Abstract
Ninety percent of cancer-mediated deaths are due to metastasis of the tumor, but the mechanisms controlling metastasis remain poorly understood. Thus, no therapy targeting this process has yet been approved. Chemokines and their receptors are mediators of chronic inflammation and have been linked to the metastasis of numerous cancers. More recently, the CXC chemokine receptor 4 (CXCR4) has emerged as a key mediator of tumor metastasis; therefore, identification of inhibitors of this receptor has the potential to abrogate metastasis. In this report, we demonstrate that acetyl-11-keto-β-boswellic acid (AKBA), a component of the therapeutic plant Boswellia serrata, can downregulate CXCR4 expression in pancreatic cancer cells. The reduction in CXCR4 induced by this terpenoid was found to be cell-type specific, as its expression was also abrogated in leukemia, myeloma, and breast cancer cell lines. Neither proteasome inhibitors nor lysosomal stabilization could prevent the AKBA-induced reduction in CXCR4 expression, and downregulation occurred at the transcriptional level. Suppression of CXCR4 by AKBA was accompanied by the inhibition of pancreatic cancer cell invasion, which is induced by CXCL12, the ligand for CXCR4. In addition, abrogation of the expression of chemokine receptor by AKBA was found in human pancreatic tissues from orthotopic animal model. AKBA also abolished breast tumor cell invasion, and this effect correlated with the disappearance of both the CXCR4 mRNA and CXCR4 protein. Overall, our results show that AKBA is a novel inhibitor of CXCR4 expression and, thus, has the potential to suppress the invasion and metastasis of cancer cells.
Keywords: CXCR4, CXCL12, AKBA, Metastasis
Introduction
The phenomenon of cancer metastasis remains poorly understood. However, metastasis still occurs in as many as 90% of cancer-associated deaths (1). A number of molecules have been linked with cancer metastasis, including matrix metalloproteases (MMPs), vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF), platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), and TWIST (2–6).
More recently, chemokines and their receptors have been identified as mediators of chronic inflammation, which plays a pivotal role in the initiation or progression of numerous cancers, such as lung, colon (7), liver, breast (8), cervix, prostate (9), bladder, ovary (10), esophagus, skin, and lymphatic cancers. Tumor growth is the result of dynamic interactions among tumor cells and between tumor cells and components of the tumor environment. In this regard, chemokines are emerging as key mediators of metastasis, not only in the homing of cancer cells to metastatic sites but also in the recruitment of a number of different cell types to the tumor microenvironment.
Several studies have suggested that cancer cells express chemokine receptors that mediate metastasis to target organs expressing their cognate chemokines. One of the most well studied chemokines in tumor cell migration and metastasis is a stromal cell-derived factor 1α (SDF-1 α, also known as CXC chemokine ligand 12 [CXCL12]) and its receptor, CXCR4 (11). Different cancers preferentially metastasize to different organs, and production of SDF-1α by an organ is responsible for the migration of cancer cells to that organ. CXCR4 has been linked with tumor metastasis in a wide variety of cancers. Because CXCR4 binds to its ligand CXCL12, leading to tumor migration, agents that can interrupt the CXCR4-CXCL12 cell-signaling pathway have the potential to suppress tumor metastasis.
Traditional medicines, which have been used for thousands of years, are still being used by the majority of people in the world today because natural products are generally considered to be safe, inexpensive, and targeted towards a number of diseases. In most cases, however, neither the active components nor their mechanisms of action are well established. Thus, identification of the active components of these traditional medicines and the signaling pathways that they modulate could validate their use in various diseases. Acetyl-11-keto-β-boswellic acid (AKBA) is a derivative of boswellic acid, which is the main component of a gum resin from Boswellia serrata. AKBA has been used traditionally in Ayurvedic systems of medicine to treat a number of inflammatory diseases, including osteoarthritis, chronic colitis, ulcerative colitis, Crohn’s disease, and bronchial asthma, but its mechanism of action is poorly understood.
Recently, it was reported that boswellic acid directly interacts with IκB kinases (12) and suppresses nuclear factor-κB–regulated gene expression (13). In addition, boswellic acid has been shown to potentiate apoptosis in several types of tumor cells, including colon cancer (14), prostate cancer (15), fibrosarcoma (16), hepatoma (17), and malignant glioma (18) cells, through caspase-8 activation (14) and death receptor 5–mediated signaling (15). However, whether AKBA can modulate tumor invasion and metastasis in general is not well established.
In the present report, we investigated whether AKBA can modulate the expression of CXCR4 and, thus, inhibit tumor cell invasion. Our results show that this pentacyclic terpenoid can downregulate constitutive CXCR4 expression in various tumor cells that overexpress this chemokine receptor. This downregulation occurred at the transcriptional level and led to the inhibition of the CXCL12-induced invasion of pancreatic and breast cancer cells.
Materials and Methods
Reagents
Purified acetyl-11-keto-β-boswellic acid (AKBA) was supplied by Sabinsa Corp. A 50 mmol/L stock solution of AKBA was prepared and then stored at −20°C as small aliquots until needed. RPMI 1640, Dulbecco’s modified Eagle’s medium (DMEM)/F12, Iscove’s modified Dulbecco’s medium (IMDM), DMEM, fetal bovine serum (FBS), 0.4% trypan blue vital stain, and an antibiotic-antimycotic mixture were obtained from Invitrogen. A rabbit polyclonal antibody to CXCR4 was obtained from Abcam. Lactacystin was obtained from Calbiochem.
