results
Rats induced with I3C had higher MAP (194±7 vs. 141±2 mmHg, P<0.001), lower renal plasma flow (RPF; 2.47±0.23 vs. 4.17±0.35 ml/min.g, P<0.001), and lower glomerular filtration rate (GFR; 1.01±0.07 vs. 1.34±0.06 ml/min.g, P=0.01) than noninduced Cyp1a1-Ren2 rats (n=5). Aliskiren administration decreased MAP (194±7 to 136±2 mmHg, P<0.001) and increased RPF (2.47±0.23 vs. 4.31±0.20 ml/min.g, P<0.001) in hypertensive but not in normotensive rats, without altering GFR.
Conclusions
Acute renin inhibition with aliskiren normalizes MAP and RPF in Cyp1a1-Ren2 rats with malignant hypertension. The normalization of MAP and RPF following acute renin inhibition indicates that renin generated by expression of the Ren2 gene is responsible for the maintenance of malignant hypertension and the associated reduction in renal hemodynamic function in Cyp1a1-Ren2 rats.
Keywords: kidney, renin-angiotensin system, malignant hypertension, renin inhibitor, renal hemodynamics
Introduction
The transgenic rat line with inducible expression of the mouse Ren2 renin gene [strain name: TGR (Cyp1a1Ren2)] was created by inserting the mouse Ren2 renin gene, fused to an 11.5 kb fragment of the cytochrome P450 1a1 (Cyp1a1) promoter, into a neutral genomic site on the Y chromosome of the Fischer 344 rat.1 Cyp1a1, which catalyzes the oxidation of a wide range of endogenous lipophilic compounds and xenobiotics, is not constitutively expressed, but is highly inducible upon exposure to various aryl hydrocarbons such as indole-3-carbinol (I3C).2,3 Induction of Cyp1a1 is mediated by the aryl hydrocarbon receptor, which is a basic helix-loop-helix-transcription factor that binds to specific DNA elements in the Cyp1a1 promoter.2 Rats transgenic for the Cyp1a1-Ren2 construct do not constitutively express the Ren2 renin gene. Rather, the Ren2 gene is expressed, primarily in the liver, only upon induction of the Cyp1a1 promoter by dietary administration of aryl hydrocarbons such as I3C.1
Administration of I3C at a dose of 0.3% wt/wt induces the development of ANG II-dependent malignant hypertension,4–6 which is a form of severe hypertension characterized by fibrinoid necrosis of arterioles and vascular damage in many tissues, including the kidney.1,5 In rats, this condition manifests as a rapid rise in systemic blood pressure accompanied by a significant loss of body weight, polyuria, polydipsia, piloerection, lethargy, and hunched posturing.1,5,6 In addition, Cyp1a1-Ren2 transgenic rats with malignant hypertension exhibit increases in plasma renin activity (PRA), plasma ANG II levels and intrarenal ANG II levels.6 Although AT1 receptor blockage prevents the development of hypertension and normalizes the elevated arterial blood pressure of hypertensive Cyp1a1-Ren2 transgenic rats,6,7 little information is available regarding the blood pressure and renal functional responses to direct inhibition of renin in the high circulating renin model of ANG II-dependent malignant hypertension.
Recently, the first direct renin inhibitor, aliskiren, was approved for the treatment of hypertension.8 Aliskiren suppresses the activity of renin to catalyze the conversion of angiotensinogen (AGT) to ANG I.9 While aliskiren was designed to inhibit human renin (IC50 = 0.6 nM), it also effectively inhibits the activity of mouse renin (IC50 = 4.5 nM).8 The compound binds the active site of both soluble and receptor-bound renin and prorenin, thus acting to suppress both plasma renin activity (PRA) and the production of the vasoactive substance ANG II.8 In human trials, chronic administration of aliskiren results in significant and sustained suppression of hypertension and PRA.10 Likewise, chronic administration of aliskiren in TG(mRen2)27 rats that constitutively express the mouse Ren2 renin gene suppresses hypertension, reduces renal and cardiac pathologies associated with increased blood pressure, and prevents the increases in both kidney and plasma ANG II levels.11,12 However, little is known about the acute effects of direct renin inhibition on blood pressure and renal hemodynamics in ANG II-dependent malignant hypertension. The present study was performed to address the hypothesis that acute direct renin inhibition with aliskiren normalizes arterial blood pressure and increase renal hemodynamic function in Cyp1a1-Ren2 transgenic rats with ANG II-dependent malignant hypertension.
