Abstract
The aim of this study was to prospectively assess the quality of life (QOL), behavioral/emotional functioning, and cognitive status of children undergoing treatment for hepatitis C virus (HCV) infection. One hundred fourteen children (5 to 18 years old) enrolled in a multi-site randomized clinical trial (Peds-C) to evaluate peginterferon alpha 2a (PEG 2a) with ribavirin (RV) or with placebo (PL) completed several standardized measures prior to treatment and at 24 weeks, 48 weeks, 6 months following treatment, and at two annual follow-up visits. After 24 weeks of treatment, mean physical QOL scores declined significantly for both groups from baseline to 24 weeks of treatment (F = 5.8, p = 0.004), although scores remained in the average range. There were no significant time or group effects for behavioral/emotional or cognitive functioning. Three children (5%) in the PEG 2a + RV group and no children in the PEG 2a + PL group had a clinically significant increase in depression symptoms. For those children who received 48 weeks of treatment, there were no significant time or group effects on any of the outcome measures (p's > 0.05). A majority of children in both the PEG 2a + RV and PEG 2a + PL groups experienced no clinically significant change in physical QOL, behavioral adjustment, depression, or cognitive functioning during or after treatment.
Conclusion
Overall QOL and psychosocial functioning are not deleteriously impacted by PEG 2a + RV or PL treatment of children with HCV.
Keywords: pediatrics, HCV, parents, clinical trial, quality of life
In the United States, an estimated 23,000 to 43,000 children are infected with hepatitis C virus (HCV), with 7,200 new cases annually.(1) While the course of HCV infection in children is generally more benign than that seen in adults, approximately 30 percent of infected children will develop symptomatic or progressive disease and be at risk for cirrhosis and hepatocellular carcinoma later in life.(2-4) However, there is emerging evidence that chronic HCV treatment in children and adolescents can yield virologic results comparable to, or even better than, those in adults.(5-7) For instance, in the largest randomized controlled trial to date, Schwarz and the Peds-C Clinical Research Network (5) showed that peginterferon alfa-2a (PEG 2a) plus ribavirin (RV) is superior to PEG 2a plus placebo (PL) for chronic HCV in children and adolescents. Sustained virologic response (SVR) was achieved for 53% of the 55 children who received PEG 2a + RV, in comparison to 21% of the 59 children randomized to PEG 2a + PL.
The chronic HCV literature is replete with findings of quality of life (QOL), psychological, and cognitive deficits in treatment-naïve adults as well as those receiving interferon and ribavirin therapy.(8-17) However, comparatively less is known about how HCV and its treatment affects these clinical parameters in children. Nydegger et al. reported in a small group of children who acquired HCV in the first year of life had lower QOL compared to non-infected children.(18) In contrast, baseline data from the Peds-C study showed that treatment-naïve children with HCV do not manifest global impairment in QOL, cognitive, behavioral, or emotional functioning.(19) Iorio et al. (20) published one of the only prospective studies examining QOL in children undergoing treatment for chronic HBV and HCV infection. They showed that while tolerability was not a limiting factor in interferon-alpha treatment in children, QOL deteriorated significantly during treatment and did not return to baseline until approximately three months after stopping treatment. They noted three- to four-fold increases in irritability and all the psychosocial dimensions of the Sickness Impact Profile (e.g. Social Interaction, Emotional Behavior, Alertness Behavior) during treatment. This finding suggests that significant QOL morbidity may accompany interferon-alpha treatment in children as it does in adults.
While QOL is emerging as an important endpoint in pediatric clinical trials of HCV treatments, there is a paucity of data on other patient-oriented outcomes that are pertinent to child development and function. The inclusion of patient-oriented outcomes such as behavioral adaptation, depression, anxiety, and cognitive functioning in HCV pediatric clinical trials is important for several reasons. The impact of HCV treatments on these parameters in children is not presently known and such information will allow health professionals to better inform patients and their parents about the range of possible side-effects. Also, identifying the complete range of morbidity associated with chronic HCV and its treatments may facilitate the development and implementation of psychotherapeutic or psychopharmacological interventions aimed at attenuating morbidity in these children.
The aim of this study was to prospectively assess the QOL, behavioral/emotional functioning, and cognitive status of children participating in the Peds-C study.(5) As part of this multi-site randomized controlled clinical trial evaluating the differential effect of PEG 2a + RV versus PEG 2a + PL, children and their parents participated in an extensive psychosocial assessment protocol at multiple time points. We hypothesized, based on findings in the adult literature on interferon-based treatments, that children in both study arms would report worsening QOL, behavioral/emotional functioning, and cognitive functioning relative to their baseline (pre-treatment) level of functioning.