Cell lines and cell culture
The KBM-5 (human chronic myeloid leukemia), U266 (multiple myeloma), MDA-MD-231 (human breast adenocarcinoma), SKBR3 (human breast adenocarcinoma), BxPC-3 (human pancreatic adenocarcinoma), PANC-28 (human pancreatic carcinoma), MIA PaCa-2 (human pancreatic carcinoma), AsPC-1 (human pancreatic adenocarcinoma), and A293 (human embryonic kidney) cells were obtained from American Type Culture Collection. Breast cancer cell lines that express different levels of HER2, including stably transfected MCF7/HER2 and their vector control, were kindly provided by Dr. D. Yu of The University of Texas MD Anderson Cancer Center. MCF7/HER2 and its control cells were cultured in DMEM/F12 supplemented with 10% FBS. U266 cells were cultured in RPMI 1640 with 10% FBS. KBM-5 cells were cultured in IMDM with 15% FBS. SKBR3 cells were cultured in McCoy 5A with 10% FBS. AsPC-1, BxPC-3, PANC-28, MIA PaCa-2, A293, and MDA-MD-231 cells were cultured in DMEM with 10% FBS. Culture media were also supplemented with 100 units/mL penicillin and 100 μg/mL streptomycin, except the media for MCF7/HER2 and its control cells. Cells were maintained at 37°C in an atmosphere of 5% CO2-95% air.
Western blotting
For detection of CXCR4 and HER2, AKBA-treated whole-cell extracts were lysed in lysis buffer (20 mmol/L Tris [pH 7.4], 250 mmol/L NaCl, 2 mmol/L EDTA [pH 8.0], 0.1% Triton X-100, 0.01 mg/mL aprotinin, 0.005 mg/mL leupeptin, 0.4 mmol/L phenylmethylsulfonyl fluoride, and 4 mmol/L NaVO4). Lysates were then centrifuged at 14,000 rpm for 10 minutes to remove insoluble material, and proteins were resolved on a 10% SDS gel. After electrophoresis, the proteins were electrotransferred onto a nitrocellulose membrane, blocked with 5% nonfat milk, and probed with anti-CXCR4 antibodies (1:3,000), anti-HER2 antibodies (1:1000) overnight at 4°C. The blot was washed, exposed to horseradish peroxidase–conjugated secondary antibodies for 2 hours, and examined by chemiluminescence (GE Healthcare).
Electrophoretic mobility shift assay
To evaluate NF-κB activation, we isolated nuclei from cells and carried out electrophoretic mobility shift assays (EMSAs) essentially as previously described (19). In brief, nuclear extracts prepared from cancer cells (1 × 106/mL) were incubated with a 32P-end-labeled 45-mer double-stranded NF-κB oligonucleotide (4 μg of protein with 16 μmol of DNA) from the human immunodeficiency virus (HIV) long terminal repeat (5′-TTGTTACAAGGGACTTTC CGCTG GGGACTTTC CAGGGA GGCGT GG-3′; boldface indicates NF-κB binding sites) for 15 minutes at 37°C. The resulting DNA-protein complex was separated from the free oligonucleotides on 6.6% native polyacrylamide gels. The dried gels were visualized, and radioactive bands were quantitated using a Phosphorimager (GE Healthcare) and ImageQuant software.
RNA analysis and reverse transcription-PCR
Total RNA was extracted using Trizol reagent according to the manufacturer’s instructions (Invitrogen). One microgram of total RNA was converted to cDNA by Superscript reverse transcriptase and then amplified by Platinum Taq polymerase using a Superscript One-Step reverse transcription–polymerase chain reaction (RT-PCR) kit (Invitrogen). The relative expression of CXCR4 was analyzed by quantitative RT-PCR with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The following forward and reverse primer set was used: CXCR4, 5′-GAAGCTGTTGGCTGAAAAGG-3′ and 5′-GAGTCGATGCTGATCCCAAT-3′ (PCR product size, 345 bp; GenBank accession no. NM_003467). The RT-PCR reaction mixture contained 12.5 μL of 2× reaction buffer, 10 μL of cDNA, 0.5 μL each of forward and reverse primers, and 1 μL of RT-Platinum Taq in a final volume of 50 μL. The reaction was done at 50°C for 30 minutes, 94°C for 2 minutes, 94°C for 30 cycles of 15 seconds each, 54°C for 30 seconds, and 72°C for 1 minute, with extension at 72°C for 10 minutes. PCR products were electrophoresed on a 2% agarose gel and then stained with ethidium bromide. Stained bands were visualized under ultraviolet light and photographed.
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was extracted from cells using TRIzol reagent (Invitrogen) following the manufacturer’s protocol. The messenger RNA (mRNA) expression of CXCR4 in PANC-28 cells was determined using real-time PCR. For mRNA quantification, cDNA was synthesized using 3 μg RNA through a RT reaction (iScriptTM cDNA Synthesis Kit, Bio-Rad). Using SYBR Green/Fluorescein PCR Master Mix (SuperArray Bioscience Corporation), cDNA was amplified using real-time PCR with a Bio-Rad MyiQ thermocycler and the SYBR green detection system (Bio-Rad). Samples were run in triplicate to ensure amplification integrity. Manufacturer-supplied (SuperArray Bioscience Corporation) primer pairs were used to measure the mRNA levels of CXCR4. The standard PCR conditions were as follows: 95 °C for 15 min, then 40 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, as recommended by the primer’s manufacturer. The expression levels of genes were normalized to the expression level of GAPDH mRNA in each sample. The threshold for positivity of real-time PCR was determined based on negative controls. For mRNA analysis, the calculations for determining the relative level of gene expression were made using the cycle threshold (Ct) method. The mean Ct values from duplicate measurements were used to calculate the expression of the target gene with normalization to a housekeeping gene used as an internal control (GAPDH) and using the formula.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was done as previously described (20) with some modifications. PANC-28 cells (2 × 106) were incubated with or without 50 μmol/L AKBA for the indicated times and immunoprecipitated with anti-p65 antibody. PCR analyses were carried out for 39 cycles with primers 5′-TCGAAAGCTTATTGCCGCCTACT-3′ (forward) and 5′-TCGAGGATCCCCAACAAACTGAAGTTTCTG-3′ (backward) for CXCR4, the amplified DNA fragment (−417 to +1), which contains two NF-κB binding sites (21).
