Abstract
NADPH oxidase (NOX) is a multicomponent enzyme that mediates electron transfer from NADPH to molecular oxygen, which leads to the production of superoxide. NOX2/gp91phox is a catalytic subunit of NOX expressed in phagocytic cells. Several homologues of NOX2, including NOX1, have been identified in non-phagocytic cells. We investigated the contributory role of NOX1 and NOX2 in hepatic fibrosis. Hepatic fibrosis was induced in wild-type (WT) mice, NOX1-knockout (NOX1KO) mice, and NOX2-knockout (NOX2KO) mice by either CCl4 injections or bile duct ligation (BDL). The functional contribution of NOX1 and NOX2 in endogenous liver cells, including hepatic stellate cells (HSCs), and bone marrow (BM) derived cells, including Kupffer cells (KCs), to hepatic reactive oxygen species (ROS) generation and hepatic fibrosis was assessed in vitro and in vivo using NOX1- or NOX2-BM chimeric mice. Hepatic NOX1 and NOX2 mRNA expression was increased in the two experimental mouse models of hepatic fibrosis. While NOX1 was expressed in HSCs but not in KCs, NOX2 was expressed in both HSCs and KCs. Hepatic fibrosis and ROS generation were attenuated in both NOX1KO and NOX2KO mice after CCl4 or BDL. Liver fibrosis in chimeric mice indicated that NOX1 mediates the profibrogenic effects in endogenous liver cells, while NOX2 mediates the profibrogenic effects in both endogenous liver cells and BM-derived cells. Multiple NOX1 and NOX2 components were upregulated in activated HSCs. Both NOX1- and NOX2-deficient HSCs had decreased ROS generation and failed to upregulate collagen α1(I) and TGF-β in response to angiotensin II.
Conclusion
Both NOX1 and NOX2 in endogenous liver cells, including HSCs, have an important role in hepatic fibrosis, while NOX2 in BM-derived cells has a lesser role.
Keywords: NADPH oxidase, oxidative stress, hepatic fibrosis, hepatic stellate cells
Introduction
Reactive oxygen species (ROS) function as key secondary messengers in numerous signaling pathways, including transcriptional regulation, differentiation, carcinogenesis, and apoptosis.(1) ROS contributes to hepatic fibrosis caused by various injuries, including alcohol abuse, hepatitis C virus infection, iron overload and chronic cholestasis.(2) Activated hepatic stellate cells (HSCs) play a key role in the pathogenesis of hepatic fibrosis by producing extracellular matrix proteins including type I collagen.(3) Free radicals or lipid peroxidation products stimulate the activation of HSCs by inducing c-myb and NF-κB which are blocked by antioxidants such as α-tocopherol.(4) ROS induce the activation of the collagen type I gene in HSCs,(5) and may act as an intracellular signaling mediator of the fibrogenic action of TGF-β.(6, 7) Hepatic ROS may be generated by multiple sources including mitochondrial respiratory chain, cytochrome p4502E1, peroxisomes, and NADPH oxidases (NOXs).(2)
NOX is a multicomponent enzyme complex originally described in phagocytes.(8) The phagocytic NOX consists of the catalytic subunit NOX2 (also termed as gp91phox) together with the regulatory subunit p22phox located in the membrane. Other regulatory components p47phox, p40phox, p67phox and the small GTPase Rac are normally located in the cytoplasm. Upon stimulation with agonists, the cytoplasmic subunits translocate to the membrane-bound complex leading to enzymatic activity.(9) Humans have six additional NOX homologues (NOX1, NOX3, NOX4, NOX5, DUOX1, and DUOX2) that may function in non-phagocytic NOX.(10) All NOX enzymes are able to catalyze the reduction of molecular oxygen to superoxide, but there are key differences in their activation, subunit composition, localization, and expression. Among the seven members of the NOX family, NOX1 is structurally and functionally similar to NOX2. While NOX1 is also p22phox-dependent, NOX1 may use NOXO1 (homologue of p47phox) to organize the enzyme assembly and NOXA1 (homologue of p67phox) for enzyme activation.(11) NOX1 is abundantly expressed in the colon, but is also detected in the uterus, prostate, stomach, and vascular smooth muscle cells.(12)
We previously demonstrated that HSCs express NOX components including NOX2 and p47phox.(13) Angiotensin II stimulates DNA synthesis, cell migration, procollagen α1(I) mRNA expression, and secretion of TGF-β1 and inflammatory cytokines in cultured HSCs. Angiotensin II activates AKT as well as MAPK components ERK, JNK, and p38 and increases AP-1 DNA binding in HSCs. All these effects were attenuated by antioxidant (N-acetylcysteine) and by diphenylene iodonium (DPI), a NOX inhibitor.(13) Angiotensin II or leptin increases intracellular ROS and fibrogenic responses in HSCs isolated from wild-type (WT) mice, but not in p47phox-deficient HSCs.(13, 14) Hepatic injury and fibrosis is also attenuated in p47phox-deficient mice after BDL, demonstrating that NOX is an important mediator in the development of hepatic fibrosis.(13)
Although NOX plays an important role in HSC activation and hepatic fibrosis, how various NOX homologues expressed in different hepatic cell types contribute to hepatic fibrogenesis is unknown. The present study investigates the contributory role of NOX1 and NOX2 in hepatic fibrosis. We evaluated the effect of genetic NOX1 and NOX2 inactivation on hepatic fibrosis in two different models of experimental fibrogenesis. We also identified NOX1- and NOX2-expressing cell types in the liver. The functional contribution of NOX1 and NOX2 in endogenous liver cells, including HSCs, and in bone marrow (BM) derived cells, including KCs, to hepatic fibrosis was assessed using either NOX1- or NOX2-BM chimeric mice. Our data indicates that both NOX1 and NOX2 in endogenous liver cells have an important role in hepatic fibrosis, while NOX2 in BM-derived cells has a lesser role. These findings may provide a new insight into the development of anti-fibrotic therapy targeting non-phagocytic NOX signaling in the liver without suppression of NOX2-mediated host defense mechanism.