Patients and Methods
Eligible study participants were 5 to 18 yr old children with documented HCV viremia on two tests ≥6 months apart and/or one positive test in a child with maternal-fetal transmission, chronic hepatitis consistent with HCV infection on liver biopsy within 36 months of screening, and compensated liver disease (Child-Pugh Grade A clinical classification). Exclusion criteria were any prior treatment with interferon or ribavirin, decompensated liver disease, major depression, or history of other severe illness. Written informed consent was obtained from the child's parent or guardian, and children over 12 years old provided written assent.
Development and implementation of the Peds-C study design has been described elsewhere.(5,21, www.clinicaltrials.gov) In brief, this was a multi-site study in which enrolled participants were randomized (stratified by site according to HCV genotype) to receive either PEG 2a + RV or PEG 2a + PL. Children received PEG 2a subcutaneously once weekly (180μg/1.73m2 body surface area) plus either RV administered orally on a daily basis (15 mg/kg) or placebo tablets. Virologic response was assessed after the first 24 weeks of treatment. For children with undetectable HCV RNA, treatment continued in their originally assigned group for an additional 24 weeks, thus resulting in 48 total weeks of therapy. For children with detectable HCV RNA in the PEG 2a + RV arm, treatment was stopped and they were considered nonresponders. For children with detectable HCV RNA in the PEG 2a + PL arm, they were also considered nonresponders but were offered “open label” PEG 2a + RV therapy for 48 weeks (although treatment was terminated if HCV RNA was detected after 24 weeks).
Measures of QOL, behavioral/emotional, and cognitive functioning were obtained for all children at baseline (pre-treatment) and at 24 weeks, 48 weeks, 6 months following treatment, and at two subsequent annual visits. A detailed description of the rationale for measurement selection and implementation is published elsewhere.(21)
Quality of Life
QOL was assessed using the Child Health Questionnaire (CHQ) – Parent Form 50 (22), a standardized measure of functional status and well-being for children 5 to 18 years old that is completed by the child's parent or guardian. The CHQ yielded two composite scores for physical health and psychosocial functioning, as well as scores for 11 different scales: physical functioning, role/social limitations (emotional, physical), general health, bodily pain/discomfort, parent impact (emotional, time), self-esteem, mental health, general behavior, and family impact. Parents answered questions based on their perceptions of the child's QOL over the past 4 weeks. Scores range from 0—100, with higher scores reflecting better QOL. T scores ≤ 40 on the Physical Summary and Psychosocial Summary composite measures are considered indicative of clinical impairment in QOL. As previously described (21), a self-report version of the CHQ was administered to children 10 years and older. However, there was more variability in its completion over time compared to the parent version, and the correspondence between parent- and child-report versions is the focus of a separate analysis and manuscript.
Behavioral/Emotional
The Child Behavior Checklist (CBCL)(23) was used to assess behavioral functioning and was completed by the child's parent or guardian. It yielded scores for 3 composite scales (Internalizing, Externalizing, and Total Behavior Problems) and 8 clinical scales: Anxious/Depressed, Withdrawn/Depressed, Somatic Problems, Social Problems, Thought Problems, Attention Problems, Rule-Breaking Behavior, and Aggressive Behavior. Raw scores were converted to T scores, with a mean of 50 and standard deviation (SD) of 10. Higher scores reflect more behavioral or emotional problems. T scores ≥ 65 are considered indicative of clinically significant behavior problems. While the CBCL was validated in children 6 to 18 years old, we used it in the current study for children as young as 5 years old to assess symptoms (e.g., social problems, thought problems) that are not part of the CBCL preschool version.
The Children's Depression Inventory (CDI)(24) was completed by the child and it assessed affective, cognitive, and behavioral symptoms of depression. A total score ≥ 19 is indicative of possible clinical depression. The CDI was administered at every patient visit to screen for depression. If the CDI score was ≥ 19, then the site investigator interviewed the child to further assess for clinical depression. If the child met criteria for major depression, s/he was referred to a mental health professional for further assessment and treatment. Study medications were discontinued if symptoms did not improve within eight weeks of initiation of treatment for depression or if other significant symptoms developed (e.g., substance abuse, recent suicide attempt or current suicidal ideation with a plan, psychosis, bipolar disorder, or inability of family to monitor child's safety).
Cognitive Functioning
Cognitive functioning was measured using the Behavior Rating Inventory of Executive Function (BRIEF)(25). The BRIEF is a parent-report measure of executive functioning in children ages 5 to 18 years. It includes two broad indices (Behavioral Regulation, Metacognition) and 8 clinical scales (Inhibit, Shift, and Emotional Control, Initiate, Working Memory, Plan/Organization, Organization of Materials, Monitor), and a Global Executive Composite score. Raw scores were converted to T scores (mean = 50, SD =10), with higher scores reflecting more cognitive impairment. T scores ≥ 65 are considered indicative of clinical impairment in executive function.
The study protocol was approved by the Institutional Review Boards at all study sites. An independent, external data and safety monitoring board (DSMB) appointed by and advisory to the National Institute of Diabetes and Digestive and Kidney Diseases reviewed and approved the study design and monitored its conduct.
