Summary
The transcription factors Foxd3 and Pax3 are important early regulators of neural crest (NC) progenitor cell properties. Homozygous mutations of Pax3 or a homozygous NC-specific deletion of Foxd3 cause marked defects in most NC derivatives, but neither loss of both Foxd3 alleles nor loss of one Pax3 allele alone greatly affects overall development of cardiac NC derivatives. In contrast, compound mutant embryos homozygous for a NC-specific Foxd3 mutation and heterozygous for Pax3 have fully penetrant persistent truncus arteriosus, severe thymus hypoplasia, and midgestation lethality. Foxd3; Pax3 compound mutant embryos have increased cell death in the neural folds and a drastic early reduction of NC cells, with an almost complete absence of NC caudal to the first pharyngeal arch. The genetic interaction between these genes implicates gene dosage-sensitive roles for Foxd3 and Pax3 in cardiac NC progenitors. Foxd3 and Pax3 act together to affect survival and maintenance of cardiac NC progenitors, and loss of these progenitors catastrophically affects key aspects of later cardiovascular development.
Keywords: truncus arteriosus, velocardiofacial syndrome, transcription factors, mouse, progenitors
INTRODUCTION
In vertebrates, the neural crest (NC) is critical for the coordinated development of multiple systems. NC cells (NCCs) form peripheral sensory and autonomic nervous system derivatives, facial skeleton, connective tissue, melanocytes, and smooth muscle of the great arteries of the heart. The cardiac NC is a subset of the NC that, in addition to contributing vascular smooth muscle and pericytes to the outflow tract (OFT) and great vessels, also forms the mesenchyme of the third pharyngeal pouch critical for signaling to the adjacent ectoderm and subsequent normal thymus development (Bockman and Kirby, 1989). Cardiac NC ablation in both chicken and mouse embryos revealed that cardiac NC is required for development of the conotruncus, aortic arch arteries, and thymus (Kirby, 1990; Porras and Brown, 2008). In humans, abnormalities in these cells are often at the root of many cardiovascular defects, including persistent truncus arteriosus (PTA), a large, single great vessel instead of the normally distinct pulmonary trunk and ascending aorta exiting the ventricles. PTA is a severe, life-threatening birth defect on its own (Bando et al., 1996); it is also one of the features of velocardiofacial syndrome (VCFS), which can include cleft palate, craniofacial abnormalities, and thymus defects, all tissues with NC contribution. Prevalence of VCFS is approximately 1 of 2,000 live births, making it one of the most common syndromic birth defects (Shprintzen, 2008). Some of the genetic causes for human congenital heart defects (CHDs) and later cardiovascular disease have been identified (Pierpont et al., 2007), but, in many cases, the presence of unknown genetic modifiers contributes considerable variability to CHD severity, and some CHDs manifest as part of a cohort of phenotypes including noncardiac defects, as in VCFS. Understanding molecular regulation of cardiac NC development is critical for a thorough understanding of the etiology of many human CHDs, cardiovascular disease, and multiorgan syndromes such as VCFS. Our data here suggest the involvement and genetic interaction of Foxd3 and Pax3 in control of cardiac NC development, particularly on early NC progenitor cells.
Foxd3 is a winged-helix transcription factor required for maintenance of multipotency and self-renewal of embryonic stem (ES) cells and trophoblast stem cells in culture (Hanna et al., 2002; Liu and Labosky, 2008; Tompers et al., 2005), and Foxd3 null mutant embryos do not survive past early gastrulation (Hanna et al., 2002). Foxd3 is one of the initial markers of the NC lineage (Labosky and Kaestner, 1998), and when removed from the NC, most NC progenitors and derivatives are markedly affected, both in vivo and in explant cultures (Teng et al., 2008; Mundell and Labosky, manuscript Submitted). In these NC-specific Foxd3 mutant embryos, many of the bones and cartilage of the developing craniofacial skeleton are absent or markedly malformed, and the face does not fuse at the midline. The peripheral nervous system and enteric nervous system (ENS) are almost completely absent, and dorsal root ganglia are reduced in size, all because of defective maintenance of the NC progenitor pool within the premigratory and early migratory NC (Teng et al., 2008). Surprisingly, the reduction in cardiac NCCs reaching the OFT in these mutants does not disrupt cardiac development, and these mutants have a low occurrence of heart defects (Teng et al., 2008).
