Abstract
Transcranial sonography (TCS) area of hyperechogenicity in the substantia nigra (aSN) is increased in idiopathic and genetic Parkinson’s disease (PD). We performed TCS in 34 LRRK2 G2019S mutation carriers manifesting PD, 24 non-manifesting mutation carriers and 28 idiopathic PD patients and compared them to 40 healthy controls (total n=126).
Compared to controls (mean 0.15 cm2), the aSN values in all other groups were increased. The mean aSN was 0.23 cm2 in non-manifesting mutation carriers (p=0.015), 0.34 cm2 in idiopathic PD patients (p<0.0001), 0.32 cm2 in LRRK2-associated PD patients (p<0.0001). and 0.33 cm2 in the overall PD group (p<.0001). LRRK2-associated PD patients had higher aSN than non-manifesting carriers (p=0.011), but there was no significant difference in aSN between patients with idiopathic and LRRK2-associated PD (p=0.439).
Our results suggest that SN pathoanatomical alterations may not be substantially different between idiopathic and LRRK2-associated PD. The findings in the non-manifesting mutation carriers suggest the presence of intermediate nigrostriatal pathology consistent with the age-dependent reduced penetrance of this mutation.
INTRODUCTION
Transcranial sonography (TCS) of the brainstem may be a helpful approach in assessing movement disorders. Approximately 90% of all patients with idiopathic Parkinson’s disease (PD) compared to only 10% of healthy control subjects show a characteristically increased area of hyperechogenicity in the substantia nigra (SN).1 Autosomal recessive and dominant genetic forms of PD are also associated with this increased echosignal. This was recently demonstrated for the Parkin (PARK2), PINK1 (PARK6), as well as LRRK2 (PARK8) and GBA (Glucocerebrosidase) genes.2–6 Single cases with similar TCS findings were reported for the DJ-1 (PARK7) and alpha-synuclein gene (PARK1/4).4, 7 Further, the extent of the SN hyperechogenicity in recessively inherited PD was reported to be similar to that in sporadic PD.2, 3 In addition to idiopathic and genetic PD, an increased area of hyperechogenicity in the SN (aSN) was also shown in asymptomatic elderly subjects with a higher degree of motor impairment and decline of the nigrostriatal function.8 Therefore, aSN echogenicity is thought to be a non-invasive imaging marker for nigrostriatal integrity with a good sensitivity but poor specificity.
As penetrance is reduced in dominantly inherited PD, not all mutation carriers develop neurological signs. For example, a proportion of LRRK2 mutation carriers remain motorically asymptomatic into late elderly life.9 This cohort of healthy subjects with a genetically determined disease risk provides a unique opportunity to study potential compensatory mechanisms that prevent or delay the onset of clinically overt PD. Furthermore, since non-manifesting carriers (NMC) may develop disease signs later in life, it is possible that markers such as hyperechogenicity on TCS may help identify those individuals in the preclinical phase of PD who will later evolve frank PD.10
Our study had two main aims: i) to evaluate the aSN in a large group of PD patients with the LRRK2 G2019S mutation in comparison with a group of idiopathic PD (IPD) patients and ii) to evaluate the contribution of this mutation to this echosignal in (unaffected) NMC in comparison with PD affected or manifesting carriers (MC) and healthy controls.
MATERIALS AND METHODS
After giving informed consent, all 126 subjects were evaluated and rated by a movement disorder trained neurologist including Unified Parkinson’s Disease Rating Scale (UPDRS) and Hoehn-Yahr stage at two study centers (New York (NY) and Lübeck, Germany (L). The number of the subjects each center evaluated were as follows: control subjects NY n=25, L n=15 (total n=40), LRRK2 NMC NY n=24 (total n=24), LRRK2 MC NY=34 (total n=34), idiopathic PD NY n=14, L n=14 (total n=28).
The clinical diagnosis of PD was made according to the Queens Square Brain Bank Criteria, with the exception that a family history of more than one affected relative was not considered an exclusion criterion.11 All PD subjects of Ashkenazi descent had been screened for LRRK2 G2019S mutations using previously described methods.12 The study was approved by the local ethics committee of both study centers (Beth Israel, Lübeck).