Statistical Analysis
Descriptive statistics were calculated to summarize the medical, sociodemographic, and outcome variables. Because children who did not respond to PEG 2 a + PL after 24 weeks were offered “open label” PEG 2a + RV therapy, a multi-level approach to analysis was necessary. In the first set of analyses, we included all children as originally randomized to examine the effect of treatment group over the first 24 weeks of therapy prior to the first treatment decision point. We used repeated measures analyses of variance to assess for treatment group and time effects on all outcomes. We then classified children into three groups based on clinical decline, improvement, or no change in scores from baseline to 24 weeks. Clinical decline was operationalized as a >1 SD change in score plus a change in score classification from no impairment at baseline to clinical impairment at 24 weeks. Clinical improvement was defined as >1 SD change in score plus a change in score classification from clinical impairment at baseline to no impairment at 24 weeks. In the second set of analyses, we used only those children with nondetectable HCV RNA at the 24-week assessment to examine whether continuation of the assigned treatment for an additional 24 weeks differentially impacted children by group assignment over time. We used repeated measures analyses of variance to examine for main and interaction effects over time. Clinically significant decline/improvement in functioning was again examined as noted above. To reduce the probability of Type I error rate, analyses initially were performed only on the composite or summary scales of the outcome measures. If a statistically significant main or interaction effect emerged, we examined for differences on the individual scales for the respective outcome measure. In the final set of analyses, we calculated Pearson correlation coefficients and T tests to examine the relationship between sociodemographic characteristics and outcomes at the different time points. Due to the large number of tests in this analysis cluster, we considered p < 0.01 as the level of statistical significance. PAWS (Version 17.0) statistical software was used for all analyses.
Results
Sample Characteristics
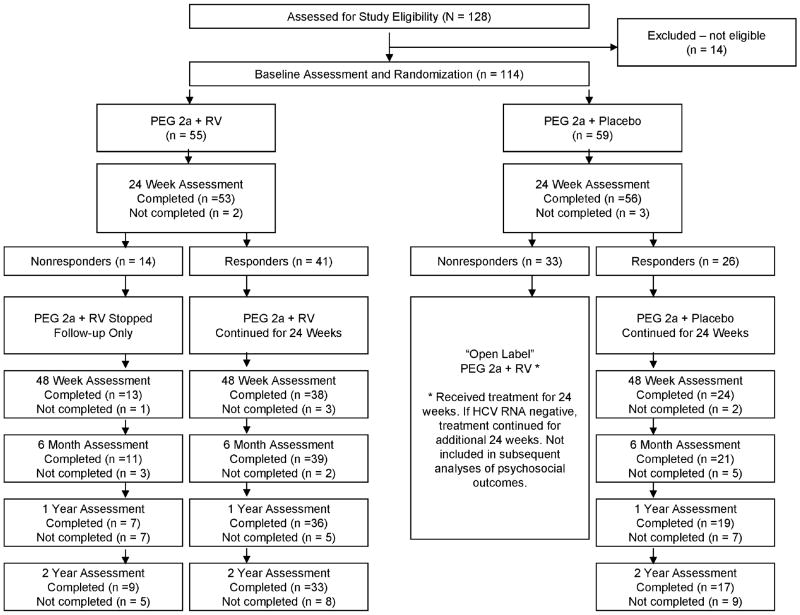
Figure 1 illustrates participant progression through the study. Participants were 114 children who met study eligibility criteria. Fifty-five children were randomized to the PEG 2a + RV group and 59 children were randomized to the PEG 2a + PL group. There were no significant differences between the two groups on baseline sociodemographic or medical characteristics (Table 1). The total sample had a mean age of 10.7 years (range 5 to 17), 75% was white, 45% was female, and 81% had HCV genotype 1.
Figure 1.
CONSORT diagram showing patient progression through the study.
Table 1.