Invasion assay
The in vitro invasion assay was done using the BD Bio-Coat Matrigel invasion assay system (BD Biosciences) according to the manufacturer’s instructions. Cancer cells (2 × 105) were suspended in medium (10% FBS-DMEM for PANC-28 and MDA-MB-231) and seeded into the Matrigel-precoated transwell chambers with polycarbonate membranes with an 8-μm pore size. After preincubation with or without AKBA (50 μmol/L) for 6 h, the transwell chambers were then placed into 24-well plates and either basal medium only or basal medium containing 100 ng/mL CXCL12 was added. After 24-hour incubation, the upper surfaces of the transwell chambers were wiped out with a cotton swab, and invading cells were fixed and stained with a Diff-Quick staining kit. The invading cells were counted in five randomly selected microscopic fields (×200).
Orthotopic implantation of PANC-28 cells in nude mice
PANC-28 cells were stably transfected with luciferase as described previously (22). Luciferase-transfected PANC-28 cells were harvested from subconfluent cultures after a brief exposure to 0.25% trypsin and 0.2% EDTA. Trypsinization was stopped with medium containing 10% FBS. The cells were washed once in serum-free medium and resuspended in PBS. Mice were anesthetized with ketamine–xylazine solution (Sigma-Aldrich, St. Louis, MO), a small left abdominal flank incision was made, and PANC-28 cells (2 × 106) in 50 μL PBS were injected into the subcapsular region of the pancreas using a 27-gauge needle and a calibrated push button-controlled dispensing device (Hamilton Syringe Co., Reno, NV).
Preparation of extract from tumor samples
Pancreatic tumor tissues (75–100 mg/mouse) from control and experimental mice were minced and homogenized using a Dounce homogenizer and the homogenate was incubated on ice for 1 hr in 0.5 mL of ice-cold buffer A[10 mmol L–1 HEPES (pH 7.9), 1.5 mmol L–1 KCl, 10 mmol L–1 MgCl2, 0.5 mmol L–1 DTT, 0.5 mmol L–1 phenylmethylsulfonyl fluoride (PMSF)]. The supernatant (cytosolic extract) was collected and stored at −80°C until use. Protein concentration was measured by the Bradford assay with BSA as the standard.
Immunohistochemical analysis of CXCR4 in tumor samples
The pancreatic cancer tumor samples were embedded in paraffin and fixed with paraformaldehyde. After being washed in DPBS, the slides were blocked with protein block solution (Dako Cytomation) for 20 min and then incubated overnight with rabbit polyclonal anti-human CXCR4 (1:100). After the incubation, the slides were washed and then incubated with biotinylated link universal antiserum followed by horseradish peroxidase-streptavidin conjugate (LSAB+ kit). The slides were rinsed, and color was developed using 3,3′-diaminobenzidine hydrochloride as a chromogen. Finally, sections were rinsed in distilled water, counterstained with Mayer’s hematoxylin and mounted with DPX mounting medium for evaluation. Pictures were captured with a Photometric Cool SNAP CF color camera (Nikon, Lewisville, TX) and Meta Morph version 4.6.5 software (Universal Imaging, Downingtown, PA).
Statistical analysis
The experiments were performed in triplicate and repeated twice. The P value was obtained after ANOVA and Student–Newman–Keul tests.
Results
The present study was designed to determine the effect of AKBA on the expression of CXCR4, the chemokine receptor that plays a critical role in tumor cell invasion and metastasis, in various tumor cells. We also determined the effect of the AKBA on CXCL12-induced pancreatic and breast cancer cell invasion.
AKBA suppresses CXCR4 in PANC-28 cells
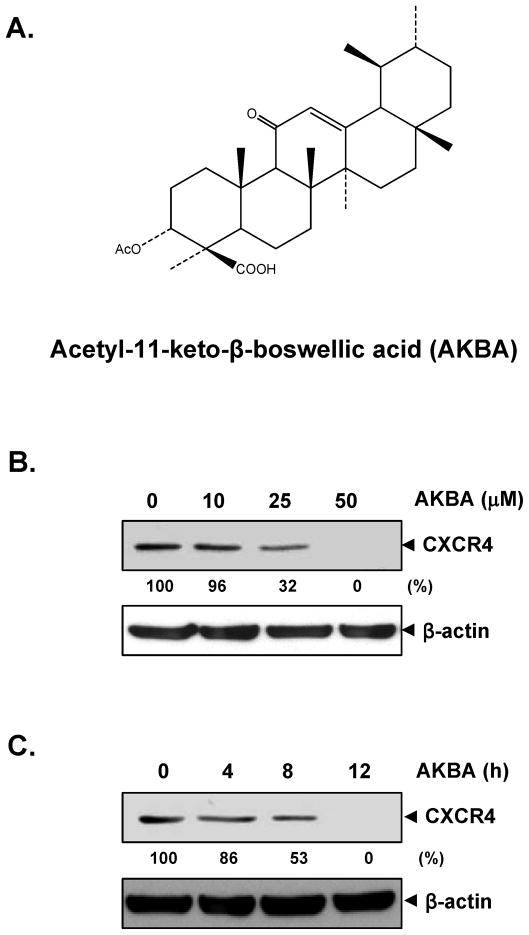
Pancreatic cancer is a highly invasive disease. Its aggressive behavior and lack of sensitivity to most treatments render this tumor a major cause of cancer-related death (23). In particular, chemokine receptor CXCR4 seems to be an important proinvasive factor, and recent reports corroborate the role of CXCR4 signaling in the local invasion and distant metastasis of pancreatic cancer (24). Our previous data also confirmed the constitutive expression of CXCR4 in several pancreatic cancer cells (unpublished data). Therefore, we investigated whether AKBA can modulate the expression of CXCR4 in PANC-28 pancreatic cancer cells. When PANC-28 cells were incubated either with different concentrations of AKBA for 12 hours or with 50 μmol/L AKBA for different time periods, AKBA suppressed the expression of CXCR4 in a dose- and time-dependent manner (Fig. 1B and C). This down-regulation was not due to a reduction in cell viability, because >90% of cells were viable under these conditions (data not shown).