Transcranial sonography
TCS was performed using the Sonos 5500 ultrasound system (Phillips) equipped with a 2.0–2.5 MHz sector transducer (S3 probe). All individuals were examined by one of two experienced sonographers (JH and KS), who were blinded to the results of the genetic status of the subjects. The examination was performed from both sides through the temporal bone window with a penetration depth of 14–16 cm. The images were adjusted for gain power, compression and time-gain compensation depending on the quality of the individual bone window. Images of the mesencephalic brainstem were digitally stored for later analysis. The area of hyperechogenicity in the ipsilateral SN was manually encircled by an independent investigator (NB) who had not been involved in the TCS examinations using a computer-based analysis (Scion Image Beta 4.02 Win software package). For statistical analysis, the larger aSN of each individual was selected or, in cases of an insufficient bone window on one side, the ipsilateral aSN of the analyzable side (aSNmax). Due to the study design, we could not assess the inter-rater reproducibility and reliability.
Statistical analysis
Demographic characteristics, echogenicity and clinical scores among groups were summarized with descriptive statistics. To account for the correlations among measurements of subjects from the same family, linear mixed effects model (LMM) was applied to compare aSN among groups of controls, NMC, MC and IPD patients, adjusting for age and gender.13 The association between echogenicity and clinical scores in the genetic and non-genetic PD patients was evaluated using LMM and a linear regression model, respectively, adjusting for age and gender. Analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, N.C).
RESULTS
Demographic data
Thirty-two LRRK2 MC harbored the common G2019S variant in the heterozygous state whereas two carried a homozygous G2019S mutation. The LRRK2 MC were older than controls (p=0.028), LRRK2 NMC (p=0.001) and IPD patients (p=0.004). There were no significant age differences among controls, LRRK2 NMC and patients with IPD. Overall 55% of the participants were male, and the distribution of gender was not different among groups of controls, NMC, MC and IPD. LRRK2 MC did not differ from IPD patients in age at onset, disease duration, and UPDRS I, II and III (Table 1).
Table 1.
Demographic and TCS data in all subgroups
| Control | LRRK2 NMC | LRRK2 MC | IPD | |
|---|---|---|---|---|
| Number (n) | 40 | 24 | 34 | 28 |
| Age (years) | 57.7±8.8 | 53.3±20.6 | 66.0±11.9 | 54.9±10.3 |
| Gender (%male) | 52.5 | 54.2 | 52.9 | 60.7 |
| Age at PD onset | - | - | 57.2±11.9* | 52.5±14.7* |
| Disease duration | - | - | 9.2±7.0* | 10.3±6.5* |
| UPDRSIII | - | - | 14.3±10.6* | 18.7±11.5* |
| aSN right (cm2) | 0.12±0.08 | 0.20±0.12 | 0.26±0.13 | 0.28±0.11 |
| aSN left (cm2) | 0.10±0.08 | 0.22±0.11 | 0.28±0.09 | 0.29±0.16 |
| aSNmax (cm2) | 0.15±0.07 | 0.23±0.12 | 0.32±0.10 | 0.34±0.15 |
not significant (LRRK2 MC vs. IPD)
TCS data
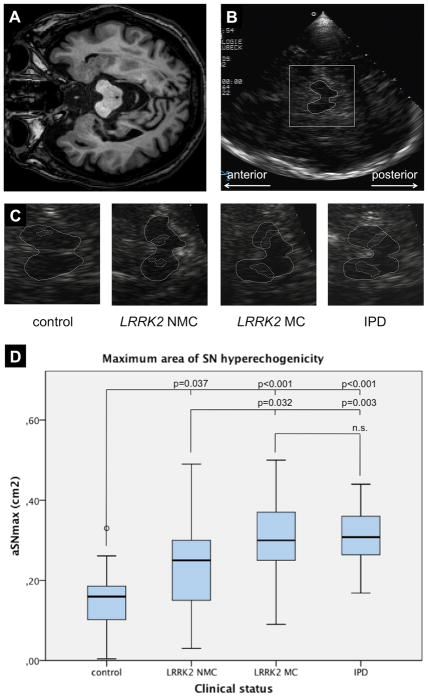
233 of the 252 examined temporal bone windows were sufficient (92.5%; right side 118/126, left side 115/126). A bilaterally insufficient bone window was present in 2/126 subjects (1.6%). Mean aSNmax was significantly lower in controls than in the LRRK2 NMC (p=0.037), LRRK2 MC (p<0.001), and IPD patients (p<0.001)(Figure 1). LRRK2 NMC had a smaller mean aSNmax than LRRK2 MC (p=0.032) and IPD patients (p=0.003). No differences in the values for mean aSNmax could be observed between LRRK2 MC and IPD patients. There were no differences between the aSN of the right and left side in any of the subgroups (Table 1). Considering all PD patients regardless of their genetic status, the disease duration showed a weak correlation with the aSNmax (rho=0.263, p=0.043). There was a trend towards higher UPDRSIII scores in patients with higher aSNmax values (rho=0.236, p=0.068).