Baseline characteristics by treatment group and for the total sample
| Characteristic | PEG 2a + RV (n = 55) |
PEG 2a + PL (n = 59) |
Total Sample (N = 114) |
|
|---|---|---|---|---|
| Age, yrs | 10.7 ± 3.3 | 10.8 ± 3.6 | 10.7 ± 3.4 | |
| 5 - 11 | 30 (54%) | 30 (51%) | 60 (53%) | |
| 12 - 18 | 25 (46%) | 29 (49%) | 54 (47%) | |
| Gender | Male | 27 (49%) | 36 (61%) | 63 (55%) |
| Female | 28 (51%) | 23 (39%) | 51 (45%) | |
| Race | White | 43 (78%) | 42 (71%) | 85 (75%) |
| Black / African American | 1 (2%) | 4 (7%) | 5 (4%) | |
| Asian | 3 (6%) | 2 (3%) | 5 (4%) | |
| American Indian / Alaska Native | 1 (2%) | 0 (0%) | 1 (1%) | |
| More than one race | 1 (2%) | 5 (9%) | 6 (5%) | |
| Unknown | 6 (11%) | 6 (10%) | 12 (11%) | |
| HCV genotype | 1 | 45 (82%) | 47 (80%) | 92 (81%) |
| 2 | 4 (7%) | 3 (5%) | 7 (6%) | |
| 3 | 6 (11%) | 7 (12%) | 13 (11%) | |
| 6 | 0 (0%) | 2 (3%) | 2 (2%) | |
| Transmission mode | Maternal-infant | 39 (71%) | 47 (80%) | 86 (75%) |
| Transfusion | 6 (11%) | 2 (3%) | 8 (7%) | |
| Other | 10 (19%) | 10 (18%) | 20 (18%) | |
| Estimated duration of infection, mos. | 105 ± 56 | 111 ± 55 | 108 ± 55 | |
| ALT (IU/L) | 49 ± 59 | 49 ± 59 | 49 ± 59 |
Note: Treatment groups did not differ significantly on any characteristic (all p-values > 0.05).
All Children: Baseline to 24 Weeks
Table 2 displays the means and standard deviations for the composite or summary scores for each outcome measure by treatment group.
Table 2.
Means ± standard deviations for primary outcomes at baseline and 24 weeks by treatment group, N = 114
| PEG 2a + RV (n = 55) | PEG 2a + PL (n = 59) | |||
|---|---|---|---|---|
| Measure | Baseline | 24 Weeks | Baseline | 24 Weeks |
| CHQ Physical Summary | 52.1 ± 4.8 | 49.8 ± 7.5 | 51.1 ± 7.1 | 49.0 ± 8.6 |
| CHQ Psychosocial Summary | 52.1 ± 7.9 | 52.3 ± 10.2 | 50.1 ± 10.4 | 52.2 ± 7.8 |
| CBCL Internalizing | 52.4 ± 8.5 | 51.0 ± 11.0 | 52.2 ± 10.9 | 50.1 ± 11.0 |
| CBCL Externalizing | 50.4 ± 9.4 | 48.8 ± 10.3 | 50.0 ± 10.4 | 48.5 ± 9.9 |
| CBCL Total Behavior Problem | 51.5 ± 9.3 | 49.7 ± 10.2 | 50.6 ± 11.6 | 48.2 ± 11.1 |
| CDI Total Score | 5.9 ± 4.2 | 6.2 ± 5.6 | 5.9 ± 4.6 | 5.1 ± 3.1 |
| BRIEF Global Executive Composite | 53.5 ± 9.9 | 52.2 ± 10.1 | 53.7 ± 12.0 | 51.6 ± 9.6 |
Note: CHQ = Child Health Questionnaire; CBCL = Child Behavior Checklist; CDI = Child Depression Inventory; BRIEF = Behavior Rating Inventory of Executive Function.
Quality of Life
There was a significant time main effect for the CHQ Physical Summary score (F = 5.81, p = 0.004), but no significant effect for treatment group or significant time by treatment group interaction effect. The mean Physical Summary score declined significantly from baseline (51.7 ± 6.0) to the 24 week assessment (49.4 ± 8.1), although both means were within the average range. Individual CHQ scale analysis showed that there was a statistically significant decrease (worsening) in Bodily Pain (82.9 ± 18.5 vs. 74.5 ± 23.0, p < 0.001) and General Health (66.6 ± 15.3 vs. 63.3 ± 18.1, p = 0.02) scores from baseline to 24 weeks. Eight (15%) children in the PEG 2a + RV group and five (9%) children in the PEG 2a + PL group had a clinically significant decline in physical QOL between baseline and 24 weeks.(Table 3) Four (7%) children in the PEG 2a + RV and three (5%) in the PEG 2a + PL group had a clinically significant decline in psychosocial QOL.
Table 3.