FIGURE 1.
AKBA suppresses CXCR4 expression in PANC-28 cells. A, The chemical structure of AKBA. B, AKBA suppresses CXCR4 levels in a dose-dependent manner. PANC-28 cells (3 × 105) were treated with the indicated concentrations of AKBA for 12 hours. Whole-cell extracts were then prepared and analyzed by western blotting with antibodies against CXCR4. C, AKBA suppresses CXCR4 levels in a time-dependent manner. PANC-28 cells (3 × 105) were treated with 50 μmol/L AKBA for the indicated times, after which western blotting was performed as described above. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. Representative results of three independent experiments are shown.
AKBA downmodulates CXCR4 in different cell types
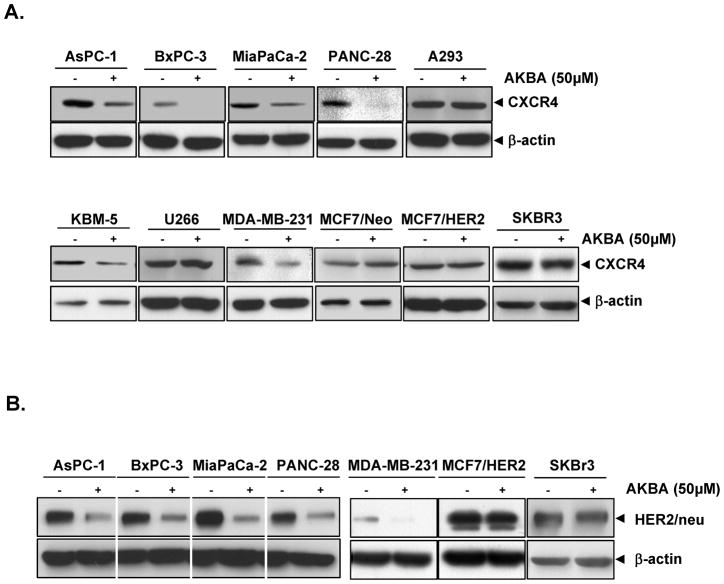
Up to this point, our studies were carried out with pancreatic PANC-28 cancer cell lines. However, CXCR4 is overexpressed in a wide variety of tumor cells. Thus, we investigated whether AKBA downregulated expression of CXCR4 in leukemia (KBM-5), kidney (A293), multiple myeloma (U266), breast (MDA-MB-231, MCF7/Neo, MCF7/HER2, SKBR3), and pancreatic (AsPC-1, BxPC-3, MIA PaCa-2 and PANC-28) cancer cell lines. These cells were treated with 50 μmol/L AKBA for 12 hours and then examined for CXCR4 expression. AKBA downregulated CXCR4 in most of these cell lines and dramatically downregulated CXCR4 in pancreatic (BxPC-3, PANC-28) cancer cells (Fig. 2A). Thus, these results suggest that CXCR4 downregulation by AKBA is not universal but depend on cell type.
FIGURE 2.
AKBA downregulates CXCR4 in different cell types. A, Different cells were incubated with or without 50 μmol/L AKBA for 12 hours. Whole-cell extracts were prepared and analyzed by western blotting with antibodies against CXCR4. B, Different cells were incubated with or without 50 μmol/L AKBA for 12 hours. Whole-cell extracts were prepared and analyzed by western blotting with antibodies against HER2. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. The results shown are representative of three independent experiments.
AKBA downmodulates HER2 expression
Since expression of HER2 has been linked with the expression of CXCR4 in pancreatic and breast cancer (25, 26), we determined whether AKBA could affect the expression of CXCR4 through the downregulation of HER2. As shown in Fig. 2B, we found that all pancreatic and breast cancer cell lines, used in our study, expressed HER2. AKBA downregulated the expression of HER2 in all pancreatic cancer cells, but not in all breast cancer cell lines. The downregulation of HER2 expression correlated with CXCR4 downregulation quite well.
Downregulation of CXCR4 by AKBA is not mediated by its degradation
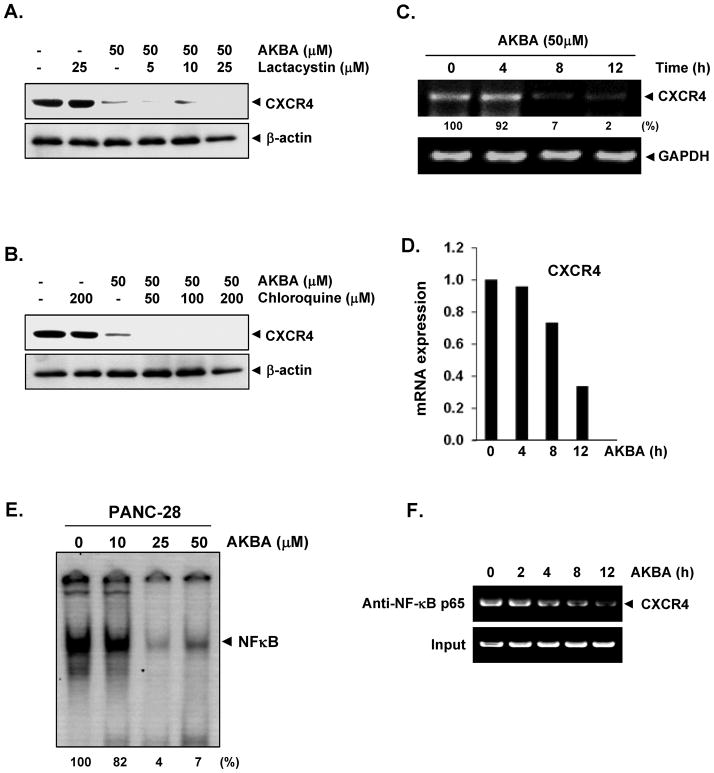
CXCR4 has been shown to be ubiquitinated at its lysine residue, which leads to its degradation (27); thus, we first investigated whether AKBA enhances the downregulation of CXCR4 through proteasomal degradation. To determine this, we treated PANC-28 cells with lactacystin, a proteasome inhibitor, 1 hour before AKBA treatment. Lactacystin had very little effect on the AKBA-induced degradation of CXCR4 (Fig. 3A), suggesting that proteasomal degradation is not likely the mechanism by which AKBA downregulates CXCR4. Because CXCR4 can undergo ligand-dependent lysosomal degradation (27), we treated cells with chloroquine, a lysosomal inhibitor, 1 hour before exposing them to AKBA. To the contrary, the chloroquine seems to exhibit slightly enhance the CXCR4 degradation (Fig. 3B). However this result also indicated that lysosomal degradation was not the primary pathway for the AKBA-mediated suppression of CXCR4 expression.