Materials and Methods
Fibrosis Induction in Mice
NOX2-knock out (NOX2KO) mice(15) on a C57BL/6 background, which lack a catalytic subunit of phagocytic NOX complex, and wild type (WT) C57BL/6 control mice were purchased from The Jackson Laboratory (Bar Habor, MA). NOX1-knock out (NOX1KO) mice(16) with C57BL/6 background were kindly gifted from Dr. John Engelhardt of the University of Iowa. Eight to ten-week old male mice were used. Liver fibrosis was induced either by intraperitoneal injection of hepatotoxin carbon tetrachloride (CCl4) or by bile duct ligation (BDL). CCl4 (Sigma Aldrich, St. Louis, MO; diluted 1:3 in corn oil) or a vehicle (corn oil) was injected on every 3rd day at a concentration of 2 μl/g body weight for 12 times. BDL was performed as previously described, and a sham operation group was used as controls.(17) Animals were sacrificed 72 hours after the last CCl4 injection or 3 weeks after BDL and blood and liver samples were collected. All animal studies have been approved by The University of California, San Diego Institutional Animal Care and Use Committee (IACUC) (protocol number: S-07088).
Bone Marrow Transplantation and Chimeric Mice Experiments
We performed bone marrow transplantation (BMT) experiments as previously described with slight modifications.(18–21) We flushed the tibias and femurs of donor mice to obtain BM. We washed BM cells twice in HBSS and injected 1×107 BM cells into the tail veins of lethally irradiated (10 Gy) recipient mice. Mice received an intravenous injection of liposomal clodronate (150 μl intraperitonealy) 2 weeks after irradiation to deplete Kupffer cells. At 8 weeks after BMT, CCl4 was injected on every 3rd day at a concentration of 2 μl/g body weight diluted in corn oil (1:3) (Sigma Aldrich). Animals were sacrificed after 10 intraperitoneal CCl4 injections and blood and liver samples were collected. Using this protocol, we achieved full replacement of KCs but not HSCs with BM-derived cells in mice transplanted with β-actin promoter-driven GFP transgenic BM, as assessed by double immunostaining.(19) All animal studies have been approved by The University of California, San Diego Institutional Animal Care and Use Committee (IACUC) (protocol number: S-07088).
Assessment of Hepatic Fibrosis
Hepatic fibrosis was assessed by morphometric analysis of the Sirius Red-stained area and measurement of hepatic hydroxyproline content, as previously described.(13, 22) Refer to the Supplementary Materials and Methods section for details.
Thiobarbituric Acid Reactive Substances (TBARS) Determination
Hepatic lipid peroxidation was assessed by TBARS formation.(23) Refer to the Supplementary Materials and Methods section for details.
Isolation of Liver Cell Fractions
Liver cells of wild-type mice were fractionated into 4 major cell populations (hepatocytes, KCs, sinusoid endothelial cells (SECs), and HSCs) as previously described.(24) Refer to the Supplementary Materials and Methods section for details.
HSCs and KCs Isolation and Cell Culture
Mouse HSCs were isolated by a 2-step collagenase-pronase perfusion of mouse livers followed by 8.2% Nycodenz (Accurate Chemical and Scientific Corp.) 2-layer discontinuous density gradient centrifugation, as previously described.(13) In vivo activated murine HSCs were isolated from mice that underwent BDL for 3 weeks by the same procedure. After isolation, HSCs were seeded on uncoated plastic tissue-culture dishes and cultured in DMEM (GIBCO BRL; Life Technologies Inc., Grand Island, NY) supplemented with 10% FBS. Mouse KCs were isolated by collagenase-pronase perfusion followed by 15% Nycodenz gradient centrifugation and subsequent positive selection of CD11b-expressing cells by MACS, as previously described.(19) Isolated KCs were cultured in DMEM supplemented with 1% FBS.
Measurement of ROS Generation in HSCs
HSCs were isolated from WT mice, NOX1KO mice, and NOX2KO mice and cultured in 96-well black plates with a transparent bottom in phenol red-free DMEM containing 10% FBS and antibiotics for five days. Cells were then changed to a serum-free media for 24 hours and subsequently loaded with the redox-sensitive dye CM-H2DCFDA (10 μM) diluted in HANKS buffer for 20 minutes at 37°C. Cells were then rinsed twice with DMEM without phenol red and stimulated with angiotensin II (10−6 M). CM-H2DCFDA fluorescence was detected at excitation and emission wavelengths of 488 nm and 520 nm.(13) ROS formation was measured in a time course of 30 minutes using a multi-well fluorescence scanner (Fluostar Optima, BMG Labtech, Cary, NC). Microscopic detection of ROS generation in HSCs is described in the Supplementary Materials and Methods section.
Other Materials and Methods
Other materials and methods for reagents, serum biochemistry, immunohistochemistry and immunofluorescence, Western blotting, quantitative real-time PCR, and assessment of superoxide production in KCs are described in the Supplementary Materials and Methods section.
Statistical Analysis
Results were expressed as the mean ± standard error of the mean. Data between groups were analysed by a 2-tailed Student's t-test. A P value of less than 0.05 was considered statistically significant.
Results
Expression of NOX1 and NOX2 are Upregulated in Fibrotic Liver
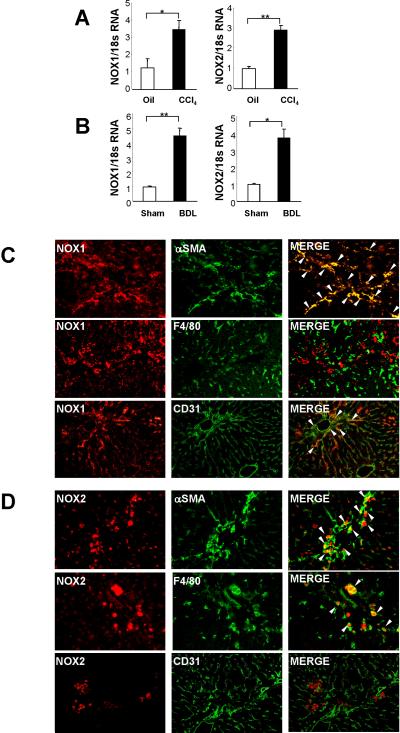
We first investigated NOX1 and NOX2 expression in normal and fibrotic liver. Expression of hepatic NOX1 and NOX2 mRNA levels were elevated in the fibrotic liver after CCl4 treatment (Fig. 1A) and BDL (Fig. 1B). We also detected NOX1 and NOX2 protein expression in the fibrotic liver by immunofluorescence (Suppl.Fig. 1A,B). We further investigated NOX1 and NOX2 expression in HSCs, KCs, and SECs, key cell types that are involved in hepatic fibrosis. We performed double immunofluorescence staining between either NOX1 or NOX2 and cell type markers α-SMA, F4/80, and CD31 for HSCs, KCs, and SECs, respectively. NOX1 expression in the fibrotic liver overlapped with α-SMA-positive HSCs and some CD31-positive SECs, but there was minimal overlap with F4/80-positive KCs (Fig. 1C). NOX2 staining overlapped with both α-SMA-positive HSCs and F4/80-positive KCs/macrophages in CCl4 treated liver (Fig. 1D).
