Abstract
Objective
Human hypothalamic hamartomas (HH) are highly associated with treatment-resistant gelastic seizures. HH are intrinsically epileptogenic, although the basic cellular mechanisms responsible for seizure activity are unknown. Altered gamma-aminobutyric acid (GABA) function can contribute to epileptogenesis in humans and animal models. Recently, functional GABAA receptor (GABAAR) rundown has been described in surgically-resected human temporal lobe epilepsy tissue. We asked whether functional GABAAR rundown also occurs in human HH neurons.
Methods
GABAAR-mediated currents were measured using perforated patch-clamp recordings in single neurons acutely dissociated from surgically-resected HH tissue. In addition, functional GABAARs were expressed in Xenopus oocytes after microinjection with membrane fractions from either HH or control hypothalamus, and were studied with two-electrode voltage-clamp recordings.
Results
Perforated patch-clamp recordings in dissociated HH neurons showed that repetitive exposure to GABA (5 consecutive exposures to 0.1 mM GABA with 1 sec duration and at 20 sec intervals) induced a time-dependent rundown of whole-cell currents in small HH neurons, while large HH neurons showed much less rundown using the same protocol. Functional rundown was not observed in HH neurons with repetitive exposure to glycine or glutamate. Two-electrode voltage-clamp recordings (6 consecutive exposures to 1 mM GABA with 10 sec duration and at 40 sec intervals) induced GABA-current rundown in Xenopus oocytes microinjected with HH membrane proteins, but not in the oocytes expressing hypothalamic membrane proteins derived from human autopsy controls. Functional rundown of GABA-currents was significantly attenuated by intracellular application of adenosine triphosphate (ATP) or the non-specific phosphatase inhibitor, okadaic acid.
Interpretation
Neurons from surgically-resected human HH demonstrate functional rundown of GABAAR-mediated transmembrane currents in response to GABA agonist exposure. Rundown may be a marker for impaired GABAergic function and a contributing mechanism for seizure genesis within HH tissue.
Keywords: γ-Aminobutyric acid (GABA), GABAA receptor, rundown, hypothalamic hamartoma, Xenopus oocytes, patch-clamp, epilepsy, gelastic seizures
Introduction
Hypothalamic hamartomas (HH) are developmental malformations of the ventral hypothalamus, associated with diverse neurological and endocrinological symptoms, including epilepsy, cognitive impairment, behavioral disturbances and central precocious puberty.1–6 HH is associated with gelastic (laughing) seizures, often beginning in early infancy, that are refractory to standard anti-epilepsy drugs (AEDs).2,7–9 Gelastic seizures arising from HH are an important human model of sub-cortical epilepsy.10,11 Seizure recordings from electrodes placed directly into the HH have demonstrated that HH tissue is intrinsically epileptogenic for gelastic seizures.12–15 However, the basic cellular and molecular mechanisms responsible for epileptogenesis within HH are unknown. Detailed understanding of these mechanisms may lead to novel treatment strategies, or insights into the pathogenesis of other forms of epilepsy.
Recent evidence suggests that functional rundown of the GABAAR may be a mechanistic component of human temporal lobe epilepsy (TLE) 16–17 and epilepsy associated with human cortical dysplasia18. Xenopus oocytes micro-injected with membrane proteins from surgically-resected TLE tissue exhibit functional rundown during repetitive exposure to GABA.16,17,19 GABAAR functional rundown may be an important mechanism in disrupting inhibitory function, contributing to excitation-inhibition imbalance and resulting in epileptic seizures. Functional rundown appears to be mediated by GABAAR desensitization (or internalization) resulting from dephosphorylation of receptor subunits.20,21 Consistent with this observation, phosphatase inhibitors significantly reduce GABAAR rundown in human TLE tissue, potentially representing a novel therapeutic approach to preventing seizures.22,23
HH most likely originate as a result of aberrant development within the ventral hypothalamus, although the specific cellular lineage of these lesions is unknown. HH lesions associated with central precocious puberty (CPP) are attached to the tuber cinereum,6 while those associated with epilepsy are attached more posteriorly near the mammillary bodies.24,25 Microscopically, HH tissue consists of normal-appearing glia and supporting cells, with clusters of small but mature neurons.26,27 Smaller numbers of large neurons are also present, resembling the principal neurons within hypothalamic nuclei, and demonstrate positive immunoreactivity to gonadotropin-releasing hormone (GnRH) and other hypothalamic markers.28–31 The hypothalamus is the ideal control tissue for experimental study of HH. However, while treatment may include resection of the HH, there is a high premium placed upon avoiding injury to the adjacent hypothalamus, and even partial resection of normal adjacent tissue is not acceptable surgical practice. An animal model for HH has not been developed. Accordingly, there is a need to utilize innovative techniques to enable controlled experimentation with HH tissue, particularly for electrophysiological methods that use freshly-resected tissue.
Previous work has demonstrated that (1) there are at least two neuron phenotypes in HH tissue, the small and the large HH neurons,32,33 (2) small HH neurons are GABAergic and exhibit intrinsic pacemaker-like spontaneous firing,32 (3) small HH neurons project synaptic connections to both large and other small HH neurons,34 and (4) large HH neurons are functionally immature, with depolarization and increased firing in response to GABA agonist administration.35,36 This line of evidence suggests that an abnormality related to GABAergic transmission could play a role in HH epileptogenesis. In this report, we examined whether functional GABAAR rundown occurs in HH tissue.
Materials and Methods
Clinical profile of study subjects
Informed consent for the use of resected tissue for research purposes was obtained according to protocols approved by the Institutional Research Board (IRB) of the Barrow Neurological Institute, St. Joseph Hospital and Medical Center, Phoenix, AZ.
Tissue samples were obtained from 23 patients treated at our institution between June 2003 and September 2008. There were 13 females (57%). The mean age at the time of surgery was 10.3 years (median age 6.0 years; range 0.8 – 36.8 years). All patients had treatment-resistant epilepsy, with onset of gelastic seizures at 12 months of age or less in 21 (91%). At the time of surgery, 21 (91%) had multiple daily seizures.
Co-morbidity included developmental delay (full-scale intelligence quotient or estimated developmental quotient <70) in 6 (26%), a history of CPP in 9 (39%), and Pallister-Hall syndrome (OMIM 146510) in one (4%). The mean HH tissue volume was 4.28 cm3 (median 2.62 cm3; range 0.22 – 38.25 cm3) according to the previously published method.23
Human control tissue
For the oocyte microinjection controls, post-mortem hypothalamic tissue derived from normal human donors was obtained from the Harvard Brain Bank (n = 2; 1 male ) and the Human Brain and Spinal Fluid Resource Center at the West Los Angeles Healthcare Center (n = 2; both males). The mean age was 59.3±7.2 years (range 47.0 to 78.0 years). Brain tissue was collected (frozen) within 4 hours after death.