FIGURE 3.
AKBA suppresses CXCR4 by reducing the mRNA level. A–B, AKBA does not suppress CXCR4 through lysosomal or proteosomal degradation. Cells were treated with the indicated concentrations of A, lactacystin or B, chloroquine for 1 hour at 37°C, followed by treatment with 50 μmol/L AKBA for 12 hours. Whole-cell extracts were prepared and analyzed by western blotting with antibodies against CXCR4. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. The results shown are representative of three independent experiments. C, AKBA suppresses the expression of CXCR4 mRNA. Cells were treated with 50 μmol/L AKBA for the indicated times. Total RNA was isolated and analyzed by an RT-PCR assay. GAPDH was used to show equal loading of total RNA. The results shown are representative of three independent experiments. D, The result of mRNA expression of CXCR4 by quantitative real-time PCR is presented after normalization to GAPDH using the Ct method. E, AKBA inhibits constitutive NF-κB activation in pancreatic cancer cells. PANC-28 cells were incubated with AKBA at the indicated concentrations for 12 hours. The nuclear extracts were assayed for NF-κB activation by electrophoretic mobility shift assay. The results shown are representative of three independent experiments. F, AKBA inhibits binding of NF-κB to the CXCR4 promoter. PANC-28 cells were pretreated with 50 μmol/L AKBA for indicated times and the proteins were cross-linked with DNA with formaldehyde and then subjected to ChIP assay with an anti- p65 antibody.
Downregulation of CXCR4 by AKBA occurs at the transcriptional level
Because AKBA did not suppress CXCR4 expression by enhancing its degradation, we investigated whether suppression occurred at the transcriptional level using both RT-PCR and quantitative PCR (real-time PCR). Cells were treated with AKBA for different times, and the mRNA level was measured. AKBA induced the downregulation of CXCR4 mRNA in a time-dependent manner (Fig. 3C and 3D).
AKBA suppresses constitutive activation of NF-κB in PANC-28 cells
The promoter of CXCR4 is known to contain several NF-κB binding sites. Moreover AKBA has been shown to inhibit NF-κB activation in various cancer cell lines. Thus, it is possible that AKBA exerts its effect on CXCR4 by suppressing NF-κB activation. We used a DNA-binding assay to explore whether AKBA could affect the constitutive NF-κB activation in PANC-28 cells. We found that treatment of PANC-28 cells with AKBA for 12h reduced NF-κB activation in a dose-dependent manner (Fig. 3E). This result suggests that AKBA may downregulate CXCR4 expression by suppressing NF-κB activation.
AKBA inhibits binding of NF-κB to the CXCR4 promoter
Whether the downregulation of CXCR4 by AKBA in PANC-28 cells was due to suppression of NF-κB activation was examined by chromatin immunoprecipitation assay targeting NF-κB binding in the CXCR4 promoter. We found that AKBA suppressed the NF-κB binding to CXCR4 promoter in a time dependent manner (Fig. 3F).
AKBA suppresses CXCL12-induced pancreatic cancer cell invasion
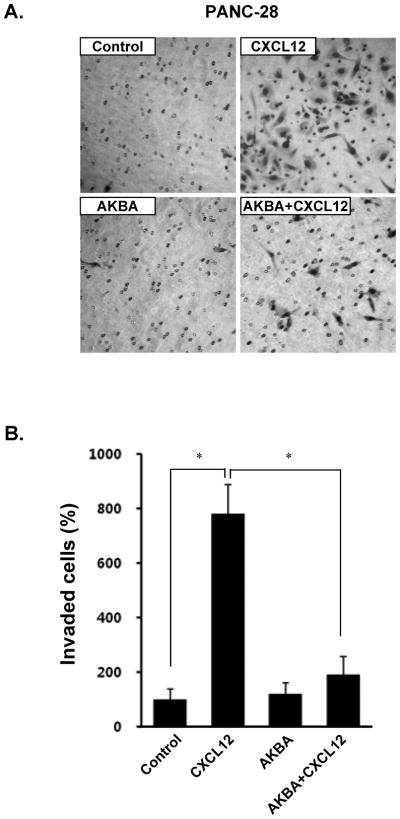
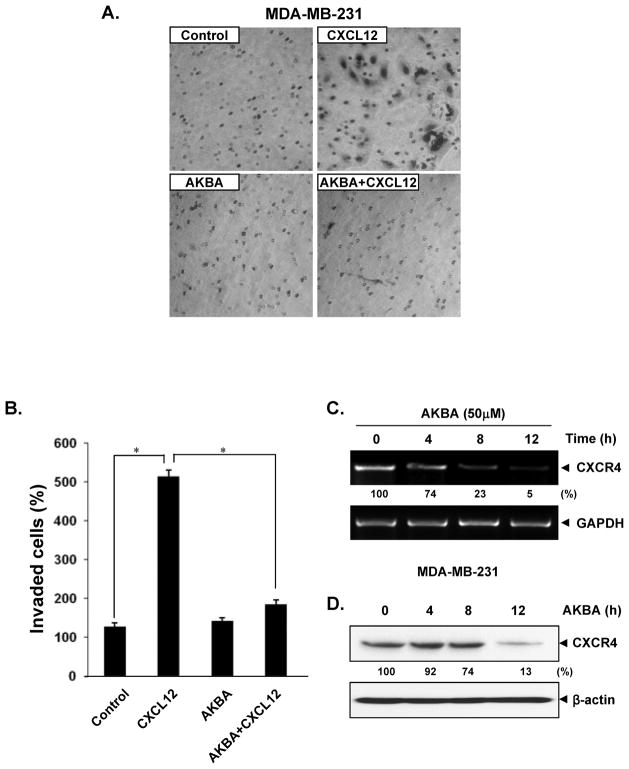
CXCL12/CXCR4 signaling has been shown to play a critical role in pancreatic cancer metastasis (28). Moreover, recent intensive research has suggested that blocking CXCR4 could reduce the metastatic potential of cancer cells (29). Whether downregulation of CXCR4 by AKBA correlates with pancreatic cancer cell migration was examined using an in vitro invasion assay. We found that CXCL12 induced the invasion of pancreatic cancer cells and that AKBA effectively abrogated the invasion (Fig. 4A and 4B).