Fig. 1. Expression of NOX1 and NOX2 are upregulated in hepatic fibrosis.
Livers were obtained from WT mice after 12 times of intraperitoneal injection of CCl4 (n=8) or 3 weeks of BDL (n=10). (A, B) Hepatic mRNA levels of NOX1 and NOX2 were measured after treatment with (A) CCl4 or (B) BDL using qPCR. (C) Double immunofluorescence staining for NOX1 (red fluorescence) and α-SMA (HSC), F4/80 (KC), or CD31 (SEC) (all green fluorescence). (D) Double Immunofluorescence staining for NOX2 (red fluorescence) and α-SMA (HSC), F4/80 (KC) or CD31 (SEC) (all green fluorescence). The liver sections were from (C, D) CCl4-treated mice. Arrow head indicates double positive cells. Data are representative of three independent experiments. *P<0.05, **P<0.01. Original magnification, ×200 (C,D).
Attenuated Hepatic Fibrosis in NOX1KO and NOX2KO Mice Compared to WT Mice after CCl4 or BDL Treatment
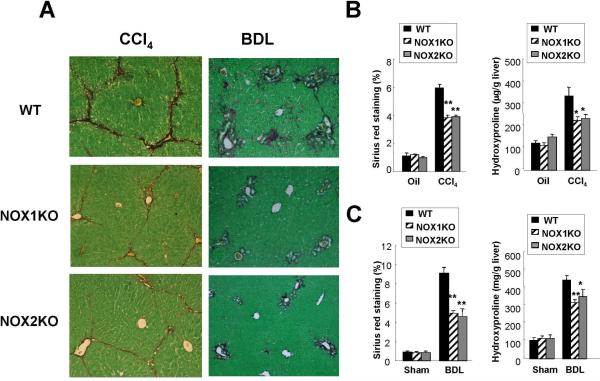
To investigate the contributing roles of NOX1 and NOX2 in hepatic fibrogenesis, we induced liver fibrosis using two different methods, CCl4 injections and BDL, in NOX1KO, NOX2KO and WT mice. In the CCl4 treatment group, serum ALT levels and the liver to body weight ratio were significantly lower in the NOX1KO and NOX2KO mice compared to WT mice (Suppl.Fig. 2A). In the BDL experiment group, there was no significant difference in serum ALT levels or total bilirubin levels between the three groups, but the liver/body weight ratio was lower in NOX1KO mice compared to WT mice (Suppl.Fig. 2B). We assessed hepatic fibrosis by morphometric analysis quantitating the Sirius Red-stained area in the liver and by measurement of hepatic hydroxyproline content. Hepatic fibrosis was significantly attenuated in NOX1KO and NOX2KO mice compared to WT mice after CCl4 injections (Fig. 2A,B). BDL-induced hepatic fibrosis was also reduced in NOX1KO and NOX2KO mice compared to WT mice (Fig. 2A,C). These results demonstrate that both NOX1 and NOX2 contribute to hepatic fibrosis induced by CCl4 and BDL in mice.
Fig. 2. Hepatic fibrosis is attenuated in NOX1KO and NOX2KO mice after CCl4 treatment or BDL.
Livers were obtained from WT mice after 12 times of intraperitoneal injection of CCl4 (n=8) or 3 weeks of BDL (n=10). (A) Representative images of Sirius Red staining in WT, NOX1KO, and NOX2KO mice after 12 CCl4 injections or 3 weeks of BDL are shown. Hepatic fibrosis was assessed by morphometric analysis of the Sirius Red-stained area in the liver or by the measurement of hepatic hydroxyproline content in (B) CCl4-treated mice or (C) BDL mice. Statistical significance relative to WT CCl4-treated mice or WT BDL mice: *P < 0.05, **P < 0.01. Original magnification, ×100 (A).
NOX1KO and NOX2KO Mice Have Reduced HSC Activation and Fibrosis-related Gene Activation after CCl4 or BDL Treatment
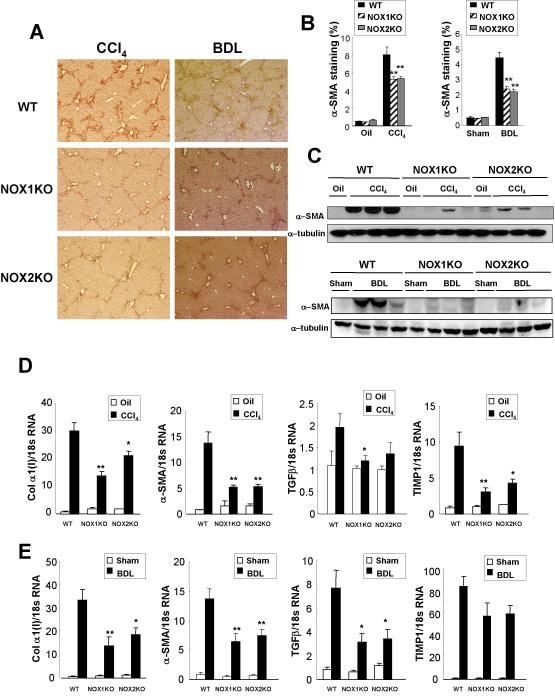
We next investigated the activation of HSCs in NOX1KO, NOX2KO, and WT mice after treatment with CCl4 or BDL. We assessed α-SMA expression in the liver by morphometric analysis and by immunoblotting. The fibrotic livers of WT mice showed high-levels of hepatic expression of α-SMA, but α-SMA expression was significantly low in NOX1KO and NOX2KO mice (Fig. 3A,B,C). In addition, the hepatic mRNA levels of fibrosis-related genes including collagen α1(I), α-SMA, and TGF-β1 were low in NOX1KO and NOX2KO mice compared to WT mice after CCl4 treatment (Fig. 3D) or BDL (Fig. 3E). There was no difference in hepatic expressions of M1 or M2 macrophage markers between WT, NOX1KO-, or NOX2KO-mice (Suppl.Fig. 3A,B). These results suggest that both NOX1 and NOX2 may be directly involved in the activation of HSCs.