Like Foxd3, Pax3 is a transcription factor critical for early NC development and NC-derived stem cell maintenance. In addition to its role in NC induction (Sato et al., 2005), Pax3 is required for maintenance and expansion of early NC progenitors and is required to maintain melanocyte stem cells (Lang et al., 2005). Pax3 homozygous-null embryos have a drastic early reduction in NC progenitors and profound defects in OFT septation (Chan et al., 2004; Morgan et al., 2008). Although Foxd3 clearly plays a paramount role in multiple progenitor lineages, little is known about the genetic and molecular pathways in which Foxd3 acts, or the factors interacting with Foxd3. Here, we describe a genetic interaction between Foxd3 and Pax3 with direct functional significance for development of the cardiac NC. Foxd3 NC mutants have subtle cardiac defects, but introducing one mutant Pax3 allele into this genetic background causes a 100% penetrant PTA. This striking complete penetrance of PTA is only observed in a handful of mouse models (Choi and Klingensmith, 2009; Choudhary et al., 2006; Jiang et al., 2002; Richarte et al., 2007; Stankunas et al., 2008), and with respect to the cardiac NC subset of NCCs, approximates an NC ablation model (Porras and Brown, 2008). The PTA observed here in this compound deletion of Foxd3 and Pax3 together is caused by a severe reduction of presumptive NCCs entering the second and third pharyngeal arches, preceded by a wave of cell death in the presumptive NC. Our results suggest a profound requirement for these two transcription factors in NC progenitor maintenance.
RESULTS
Pax3Cre-Mediated Deletion of Foxd3 From the NC Results in Craniofacial Defects and Embryonic Lethality
Cardiac NCCs migrate along the third, fourth, and sixth pharyngeal arch arteries, facilitating remodeling of these arteries and septation of the OFT into the distinct aortic and pulmonary arteries (Snider et al., 2007). A Wnt1-Cre-mediated NC deletion of Foxd3 results in severe defects in almost all NC lineages, but cardiac NC derivatives are minimally affected, and most Wnt1-Cre; Foxd3flox/− mutant embryos survive until birth (Teng et al., 2008). (Mice and their genotypes, MGI allele names, and references are in Table 1; breeding schemes are in Supplemental Fig. 1.) This minimal cardiac phenotype is the result of a small remaining population of cardiac NCCs sufficient for cardiac NC function in the majority of Wnt1-Cre; Foxd3flox/− mutant embryos. To test if this somewhat surprising result was due to an early population of NCCs that did not yet express Wnt1-Cre and, therefore, maintained Foxd3 expression, an alternative, earlier NC-expressed Cre recombinase, Pax3-Cre, was used. This Pax3Cre allele was generated by insertion of a Cre cassette into the Pax3 locus, resulting in a null allele (Engleka et al., 2005). Pax3 is expressed in the dorsal neural tube, early NC, and presomitic mesoderm. The effect of Pax3Cre in deleting Foxd3 in this mouse model is limited to the NC, because Foxd3 is not expressed in the presomitic mesoderm of the mouse embryo (Labosky and Kaestner, 1998; Tompers et al., 2005). Foxd3 is expressed in murine premigratory NC starting slightly before 8.5 days post coitum (Labosky and Kaestner, 1998). Foxd3 is maintained in migratory NCCs as they leave the neural tube, but its expression is extinguished in many NCCs as they begin to differentiate toward other lineages (see Supplemental Fig. 2) and, by 10.5 dpc, is only maintained in ENS precursors, future spinal ganglia and sympathetic ganglia, and is not expressed in cells entering the developing OFT (Labosky and Kaestner, 1998; Mundell and Labosky, manuscript submitted).
Table 1.
Summary of Alleles Useda
| Allele | Type of allele | Full name | MGI | Reference |
|---|---|---|---|---|
| Foxd3flox | Conditional | Foxd3tm3Lby | 1347473 | Teng et al. (2008) |
| Foxd3− | Deletion (by GFP insertion) | Foxd3tm2Lby | 1347473 | Hanna et al. (2002) |
| Pax3Cre | Insertion | Pax3tm1(cre)Joe | 97487 | Engleka et al. (2005) |
| Pax3Sp | Spontaneous splice-site mutation (null) | Pax3Splotch | 97487 | Chalepakis et al. (1994) |
| Pax3Sp-d | Missense mutation | Pax3Splotch-delayed | 97487 | Vogan et al. (1993) |
| Wnt1-Cre | Transgene | Tg(Wnt1-Cre)11Rth | 98953 | Danielian et al. (1998) |
| R26RlacZ | Reporter | Gt(ROSA)26Sortm1Sor | 104735 | Soriano (1999) |
Details are in Methods section.