FIGURE 4.
AKBA suppresses invasion in pancreatic cancer cells. A, PANC-28 cells (3 × 104 cells/well) were seeded in the top chamber of a Matrigel. After preincubation with or without AKBA (50 μmol/L) for 6 hours, the transwell chambers were placed into 24-well plates with basal medium only or basal medium containing 100 ng/mL CXCL12. After incubation, the cell invasion assay was done as described in Materials and Methods. B, Columns mean number of invaded cells, bars SE. *P<0.01
AKBA inhibits CXCL12-induced breast cancer cell invasion
We next determined whether suppression of cell invasion by AKBA is specific to pancreatic cancer cells. It has been reported that the motility and migration of breast cancer cells can be induced when the cells are exposed to their ligand, CXCL12 (8). In addition, breast cancer cell metastasis can be inhibited by silencing CXCR4 (30). To elucidate whether AKBA has an effect on breast cancer cell metastasis, we examined the effect of AKBA on CXCL12-induced cell invasion. Treatment of breast cancer MDA-MB-231 cells with AKBA suppressed the CXCL12-induced invasion of these cells (Fig. 5A and 5B). We also found that the AKBA-mediated downregulation of CXCR4 occurred at both the mRNA (Fig. 5C) and protein levels (Fig. 5D).
FIGURE 5.
AKBA suppresses CXCR4 and breast cancer cell invasion. A, MDA-MB-231 cells (5 × 104 cells/well) were seeded in the top chamber of a Matrigel. After preincubation with or without AKBA (50 μmol/L) for 6 hours, the transwell chambers were placed into 24-well plates with basal medium only or basal medium containing 100 ng/mL CXCL12. B, Columns mean number of invaded cells, bars SE. *P<0.01 C, AKBA suppresses expression of CXCR4 mRNA. MDA-MB-231 cells were treated with 50 μmol/L AKBA for the indicated times. Total RNA was isolated and analyzed by an RT-PCR assay. GAPDH was used to show equal loading of total RNA. D, Cells were incubated with 50 μmol/L AKBA for 12 hours. Whole-cell extracts were prepared and analyzed by western blotting with antibodies against CXCR4. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. Representative results of three independent experiments are shown.
AKBA inhibits CXCR4 expression in orthotopic pancreatic cancer animal model
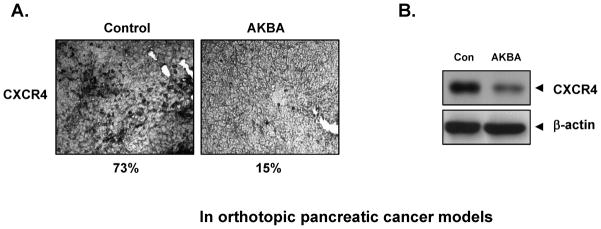
PANC-28 cells were implanted in the pancreas tails of nude mice. After a week, based on the initial IVIS image we randomized animals into 2 groups and started the treatment per the experimental protocol. The treatment was continued for 4 weeks and animals were sacrificed 6 weeks after tumor cell injection. The result of immunohistochemical analysis showed that AKBA suppressed the expression of CXCR4 in human pancreatic tumor tissues (from 73% to 15%, Fig. 6A). Western blotting data further confirmed that AKBA decreased the expression of CXCR4 in pancreatic tumor tissues from mice (Fig. 6B). Under these conditions, where AKBA reduced tumor growth by 50% and metastasis to spleen, liver and lung was significantly reduced (data not shown).
FIGURE 6.
AKBA suppresses CXCR4 in vivo. A, Immunohistochemical analysis showed the inhibition of CXCR4 by AKBA. Percentage, positive staining for the given biomarker. The photographs were taken at the magnification of ×40. B, Western blot showed that AKBA inhibited the expression of CXCR4 in pancreatic tumor tissues.
Discussion
Numerous studies have suggested that the CXCR4-CXCL12 axis plays a pivotal role in triggering tumor metastasis. The goal of the present study was to determine whether AKBA could suppress the expression of CXCR4, a chemokine receptor that has been closely linked with cancer cell growth, invasion, angiogenesis, and metastasis.
We have shown for the first time that AKBA abolishes the expression of CXCR4 in a wide variety of tumor cell types, especially in pancreatic cancer cell lines. However, AKBA didn’t affect the expression of CXCR4 in all cell types. This result suggested that CXCR4 downregulation by AKBA may depend on cell type.
To investigate the mechanism of CXCR4 downregulation by AKBA, we examined the expression of HER2, which has been linked with CXCR4 expression (25, 26). AKBA suppressed the expression of HER2 in all pancreatic cancer cells and MDA-MB-231 breast cancer cells, but didn’t show any inhibition in MCF7/HER2 (HER2 stably transfected) and SKBR3 (express endogenous HER2) breast cancer cells. Downregulation of HER2 correlated with the CXCR4 downregulation by AKBA.