Fig. 3. Activation of HSCs and expression of profibrogenic genes are decreased in NOX1KO and NOX2KO mice after CCl4 treatment or BDL.
(A) Representative images of α-SMA immunohistochemical staining in WT, NOX1KO and NOX2KO mice after 12 intraperitoneal injections of CCl4 (n=8) or 3 weeks of BDL (n=10) are shown. Activation of HSCs was assessed by (B) morphometric measurement of α-SMA stained area in the liver or (C) immunoblotting for α-SMA. (D, E) Hepatic mRNA levels of collagen α1(I), α-SMA, TGFβ, and TIMP1 were measured in WT, NOX1KO, and NOX2KO mice after (D) 12 CCl4 injections (n=8) or (E) 3 weeks of BDL (n=10) by quantitative real-time PCR. Statistical significance relative to WT CCl4-treated mice or WT BDL mice: *P < 0.05, **P < 0.01. Original magnification, ×50 (A).
NOX1KO and NOX2KO Mice Have Reduced Lipid Peroxidation Compared to WT Mice after CCl4 or BDL Treatment
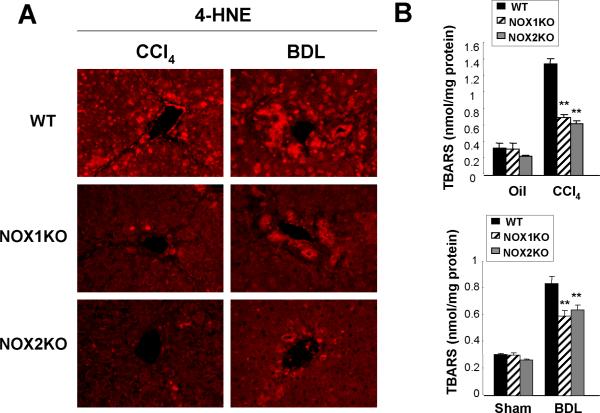
We measured lipid peroxidation products 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) as indicators of oxidative stress in the liver in NOX1KO, NOX2KO, and WT mice after CCl4 or BDL treatment. Immunofluorescence staining showed lower hepatic 4-HNE levels in NOX1KO and NOX2KO mice compared to WT mice after CCl4 or BDL treatment (Fig. 4A). Measurement of MDA using thiobarbituric acid reactive substances (TBARS) showed that NOX1KO and NOX2KO mice have lower levels of lipid peroxidation compared to WT mice after CCl4 or BDL treatment (Fig. 4B,C), suggesting that both NOX1 and NOX2 play an important role in the generation of hepatic oxidative stress in response to CCl4 or BDL in mice.
Fig. 4. Lipid peroxidation is reduced in NOX1KO and NOX2KO mice compared to WT mice after CCl4 treatment or BDL.
(A) Representative images of 4-hydroxynonenal (4-HNE) immunofluorescent staining in WT, NOX1KO, and NOX2KO mice after 12 intraperitoneal injections of CCl4 (n=8) or 3 week of BDL (n=10) are shown. (B) Hepatic malondialdehyde levels were measured using thiobarbituric acid reactive substances (TBARS) assay in WT, NOX1KO and NOX2KO mice after 12 CCl4 injections (n=8) or 3 weeks of BDL (n=10). Statistical significance relative to WT CCl4-injected mice or WT BDL mice: *P < 0.05, **P < 0.01. Original magnification, ×200 (A).
NOX1 and NOX2 in Endogenous Liver Cells Mediate Hepatic Fibrosis
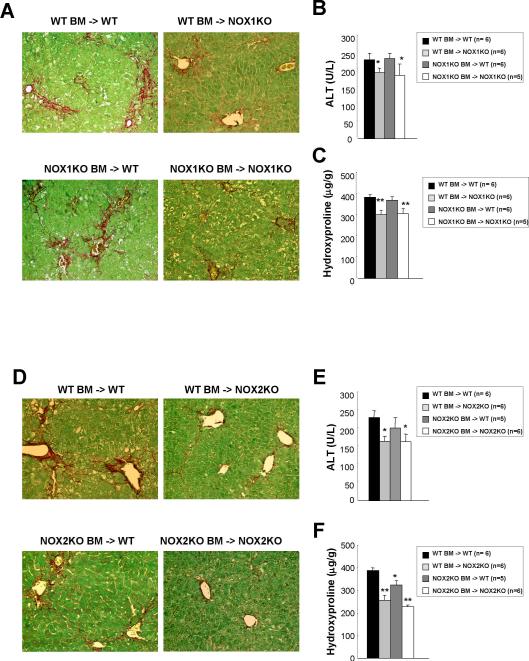
To characterize the contributory roles of NOX1 and NOX2 in hepatic fibrosis in different liver cell populations, we generated NOX1- and NOX2-BM chimeric mice using a combination of lethal irradiation, KCs depletion by clodronate injection, and BMT. This combination generates complete substitution of Kupffer cells and other BM-derived cells, but not of resident hepatic cell populations, including HSCs and SECs.(18, 19) Eight weeks after BMT, hepatic fibrosis was induced by CCl4 for 1 month. Serum ALT levels were lower in NOX1-chimeric mice with NOX1 deficient endogenous liver cells (WT BM -> NOX1KO and NOX1KO BM -> NOX1KO) compared to WT mice transplanted with WT BM (Fig. 5B). As expected, NOX1KO mice transplanted with NOX1KO BM had reduced hepatic fibrosis compared to WT mice transplanted with WT BM. NOX1-chimeric mice that express NOX1 in BM-derived cells but not endogenous liver cells (WT BM -> NOX1KO) showed the similar reduction of hepatic fibrosis as mice with complete NOX1 deficiency. However, NOX1-chimeric mice that express NOX1 in endogenous liver cells, but not BM-derived cells (NOX1KO BM -> WT) showed the same levels of fibrosis as WT mice (Fig. 5A,C).