Although Pax3Cre/+; Foxd3flox/+ control embryos had no discernible defects, Pax3Cre/+; Foxd3flox/− compound mutant embryos had a fully penetrant cleft face phenotype from 11.5 dpc and onward (Fig. 1a compared with 1b) and loss of NCCs in the ENS (Supplemental Fig. 3), indicating similar loss of cranial and trunk NC derivatives compared with the Wnt1-Cre-driven deletion of Foxd3 (Teng et al., 2008). Control embryos (Pax3Cre/+; Foxd3flox/+) at these stages had a 100% survival rate (Table 2). In contrast, littermate compound mutant embryos (Pax3Cre/+; Foxd3flox/−) died at midgestation as early as 11.5 dpc, with the majority (71%) dead by 13.5 dpc, and 100% by 14.5 dpc (Table 2). Only compound Pax3; Foxd3 mutants exhibited midgestation lethality or craniofacial defects. Much like Wnt1-Cre; Foxd3flox/− mutant embryos, Foxd3 protein was efficiently deleted to levels beyond detection within the NC by 9.5 dpc (Supplemental Fig. 2).
FIG. 1.
Pax3Cre/+; Foxd3flox/− compound mutant embryos have craniofacial defects and persistent truncus arteriosus. (a,b) Frontal views of littermate control and compound mutant embryos at 12.5 dpc highlight craniofacial defects in the compound mutants. (a) In controls, the two sides of the face have met at the midline and fused. (b) The Pax3Cre/+; Foxd3flox/− compound mutant embryo has a characteristic cleft face. (c,d) Higher magnification ventral views of the developing hearts of the same embryos shown in (a) and (b), after dissection of body cavity and injection of India ink into the right ventricle. (c) In control embryos, the common truncus was septated to form the ascending aorta and pulmonary trunk. (d) In Pax3Cre/+; Foxd3flox/− embryos, the developing truncus arteriosus is not septated and persists as a single tube with less leftward rotation than that of the control pulmonary trunk as it exits the right ventricle. (e,f) Microresin casts of OFTs from 13.5 dpc control and mutant embryos. (e) Pax3Cre/+; Foxd3flox/+ control embryos have normal OFT morphology. (f) Mutant embryos lack the ductus arteriosus, but have normal patterning of the aortic arch arteries. The two pulmonary arteries emanating from the pulmonary trunk appear stunted with attenuated branching (arrowhead). (g,h) Outline trace of resin casts from (e) and (f), respectively. A, atria; AA, ascending aorta; BCA, brachiocephalic artery; CFD, craniofacial defect; DA, ductus arteriosus; LCA, left carotid artery; LSA, left subclavian artery; LV, left ventricle; PA, pulmonary artery; PT, pulmonary trunk; PTA, persistent truncus arteriosus; RV, right ventricle.
Table 2.
Pax3Cre/+; Foxd3flox/+ Compound Mutant Embryos Die at Midgestation
| Control (Pax3Cre/+; Foxd3flox/+) |
Mutant (Pax3Cre/+; Foxd3flox/−) |
|||||
|---|---|---|---|---|---|---|
| Alive/total | % Alive |
PTA | Alive/total | % Alive |
PTA | |
| 10.5 dpc | 14/14 | 100 | N/A | 16/16 | 100 | N/A |
| 11.5 dpc | 26/26 | 100 | N/A | 17/25 | 68 | N/A |
| 12.5 dpc | 32/33 | 97 | 0% | 24/40 | 60 | 100% |
| 13.5 dpc | 13/13 | 100 | 0% | 4/14 | 29 | 100% |
| ≥ 14.5 dpc | 12/12 | 100 | 0% | 0/11 | 0 | 100% |
Deletion of Foxd3 from the NC in a Pax3 heterozygous null (Pax3Cre/+) background resulted in embryonic lethality beginning at 11.5 dpc; all embryos were dead by 14.5 dpc. Control embryos did not exhibit embryonic lethality with the exception of one 12.5 dpc embryo that most likely died because of an unrelated cause; this embryo did not have a craniofacial or OFT defect. All mutant embryos examined had a PTA. N/A, not applicable.