Methods
The experimental procedures in this study conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Tulane University Health Sciences Center. Experiments were performed on adult male transgenic rats [TGR(Cyp1a1Ren2)] with inducible expression of the mouse Ren2 renin gene1. All transgenic rats used in the present study were bred at Tulane University School of Medicine from stock animals supplied by Harlan UK Limited, Bicester, UK. The experimental animals were divided into two groups. Group 1 (Noninduced; n=5) were maintained on a normal, non-I3C rat diet (diet TD 99414, Harlan-Teklad, Madison, WI) for 10 days. Group 2 (Hypertensive; n=5) were littermates fed a normal rat diet containing 0.3% I3C (wt/wt; diet TD 05381, Harlan-Teklad) for 10 days to induce ANG II-dependent malignant hypertension. The rats were allowed free access to food and tap water until the day of the experiment. Body weight was measured throughout the course of the study.
At the conclusion of the treatment period, all animals were surgically prepared for assessment of renal hemodynamics and continuous measurement of arterial blood pressure as described previously.6 Briefly, the rats were anesthetized with pentobarbital sodium (50 mg/kg, ip) and placed on a surgical table thermostatically controlled to maintain body temperature at 37°C. A tracheostomy was performed, and the animals were allowed to breathe humidified air enriched with oxygen. The left jugular vein was cannulated to allow infusion of solutions and additional anesthetic. The rats were infused at a constant rate of 1.2 ml/h with isotonic saline containing 6% albumin (bovine; Sigma Chemical, St. Louis, MO) during surgery and thereafter with isotonic saline containing 1% albumin, 7.5% polyfructosan (Inutest; Laevosan Geselschaft, Linz, Austria), and 1.5% p-aminohippurate sodium (PAH; Merck Sharp & Dohme, West Point, PA). A catheter was inserted into the left femoral artery to allow monitoring of arterial blood pressure and to facilitate collection of blood samples. Blood pressure was monitored with a Statham pressure transducer (model P23DC) and recorded using a computerized data-acquisition system (MP100 System; BIOPAC Systems, Santa Barbara, CA) with the AcqKnowledge Software Package (version 3.7.3, BIOPAC). The left kidney was exposed via a flank incision, freed from surrounding tissue, and placed in a Lucite cup. The left ureter was cannulated to allow timed urine collections to be obtained.
After a 45 minute recovery period, urine was collected during two 30-minute control periods, followed by a control blood sample (~250 µL). Aliskiren (Novartis Pharma AG, Basel, Switzerland; 10 mg/kg) was administered by iv bolus injection into the left jugular vein. The dose of aliskiren used in the present study was chosen on the basis of the previous observation that chronic administration of this dose of aliskiren elicited a pronounced and prolonged decrease in blood pressure in hypertensive TGR(mRen2)27 transgenic rats, which constitutively express high levels of the mouse Ren2 renin gene.13 After a 15-minute equilibration period, urine was collected during two 30-minute experimental periods, followed by collection of an additional blood sample (~250 µL). At the conclusion of the protocol, the left kidney was excised, blotted dry, and weighed.
Urine volume was determined gravimetrically. Sodium concentration in urine and plasma were measured using flame photometry. Inulin and PAH concentrations in both urine and plasma were measured by standard spectrophotometry. Glomerular filtration rate (GFR) and renal plasma flow (RPF) were estimated from the clearance of inulin and PAH, respectively. Renal blood flow (RBF) was calculated as RPF/(1-Hct). Renal vascular resistance (RVR) was determined from the quotient of mean arterial pressure (MAP) and calculated renal blood flow.
Statistical analyses were performed within groups using one-way repeated measures ANOVA followed by Student-Newman-Keuls test where appropriate and between groups using one-way ANOVA followed by Student-Newman-Keuls test where appropriate. All statistical analyses were performed using SigmaPlot for Windows (version 11; Systat Software Inc., San Jose, CA). Statistical significance was defined as P<0.05. All data are expressed as means ± SE.