Number (%) of children in each treatment group with Clinical Improvement, Clinical Decline, or No Clinical Change after 24 weeks of treatment, N = 114
| PEG 2a + RV (n = 55) | PEG 2a + PL (n = 59) | |||||
|---|---|---|---|---|---|---|
| Measure | Improved | Declined | No Change | Improved | Declined | No Change |
| CHQ Physical Summary | 0 (0%) | 8 (15%) | 47 (86%) | 3 (5%) | 5 (9%) | 51 (86%) |
| CHQ Psychosocial Summary | 3 (5%) | 4 (7%) | 48 (88%) | 5 (9%) | 3 (5%) | 51 (86%) |
| CBCL Internalizing | 2 (4%) | 3 (5%) | 50 (91%) | 4 (7%) | 3 (5%) | 52 (88%) |
| CBCL Externalizing | 1 (2%) | 3 (5%) | 51 (93%) | 2 (3%) | 0 (0%) | 57 (97%) |
| CBCL Total Behavior Problem | 1 (2%) | 2 (4%) | 52 (94%) | 3 (5%) | 2 (3%) | 54 (92%) |
| CDI Total Score | 0 (0%) | 3 (5%) | 52 (95%) | 2 (3%) | 0 (0%) | 57 (97%) |
| BRIEF Global Executive Composite | 3 (5%) | 3 (5%) | 49 (90%) | 3 (5%) | 0 (0%) | 56 (95%) |
Note: CHQ = Child Health Questionnaire; CBCL = Child Behavior Checklist; CDI = Child Depression Inventory; BRIEF = Behavior Rating Inventory of Executive Function
Behavioral/Emotional
There were no significant effects for time, treatment group, or time by treatment group interaction for the CBCL Internalizing, Externalizing, or Total Behavior Problems scores or for the CDI Total score (p's > 0.05). Six (3 PEG 2a + RV, 3 PEG 2a + PL) and three (all PEG 2a + RV) children had clinically significant worsening of internalizing and externalizing behaviors, respectively, between baseline and 24 weeks.(Table 3) Examination of CDI scores showed that three (5%) children in the PEG 2a + RV group and no children in the PEG 2a + PL group experienced a clinically significant increase in depression symptoms from baseline to 24 weeks. One child in the PEG 2a + RV group was withdrawn from treatment due to a suicidal gesture and subsequent hospitalization, and one child in the PEG 2a + PL group was withdrawn from the study due to an increase in aggressive behaviors.
Cognitive Functioning
There were no significant effects for time, treatment group, or time by treatment group interaction for the BRIEF composite scores (p's > 0.05). Three children receiving PEG 2a + RV had significant clinical deterioration in their Global Executive functioning from baseline to 24 weeks.(Table 3) No children other than the two noted above were removed from the study due to changes in cognitive or neuropsychological functioning.
Children with Nondetectable HCV RNA Who Received 48 Weeks of Treatment
At the 24 week assessment, 41 children in the PEG 2a + RV group and 26 children in the PEG 2a + PL group had nondetectable HCV RNA and continued treatment in their original randomized condition for an additional 24 weeks. Table 4 presents the means and standard deviations for the outcome measures for this subsample of children. Repeated measures analyses of variance showed that there were no statistically significant time, treatment group, or time by treatment group effects for any of the outcome measures during the 48 weeks of treatment or at the 6-month follow-up assessment (p's > 0.05).
Table 4.
Means ± standard deviations for primary outcomes at baseline, mid-treatment (24 weeks), treatment end (48 weeks), and first follow-up (6 months) for children with nondetectable HCV RNA at 24 weeks who continued their originally assigned treatment for an additional 24 weeks, n = 67.
| PEG 2a + RV (n = 41) | PEG 2a + PL (n = 26) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 24 Weeks | 48 Weeks | 6 Months | Baseline | 24 Weeks | 48 Weeks | 6 Months | |
| CHQ Physical Summary | 52.5 ± 4.2 | 49.3 ± 7.6 | 50.7 ± 8.0 | 51.9 ± 7.5 | 50.2 ± 7.6 | 47.2 ± 10.4 | 48.8 ± 9.3 | 51.8 ± 8.1 |
| CHQ Psychosocial Summary | 52.3 ± 8.1 | 52.0 ± 9.3 | 51.9 ± 8.4 | 52.9 ± 9.3 | 53.2 ± 7.9 | 51.2 ± 6.3 | 50.7 ± 10.6 | 52.9 ± 7.6 |
| CBCL Internalizing | 53.9 ± 8.4 | 50.9 ± 11.3 | 49.7 ± 10.4 | 49.1 ± 10.8 | 51.3 ± 11.3 | 51.6 ± 10.5 | 54.3 ± 14.5 | 51.8 ± 7.6 |
| CBCL Externalizing | 51.9 ± 9.0 | 49.9 ± 9.9 | 49.4 ± 9.5 | 48.5 ± 10.5 | 49.4 ± 9.8 | 48.8 ± 9.3 | 50.7 ± 10.4 | 51.6 ± 11.0 |
| CBCL Total Behavior Problem | 52.8 ± 8.5 | 50.4 ± 10.1 | 50.0 ± 10.3 | 48.5 ± 11.9 | 49.8 ± 9.9 | 50.1 ± 9.7 | 51.7 ± 13.5 | 51.5 ± 9.7 |
| CDI Total Score | 6.2 ± 4.4 | 6.1 ± 5.0 | 5.7 ± 3.8 | 4.7 ± 3.3 | 5.9 ± 4.5 | 5.1 ± 3.2 | 5.6 ± 4.1 | 6.9 ± 7.3 |
| BRIEF Global Executive Composite | 53.1 ± 10.5 | 52.5 ± 9.7 | 52.4 ± 12.1 | 51.8 ± 11.1 | 51.0 ± 11.5 | 51.4 ± 9.3 | 51.9 ± 10.4 | 52.0 ± 10.9 |
Note: CHQ = Child Health Questionnaire; CBCL = Child Behavior Checklist; CDI = Child Depression Inventory; BRIEF = Behavior Rating Inventory of Executive Function.