Freshly dissociated neurons from HH tissue
Preparation of acutely dissociated single HH neurons was performed according to previously published methods.32,35,37,38 Briefly, HH tissue obtained at the time of surgical resection was placed immediately into ice-cold dissection solution (2–4°C) that contained (in mM): 136.7 NaCl, 5 KCl, 0.1 Na2HPO4, 0.2 KH2PO4, 9.84 HEPES, 16.6 D-glucose, and 21.9 sucrose; pH 7.3; 330 mOsm; oxygenated with 100% O2. Tissue sections of 350–400 µm were cut using a VF-100 sliding microtome (Precisionary Instruments Inc., Greenville, NC), and were transferred immediately to a recovering bath containing (in mM): 125 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 26 NaHCO3 and 11 glucose for at least 1 hour before dissociation (at 21–22°C). Tissue sections were then treated with 4–6 mg/ml papain (Sigma Chemical Co., St. Louis, MO) for 50–60 minutes (at 31°C). Each tissue section was then mechanically dissociated using fire-polished micro-Pasteur pipettes with oxygenated standard extracellular solution containing (in mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES; pH 7.4 (with Tris-base). Isolated single neurons usually adhered to the bottom of the dish within 30 minutes and were viable for 2–6 hours.
Patch-clamp recordings in dissociated HH neurons
Perforated-patch whole-cell recordings, combined with a U-tube fast application system, allowing for both quick application and removal of drug, were employed in dissociated HH neurons using previously described techniques.32,35,38 The perforated-patch pipette solution contained (in mM): 150 CsCl, 4 MgCl2 and 10 HEPES, adjusted to a pH of 7.2 using Tris-OH (CsCl electrode). Amphotericin B or gramicidin was dissolved in DMSO (40 mg/ml for amphotericin B and 10 mg/ml for gramicidin as a stock) and diluted with internal (patch-pipette) solution to a final concentration of 200–250 µg/ml (amphotericin B) or 70–100 µg/ml (gramicidin) immediately before use. GABA-induced currents were recorded using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). Whole-cell access resistance less than 60 MΩ was accepted for experiments. In 25 HH neurons that exhibited GABA current rundown, the average whole-cell access resistance was 30.3±2.4 MΩ. The series resistance was not compensated in this study. Typically, data were acquired at 10 kHz, filtered at 2 kHz, displayed and digitized on-line (Digidata 1322 series A/D board, Axon Instruments). All experiments were performed at room temperature. All drugs used in this study were purchased from Sigma-Aldrich (St. Louis, MO) and Tocris Cookson, Inc (Ellisville, MO).
Human tissue membrane preparation, injection procedures, and two-electrode voltage-clamp recording from oocytes
The protocol for the preparation of Xenopus oocytes was approved by the Institutional Animal Care and Use Committee of the Barrow Neurological Institute, St. Joseph’s hospital and Medical Center, Phoenix, AZ.
Membrane proteins from HH tissue and normal human hypothalamus were prepared and injected into oocytes according to published protocols.19 In brief, tissue frozen in liquid nitrogen was homogenized in glycine buffer by using a glass homogenizer. The filtrate was centrifuged for 15 minutes at 9,600 × g in an Eppendorf Centrifuge 5417R–F45-30-11 rotor. The supernatant was then centrifuged for 2 hours at 100,000 ×g in a Beckman L-7 Ultracentrifuge SW61 Ti rotor at 4°C. The pellet was washed, re-suspended in 5 mM glycine, and utilized immediately, or frozen at −80 °C for later use. The incubation solution for Xenopus oocytes contains (in mM): 82.5 NaCl, 2.5 KCl, 1 MgCl2, 1 CaCl2, 1 Na2HPO4, 2.5 sodium pyruvate, 0.6 theophylline and 5 HEPES, adjusted to pH 7.5 with 2M NaOH at 24–25°C, sterile filtered with a NALGENE filter (Nalge Nunc International Corporation) with added antibiotics (penicillin − streptomycin − neomycin solution, P4083).
GABAA-currents were elicited by 1 mM GABA. The GABA-current rundown was defined and recorded as previously described.18 The oocyte Ringer solution (OR2) has the following composition (in mM): 92.5 NaCl, 2.5 KCl, 1 MgCl2, 1 CaCl2 and 5 HEPES, adjusted to pH 7.5 with 2M NaOH at 24–25°C.
Statistics
The results were expressed as mean ±SEM. Two-tailed Student’s t-test was used to determine statistical differences (significance p<0.05). Significant p values for each experiment are provided unless p<0.001.
Results
Functional rundown of GABAA-receptors in dissociated single HH neurons
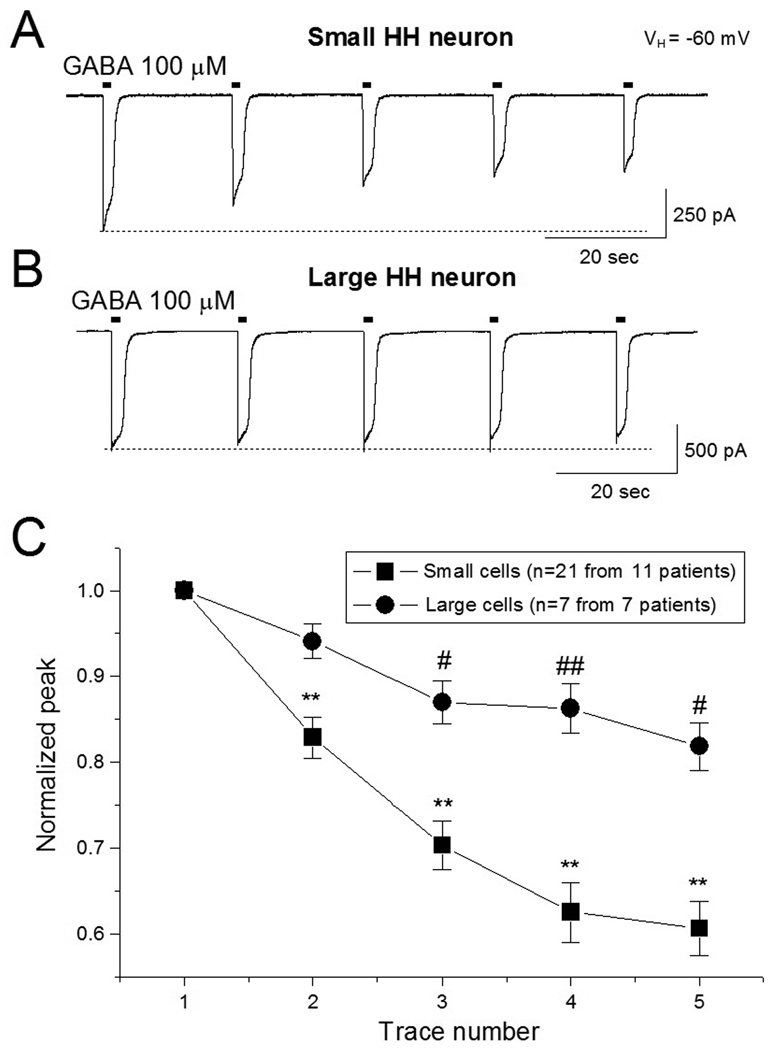
Our initial experiments were designed to determine if GABAAR-mediated current (GABA-current) rundown occurs in dissociated small and large HH neurons. In 21 small HH neurons dissociated from 11 patients (age = 7.8 ± 2.0 years), repetitive exposure with 100 µM GABA (5 exposures of 1 sec duration at 20 sec intervals) induced a progressive decline in the peak amplitude of the GABA-current. The normalized ratio of 5th/1st (1st as 100%) of peak amplitude of the GABA-current was 60.6 ± 3.8% (n=21, p<0.001). In 7 large HH neurons dissociated from 7 HH patients (age = 7.5 ± 2.6 years), the same experimental protocol induced much less decline of GABA-current (Fig. 1B). The normalized ratio (5th/1st) of peak amplitude for GABA-current was 81.8 ± 2.9% (n=7, p=0.293). The difference of the ratio (5th/1st) of GABA-current between small and large HH neurons was significant (p=0.012). Figure 1C summarizes our finding on functional rundown of GABA-currents in small and large HH neurons.