Results
Chronic dietary administration of 0.3% I3C to Cyp1a1-Ren2 rats (n=5) for 10 days resulted in the development of severe hypertension (194±7 vs. 141±2 mmHg, P<0.001; Fig. 1). The development of hypertension was associated with a marked decrease in body weight (261±14 to 200±17 g, P<0.001), which was not seen in the animals maintained on a non-I3C containing diet. The hypertensive rats also demonstrated severe lethargy, piloerection and assumption of hunched posture, which are manifestations of malignant hypertension in the rat.1,5,6 Thus, in the present study, chronic dietary administration of 0.3% I3C induced malignant hypertension in the Cyp1a1-Ren2 rats, as previously observed.1,5,6 As shown in Fig. 1, aliskiren administration elicited a pronounced decrease in MAP in the hypertensive rats (194±7 to 136±2 mmHg, P<0.001), but not in the noninduced rats (141±2 to 136±4, NS).
FIG. 1.
Mean arterial blood pressure in anesthetized noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions and after acute bolus injection of aliskiren (10 mg/kg, iv); *P<0.001 vs. Noninduced; #P<0.001 vs. Corresponding control.
As shown in Figs. 2 and 3, basal values for GFR and RPF in the hypertensive rats were lower than the corresponding values in the noninduced normotensive rats (1.01±0.07 vs. 1.34±0.06 ml/min.g, P=0.01, and 2.47±0.23 vs. 4.17±0.35 ml/min.g, P<0.001, respectively). Aliskiren administration increased GFR (1.01±0.07 to 1.18±0.08, P<0.05) and RPF (2.47±0.23 to 4.31±0.20 ml/min.g, P<0.001) in the hypertensive rats but not in the noninduced rats. However, GFR in the aliskiren-treated hypertensive rats remained significantly lower than that observed in the aliskiren-treated normotensive control rats (1.18±0.08 vs. 1.50±0.06 ml/min.g, P<0.01). Although there was a tendency for dietary administration of 0.3% I3C to raise filtration fraction, the increase did not achieve statistical significance (42±5 vs. 33±2%, NS). Upon systemic administration of aliskiren, filtration fraction significantly decreased in hypertensive rats (42±5 to 28±1%, P<0.05) but remained unaltered in the noninduced control rats (33±2 to 35±2, NS). As shown in Fig. 4, the basal value for RVR in the hypertensive rats was markedly greater than that in the noninduced rats (41.0±3.2 vs. 17.7±1.3 mmHg/ml/min.g, P<0.001). Aliskiren administration normalized RVR in the hypertensive rats (41.0±3.2 to 17.6±1.6 mmHg/ml/min.g, P<0.001) but did not alter RVR in the normotensive rats.
FIG. 2.
Glomerular filtration rate in anesthetized noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions and after acute bolus injection of aliskiren (10 mg/kg, iv). *P<0.05 vs. Noninduced; #P<0.05 vs. Corresponding control.
FIG. 3.
Renal plasma flow in anesthetized noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions and after acute bolus injection of aliskiren (10 mg/kg, iv); *P<0.001 vs. Noninduced; #P<0.001 vs. Corresponding control.
FIG. 4.
Renal vascular resistance in anesthetized noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions and after acute bolus injection of aliskiren (10 mg/kg, iv); *P<0.001 vs. Noninduced; #P<0.001 vs. Corresponding control.