Among the 41 children who continued on PEG 2a + RV therapy, 34 (83%) experienced no clinically significant change in physical QOL during treatment. Two (5%) children experienced a clinical decline in physical QOL at 24 weeks but returned to baseline levels by the end of treatment, and five (12%) experienced an early clinical decline that persisted through the end of treatment. However, three of these five children returned to baseline QOL levels by the 6-month post-treatment assessment. Most children experienced no clinically significant change in internalizing behaviors (95%), externalizing behaviors (95%), or total behavior problems (93%) during the 48 weeks of treatment. On the CDI, two children (5%) had a clinically significant increase in depression symptoms during treatment. One child was removed from the study (as noted above) and the other child's symptoms remitted by the end of treatment. Finally, one child experienced a clinically significant decline in executive functioning at 24 weeks, which persisted through the duration of treatment and the 6-month follow-up assessment.
Of the 26 children who continued on PEG 2a + PL for 48 weeks, 21 (81%) did not have any clinically significant decline in physical QOL. Three (12%) children experienced a clinical decline in physical QOL at 24 weeks but returned to baseline levels by the end of treatment. Only one (4%) child had an early clinical decline that persisted through the end of treatment but this returned to baseline level after treatment was terminated. The majority had no significant clinical change in internalizing (77%), externalizing (92%), or total behavior problems (88%) during treatment. None of the children in this group had a clinically significant increase in depression symptoms (CDI) or executive functioning problems (BRIEF) during 48 weeks of treatment or the 6-month follow-up period.
Long-term Follow-up
For all children who completed 48 weeks of treatment, scores on all outcome measures obtained at the 1 yr and 2 yr follow-up assessments did not differ significantly from baseline scores (p's > 0.05). Very few children had clinical elevations on the CBCL, no child had a clinically high depression score at 1 year, and only one child (PEG 2a + RV) had a clinically elevated depression score at the 2-yr follow-up assessment.
Relationship Between Sociodemographic Characteristics and Outcomes
Age, gender, race, transmission mode, and baseline ALT were not significantly associated with QOL, behavioral/emotional, or cognitive outcomes at any time.
Discussion
A sustained virologic response, defined by undetectable serum HCV RNA 24 weeks after treatment cessation, is the primary objective of chronic HCV treatment. However, the virologic benefits of HCV treatment may be attenuated by negative changes along other clinical parameters that are of importance to patients and their caregivers, including QOL and psychological functioning. For instance, compromised QOL, fatigue, and depression increases the likelihood that adults will discontinue HCV treatment before realizing health benefits.(8,17,26) Findings from the current study, however, indicate that few children experience clinically significant changes in QOL, behavior problems, depression, or cognitive functioning during or after HCV treatment. Previously, we reported that pre-treatment scores for this HCV pediatric cohort were comparable to that of healthy children (19), and now we provide evidence that this remains largely unchanged after 48 weeks of peginterferon with ribavirin (or placebo) treatment and for two years after cessation of therapy. As the first randomized pediatric study to examine the QOL, psychological functioning, and cognitive impact of both peginterferon alone and with ribavirin, these findings have important clinical implications.
Overall QOL, behavior, depression, or executive function did not change for children over time, regardless of whether they received PEG 2a + RV or PEG 2a + PL. On the surface, this finding differs from both pediatric and adult studies, which have reported declines in QOL and other functional or psychological parameters during HCV treatments.(20,27) For instance, while PEG 2a has fewer QOL side-effects than unmodified interferon (28,29), the addition of RV has been shown to substantially impair QOL in adults.(27) Relative to adults, children may have less severe disease, fewer medical and psychiatric co-morbidities, and more available and stable social support systems. These factors may account for the lower incidence of QOL and behavioral/emotional impairment observed in the current study.
Iorio et al.(20) showed that the QOL of children deteriorates in the first month of interferon-alpha treatment, although QOL returned to baseline within three months of concluding treatment. We did not conduct QOL assessments during the first 6 months of treatment, so we are unable to determine whether impairments were present in our cohort during the early part of treatment. However, we did find that, for the few children who experienced clinically significant changes in QOL and behavioral/emotional functioning, almost all of them returned to healthy baseline levels by the end of treatment or by the 6-month follow-up assessment. It is important to note the different methodological and treatment characteristics between our study and the Iorio et al. study when evaluating these findings. For instance, in contrast to our study, the Iorio et al. study included children with different viral infections (HBV and HCV) and treated with various interferon types, schedules, and routes of administration. Also, Iorio et al. measured QOL using a modified version of the Sickness Impact Profile (SIP), which is an adult-based health status measure that has not been standardized on pediatric samples. In contrast, our patient cohort was homogeneous, children received a standardized antiviral regimen, and we used QOL instruments that have been developed and validated specifically for pediatric populations.