Figure 1.
GABA-current rundown in small and large HH neurons. Under perforated patch recording (amphotericin B, 150 mM CsCl in the pipette solution), GABA was repetitively applied to HH neurons (100 µM, 1 sec exposure at an interval of 20 sec) as shown in the short horizontal bars above each trace (A and B). C: Comparison of GABA-current rundown between small and large HH neurons. In this and all following figures, the vertical bar represents ± SEM. Comparison of rundown between 1st trace (as 100%) and subsequent traces: * p<0.05, ** p<0.01. Comparison of rundown between HH and control: # p<0.05, ## p<0.01.
Functional rundown in HH neurons is specific to GABAA-receptors
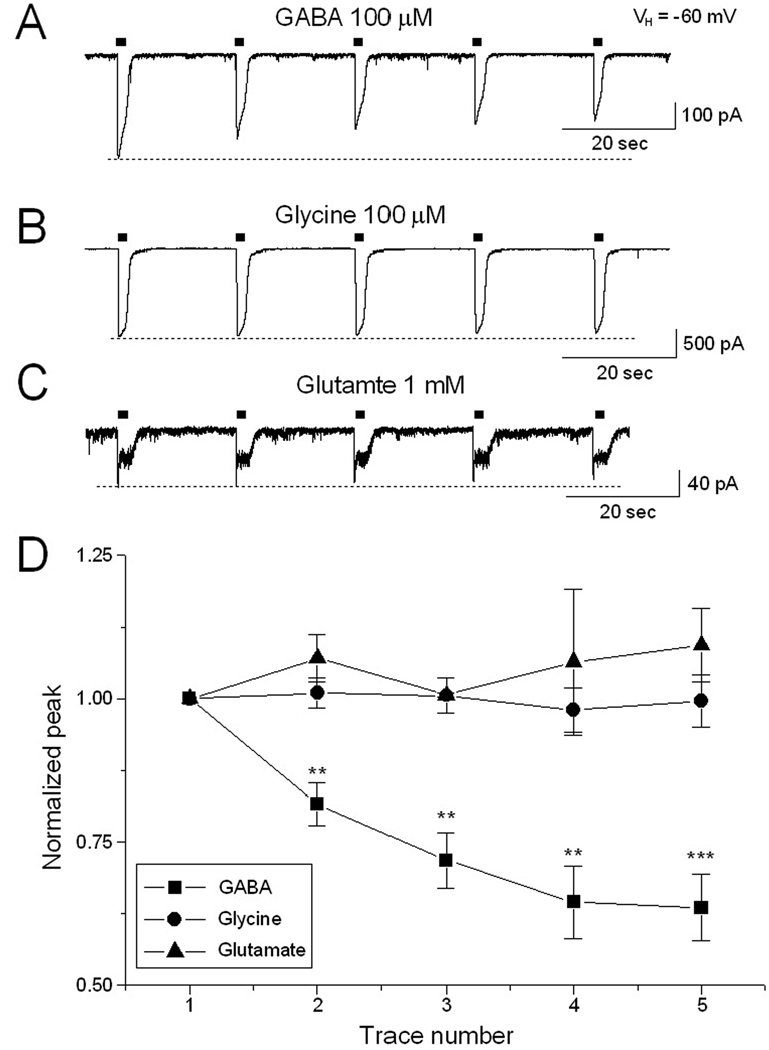
We asked whether functional rundown of ligand-gated transmembrane currents is specific to GABA by examining the effect of repetitive exposure to other receptor agonists (glycine and glutamate) in small HH neurons. Figure 2A shows GABA-current rundown in 7 small HH neurons tested (dissociated from 3 HH patients). In these same HH neurons, repetitive exposure to 100 µM glycine (Fig. 2B, n=7) and 1 mM glutamate (Fig. 2C, n=3) did not result in functional rundown of GABA currents. The normalized ratio (5th/1st) of peak current amplitude was 63.1 ±6.1% for GABA-current (n=7, p<0.001), 99.2 ± 5.0% for glycine-current (n=7, p=0.943) and 107.1 ± 5.2% for glutamate current (n=3, p=0.281), respectively. These results suggest that functional rundown of GABAAR mediated current is a relatively specific event in small HH neurons.
Figure 2.
Comparison of functional rundown observed for different ligand-gated channels. Using the same protocol, functional rundown occurred with GABA-induced currents (A) but not with glycine- (B) or glutamate-induced currents (C). D: Comparison of rundown responses for GABA, glycine, and glutamate exposure.