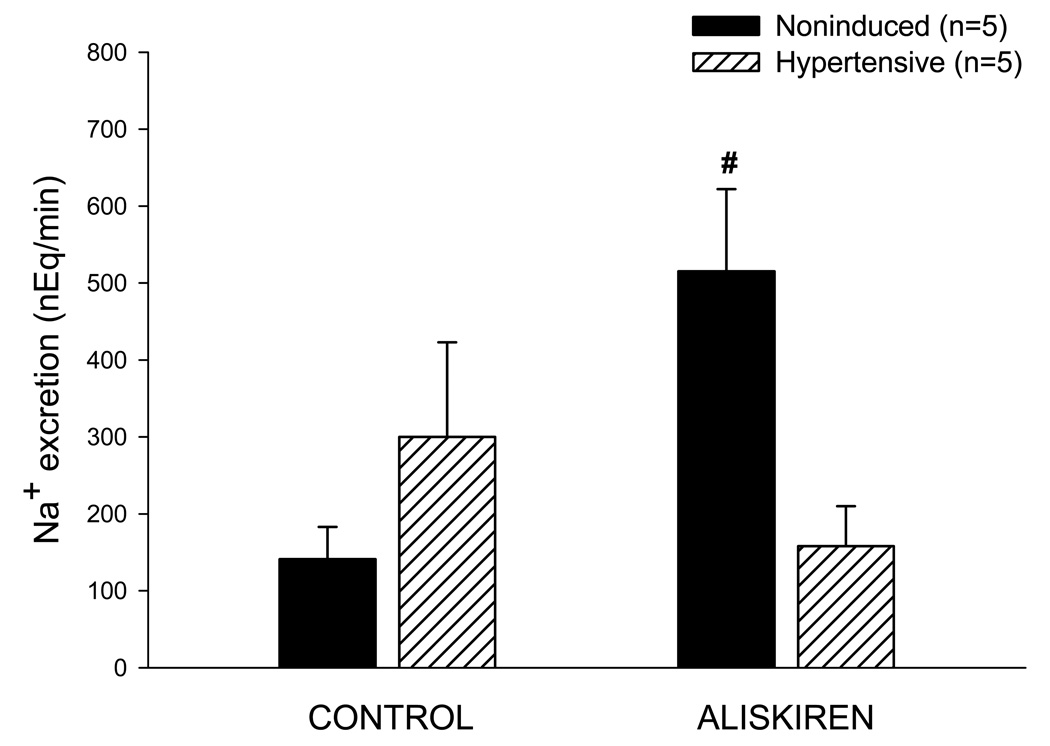
As shown in Fig. 5, control values for urine flow in the hypertensive rats were significantly greater than those observed in the noninduced rats (12.08±0.89 vs. 6.73±0.44 µL/min, P<0.001). However, the basal value for urinary sodium excretion in the hypertensive rats was not different from the corresponding value in the noninduced normotensive rats (300±123 vs. 141±42 nEq/min) (Fig. 6). Urine flow remained unaltered following aliskiren administration in both groups (Fig. 5). In contrast, aliskiren increased urinary sodium excretion in the normotensive rats (141±42 to 515±107 nEq/min, P<0.05), but not in the hypertensive rats (300±123 to 158±52).
FIG. 5.
Urine flow in anesthetized noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions and after acute bolus injection of aliskiren (10 mg/kg, iv); *P<0.01 vs. Noninduced.
FIG. 6.
Urinary sodium excretion in anesthetized noninduced and hypertensive Cyp1a1-Ren2 rats during control conditions and after acute bolus injection of aliskiren (10 mg/kg, iv); #P<0.05 vs. Corresponding control.
Discussion
The present study was performed to evaluate the effects of acute direct renin inhibition with aliskiren on blood pressure and renal hemodynamics and excretory function in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. In the present study, induction of the Ren2 renin gene by dietary administration of 0.3% I3C for 10 days resulted in the development of hypertension. The hypertension was associated with a pronounced decrease in body weight, and the rats also exhibited extreme lethargy, assumption of a hunched posture, and piloerection, which are clinical manifestations of malignant hypertension in the rat.1,5,6 Acute administration of the direct renin inhibitor, aliskiren, resulted in a rapid normalization of mean arterial blood pressure in the five rats studied. This finding indicates that renin generated as a consequence of expression of the Ren2 renin gene is responsible for the maintenance of malignant hypertension in Cyp1a1-Ren2 transgenic rats.
Cyp1a1-Ren2 transgenic rats exhibited significantly decreased values for GFR and RPF and significantly elevated RVR. These findings are consistent with our previous observations that GFR and RPF are maintained within or slightly below the normal range and that RVR is significantly elevated in Cyp1a1-Ren2 transgenic rats with malignant hypertension.6 The present study does not allow determination of the relative contribution of the autoregulatory response to the increased arterial pressure or of the direct pre- and post-glomerular vasoconstrictor effects of ANG II to the reduced GFR and RPF and elevated RVR in Cyp1a1-Ren2 transgenic rats with malignant hypertension. Whatever the mechanism, the present data confirm our previous findings that the ability of the pre-glomerular vasculature to prevent the transmission of the systemic hypertension to the glomerular capillaries appears to be intact at the stage of the pathogenesis of malignant hypertension.5
In the present study, aliskiren administration increased GFR and RPF and decreased RVR and filtration fraction in the hypertensive rats but not in the noninduced rats. The observation that acute aliskiren administration significantly reduced filtration fraction in the hypertensive rats is consistent with our previous observation that chronic administration of the AT1 receptor antagonist, candesartan, prevented the increase in filtration fraction in rats induced with 0.3% I3C for 11–12 days.6 Collectively, these findings indicate that ANG II generated as a consequence of Ren2 renin gene expression contributes to an elevated filtration fraction in Cyp1a1-Ren2 rats with malignant hypertension.