Figure 1.
MRI (A.) and sonographic images (B.) of an axial section at the midbrain level upon TCS. The rectangle in (B.) denotes the area to be zoomed in for evaluation of the midbrain structures as is shown in the lower row. The butterfly-shaped midbrain and the SN echogenicity have been encircled in representative TCS images of each subgroup (C.). The diagram in (D.) shows the mean values for the aSNmax according to the clinical and mutational status. Age did not influence the aSN differences between the subgroups.
NMC – non-manifesting mutation carrier, MC – manifesting mutation carrier
DISCUSSION
This is largest study to date assessing TCS in LRRK2-associated PD. Similar to other TCS analyses of genetic and non-genetic forms of PD, we found an increased aSN in PD patients with LRRK2 mutations and a comparable increase in those with IPD.2–5 Thus, an increased aSN is a neuroimaging feature of levodopa-responsive neurodegenerative parkinsonism irrespective of the etiology. In contrast, most forms of atypical parkinsonism without or only a poor response to levodopa do not share this echofeature.14 This is also true for Kufor-Rakeb syndrome (PARK9), a rare recessively inherited form of parkinsonism with atypical features such as supranuclear palsy and dementia. PARK9-associated parkinsonism is caused by mutations in the ATP13A2 gene; neither carriers of one or two mutated alleles show an increased aSN.15
The extent of SN hyperechogenicity in asymptomatic carriers of mutations in PD-causing genes was currently described in recessively inherited forms only.2, 3 We demonstrate an increased aSN also in LRRK2 NMC comparable to asymptomatic carriers of single mutations in the Parkin and PINK1 gene with values ranging between those of controls and MC. In this context, SN hyperechogenicity may be an early manifestation of mutant LRRK2 expression, which occurs independently of developing PD. Alternatively, the hyperechogenicity may be a marker of increased susceptibility in a subset of mutation carriers who are at higher risk to develop PD. This assumption is supported by PET studies, one of them in a small cohort of heterozygous Parkin mutation carriers5, that revealed a reduced striatal 18F-Dopa uptake in healthy subjects with an increased aSN pointing towards subclinical nigrostriatal degeneration.16, 17 In LRRK2-associated PD, the penetrance of the G2019S mutation is known to be age-dependent, increasing from about 20% at age 50 years to 30–80% at age 70 years.18, 19 That is, a proportion of (younger) NMC will develop PD later in life while others will not due to the overall reduced penetrance. One can speculate that some of the yet asymptomatic subjects (that will develop the disease) already show this echofeature as a sign of ongoing nigral damage. Alternatively, an increasing aSN may be an imaging marker of adaptive and compensatory mechanisms in response to a latent nigrostriatal dysfunction in asymptomatic subjects carrying showing this echofeature. In contrast to others20, we found a slight correlation of the aSN with disease duration in PD cases, which may suggest ongoing structural alterations over the disease course. Only substantial longitudinal examinations (performed over decades) will demonstrate who among the NMC will develop PD and who will not and whether these subgroups had different values for the aSN, retrospectively. However, recent reports suggest increased aSN values even in unaffected family members of sporadic PD patients.2, 21 This, in turn, argues for the presence of intrafamilial factors other than monogenic variants and raises the question whether the higher aSN values in the NMC can be solely ascribed to the LRRK2 G2019S mutation.