Findings reported herein must be considered in the context of several important study limitations. First, we present data based primarily on parent or guardian report. Such reports can be biased for many different reasons and, therefore, may not accurately capture the true functional status of the child. For instance, parents who are more stressed and worried about their child's medical status may respond different to questions about their child's functioning, compared to parents who are coping well with their child's condition. Second, while the sample size was large compared to published literature, the number of children who remained in their assigned treatment through the full 48 weeks of treatment was relatively small and this may have limited our ability to detect small changes in QOL functioning over time. Third, we did not assess QOL and the other psychosocial outcomes until 24 weeks after treatment initiation. It is possible that changes in these parameters occurred in the early stages of treatment and subsequently recovered by the time of our 24 week assessment.
In conclusion, PEG 2a + RV has been shown to be superior to PEG alone in achieving early and sustained virologic response in children and adolescents.(5) Data from the current study show that overall QOL, behavioral/emotional functioning, and cognitive functioning are not deleteriously impacted by the inclusion of RV. Nevertheless, because some individual children may experience clinical changes over time, we recommend close monitoring of these patient-oriented outcomes for those children receiving PEG 2a, with or without RV.
Acknowledgments
Grant Support: This study is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, grant number 1UO1DK067767, with additional support from the Office of Orphan Products Development, Food and Drug Administration. Contents herein are the authors' sole responsibility and do not necessarily represent official NIH views. Additional support is provided by Hoffman-La Roche for the study medications, data coordinating center, and central laboratory costs.
List of Abbreviations
- HCV
hepatitis C virus
- HBV
hepatitis B virus
- PEG
peginterferon
- RV
ribavirin
- PL
placebo
- QOL
quality of life
- CHQ
Child Health Questionnaire
- BRIEF
Behavior Rating Inventory of Executive Function
- CBCL
Child Behavior Checklist
- CDI
Child Depression Inventory
- ALT
Alanine aminotransferase
- SD
standard deviation
Footnotes
Conflicts of Interest: None
References
- 1.Jhaveri R, Grant W, Kauf TL, McHutchison J. The burden of hepatitis C virus infection in children: estimated direct medical costs over a ten-year period. J Pediatr. 2006;148:353–358. doi: 10.1016/j.jpeds.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 2.European Paediatric Hepatitis C Virus Network Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. 2005;41:45–51. doi: 10.1086/430601. [DOI] [PubMed] [Google Scholar]
- 3.Murray KF, Finn LS, Taylor SL, et al. Liver histology and alanine aminotransferase levels in children and adults with chronic hepatitis C infection. J Pediatr Gastroenterol Nutr. 2005;41:634–638. doi: 10.1097/01.mpg.0000179758.82919.1f. [DOI] [PubMed] [Google Scholar]
- 4.González-Peralta RP, Langham MR, Andres JM, et al. Hepatocellular carcinoma in two young adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr. 2009;48:630–635. doi: 10.1097/MPG.0b013e318170af04. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz KB, Gonzalez-Peralta RP, Murray KF, et al. for the Peds-C Clinical Research Network. A randomized trial of Peginterferon with or without ribavirin for chronic hepatitis C in children and adolescents. Gastroenterology. 2010 in press. [Google Scholar]
- 6.Gonzalez-Peralta RP, Kelly DA, Haber B, et al. Interferon Alfa-2b in Combination with Ribavirin for the Treatment of Chronic Hepatitis C in Children: Efficacy, Safety, and Pharmacokinetics. Hepatology. 2005;42:1010–1018. doi: 10.1002/hep.20884. [DOI] [PubMed] [Google Scholar]
- 7.Wirth S, Lang T, Gehring S, Gerner P. Recombinant alfa-interferon plus ribavirin therapy in children and adolescents with chronic hepatitis C. Hepatology. 2002;36:1280–1284. doi: 10.1053/jhep.2002.36495. [DOI] [PubMed] [Google Scholar]
- 8.Younossi Z, Kallman J, Kincaid J. The effects of HCV infection and management on health-related quality of life. Hepatology. 2007;45:806–816. doi: 10.1002/hep.21565. [DOI] [PubMed] [Google Scholar]
- 9.Kallman J, O'Neil MM, Larive B, et al. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig Dis Sci. 2007;52:2531–2539. doi: 10.1007/s10620-006-9708-x. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel BM, Younossi ZM, Hays RD, et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41:790–800. doi: 10.1002/hep.20659. [DOI] [PubMed] [Google Scholar]