Fig. 5. Chimeric mice with NOX1- or NOX2-deficient endogenous liver cells show decreased hepatic fibrosis after CCl4 treatment.
After the lethal irradiation, BMT was performed to generate chimeric mice as follows: WT mice with WT BM, WT mice with NOX1KO or NOX2KO BM, NOX1KO mice with WT or NOX1KO BM, and NOX2KO mice with WT or NOX2KO BM. Liposomal clodronate was injected intraperitoneally to deplete resident KCs after 2 weeks of BMT. Mice underwent 10 times of intraperitoneal CCl4 injections from 8 weeks after BMT. (A and D) Representative images of Sirius Red staining are shown (original magnification, ×100). (B and E) Liver injury was assessed by measurement of serum ALT levels. (C and F) Hepatic fibrosis was assessed by measurement of hepatic hydroxyproline content. Statistical significance relative to WT mice with WT BM: *P < 0.05, **P < 0.01.
Serum ALT levels were reduced in NOX2-chimeric mice with NOX2 deficient endogenous liver cells (WT BM -> NOX2KO and NOX2KO BM ->NOX2KO) compared to WT mice transplanted with WT BM (Fig. 5E). NOX2KO mice transplanted with NOX2-deficient BM had reduced hepatic fibrosis compared to WT mice transplanted with WT BM. NOX2 chimeric mice that express NOX2 in BM-derived cells, but not endogenous liver cells (WT BM -> NOX2KO), showed a similar reduction in hepatic fibrosis as those with complete NOX2 deficiency. NOX2 chimeric mice that express NOX2 in endogenous liver cells, but not BM-derived cells (NOX2KO BM -> WT), showed a modest reduction in fibrosis compared to WT mice (Fig. 5D,F). We confirmed that NOX2 BM chimeric mice harboring NOX2KO BM-derived macrophages (NOX2KO BM -> WT and NOX2KO BM -> NOX2KO) express no NOX2 in F4/80 positive cells in the liver (Suppl.Fig. 4). Taken together, the experiments using chimeric mice suggest that both NOX1 and NOX2 in endogenous liver cells, including HSCs, mediate hepatic fibrosis, while NOX2 in BM derived cells, including KCs/macrophages, has a lesser role in hepatic fibrosis.
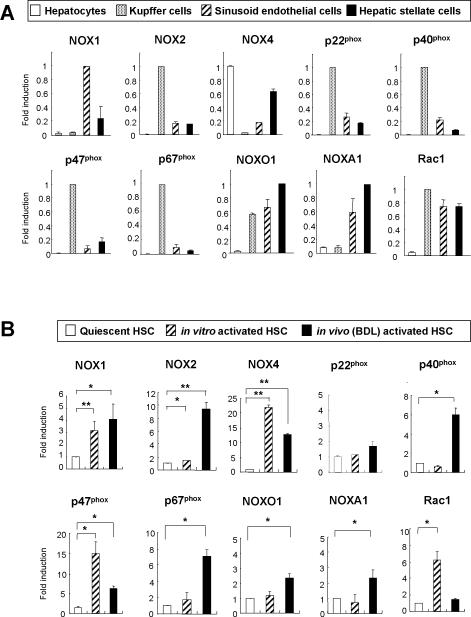
Both Phagocytic and Nonphagocytic NOX Components Are Upregulated in Activated HSCs Compared to Quiescent HSCs
To investigate the expression of NOX components in different liver cell populations, we assessed the mRNA levels of NOX components in the 4 major liver cell fractions (hepatocytes, KCs, SECs and HSCs) from WT mice. The phagocytic NOX components, including NOX2, p47phox, and p67phox, are mainly expressed in KCs. The nonphagocytic NOX components such as NOX1, NOXO1, and NOXA1 are mainly expressed in HSCs and SECs. The mRNA expression of NOX4, another nonphagocytic NOX, was observed in hepatocytes, SECs, and HSCs (Fig. 6A). Next, we investigated the expression of NOX components in quiescent and activated HSCs. mRNAs of the phagocytic NOX catalytic subunit NOX2 and nonphagocytic NOX catalytic subunits NOX1 and NOX4 were upregulated in in vitro and in vivo(BDL)-activated HSCs compared to quiescent HSCs. Other NOX components, including p40phox, p47phox, p67phox, NOXO1, NOXA1, and Rac1, were also upregulated in activated HSCs (Fig. 6B). We found that NOX2 and its regulators such as p40phox, p47phox, and p67phox were strongly upregulated in in vivo(CCl4)-activated HSCs compared to quiescent HSCs (Supple.Fig. 5). We confirmed that NOX1 and NOX2 proteins were expressed in the activated human HSC line LX-2 (Suppl.Fig. 6A,B). These findings provide further evidence that non-phagocytic NOX, including NOX1, as well as phagocytic NOX2 may be involved in hepatic fibrogenesis.
Fig. 6. Expression of NOX components in isolated liver cell fractions.
mRNA levels of NOX1, NOX2, NOX4, p22phox, p40phox, p47phox, p67phox, NOXO1, NOXA1, and Rac1 were evaluated by quantitative real-time PCR in (A) isolated liver cell fractions from WT mice including hepatocytes, KCs, SECs, and HSCs, and in (B) quiescent (1 day in culture) HSCs, in vitro activated (7 days in culture) HSCs and in vivo activated (isolated from WT mice after 3 weeks of BDL) HSCs. Ribosomal 18S was used as an internal control. Data were from 2 independent experiments performed in triplicates. Statistical significance compared to quiescent HSCs: *P < 0.05, **P < 0.01.