There are potential limitations to our study. First, the subgroup of manifesting LRRK2 mutation carriers were older than individuals of the other subgroups, although we do not assume that this had a relevant influence on the TCS data in patients once affected with PD. Second, we did not assess interrater reproducibility. However, we had a limited number of raters, and raters were not differentially examining certain groups.
In conclusion, our results suggest that the TCS findings of patients with the common G2019S mutation in the LRRK2 gene are not substantially different from idiopathic PD. The increased SN hyperechogenicity in both PD groups may be caused by similar alterations in the midbrain iron metabolism regardless of the genetic state. However, it remains elusive whether an increased aSN is also related to a distinct histopathological pattern. All LRRK2 mutation subjects have demonstrated neuronal loss and gliosis in the substantia nigra, and similar to idiopathic PD, 80% of brains of LRRK2 G2019S mutation carriers demonstrate Lewy bodies (LB), either in the brain or the olfactory bulb.22 While the minority of PD patients with LRRK2 mutations other than G2019S show LB pathology22, the significance of this finding is uncertain as there are few cases overall, and most are ascertained from a very small number of families. Post mortem investigations in atypical parkinsonian syndromes such as progressive supranuclear palsy and multiple system atrophy also reveal a lack of LB pathology. These syndromes typically present a normal echogenic substantia nigra. Therefore, future studies should address the aSN in carriers of LRRK2 mutations other than the common G2019S variant. Moreover, combined assessment of the aSN upon TCS during lifetime and post mortem histopathological examinations in typical and atypical, genetic and non-genetic forms of parkinsonism are urgently needed to determine the underlying morphological changes that are responsible for this midbrain echofeature.
The findings in the NMC point towards nigrostriatal pathology in at least a subset and may reflect the age-dependent reduced penetrance of LRRK2-associated PD.
Acknowledgments
This work was supported by research grants from the Michael J. Fox Foundation, the National Institute of Neurological Disorders and Stroke (K23NS047256, RSP), the Thomas Hartman Foundation for Parkinson’s Research, the Volkswagen Foundation and the Hermann and Lilly Schilling Foundation (CK).
FINANCIAL DISCLOSURE
| Norbert Brüggemann, MD | |
| Stock Ownership in medically-related fields | None |
| Consultancies | None |
| Advisory Boards | None |
| Partnerships | None |
| Honoraria | None |
| Grants | Research Grant by the University of Lübeck (grant # E17-2009, January 2009 – December 2010) |
| Intellectual Property Rights | None |
| Expert Testimony | None |
| Employment | University of Lübeck |
| Contracts | None |
| Royalties | None |
| Other | None |
| Johann Hagenah, MD | |
| Stock Ownership in medically-related fields | None |
| Consultancies | None |
| Advisory Boards | None |
| Partnerships | None |
| Honoraria | GlaxoSmithKline |
| Grants | Research Grant from the Bachmann-Strauss Dystonia Parkinson Foundation |
| Intellectual Property Rights | None |
| Expert Testimony | None |
| Employment | University of Lübeck |
| Contracts | None |
| Royalties | None |
| Other | None |
| Kaili Stanley, MA | |
| Stock Ownership in medically-related fields | None |
| Consultancies | None |
| Advisory Boards | None |
| Partnerships | None |
| Honoraria | None |