- 11.Hilsabeck RC, Hassanein TI, Carlson MD, et al. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2003;9:847–854. doi: 10.1017/S1355617703960048. [DOI] [PubMed] [Google Scholar]
- 12.Goulding C, O'Connell P, Murray FE. Prevalence of fibromyalgia, anxiety and depression in chronic hepatitis C virus infection: relationship to RT-PCR status and mode of acquisition. Eur J Gastroenterol Hepatol. 2001;13:507–511. doi: 10.1097/00042737-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Schäfer A, Wittchen HU, Seufert J, Kraus MR. Methodological approaches in the assessment of interferon-alfa-induced depression in patients with chronic hepatitis C - a critical review. Int J Methods Psychiatr Res. 2007;16:186–201. doi: 10.1002/mpr.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forton DM, Allsop JM, Cox IJ, Hamilton G, Wesnes K, Thomas HC, Taylor-Robinson SD. A review of cognitive impairment and cerebral metabolite abnormalities in patients with hepatitis C infection. AIDS. 2005;19 3:S53–S63. doi: 10.1097/01.aids.0000192071.72948.77. [DOI] [PubMed] [Google Scholar]
- 15.Lieb K, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Janssen G, Schaefer M. Cognitive impairment in patients with chronic hepatitis treated with interferon alpha (IFNalpha): results from a prospective study. Eur Psychiatry. 2006;21:204–210. doi: 10.1016/j.eurpsy.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Reichenberg A, Gorman JM, Dieterich DT. Interferon-induced depression and cognitive impairment in hepatitis C virus patients: a 72 week prospective study. AIDS. 2005;19:S174–S178. doi: 10.1097/01.aids.0000192087.64432.ae. [DOI] [PubMed] [Google Scholar]
- 17.Leutscher PDC, Laggin M, Rauning Buhl M, et al. Evaluation of depression as a risk factor for treatment failure in chronic hepatitis C. Hepatology. 2010;52:430–435. doi: 10.1002/hep.23699. [DOI] [PubMed] [Google Scholar]
- 18.Nydegger A, Srivastava A, Wake M, et al. Health-related quality of life in children with hepatitis C acquired in the first year of life. Hepatology. 2008;23:226–230. doi: 10.1111/j.1440-1746.2007.04859.x. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, Murray KF, Narkewicz MR, Rosenthal P, Smith LJ, Schwarz KB, Robuck P, Barton B, González-Peralta RP. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr. 2009;48:341–347. doi: 10.1097/MPG.0b013e318185998f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iorio R, Pensati P, Botta S, et al. Side effects of alpha-interferon therapy and impact on health-related quality of life in children with chronic viral hepatitis. Pediatr Infect Dis J. 1997;16:984–990. doi: 10.1097/00006454-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Murray KF, Rodrigue JR, González-Peralta RP, et al. Design of the PEDS-C Trial: Pegylated Interferon +/- Ribavirin for children with chronic hepatitis C viral infection. Clin Trials. 2007;4:661–673. doi: 10.1177/1740774507085445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landgraf JM, Abetz L, Ware JE. The CHQ User's Manual. The Health Institute, New England Medical Center; Boston: 1996. [Google Scholar]
- 23.Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2001. [Google Scholar]
- 24.Kovacs M. Children's Depression Inventory manual. Multi-Health Systems Inc.; North Tonawanda, NY: 1992. [Google Scholar]
- 25.Gioia GA, Isquith PK, Guy SC, et al. Behavior rating inventory of executive function. Psychological Assessment Resources; Odessa FL: 2000. [Google Scholar]
- 26.Bernstein D, Kleinman L, Barker CM, et al. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35:704–708. doi: 10.1053/jhep.2002.31311. [DOI] [PubMed] [Google Scholar]
- 27.Hassanein T, Cooksley G, Sulkowski M, Smith C, Marinos G, Lai MY, Pastore G, Trejo-Estrada R, Horta E Vale A, Wintfeld N, Green J. The impact of peginterferon alfa-2a plus ribavirin combination therapy on health-related quality of life in chronic hepatitis C. J Hepatol. 2004;40:675–681. doi: 10.1016/j.jhep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Perrillo R, Rothstein KD, Rubin R, Alam I, Imperial J, Harb G, Hu S, Klaskala W. Comparison of quality of life, work productivity and medical resource utilization of peginterferon alpha 2a vs the combination of interferon alpha 2b plus ribavirin as initial treatment in patients with chronic hepatitis C. J Viral Hepat. 2004;11:157–165. doi: 10.1046/j.1365-2893.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- 29.Rasenack J, Zeuzem S, Feinman SV, Heathcote EJ, Manns M, Yoshida EM, Swain MG, Gane E, Diago M, Revicki DA, Lin A, Wintfeld N, Green J. Peginterferon alpha-2a (40kD) [Pegasys] improves HR-QOL outcomes compared with unmodified interferon alpha-2a [Roferon-A]: in patients with chronic hepatitis C. Pharmacoeconomics. 2003;21:341–349. doi: 10.2165/00019053-200321050-00005. [DOI] [PubMed] [Google Scholar]