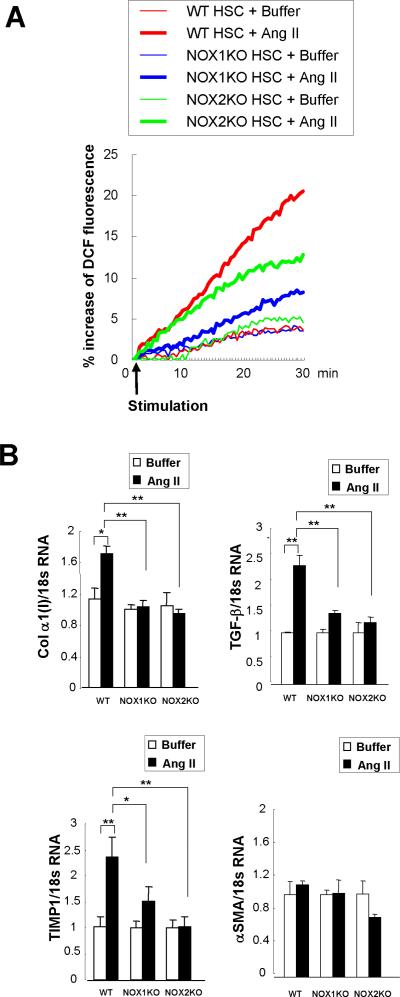
NOX1 and NOX2 Mediate Angiotensin II-induced ROS Production and Fibrogenic Responses in HSCs
To identify the NOX components required for ROS generation in HSCs, we assessed ROS generation in HSCs from WT, NOX1KO, and NOX2KO mice. We quantitated the ROS generation in CM-H2DCFDA loaded HSCs after treatment with angiotensin II, a known NOX agonist. Cells treated with buffer showed a 3% to 4% increase, representing basal ROS production. Angiotensin II induced an 18–20% increase in ROS production in WT HSCs, a 12–13% increase in NOX2KO HSCs, and only a 7–8% increase in NOX1KO HSCs (Fig. 7A). Angiotensin II (10−6 M) treatment induced a strong fluorescent signals in both diffuse and dot patterns in the cytoplasm, followed by cell contraction in WT HSCs, weak signals only in the dot pattern in NOX2KO HSCs, and almost no detectable signal in NOX1KO HSCs (Suppl. Fig. 7). These data suggest that both NOX1 and NOX2 contribute to angiotensin II-induced ROS generation in HSCs, and NOX1 contributes more than NOX2. As a positive control, we also measured superoxide production in isolated KCs from WT, NOX1KO, and NOX2KO mice. After stimulation of phorbol 12-myristate 13-acetate (PMA), a NOX activator, WT and NOX1KO KCs produced comparable amounts of superoxide. As expected, NOX2KO KCs failed to produce superoxide (Suppl. Fig. 8A,B). These data corroborate the finding that KCs express NOX2 but not NOX1.
Fig. 7. Both NOX1 and NOX2 mediate ROS production and fibrogenic responses in HSCs.
(A) HSCs from WT, NOX1KO and NOX2KO mice were loaded with redox-sensitive dye CMH2DCFDA (10 μM) for 20 minutes. Cells were then washed twice and subsequently stimulated with angiotensin II (10−6 M). Fluorescent signals were quantified continuously for 30 minutes using a fluorometer. (B) mRNA expression of collagen α1(I), TGF-β, TIMP1, and α-SMA were measured in WT, NOX1KO, and NOX2KO mice 24 hours after angiotensin II (10−6 M) stimulation by qPCR. Data were from 2 independent experiments performed in triplicates. *P < 0.05, **P < 0.01.
Finally, we tested whether NOX1 and NOX2 are involved in fibrogenic responses in HSCs. We measured expression of fibrogenic genes (collagen α1(I), TGF-β, TIMP1, α-SMA) in response to angiotensin II in HSCs that were isolated from WT, NOX1KO, and NOX2KO mice (Fig. 7B). Angiotensin II induced the upregulation of these fibrogenic genes except α-SMA in WT HSCs. In contrast, the expression of these fibrogenic genes were not elevated in NOX1KO and NOX2KO HSCs after angiotensin II stimulation, indicating both NOX1 and NOX2 mediate fibrogenic responses in response to angiotensin II in HSCs.
Discussion
Several reports have documented that NOX is important in the pathogenesis of hepatic fibrosis.(13, 25–27) We previously demonstrated human HSCs express mRNA for phagocytic NOX components including NOX2 and p47phox.(13) NOXs in HSCs are functionally active in ROS generation in response to agonists such as angiotensin II, PDGF and leptin.(13, 14, 28) HSCs from p47phox-deficient mice fail to generate ROS in response to angiotensin II or leptin, and p47phox-deficient mice show decreased hepatic fibrosis, demonstrating NOXs play an important role in hepatic fibrosis.(13, 14, 26) Recently, it was reported that NOX2-deficient mice have reduced hepatic fibrosis after CCl4 treatment.(25, 27) However, the contributory role of NOX homologues in different cell types in the liver in the development of hepatic fibrosis is not understood.
Our current study provides compelling evidence that both NOX1 and NOX2 have an important role in hepatic fibrogenesis. Mice deficient for either NOX1 or NOX2 displayed a significant reduction of hepatic fibrosis in two different models of liver injury, CCl4 and BDL. We found that both NOX1 and NOX2 were upregulated in the fibrotic liver. Through double immunofluorescent staining, we demonstrated that NOX1 is expressed in HSCs and SECs, whereas NOX2 is expressed in HSCs and KCs in the fibrotic liver. Interestingly, NOX1 is expressed in almost all αSMA-expressing HSCs, but NOX2 is expressed in some HSCs in the fibrotic liver. Recently, Jiang et al. reported that phagocytosis of apoptotic hepatocytes directly induce HSC activation and collagen production via NOX2.(27) Perhaps, NOX2 expression in HSCs reflects the phagocytic function of HSCs.(29,30) In response to angiotensin II, we observed minimal ROS generation in NOX1KO HSCs, whereas NOX2KO HSCs generated a decreased but detectable ROS. These data suggest that NOX1 may be a major NOX form in HSCs, and NOX2 may act in specific circumstances such as apoptotic body-induced HSCs activation.