Comparison of functional rundown of GABAA-receptors between HH and normal human hypothalamus
To determine if functional GABAAR rundown is also present in normal human hypothalamus, we employed a Xenopus oocyte microinjection approach reported by Miledi and colleagues.19 In these experiments, the oocytes injected with either HH or normal human hypothalamic membrane fractions were maintained in incubation solution on a shaker at 18° C. As early as 12 hours after membrane injection, GABAAR-mediated currents can be elicited by 1 mM GABA, suggesting that functional GABAARs have been expressed on the surface of the oocytes, consistent with previous reports.16,23
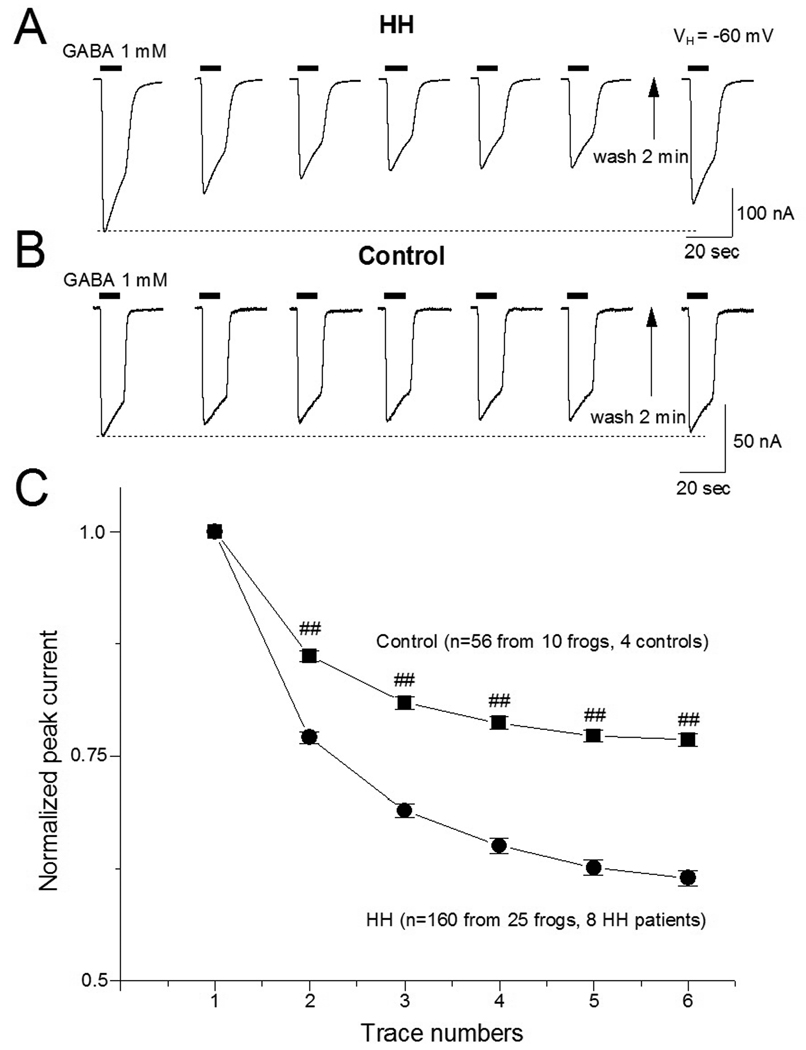
In 160 oocytes (from 25 frogs) injected from 8 HH patients, the mean peak amplitude of GABAA-R-mediated current was 100 ± 65 nA at a holding potential of −60 mV. With the rundown protocol utilized for oocytes (6 consecutive exposures to 1 mM GABA for 10 sec duration at 40 sec intervals),17 the peak amplitude of the GABA-currents in the oocytes injected with HH membrane fractions declined in a time-dependent manner (Fig. 3A). After a 2 min washout, the reduced peak currents recovered to pre-exposure baseline values (Fig. 3A). The normalized ratio (6th/1st) of GABA-current was 61.5 ± 0.8% (n=160, p<0.001).
Figure 3.
GABA-current rundown in oocytes injected with HH and control tissue membrane fractions. A: Typical trace shows an inward current mediated by human HH GABAAR expressed in oocytes. Under the rundown protocol for oocytes (1 mM GABA, 10 sec exposures at 40 sec intervals),55, peak GABA-current declined with repeated exposures. Two minutes after the last GABA exposure, the peak GABA-current response has largely recovered. B. This protocol was repeated with oocytes injected with membrane fractions derived from normal human autopsy controls. C: Cumulative time course of functional rundown in oocytes injected with HH membrane fractions from eight patients (circles; 160 oocytes) and from four normal controls (squares; 56 oocytes). All peak currents were normalized to the first GABA exposure response. Compared to 1st response (trace), subsequent responses (traces 2–6) showed rundown in both HH and control tissues. However, the difference in the peak amplitude rundown response between HH and controls was highly significant (p<0.01 for traces 2–6 indicated by ##).
GABAAR-mediated currents in the oocytes injected with membrane protein fractions derived from normal human autopsy controls (n=4 subjects) showed less rundown (Fig. 3B, n=56). The normalized ratio (6th/1st) of GABA-current was 76.8 ± 0.7% (p=0.046). The difference in altered ratio (6th/1st peak amplitude) between HH and control groups is statistically significant (61.5 ± 0.8% vs. 76.8 ± 0.7%, p<0.001). Figure 3C compares GABA-current rundown between HH and control tissues.
Functional rundown of GABAAR in HH and normal control tissue is not age-dependent
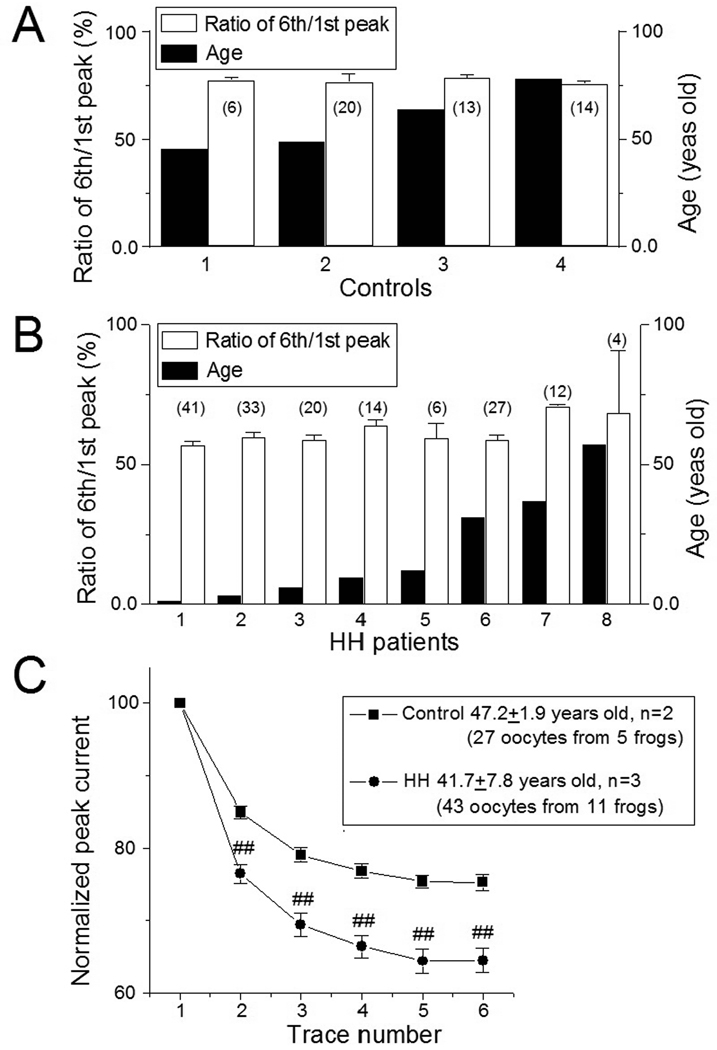
Considering the age difference between the cohort of HH patients included in the oocyte microinjection experiments (mean age 28.0 ± 8.2 years, n=8) and controls (mean age 59.3 ± 7.2 years, n=4, p<0.001), we analyzed the possible influence of age on GABA-mediated functional rundown. There was no significant difference in GABA-current rundown versus age within either the HH or control cohorts (Fig. 4A, B). We also examined functional rundown between the HH and control cohorts on an age-matched basis. In 27 oocytes expressed from 3 HH patients (41.3 ± 8.0 years), GABA induced more functional rundown in comparison to age-marched controls (43 oocytes expressed from 2 controls, 48.0 ± 1.0 years; Fig. 4C). While the difference in age is not significant (p=0.561), the difference in the peak amplitude ratios (6th/1st) of GABA-currents is significantly different (64.6 ± 1.7% for HH group; 75.3 ± 1.1% for control group; p<0.001). These results suggest that the difference in functional rundown of GABA-current between the HH and normal hypothalamus control groups is related to pathology rather than age.
Figure 4.