Although aliskiren treatment increased GFR in the hypertensive rats, GFR in the aliskiren-treated hypertensive rats remained significantly lower than the GFR in the aliskiren-treated noninduced normotensive rats (Fig. 2). It is possible that the sustained reduction of GFR in the aliskiren-treated hypertensive rats reflects the glomerular damage that occurred during the development of malignant hypertension. In this regard, we have previously demonstrated that the renal pathological changes that occur during the development of malignant hypertension in Cyp1a1-Ren2 rats are characterized by myointimal hyperplasia and tubular dilation, inflammation and cellular proliferation in the cortical vessels and tubulointerstitium, and pronounced glomerulosclerosis.5 One would predict that acute aliskiren administration would be unlikely to reverse such renal vascular and glomerular injury and that this likely contributed to the inability of acute aliskiren treatment to restore GFR to levels observed in the aliskiren-treated noninduced control rats. Nevertheless, it is likely that the increases in GFR and RPF, and the reductions in total RVR and filtration fraction after acute aliskiren administration occurred as a consequence of vasodilation of both pre- and post-glomerular vascular resistance elements. Whatever the relative contribution of pre- and post-glomerular vasodilation to the elevated GFR and RPF, the observation that GFR and RPF increased significantly following aliskiren administration indicates that the glomerular vascular resistance vessels can vasodilate in response to direct renin inhibition and to subsequent reduction of the renal vasoconstrictor actions of ANG II.
Despite the markedly elevated arterial blood pressure, the control values for urinary sodium excretion in the hypertensive rats were not significantly different from those observed in the noninduced normotensive rats. However, the basal values for urine flow in the hypertensive rats were higher than those in the normotensive rats, presumably reflecting the pressure diuretic effects of the elevated blood pressure. In contrast, the finding that basal values of sodium excretion in the hypertensive rats were not different from those in normotensive rats indicates that the kidneys of Cyp1a1-Ren2 rats with malignant hypertension exhibit an impaired natriuretic response to elevated arterial blood pressure and can maintain normal rates of sodium excretion only at hypertensive arterial pressures. Administration of aliskiren increased urinary sodium excretion in the noninduced normotensive rats but not in the hypertensive rats. In contrast, we previously observed that chronic administration of the AT1 receptor antagonist, candesartan, increased sodium excretion in Cyp1a1-Ren2 rats induced with 0.3% I3C.6 The reasons for these apparent disparate findings remain unclear but are likely related to the fact that in the present study the aliskiren-treated hypertensive rats exhibited a rapid and pronounced (~60 mmHg) decrease in arterial blood pressure which would have masked any effect of acute direct renin inhibition to increase sodium excretion. In contrast, chronic AT1 receptor blockade with candesartan prevented the development of malignant hypertension rather than rapidly normalizing a markedly elevated arterial blood pressure6 which would likely allow manifestation of the natriuretic effects of chronic AT1 receptor blockade in this model. In the present study, the aliskiren-induced pronounced decrease in arterial blood pressure likely acted to offset any renal natriuretic effects of direct renin inhibition by aliskiren.
It has been demonstrated that in the kidney, aliskiren localizes extensively in the glomeruli, the JG cells of the afferent arteriole and the larger caliber intrarenal arteries.8 Thus, it is possible that in addition to inhibiting circulating renin, acute aliskiren administration also inhibited renal renin and that this contributed in part to the renal functional responses to acute aliskiren administration. However, the present study does not allow discrimination between the relative contributions of inhibition of circulating versus kidney tissue renin to the observed renal functional responses to acute aliskiren administration. Further studies are required to address this issue.
It has been reported that aliskiren is able to bind both circulating and receptor bound prorenin to decrease ANG I and II generation.8 Thus it is possible that prorenin contributed to the hypertensive phenotype in Cyp1a1-Ren2 transgenic rats and that the blood pressure and renal functional responses to acute aliskiren administration observed in the present study occurred, in part, as a consequence of inhibition of both circulating and receptor bound prorenin. However, the present data do not allow determination of the relative contribution of prorenin inhibition to the blood pressure and renal functional responses to acute aliskiren administration. Additional studies are required to address this issue.