The degree of fibrosis reduction in NOX1KO and NOX2KO mice was less than that observed in p47phox-deficient mice after BDL.(13, 26) NOX organizer 1 (NOXO1) is a p47phox homologue in the NOX1 complex.(11) The NOX organizers (p47phox and NOXO1) share common structures and functional properties except that NOXO1 lacks a sequence homologous to the C-terminal “auto-inhibitory (AIR)” domain of p47phox.(9) However, activities of NOX1 as well as NOX2 can be regulated by p47phox in some cell types.(31) Studies in vascular smooth muscle cells from normal and p47phox-deficient mice suggest that p47phox participates in an oxidative response which involves NOX1 as the core catalytic oxidase component in these cells.(32, 33) Moreover, co-expression of NOX1 with NOXO1 and NOXA1 leads to stimulus-independent high level superoxide generation whereas stimulus dependence of NOX1 was restored when p47phox was used to replace its homologue NOXO1.(11) Thus, p47phox appears to involve a functional partnership with both NOX2 and NOX1 in the liver, resulting in hepatic ROS generation and fibrosis.
Compared to WT mice, NOX1KO and NOX2KO mice showed weak hepatic fibrosis after both CCl4 and BDL treatments. However, low serum ALT levels were only observed in CCl4-treated NOX1KO and NOX2KO mice, but not in those treated with BDL. NOX1KO and NOX2KO mice showed low hepatic lipid peroxidation after both CCl4 and BDL treatments. Similarly to liver injury, lipid peroxidation in NOX1KO and NOX2KO mice was more evidently reduced after CCl4 treatment than BDL. We found strong upregulation of NOX2 and its regulators such as p40phox, p47phox, p67phox in in vivo-activated HSCs by CCl4 compared to quiescent HSCs, suggesting a stronger participation of NOX in CCl4-induced liver fibrosis than in BDL. Hydrophobic bile acids which accumulate during cholestasis stimulate the generation of ROS in hepatocyte mitochondria through induction of mitochondrial membrane transition (MPT).(34) NOX-independent ROS such as mitochondria-produced ROS might play a more important role in the generation of hepatic lipid peroxidation in BDL than in CCl4.
Our current study characterizes the functional contribution of different NOX1- and NOX2-expressing cell populations to hepatic fibrosis. Through experiments using NOX1- and NOX2-BM chimeric mice, we demonstrate that NOX1 mediates fibrogenic effects in endogenous liver cells, and NOX2 mediates fibrogenic effects in both endogenous liver cells and BM-derived cells. In this study, NOX2 BM chimeric mice that express NOX2 in endogenous liver cells, but not BM-derived cells (NOX2KO BM -> WT), showed a modest but significant reduction of fibrosis compared to WT mice. These results are consistent with our previous study using p47phox BM chimeric mice. p47phox BM chimeric mice that express p47phox in endogenous liver cells, but not BM-derived cells (p47phoxKO BM -> WT), showed a ~25% reduction in fibrosis, whereas chimeric mice with WT BM-derived cells and p47phoxKO endogenous liver cells (WT BM -> p47phoxKO) showed a ~60% reduction in fibrosis.(26) Taken together, NOX2 in both endogenous liver cells and BM-derived cells contributes to liver fibrosis, with the endogenous liver cells making the greater contribution.
Phagocytic NOX is expressed in all professional phagocytes (macrophage, neutrophils and eosinophils) and also in B and T lymphocytes. The role of NOX expressed in non-phagocytic inflammatory cells such as lymphocytes, NK cells and NKT cells in hepatic fibrogenesis is unknown. T lymphocytes express a phagocyte-type NOX, that functions in T cells to produce ROS in response to stimulation via the TCR.(35) When we assessed the expression of M1 and M2 macrophage markers in the fibrotic liver, there was no significant difference between WT and NOX2KO-mice, suggesting the less important role of other NOX2-expressing BM derived immune cells in hepatic fibrosis.
Analysis of expression of NOX components in isolated liver cell fractions from control mice demonstrate that phagocytic NOX components such as NOX2, p40phox, p47phox, and p67phox are mainly expressed in KCs, whereas nonphagocytic NOX components including NOX1, NOXO1, and NOXA1 are expressed in HSCs and SECs. In addition, both NOX1 and NOX2 components are upregulated in activated HSCs compared to quiescent cells. We confirmed the expression of NOX1 and NOX2 proteins in mouse HSCs as well as in the human activated HSC line LX-2. We demonstrated that angiotensin II-induced ROS production and fibrogenic responses in NOX1- or NOX2-deficient HSCs are attenuated compared to WT HSCs, indicating that both NOX1 and NOX2 are important in NOX-mediated ROS generation and fibrogenic responses in HSCs. Taken all together, HSCs appear to be the primary cell type for NOX1- and NOX2-mediated hepatic fibrosis. We also found that NOX1 is expressed in the minority of CD31-positive SECs in the fibrotic liver. We suggest that NOX1-mediated low levels of O2.− production in SECs may have some regulatory function in the liver and warrants further study.
ROS has diverse effects with respect to different kinds, concentration, and cell types. H2O2 and superoxide have different physiological characteristics in that H2O2 can easily diffuse across plasma membrane and throughout the cell, whereas superoxide diffuses poorly across cell membranes.(36) Although higher concentration of ROS is cytotoxic, lower concentration of ROS serves as second messengers during cellular responses to a variety of physiologic stimuli. A low dose of H2O2 has mitogenic effects and can mimic the function of growth factors.(37) Regarding the effects of ROS on HSCs, the contradictory results have been reported: both mitogenic and cell-death inducing properties. Non-toxic levels of ROS or lipid peroxidation products stimulate the activation, proliferation and collagen production of HSCs, but high concentration of ROS induce HSC death.(4, 5, 38) We speculate that NOX2-mediated robust production of superoxide in KCs acts mainly for the host defense, while NOX1- and NOX2-mediated ROS generation in HSCs may act as an important secondary messenger to activate HSCs in hepatic fibrosis.