GABA-current rundown is not age-dependent. A: In four controls with different ages (range 45.3 to 78 years), there is no significant difference in GABA-current rundown. B: Likewise, there is no significant difference in GABA-mediated rundown in the cohort of HH patients (range 1.1 to 57 years). The number in each open bar in panels A and B indicate the number of oocytes tested for each subject. C: An age-matched comparison between the HH (n=3; mean age 41.7 ± 7.8 years) and control (n=2; mean ages 47.2 ± 1.9 years) cohorts. These age-matched results show significantly greater functional rundown in the HH cohort than in control cohort (##p<0.01).
Intracellular ATP eliminates GABAA-receptor rundown in small HH neurons and microinjected oocytes
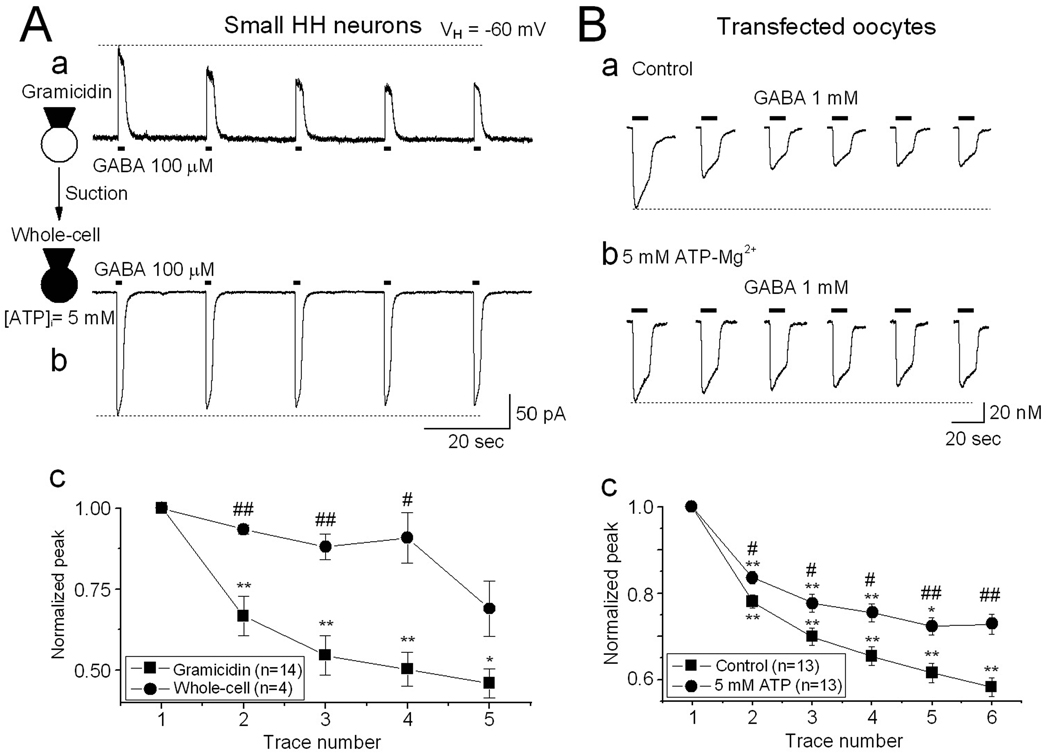
Functional run-down of GABAAR-mediated current is related to the intracellular ATP level.35,36 To test the effects of intracellular ATP on GABAAR rundown in small HH neurons, we added 5 mM ATP-Mg2+ into the recording pipette solution. We initially performed the rundown protocol with acutely-dissociated small HH neurons while utilizing the perforated patch (gramicidin) recording technique, and then converted the perforated patch into conventional whole-cell patch configuration by brief suction (Fig. 5Aa,b). This approach allows ATP-Mg2+ and a high concentration of Cl− (using 150 mM CsCl pipette solution) to diffuse into the recorded neuron. After conventional whole-cell recording for 5–10 min, we repeated the rundown protocol, and compared functional rundown before and after ATP infusion. Under perforated patch conditions, repetitive exposure to GABA induced GABA-current rundown (Fig. 5Aa, n=14), while after intracellular infusion of 5 mM ATP-Mg2+, functional GABAAR rundown was significantly reduced (n=4, Fig. 5Ab,c).
Figure 5.
Increased intracellular ATP prevents GABA-current rundown. A: To compare the effects of intracellular ATP on GABA-current rundown in the same neuron, the rundown protocol was initially performed under perforated patch conditions (gramicidin, 150 mM CsCl and 5 mM ATP-Mg2+ in the pipette solution), in which GABA induced an outward current since the gramicidin pore is not permeable to Cl− ion, and thus external Cl− enters the cell during GABAAR activation. Under this condition, GABA-current rundown occurred (Aa). Brief suction was then applied to convert the recorded neuron to conventional whole-cell mode, and in this way, ATP and Cl− were infused into the cell. After 10 min, the rundown protocol was repeated. Under whole-cell conditions (intracellular 5 mM ATP and 150 mM Cl−), Cl− exits the cell in response to GABA, resulting in an inward current, and GABA-currents showed much less rundown (Ab). Ac: Line graph summarizes the data from A and B, and shows that an increase of intracellular ATP-Mg2+ significantly prevents GABA-current rundown in dissociated small HH neurons. B: Similar results were also obtained from oocytes injected with HH membrane fractions without (Ba) and with (Bb) injection of 5 mM ATP-Mg2+, showing a significant reduction of GABA-current rundown with ATP exposure (Bc).
Oocytes expressing membrane proteins from HH tissue were injected with 5 mM ATP-Mg2+, and GABAAR-mediated currents were measured after 8–10 minute. Figure 5Ba shows that oocytes with elevated intracellular ATP levels demonstrated significantly less GABA-current rundown (6th/1st ratio 72.8 ± 2.3%; n=13) versus oocytes without injected ATP (6th/1st ratio 58.2 ± 2.2%; n=13; p=0.005 between ATP and no ATP groups). These results suggest that intracellular ATP concentrations can mediate GABA-current rundown.