| Grants | Michael J. Fox Foundation for Parkinson’s Research and Bachmann-Strauss Dystonia & Parkinson Foundation and has received research support from the NIH. |
| Intellectual Property Rights | None |
| Expert Testimony | None |
| Employment | Beth Israel Medical Center, New York |
| Contracts | None |
| Royalties | None |
| Other | None |
| Christine Klein, MD | |
| Stock Ownership in medically-related fields | None |
| Consultancies | Boehringer Ingelheim, Centogene |
| Advisory Boards | None |
| Partnerships | None |
| Honoraria | Boehringer Ingelheim, Merz Pharma |
| Grants | Recipient of a career development award from the Hermann and Lilly Schilling Foundation. Funded by the Volkswagen Foundation, the Deutsche Forschungsgemeinschaft, the Possehl Foundation. Received institutional support from the University of Lübeck for genetics research |
| Intellectual Property Rights | None |
| Expert Testimony | None |
| Employment | Schilling Section of Clinical and Molecular Neurogenetics, University of Lübeck |
| Contracts | None |
| Royalties | None |
| Other | None |
| Deborah Raymond, MS | |
| Stock Ownership in medically-related fields | None |
| Consultancies | None |
| Advisory Boards | None |
| Partnerships | None |
| Honoraria | None |
| Grants | Bachmann-Strauss Dystonia & Parkinson Foundation, and the Michael J. Fox Foundation for Parkinson’s Research. |
| Intellectual Property Rights | None |
| Expert Testimony | None |
| Employment | Beth Israel Medical Center, New York |
| Contracts | None |
| Royalties | None |
| Other | None |
| Laurie Ozelius, PhD | |
| Stock Ownership in medically-related fields | None |
| Consultancies | None |
| Advisory Boards | None |
| Partnerships | None |
| Honoraria | None |
| Grants | Bachmann-Strauss Dystonia & Parkinson’s Foundation, the Dystonia Medical Research Foundation and the NIH |
| Intellectual Property Rights | None |
| Expert Testimony | None |
| Employment | Mount Sinai School of Medicine, New York |
| Contracts | None |
| Royalties | None |
| Other | None |
| Susan Bressman, MD | |
| Stock Ownership in medically-related fields | None |
| Consultancies | None |
| Advisory Boards | None |
| Partnerships | None |
| Honoraria | None |
| Grants | Michael J. Fox Foundation for Parkinson’s Research, the NIH (NINDS RO1-NS046340-01A1 and NINDS via University of Rochester Parkinson Study Group 5 U01 NS050095-02), and the Bachmann-Strauss Dystonia Parkinson Foundation |
| Intellectual Property Rights | None |
| Expert Testimony | None |
| Employment | Beth Israel Medical Center, New York |
| Contracts | None |
| Royalties | None |
| Other | None |
| Rachel Saunders-Pullman, MD | |
| Stock Ownership in medically-related fields | None |
| Consultancies | consultation fees from GE |
| Advisory Boards | None |
| Partnerships | None |
| Honoraria | None |
| Grants | NIH [NINDS K23-NS047256(PI)], research support from the Michael J. Fox Foundation for Parkinson’s Research, funding from the Thomas Hartman Foundation for Parkinson’s Research and the Bachmann-Strauss Dystonia and Parkinson Foundation |
| Intellectual Property Rights | None |
| Expert Testimony | None |
| Employment | Beth Israel Medical Center, New York |
| Contracts | None |
| Royalties | None |
| Other | None |
AUTHOR ROLES
Norbert Brüggemann, 1A, 1B, 1C, 2A, 2B, 2C, 3A
Johann Hagenah, 1A, 1B, 1C, 2C, 3B
Kaili Stanley, 1B, 1C, 2C, 3B
Christine Klein, 1C, 3B
Cuiling Wang, 2A, 2B, 3B
Deborah Raymond, 1C, 3B
Laurie Ozelius, 1C, 3B
Susan Bressman, 1C, 3B
Rachel Saunders-Pullman, 1A, 1B, 1C, 2A, 2C, 3B
1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique.