In conclusion, our study demonstrates the contributory roles of NOX1 and NOX2 in hepatic fibrosis. Mice lacking NOX1 or NOX2 show attenuated hepatic ROS generation and liver fibrosis. Chimeric BM mice demonstrate both NOX1 and NOX2 in endogenous liver cells including HSCs have an important role in hepatic fibrosis, while NOX2 in BM-derived cells has a lesser role. Activated HSCs have upregulated expression of components of NOX1 and NOX2, and both NOX1 and NOX2 mediate ROS generation and fibrogenic responses in HSCs. Our study provides the rationale to target specific components of non-phagocytic NOX as novel therapies for hepatic fibrosis without suppression of NOX2-mediated host defense.
Supplementary Material
Acknowledgments
The authors thank Ms. Karin Diggle (UC San Diego) for her technical assistance and Dr Jung Ho Lee (UC San Diego) for technical assistance for fluorescent microscopy and helpful discussion.
Grant Support: This study was supported by grants from the Korea Research Foundation (KRF-2009 E00006), a grant from the Good Health R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (No. A050021), and a faculty research grant of Yonsei University College of Medicine for 2010 (6-2010-0168) (to Y.H.P), a Liver Scholar Award from the American Association for the Study of Liver Diseases/American Liver Foundation and a research grant from ABMRF (to E.S.), and the National Institute of Health (NIH RO1 DK072237-06, NIH RO1 GM041804-23, NIH P50 AA011999-11) (to D.A.B).
Abbreviations
- Ang II
angiotensin II
- α-SMA
alpha-smooth muscle actin
- BDL
bile duct ligation
- BM
bone marrow
- BMT
bone marrow transplantation
- CCl4
carbon tetrachloride
- CM-H2DCFDA
2'7'-dichlorofluorescein diacetate
- DPI
diphenylene iodonium
- HEP
hepatocytes
- 4-HNE
4-hydroxynonenal
- HSC
hepatic stellate cell
- KC
Kupffer cell
- SEC
sinusoid endothelial cell
- KO
knock out
- NADPH
nicotinamide adenine dinucleotide phosphate
- NOX
NADPH oxidase
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
- WT
wild-type
Footnotes
Disclosures: The authors have no conflicts of interest
References
- 1.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 2.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, Cederbaum AI. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem. 2000;275:20136–20145. doi: 10.1074/jbc.M001422200. [DOI] [PubMed] [Google Scholar]
- 6.Maher JJ, Tzagarakis C, Gimenez A. Malondialdehyde stimulates collagen production by hepatic lipocytes only upon activation in primary culture. Alcohol Alcohol. 1994;29:605–610. [PubMed] [Google Scholar]
- 7.De Bleser PJ, Xu G, Rombouts K, Rogiers V, Geerts A. Glutathione levels discriminate between oxidative stress and transforming growth factor-beta signaling in activated rat hepatic stellate cells. J Biol Chem. 1999;274:33881–33887. doi: 10.1074/jbc.274.48.33881. [DOI] [PubMed] [Google Scholar]
- 8.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 9.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 11.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 12.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 13.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Minicis S, Seki E, Oesterreicher C, Schnabl B, Schwabe RF, Brenner DA. Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology. 2008;48:2016–2026. doi: 10.1002/hep.22560. [DOI] [PubMed] [Google Scholar]
- 15.Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, Engelhardt JF. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 17.Uchinami H, Seki E, Brenner DA, D'Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44:420–429. doi: 10.1002/hep.21268. [DOI] [PubMed] [Google Scholar]
- 18.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 20.Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodama Y, Kisseleva T, Iwaisako K, Miura K, Taura K, De Minicis S, Osterreicher CH, et al. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137:1467–1477. e1465. doi: 10.1053/j.gastro.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paik YH, Kim JK, Lee JI, Kang SH, Kim DY, An SH, Lee SJ, et al. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut. 2009;58:1517–1527. doi: 10.1136/gut.2008.157420. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, Kodama Y, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–1738. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 25.Aram G, Potter JJ, Liu X, Wang L, Torbenson MS, Mezey E. Deficiency of nicotinamide adenine dinucleotide phosphate, reduced form oxidase enhances hepatocellular injury but attenuates fibrosis after chronic carbon tetrachloride administration. Hepatology. 2009;49:911–919. doi: 10.1002/hep.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Minicis S, Seki E, Paik YH, Osterreicher CH, Kodama Y, Kluwe J, Torozzi L, et al. Role and cellular source of nicotinamide adenine dinucleotide phosphate oxidase in hepatic fibrosis. Hepatology. 2010;52:1420–1430. doi: 10.1002/hep.23804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, Li Y, Adamson R, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010;139:1375–1384. doi: 10.1053/j.gastro.2010.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, Kawata S. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology. 2005;41:1272–1281. doi: 10.1002/hep.20719. [DOI] [PubMed] [Google Scholar]
- 29.Vinas O, Bataller R, Sancho-Bru P, Gines P, Berenguer C, Enrich C, Nicolas JM, et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38:919–929. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- 30.Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, Torok NJ. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–443. doi: 10.1002/hep.21093. [DOI] [PubMed] [Google Scholar]
- 31.Geiszt M, Lekstrom K, Brenner S, Hewitt SM, Dana R, Malech HL, Leto TL. NAD(P)H oxidase 1, a product of differentiated colon epithelial cells, can partially replace glycoprotein 91phox in the regulated production of superoxide by phagocytes. J Immunol. 2003;171:299–306. doi: 10.4049/jimmunol.171.1.299. [DOI] [PubMed] [Google Scholar]
- 32.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, et al. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med. 1998;4:165–178. [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 36.Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 37.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 38.Novo E, Marra F, Zamara E, Valfre di Bonzo L, Caligiuri A, Cannito S, Antonaci C, et al. Dose dependent and divergent effects of superoxide anion on cell death, proliferation, and migration of activated human hepatic stellate cells. Gut. 2006;55:90–97. doi: 10.1136/gut.2005.069633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.