Phosphatase inhibitor okadaic acid (OA) reduces GABA-current rundown in small HH neurons and microinjected oocytes
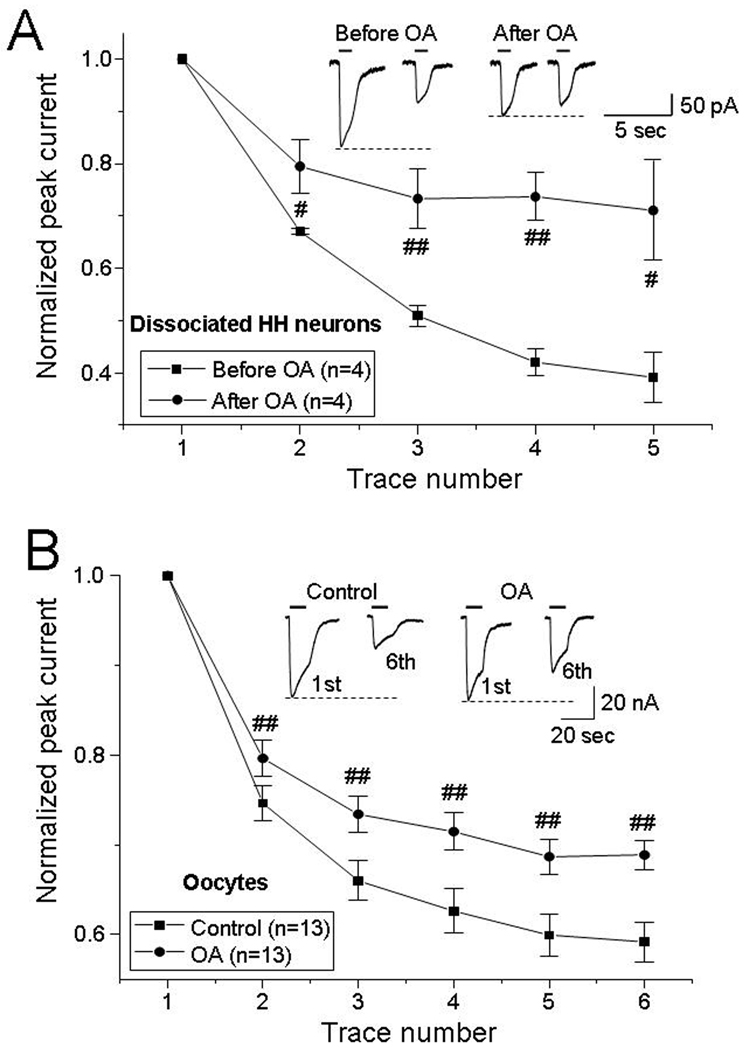
GABAAR function, including GABA-induced rundown, is influenced by phosphorylation of the GABAAR.39–41 To explore whether phosphorylation affects functional rundown in small HH neurons, the nonspecific phosphatase inhibitor okadaic acid (OA) was applied. In 4 dissociated small HH neurons (from 3 HH patients), bath perfusion of 20 nM OA for 10 min significantly eliminated GABA-current rundown (Fig. 6A). In these same small HH neurons, the normalized ratio (5th/1st) of peak GABA-currents was 39 ± 5% prior to OA perfusion, and 71 ± 9% in the presence of OA (n=4, p=0.047).
Figure 6.
Reversal of GABA-current rundown by the non-specific phosphatase inhibitor okadaic acid (OA). In dissociated small HH neurons, GABA-current rundown was reduced after bath application of 20 nM OA for 10 min (panel A; typical tracings are illustrated in the insets; p=0.047). This effect was also observed in oocytes microinjected with membrane fractions of HH tissue, and infused with 2 nM OA (panel B; typical tracings are illustrated in the insets; p=0.002).
GABA-mediated rundown was also significantly reduced in oocytes expressing HH membrane proteins, following the intracellular injection of 2 nM OA (Fig. 6B; n=13 oocytes from 4 HH patients). In these same oocytes, the normalized ratio (6th/1st) of GABA-current was 59.2 ± 2.2% prior to OA injection and 68.9 ± 1.6% after 6–8 min of OA exposure (n=13, p=0.002). These results suggest that dephosphorylation of GABAARs (or closely associated proteins that mediate GABAAR function) contributes to GABAAR rundown in HH tissue.
Discussion
Functional GABAAR rundown in human HH tissue
These findings demonstrate that GABA-mediated functional rundown occurs in surgically-resected epileptogenic HH tissue. In the absence of the availability of surgically-resected normal human hypothalamus, we have utilized Xenopus oocytes microinjected with membrane proteins from HH and normal human autopsy controls, and demonstrated that significantly less GABA-current rundown occurred in the control group under our experimental protocol. Small HH neurons (the most abundant neuron in HH tissue33) demonstrate a significantly greater degree of functional rundown in comparison to large HH neurons, and this appears to be ligand-specific, as it was observed with GABA administration but not following exposure to either glycine or glutamate. Lastly, GABA-mediated rundown in small HH neurons was attenuated by increased intracellular concentrations of ATP, and was largely reversed by a phosphatase inhibitor (okadaic acid). These findings are consistent with the hypothesis that rundown is mediated by dephosphorylation of the GABAAR.
The role of GABAAR rundown in epileptogenesis
Imbalance between cerebral excitation and inhibition caused by functional impairment of GABAergic signaling can result in epileptic seizures.39–43 Functional rundown of the GABAAR is one mechanism by which the inhibitory effects of GABA may be reduced. GABAAR functional rundown, a phenomenon characterized by exposure-dependent decreases in transmembrane Cl− current in response to GABA agonists, has been observed during patch-clamp recordings21,40,44,45 under pathological conditions such as ischemia46 and epilepsy.16,17,19 Recent evidence indicates that functional GABAAR rundown occurs in human epileptic temporal lobe tissue, 16,17,19 and human cortical dysplasia,18 suggesting a possible mechanistic role for GABAAR rundown in epileptogenesis.18
Two neuron phenotypes have been identified in surgically-resected HH tissue: the small and the large HH neuron.32–35,38,47 Approximately 90% of HH neurons are small neurons, which exhibit immunocytochemical staining consistent with a GABAergic phenotype, and demonstrate intrinsic pacemaker-like firing.32 Conversely, most large HH neurons have a functionally immature phenotype with depolarization and enhanced firing in response to GABA agonist administration.35,36,47 Small HH neurons are known to project to large HH neurons34 and may therefore paradoxically excite these neurons, which may mediate the output from HH tissue. Small HH neurons also project to other small HH neurons with inhibitory synapses34 that may act to modulate or perhaps synchronize small HH neuron pacemaker-like firing. This small neuron-large neuron network, driven by GABAergic activity, may be the functional unit for intrinsic seizure genesis within HH.
In this report, we find another electrophysiological difference between small and large HH neurons: small HH neurons exhibit much more GABAAR rundown than large HH neurons. We hypothesize that the difference of GABAAR rundown between small and large HH neurons might contribute to epileptogenesis within HH tissue. One possibility is that functional rundown of the GABA-current may alter inhibitory control over small HH neurons, resulting in more net GABA release to large projection neurons (resulting in depolarization and excitation of the large neurons). Alternatively, rundown may enhance synchrony within small neuron clusters. GABAARs on large HH neurons exhibit less functional rundown, thereby possibly maintaining a high level of excitatory output from the HH to brain circuitry responsible for expressing gelastic seizures.
GABAAR rundown as a potential therapeutic target
Although the precise mechanisms are still unclear, the current hypothesis is that functional ligand-gated receptor rundown reflects a change in the tonic activity of an intracellular modulator (or modulators) of receptor-ion channel activity. Intracellular ATP21,40,44–46,48 and Ca2+ 49 play a role in mediating GABAAR rundown. Previous studies on wild-type receptors have suggested that an increase (or maintenance) in intracellular ATP levels limits rundown of the GABAAR by acting as a substrate for protein kinases.39,40,47,50 Phosphorylation/dephosphorylation is a common mechanism for regulating receptor-ion channel function.51
Selective enhancement of inhibitory drive is a potential strategy to modulate cortical hyperexcitability. Many commonly used anti-epilepsy drugs (AEDs) act via the GABAAR. In this study, we found that an increase of intracellular ATP or pharmacological block of protein phosphatase activity largely reversed GABAAR rundown in HH neurons, suggesting a novel therapeutic strategy for controlling gelastic seizures arising from HH tissue. It has recently been reported that levetiracetam stabilizes GABAARs derived from human epileptic tissue, either by preventing GABAAR rundown or by accelerating GABAAR recovery from rundown.52–55 The possibility that levetiracetam is a disease-specific therapy for gelastic seizures associated with HH should be addressed with a controlled treatment trial.