In summary, the present data demonstrate that acute direct renin inhibition with aliskiren normalizes mean arterial blood pressure, increases GFR and RPF, and decreases RVR and filtration fraction in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. The normalization of arterial blood pressure, the increases in GFR and RPF, and the decreases in RVR and filtration fraction indicate that renin generated as a consequence of expression of the Ren2 Renin gene is responsible for the maintenance of malignant hypertension and reduced renal hemodynamic function in Cyp1a1-Ren2 transgenic rats.
Acknowledgments
The authors would like to thank Porcha D. Davis for excellent technical assistance. We also thank Dr. L. Gabriel Navar for helpful comments and suggestions, and Dr. Barb Mickelson, Harlan-Teklad, for help with the design and production of the I3C-containting rat diet. This study was supported by the Tulane COBRE in Hypertension and Renal Biology (NCRR 2P20RR017659-06), by NHLBI grant HL26371, and by a grant from Novartis Pharmaceuticals Corp. (CSPP100A-US21T).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kantachuvesiri S, Fleming S, Peters J, et al. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem. 2001;276(39):36727–36733. doi: 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 2.Campbell SJ, Carlotti F, Hall PA, et al. Regulation of the CYP1A1 promoter in transgenic mice: an exquisitely sensitive on-off system for cell specific gene regulation. J Cell Sci. 1996;109(11):2619–2625. doi: 10.1242/jcs.109.11.2619. [DOI] [PubMed] [Google Scholar]
- 3.Loub WD, Wattenberg LW, Davis DW. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J Natl Cancer Inst. 1975;54(4):985–988. [PubMed] [Google Scholar]
- 4.Vanourková Z, Kramer HJ, Erbanová M, et al. Endothelin receptor blockade does not affect blood pressure or angiotensin II levels in CYP1A1-Ren-2 transgenic rats with acutely induced hypertension. Vascul Pharmacol. 2009;50(5–6):194–199. doi: 10.1016/j.vph.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Graciano ML, Mouton CR, Patterson ME, et al. Renal vascular and tubulointerstitial inflammation and proliferation in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol. 2007;292(6):F1858–F1866. doi: 10.1152/ajprenal.00469.2006. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell KD, Bagatell SJ, Miller CS, et al. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal Ang II levels of graded severity in Cyp1a1-Ren2 transgenic rats. J Renin Angiotensin Aldosterone Syst. 2006;7(2):74–86. doi: 10.3317/jraas.2006.013. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell KD, Mullins JJ. Enhanced tubuloglomerular feedback in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol. 2005;289(6):F1210–F1216. doi: 10.1152/ajprenal.00461.2004. [DOI] [PubMed] [Google Scholar]
- 8.Feldman DL. New insights into the renoprotective actions of the renin inhibitor aliskiren in experimental renal disease. Hypertens Res. 2010;33(4):279–287. doi: 10.1038/hr.2010.19. [DOI] [PubMed] [Google Scholar]
- 9.Pilz B, Shagdarsuren E, Wellner M, et al. Aliskiren, a human renin inhibitor, ameliorates cardiac and renal damage in double-transgenic rats. Hypertension. 2005;46(3):569–576. doi: 10.1161/01.HYP.0000179573.91016.3f. [DOI] [PubMed] [Google Scholar]
- 10.Nussberger J, Gradman AH, Schmieder RE, et al. Plasma renin and the antihypertensive effect of the orally active renin inhibitor aliskiren in clinical hypertension. Int J Clin Pract. 2007;61(9):1461–1468. doi: 10.1111/j.1742-1241.2007.01473.x. [DOI] [PubMed] [Google Scholar]
- 11.Whaley-Connell A, Habibi J, Cooper SA, et al. Effect of renin inhibition and AT1R blockade on myocardial remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol Metab. 2008;295(1):E103–E109. doi: 10.1152/ajpendo.00752.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakusan D, Kujal P, Kramer HJ, et al. Persistent antihypertensive effect of aliskiren is accompanied by reduced proteinuria and normalization of glomerular area in Ren-2 transgenic rats. Am J Physiol Renal Physiol. 2010;299(4):F758–F766. doi: 10.1152/ajprenal.00259.2010. [DOI] [PubMed] [Google Scholar]
- 13.Feldman DL, Jin L, Xuan H, et al. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52(1):130–136. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]