Our results also showed that downregulation of CXCR4 did not occur through proteolytic degradation of the receptor but rather through the inhibition of transcription. Furthermore, suppression of receptor expression led to reduced cell invasion induced by CXCL12.
CXCR4 has been reported to be overexpressed in more than 20 different tumors, including pancreatic and breast cancer cells. Inflammatory cytokines such as TNF (31) and VEGF (32) can induce the expression of CXCR4. Recent reports have documented the ligand-dependent downregulation of CXCR4 expression by lysosomal degradation (33), which involves atrophin-interacting protein-4–mediated ubiquitination and degradation (27). Our results, however, suggest that downregulation of CXCR4 by AKBA is not mediated by ligand-dependent lysosomal degradation but instead is controlled at the transcriptional level.
The transcription factors HIF-1α (34), PPARγ (35), and NF-κB (36) have been suggested to upregulate CXCR4 expression in human cancer cells. AKBA has been documented to downregulate NF-κB activation. Therefore, it is possible that downregulation of CXCR4 occurs through the downregulation of NF-κB activation. Our results showed that AKBA inhibited constitutive NF-κB activity in pancreatic PANC-28 cancer cells. However, the possibility that mechanisms other than suppression of NF-κB activation are involved in the downregulation of CXCR4 by AKBA cannot be ruled out.
In addition to inducing CXCR4 expression, the activation of NF-κB also induces the expression of various adhesion molecules, including intracellular adhesion molecule-1, vascular cell adhesion molecule-1, and endothelial leukocyte adhesion molecule-1, which are also linked with cancer cell metastasis to other organs. Because AKBA can inhibit constitutive NF-κB activation, it is possible that AKBA can suppress the expression of these adhesion molecules as well.
We also found that AKBA suppressed the ligand-induced invasion of both pancreatic and breast cancer cells. This shows the critical role of the CXCR4 receptor in tumor invasion and the potential of AKBA to downregulate the expression or the activity of CXCR4. Elevated levels of CXCR4 have been demonstrated with nodal metastasis of human breast cancer (37). AKBA has been shown to inhibit VEGFR2-mediated angiogenesis (38), cellular proliferation and survival (39), and osteoclastogenesis (13) and to potentiate apoptosis and cytostatic and cytotoxic action (18, 40). Thus, it is possible that some of these antitumor effects of AKBA are also mediated through CXCR4 regulation.
The use of CXCR4 antagonists as cancer therapeutics was recently reviewed (41). One of these CXCR4 antagonists, BKT140, also called AMD3100 and plerixafor, was approved by the Food and Drug Administration (42). Plerixafor was approved not for the prevention of cancer metastasis but as a mobilizer of peripheral blood stem cells. However, this inhibitor has been shown to suppress tumor growth (43), cell adhesion (44), and angiogenesis (45). AKBA is currently in clinical trials. Recently it was reported that 5-Loxin, a novel Boswellia serrata extract enriched with 30% 3-O-acetyl-11-keto-beta-boswellic acid (AKBA), reduces pain and improves physical functioning significantly in osteoarthritis (OA) patients; and it is safe for human consumption (46).
Taken together, our data suggest that AKBA can downregulate the expression of CXCR4, a key receptor involved in the cross-talk between tumor cells and the microenvironment, which contributes to its anti-invasive activity. Thus, AKBA may be a useful anti-cancer therapeutic agent. Further in vivo studies are planned to show the relevance of these observations to cancer treatment.
Acknowledgments
Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from a program project grant from National Institutes of Health (NIH CA-124787-01A2), and a grant from Clayton Foundation for Research, USA.
References
- 1.Gupta PB, Mani S, Yang J, Hartwell K, Weinberg RA. The evolving portrait of cancer metastasis. Cold Spring Harb Symp Quant Biol. 2005;70:291–7. doi: 10.1101/sqb.2005.70.033. [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Leivonen SK, Kahari VM. Transforming growth factor-β signaling in cancer invasion and metastasis. Int J Cancer. 2007;121:2119–24. doi: 10.1002/ijc.23113. [DOI] [PubMed] [Google Scholar]
- 4.van Kempen LC, Coussens LM. MMP9 potentiates pulmonary metastasis formation. Cancer Cell. 2002;2:251–2. doi: 10.1016/s1535-6108(02)00157-5. [DOI] [PubMed] [Google Scholar]
- 5.Orosz P, Echtenacher B, Falk W, Ruschoff J, Weber D, Mannel DN. Enhancement of experimental metastasis by tumor necrosis factor. J Exp Med. 1993;177:1391–8. doi: 10.1084/jem.177.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 7.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–3839. [PubMed] [Google Scholar]
- 8.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 9.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 10.Porcile C, Bajetto A, Barbero S, Pirani P, Schettini G. CXCR4 activation induces epidermal growth factor receptor transactivation in an ovarian cancer cell line. Ann N Y Acad Sci. 2004;1030:162–9. doi: 10.1196/annals.1329.021. [DOI] [PubMed] [Google Scholar]
- 11.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–65. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syrovets T, Buchele B, Krauss C, Laumonnier Y, Simmet T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-α induction in monocytes by direct interaction with IκB kinases. J Immunol. 2005;174:498–506. doi: 10.4049/jimmunol.174.1.498. [DOI] [PubMed] [Google Scholar]
- 13.Takada Y, Ichikawa H, Badmaev V, Aggarwal BB. Acetyl-11-keto-β-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-κB and NF-κB-regulated gene expression. J Immunol. 2006;176:3127–40. doi: 10.4049/jimmunol.176.5.3127. [DOI] [PubMed] [Google Scholar]
- 14.Liu JJ, Nilsson A, Oredsson S, Badmaev V, Zhao WZ, Duan RD. Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis. 2002;23:2087–93. doi: 10.1093/carcin/23.12.2087. [DOI] [PubMed] [Google Scholar]