Acknowledgements
The authors wish to acknowledge our HH patients and their families for their willingness to allow scientific use of surgically-resected tissue. We are grateful to the Harvard Brain Tissue Resource Center, which was supported in part by a R24 MH 068855, and the Human Brain and Spinal Fluid Resource Center (West Los Angeles Healthcare Center) for providing normal human hypothalamic tissue. This work was supported by an NIH grant (NS-056104, J.W., Y.C.) and the grants from the Foundation and Women’s Board of the Barrow Neurological Institute (J.W., J.F.K.).
References
- 1.List CF, Dowman CE, Bagchi BK, et al. Posterior hypothalamic hamartomas and gangliogliomas causing precocious puberty. Neurology. 1958;(3):164–174. doi: 10.1212/wnl.8.3.164. [DOI] [PubMed] [Google Scholar]
- 2.Berkovic SF, Andermann F, Melanson D, et al. Hypothalamic hamartomas and ictal laughter: evolution of a characteristic epileptic syndrome and diagnostic value of magnetic resonance imaging. Ann Neurol. 1988;23(5):429–439. doi: 10.1002/ana.410230502. [DOI] [PubMed] [Google Scholar]
- 3.Iannetti P, Chessa L, Raucci U, et al. Gelastic epilepsy. A clinical contribution. Clin Pediatr (Phila) 1992;31(8):467–470. doi: 10.1177/000992289203100804. [DOI] [PubMed] [Google Scholar]
- 4.Deonna T, Ziegler AL. Hypothalamic hamartoma, precocious puberty and gelastic seizures: a special model of "epileptic" developmental disorder. Epileptic Disord. 2000;2(1):33–37. [PubMed] [Google Scholar]
- 5.Weissenberger AA, Dell ML, Liow K, et al. Aggression and psychiatric comorbidity in children with hypothalamic hamartomas and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2001;40(6):696–703. doi: 10.1097/00004583-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Freeman JL, Zacharin M, Rosenfeld JV, et al. The endocrinology of hypothalamic hamartoma surgery for intractable epilepsy. Epileptic Disord. 2003;5(4):239–247. [PubMed] [Google Scholar]
- 7.Valdueza JM, Cristante L, Dammann O, et al. Hypothalamic hamartomas: with special reference to gelastic epilepsy and surgery. Neurosurgery. 1994;34(6):949–958. doi: 10.1227/00006123-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Leal AJ, Passão V, Calado E, et al. Interictal spike EEG source analysis in hypothalamic hamartoma epilepsy. Clin Neurophysiol. 2002;113(12):1961–1969. doi: 10.1016/s1388-2457(02)00253-5. [DOI] [PubMed] [Google Scholar]
- 9.Andermann F, Arzimanoglou A, Berkovic SF. Hypothalamic hamartoma and epilepsy: the pathway of discovery. Epileptic Disord. 2003;5(4):173–175. [PubMed] [Google Scholar]
- 10.Berkovic SF, Kuzniecky RI, Andermann F. Human epileptogenesis and hypothalamic hamartomas: new lessons from an experiment of nature. Epilepsia. 1997;38(1):1–3. doi: 10.1111/j.1528-1157.1997.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 11.Kerrigan JF, Ng YT, Chung S, et al. The hypothalamic hamartoma: a model of subcortical epileptogenesis and encephalopathy. Semin Pediatr Neurol. 2005;12(2):119–131. doi: 10.1016/j.spen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Munari C, Kahane P, Francione S, et al. Role of the hypothalamic hamartoma in the genesis of gelastic fits (a video-stereo-EEG study) Electroencephalogr Clin Neurophysiol. 1995;95(3):154–160. doi: 10.1016/0013-4694(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 13.Palmini A, Chandler C, Andermann F, et al. Resection of the lesion in patients with hypothalamic hamartomas and catastrophic epilepsy. Neurology. 2002;58(9):1338–1347. doi: 10.1212/wnl.58.9.1338. [DOI] [PubMed] [Google Scholar]
- 14.Kuzniecky R, Guthrie B, Mountz J, et al. Intrinsic epileptogenesis of hypothalamic hamartomas in gelastic epilepsy. Ann Neurol. 1997;42(1):60–67. doi: 10.1002/ana.410420111. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda M, Kameyama S, Wachi M, et al. Stereotaxy for hypothalamic hamartoma with intractable gelastic seizures: technical case report. Neurosurgery. 1999;44(6):1347–1350. doi: 10.1097/00006123-199906000-00115. [DOI] [PubMed] [Google Scholar]
- 16.Palma E, Spinelli G, Torchia G, et al. Abnormal GABAARs from the human epileptic hippocampal subiculum microtransplanted to Xenopus oocytes. Proc Natl Acad Sci U S A. 2005;102(7):2514–2518. doi: 10.1073/pnas.0409687102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ragozzino D, Palma E, Di Angelantonio S, et al. Rundown of GABA type A receptors is a dysfunction associated with human drug-resistant mesial temporal lobe epilepsy. Proc Natl Acad Sci U S A. 2005;102(42):15219–15223. doi: 10.1073/pnas.0507339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansen LA, Peugh LD, Ojemann JG. GABAA receptor properties in catastrophic infantile epilepsy. Epilepsy Res. 2008;81:188–197. doi: 10.1016/j.eplepsyres.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miledi R, Palma E, Eusebi F. Microtransplantation of neurotransmitter receptors from cells to Xenopus oocyte membranes: new procedure for ion channel studies. Methods Mol Biol. 2006;322:347–355. doi: 10.1007/978-1-59745-000-3_24. [DOI] [PubMed] [Google Scholar]
- 20.Chen PL, Scully P, Shew JY, et al. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- 21.Shirasaki T, Aibara K, Akaike N. Direct modulation of GABAAR by intracellular ATP in dissociated nucleus tractus solitarii neurones of rat. J Physiol. 1992;449:551–572. doi: 10.1113/jphysiol.1992.sp019101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palma E, Torchia G, Limatola C, et al. BDNF modulates GABAARs microtransplanted from the human epileptic brain to Xenopus oocytes. Proc Natl Acad Sci U S A. 2005;102(5):1667–1672. doi: 10.1073/pnas.0409442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palma E, Ragozzino DA, Di Angelantonio S, et al. Phosphatase inhibitors remove the run-down of gamma-aminobutyric acid type A receptors in the human epileptic brain. Proc Natl Acad Sci U S A. 2004;101(27):10183–10188. doi: 10.1073/pnas.0403683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diebler C, Ponsot G. Hamartomas of the tuber cinereum. Neuroradiology. 1983;25(2):93–101. doi: 10.1007/BF00333299. [DOI] [PubMed] [Google Scholar]