References
- 1.Walter U, Behnke S, Eyding J, et al. Transcranial brain parenchyma sonography in movement disorders: state of the art. Ultrasound Med Biol. 2007;33(1):15–25. doi: 10.1016/j.ultrasmedbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Hagenah JM, Becker B, Brüggemann N, et al. Transcranial sonography findings in a large family with homozygous and heterozygous PINK1 mutations. J Neurol Neurosurg Psychiatry. 2008;79(9):1071–1074. doi: 10.1136/jnnp.2007.142174. [DOI] [PubMed] [Google Scholar]
- 3.Hagenah JM, Konig IR, Becker B, et al. Substantia nigra hyperechogenicity correlates with clinical status and number of Parkin mutated alleles. J Neurol. 2007;254(10):1407–1413. doi: 10.1007/s00415-007-0567-y. [DOI] [PubMed] [Google Scholar]
- 4.Schweitzer KJ, Brussel T, Leitner P, et al. Transcranial ultrasound in different monogenetic subtypes of Parkinson’s disease. J Neurol. 2007;254(5):613–616. doi: 10.1007/s00415-006-0369-7. [DOI] [PubMed] [Google Scholar]
- 5.Walter U, Klein C, Hilker R, Benecke R, Pramstaller PP, Dressler D. Brain parenchyma sonography detects preclinical parkinsonism. Mov Disord. 2004;19(12):1445–1449. doi: 10.1002/mds.20232. [DOI] [PubMed] [Google Scholar]
- 6.Saunders-Pullman R, Hagenah J, Dhawan V, et al. Gaucher ascertained through a Parkinson’s Center: Imaging and Clinical Characterization. Mov Disord. doi: 10.1002/mds.23046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brueggemann N, Odin P, Gruenewald A, et al. Re: Alpha-synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;71(16):1294. doi: 10.1212/01.wnl.0000338439.00992.c7. author reply 1294. [DOI] [PubMed] [Google Scholar]
- 8.Behnke S, Double KL, Duma S, et al. Substantia nigra echomorphology in the healthy very old: Correlation with motor slowing. Neuroimage. 2007;34(3):1054–1059. doi: 10.1016/j.neuroimage.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 9.San Luciano M, Lipton RB, Wang C, et al. Clinical LRRK2 expression in the elderly. Mov Disord. doi: 10.1002/mds.23330. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommer U, Hummel T, Cormann K, et al. Detection of presymptomatic Parkinson’s disease: combining smell tests, transcranial sonography, and SPECT. Mov Disord. 2004;19(10):1196–1202. doi: 10.1002/mds.20141. [DOI] [PubMed] [Google Scholar]
- 11.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2006;354(4):424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 13.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 14.Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D. Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology. 2003;60(1):74–77. doi: 10.1212/wnl.60.1.74. [DOI] [PubMed] [Google Scholar]
- 15.Brüggemann N, Hagenah J, Reetz K, et al. Recessively inherited parkinsonism: effect of ATP13A2 mutations on the clinical and neuroimaging phenotype. Arch Neurol. doi: 10.1001/archneurol.2010.281. accepted. [DOI] [PubMed] [Google Scholar]
- 16.Berg D, Becker G, Zeiler B, et al. Vulnerability of the nigrostriatal system as detected by transcranial ultrasound. Neurology. 1999;53(5):1026–1031. doi: 10.1212/wnl.53.5.1026. [DOI] [PubMed] [Google Scholar]
- 17.Berg D, Roggendorf W, Schroder U, et al. Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol. 2002;59(6):999–1005. doi: 10.1001/archneur.59.6.999. [DOI] [PubMed] [Google Scholar]
- 18.Healy DG, Falchi M, O’Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7(7):583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldwurm S, Zini M, Mariani L, et al. Evaluation of LRRK2 G2019S penetrance: relevance for genetic counseling in Parkinson disease. Neurology. 2007;68(14):1141–1143. doi: 10.1212/01.wnl.0000254483.19854.ef. [DOI] [PubMed] [Google Scholar]
- 20.Walter U, Dressler D, Wolters A, et al. Transcranial brain sonography findings in clinical subgroups of idiopathic Parkinson’s disease. Mov Disord. 2007;22(1):48–54. doi: 10.1002/mds.21197. [DOI] [PubMed] [Google Scholar]
- 21.Ruprecht-Dorfler P, Berg D, Tucha O, et al. Echogenicity of the substantia nigra in relatives of patients with sporadic Parkinson’s disease. Neuroimage. 2003;18(2):416–422. doi: 10.1016/s1053-8119(02)00035-6. [DOI] [PubMed] [Google Scholar]
- 22.Wider C, Dickson DW, Wszolek ZK. Leucine-rich repeat kinase 2 gene-associated disease: redefining genotype-phenotype correlation. Neurodegen Dis. 2010;7:175–9. doi: 10.1159/000289232. [DOI] [PMC free article] [PubMed] [Google Scholar]