- 15.Lu M, Xia L, Hua H, Jing Y. Acetyl-keto-β-boswellic acid induces apoptosis through a death receptor 5-mediated pathway in prostate cancer cells. Cancer Res. 2008;68:1180–6. doi: 10.1158/0008-5472.CAN-07-2978. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W, Entschladen F, Liu H, Niggemann B, Fang Q, Zaenker KS, Han R. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cells. Cancer Detect Prev. 2003;27:67–75. doi: 10.1016/s0361-090x(02)00170-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu JJ, Nilsson A, Oredsson S, Badmaev V, Duan RD. Keto- and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in Hep G2 cells via a caspase-8 dependent pathway. Int J Mol Med. 2002;10:501–5. [PubMed] [Google Scholar]
- 18.Hostanska K, Daum G, Saller R. Cytostatic and apoptosis-inducing activity of boswellic acids toward malignant cell lines in vitro. Anticancer Res. 2002;22:2853–62. [PubMed] [Google Scholar]
- 19.Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24:6957–69. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]
- 20.Peters AH, Kubicek S, Mechtler K, O’Sullivan RJ, Derijck AA, Perez-Burgos L, Kohlmaier A, Opravil S, Tachibana M, Shinkai Y, Martens JH, Jenuwein T. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell. 2003;12:1577–1589. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 21.Maroni P, Bendinelli P, Matteucci E, Desiderio MA. HGF induces CXCR4 and CXCL12-mediated tumor invasion through Ets1 and NF-kappaB. Carcinogenesis. 2007;28:267–279. doi: 10.1093/carcin/bgl129. [DOI] [PubMed] [Google Scholar]
- 22.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–18. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans DB, Lee JE, Pisters PW, Charnsangavej C, Ellis LM, Chiao PJ, Lenzi R, Abbruzzese JL. Advances in the diagnosis and treatment of adenocarcinoma of the pancreas. Cancer Treat Res. 1997;90:109–25. doi: 10.1007/978-1-4615-6165-1_6. [DOI] [PubMed] [Google Scholar]
- 24.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, Kawaguchi M, Kobayashi H, Doi R, Hori T, Fujii N, Imamura M. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–3535. [PubMed] [Google Scholar]
- 25.Day JD, DiGiuseppe JA, Yao C, Lai-Goldman MC, Anderson SM, Goodman SN, Kern SE, Hruban RH. Immunohistochemical evaluation of HER2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum Pathol. 1996;2:119–124. doi: 10.1016/s0046-8177(96)90364-0. [DOI] [PubMed] [Google Scholar]
- 26.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J Biol Chem. 2007;282:36971–36979. doi: 10.1074/jbc.M705085200. [DOI] [PubMed] [Google Scholar]
- 28.Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, Mantovani A, Allavena P. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–8427. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 29.Guleng B, Tateishi K, Ohta M, Kanai F, Jazag A, Ijichi H, Tanaka Y, Washida M, Morikane K, Fukushima Y, Yamori T, Tsuruo T, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005;65:5864–71. doi: 10.1158/0008-5472.CAN-04-3833. [DOI] [PubMed] [Google Scholar]
- 30.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–12. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 31.Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL. The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res. 2005;65:10355–10362. doi: 10.1158/0008-5472.CAN-05-0957. [DOI] [PubMed] [Google Scholar]
- 32.Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62:7203–7206. [PubMed] [Google Scholar]
- 33.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 34.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 down-regulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–11. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 35.Richard CL, Blay J. Thiazolidinedione drugs down-regulate CXCR4 expression on human colorectal cancer cells in a peroxisome proliferator activated receptor γ-dependent manner. Int J Oncol. 2007;30:1215–22. [PubMed] [Google Scholar]
- 36.Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res. 2005;65:9891–9898. doi: 10.1158/0008-5472.CAN-05-1293. [DOI] [PubMed] [Google Scholar]
- 37.Cabioglu N, Sahin A, Doucet M, Yavuz E, Igci A, O’Yildirim E, Aktas E, Bilgic S, Kiran B, Deniz G, Price JE. Chemokine receptor CXCR4 expression in breast cancer as a potential predictive marker of isolated tumor cells in bone marrow. Clin Exp Metastasis. 2005;22:39–46. doi: 10.1007/s10585-005-3222-y. [DOI] [PubMed] [Google Scholar]
- 38.Pang X, Yi Z, Zhang X, Sung B, Qu W, Lian X, Aggarwal BB, Liu M. Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res. 2009;69:5893–900. doi: 10.1158/0008-5472.CAN-09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunnumakkara AB, Nair AS, Sung B, Pandey MK, Aggarwal BB. Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Mol Cancer Res. 2009;7:118–28. doi: 10.1158/1541-7786.MCR-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Park YS, Lee JH, Bondar J, Harwalkar JA, Safayhi H, Golubic M. Cytotoxic action of acetyl-11-keto-beta-boswellic acid (AKBA) on meningioma cells. Planta Med. 2002;68:397–401. doi: 10.1055/s-2002-32090. [DOI] [PubMed] [Google Scholar]
- 41.Teicher BA. Antibody-drug conjugate targets. Curr Cancer Drug Targets. 2009;9:982–1004. doi: 10.2174/156800909790192365. [DOI] [PubMed] [Google Scholar]
- 42.Burger JA, Stewart DJ. CXCR4 chemokine receptor antagonists: perspectives in SCLC. Expert Opin Investig Drugs. 2009;18:481–90. doi: 10.1517/13543780902804249. [DOI] [PubMed] [Google Scholar]
- 43.Barbero S, Bonavia R, Bajetto A, Porcile C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T, Schettini G. Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 2003;63:1969–74. [PubMed] [Google Scholar]
- 44.Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood. 2001;97:3342–8. doi: 10.1182/blood.v97.11.3342. [DOI] [PubMed] [Google Scholar]
- 45.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sengupta K, Alluri KV, Satish AR, Mishra S, Golakoti T, Sarma KV, Dey D, Raychaudhuri SP. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res Ther. 2008;10(4):R85. doi: 10.1186/ar2461. [DOI] [PMC free article] [PubMed] [Google Scholar]