- 25.Diaz LL, Grech KF, Prados MD. Hypothalamic hamartoma associated with Laurence-Moon-Biedl syndrome. Case report and review of the literature. Pediatr Neurosurg. 1991–1992;17(1):30–33. doi: 10.1159/000120563. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld JV, Harvey AS, Wrennall J, et al. Transcallosal resection of hypothalamic hamartomas, with control of seizures, in children with gelastic epilepsy. Neurosurgery. 2001 Jan;48(1):108–118. doi: 10.1097/00006123-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Boyko OB, Curnes JT, Oakes WJ, et al. Hamartomas of the tuber cinereum: CT, MR, and pathologic findings. AJNR Am J Neuroradiol. 1991;12(2):309–314. [PMC free article] [PubMed] [Google Scholar]
- 28.Judge DM, Kulin HE, Page R, et al. Hypothalamic hamartoma: a source of luteinizing-hormone-releasing factor in precocious puberty. N Engl J Med. 1977;296(1):7–10. doi: 10.1056/NEJM197701062960102. [DOI] [PubMed] [Google Scholar]
- 29.Culler FL, James HE, Simon ML, et al. Identification of gonadotropin-releasing hormone in neurons of a hypothalamic hamartoma in a boy with precocious puberty. Neurosurgery. 1985;17:408–412. doi: 10.1097/00006123-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Price RA, Lee PA, Albright AL, et al. Treatment of sexual precocity by removal of a luteinizing hormone-releasing hormone secreting hamartoma. JAMA. 1984;251(17):2247–2249. [PubMed] [Google Scholar]
- 31.Hochman HI, Judge DM, Reichlin S. Precocious puberty and hypothalamic hamartoma. Pediatrics. 1981;67:236–244. [PubMed] [Google Scholar]
- 32.Wu J, Xu L, Kim DY, et al. Electrophysiological properties of human hypothalamic hamartomas. Ann Neurol. 2005;58(3):371–382. doi: 10.1002/ana.20580. [DOI] [PubMed] [Google Scholar]
- 33.Coons SW, Rekate HL, Prenger EC, et al. The histopathology of hypothalamic hamartomas: study of 57 cases. J Neuropathol Exp Neurol. 2007;66:131–141. doi: 10.1097/nen.0b013e3180302090. [DOI] [PubMed] [Google Scholar]
- 34.Beggs J, Nakada S, Fenoglio K, et al. Hypothalamic hamartomas associated with epilepsy: ultrastructural features. J Neuropathol Exp Neurol. 2008;67:657–668. doi: 10.1097/NEN.0b013e31817d8085. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, DeChon J, Xue F, et al. GABA(A) receptor-mediated excitation in dissociated neurons from human hypothalamic hamartomas. Exp Neurol. 2008;213(2):397–404. doi: 10.1016/j.expneurol.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DY, Fenoglio KA, Simeone TA, et al. GABAA receptor-mediated activation of L-type calcium channels induces neuronal excitation in surgically resected human hypothalamic hamartomas. Epilepsia. 2008;49:861–871. doi: 10.1111/j.1528-1167.2007.01455.x. [DOI] [PubMed] [Google Scholar]
- 37.Ng YT, Rekate HL, Prenger EC, et al. Transcallosal resection of hypothalamic hamartoma for intractable epilepsy. Epilepsia. 2006;47(7):1192–1202. doi: 10.1111/j.1528-1167.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 38.Wu J, Chang Y, Li G, et al. Electrophysiological properties and subunit composition of GABAARs in patients with gelastic seizures and hypothalamic hamartoma. J Neurophysiol. 2007;98(1):5–15. doi: 10.1152/jn.00165.2007. [DOI] [PubMed] [Google Scholar]
- 39.Stelzer A, Kay AR, Wong RK. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- 40.Chen QX, Stelzer A, Kay AR, et al. GABAAR function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurons. J Physiol. 1990;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terunuma M, Xu J, Vithlani M, et al. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28(2):376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laschet JJ, Kurcewicz I, Minier F, et al. Dysfunction of GABAAR glycolysis-dependent modulation in human partial epilepsy. Proc Natl Acad Sci U S A. 2007;104(9):3472–3477. doi: 10.1073/pnas.0606451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sperk G, Furtinger S, Schwarzer C, et al. GABA and its receptors in epilepsy. Adv Exp Med Biol. 2004;548:92–103. doi: 10.1007/978-1-4757-6376-8_7. [DOI] [PubMed] [Google Scholar]
- 44.Gyenes M, Farrant M, Farb DH. "Run-down" of gamma-aminobutyric acidA receptor function during whole-cell recording: a possible role for phosphorylation. Mol Pharmacol. 1988;34(6):719–723. [PubMed] [Google Scholar]
- 45.Gyenes M, Wang Q, Gibbs TT, et al. Phosphorylation factors control neurotransmitter and neuromodulator actions at the gamma-aminobutyric acid type A receptor. Mol Pharmacol. 1994;46(3):542–549. [PubMed] [Google Scholar]
- 46.Harata N, Wu J, Ishibashi H, et al. Run-down of the GABAA response under experimental ischaemia in acutely dissociated CA1 pyramidal neurones of the rat. J Physiol. 1997;500(Pt 3):673–688. doi: 10.1113/jphysiol.1997.sp022052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim DY, Fenoglio KA, Kerrigan JF, Rho JM. Bicarbonate contributes to GABAA receptor-mediated neuronal excitation in surgically-resected human hypothalamic hamartomas. Epilepsy Res. 2009;83:89–93. doi: 10.1016/j.eplepsyres.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang RQ, Dillon GH. Maintenance of recombinant type A gamma-aminobutyric acid receptor function: role of protein tyrosine phosphorylation and calcineurin. J Pharmacol Exp Ther. 1998;286(1):243–255. [PubMed] [Google Scholar]
- 49.Mouginot D, Feltz P, Schlichter R. Modulation of GABA-gated chloride currents by intracellular Ca2+ in cultured porcine melanotrophs. J Physiol. 1991;437:109–132. doi: 10.1113/jphysiol.1991.sp018587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maglóczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28(6):334–340. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- 52.Hovinga CA. Levetiracetam: a novel antiepileptic drug. Pharmacotherapy. 2001;21(11):1375–1388. doi: 10.1592/phco.21.17.1375.34432. [DOI] [PubMed] [Google Scholar]
- 53.Nash EM, Sangha KS. Levetiracetam. Am J Health Syst Pharm. 2001;58(13):1195–1199. doi: 10.1093/ajhp/58.13.1195. [DOI] [PubMed] [Google Scholar]
- 54.Rocamora R, Wagner K, Schulze-Bonhage A. Levetiracetam reduces frequency and duration of epileptic activity in patients with refractory primary generalized epilepsy. Seizure. 2006;15(6):428–433. doi: 10.1016/j.seizure.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Palma E, Ragozzino D, Di Angelantonio S, et al. The antiepileptic drug levetiracetam stabilizes the human epileptic GABAARs upon repetitive activation. Epilepsia. 2007;48(10):1842–1849. doi: 10.1111/j.1528-1167.2007.01131.x. [DOI] [PubMed] [Google Scholar]