Pax3Cre-Mediated Deletion of Foxd3 From the NC Results in PTA
Cardiac NCCs migrate ventrally from dorsal neural tube regions corresponding to rhombomeres 6, 7, and 8, and travel along the pharyngeal arch arteries, facilitating remodeling of these arteries. A wedge of cardiac NCCs continues migration, forming a septation complex dividing the common truncus into the dorsal aorta and the pulmonary trunk. All control embryo OFTs at 12.5 and 13.5 dpc had clearly septated into aortic and pulmonary arteries (Figs. 1c, 2a, and 3a). In contrast, a failure to septate the OFT, resulting in PTA, was first evident in compound mutants at 12.5 dpc, when all wild-type and littermate control embryos had completed septation. All compound mutant embryos examined at 12.5 and 13.5 dpc had an unseptated truncus (Figs. 1d, 2b, and 3b). Therefore, in stark contrast to Wnt1-Cre; Foxd3flox/− mutant embryos, all Pax3Cre/+; Foxd3flox/− compound mutant embryos had a 100% penetrant PTA (n = 25).
FIG. 2.
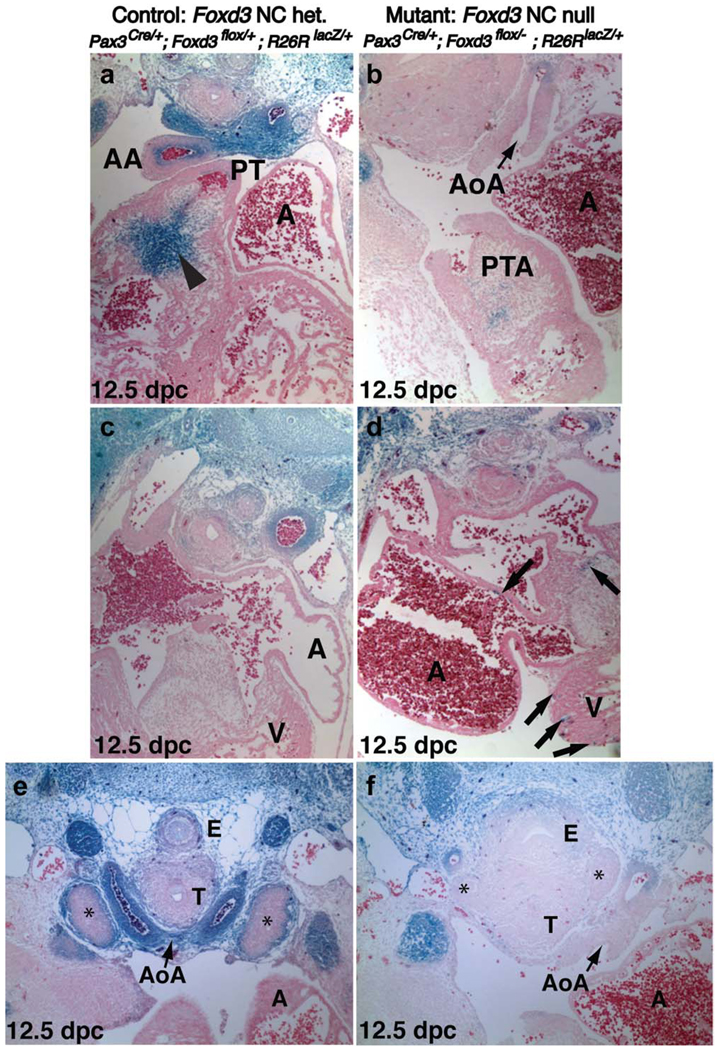
Lineage analysis of NC in control and compound mutant embryos reveals a reduction in the population of Pax3-lineage NCCs migrating into the OFT cushions and aortic arch arteries. (a–d) Transverse sections of X-gal-stained hearts from 12.5 dpc littermate control embryos (a,c) and compound mutant embryos (b,d). Sections (a) and (b), and (c) and (d) are at the same levels. (a) In controls, Pax3-lineage-labeled cells enter the OFT cushions as a large wedge in the pulmonary trunk and the inner layer of the ascending aorta (arrowhead). The majority of cells along the aortic arch are labeled. The ascending aorta and pulmonary trunk are clearly septated. (b) In mutants, the aortic arch (AoA) is mostly devoid of labeled cells. The truncus arteriosus has some X-gal-positive cells present, but remains a single vessel with no septation between the ascending aorta and pulmonary trunk (PTA). (c) In controls, Pax3-lineage-labeled cells are only rarely detected in the ventricles or elsewhere within the heart. (d) In mutants, labeled cells are found ectopically within the ventricles and sometimes in the atria, indicative of abnormal Pax3-lineage contribution and migratory behavior (arrows). (e,f) X-gal-labeled transverse sections of 12.5 dpc Pax3Cre/+; Foxd3flox/−; R26RLacZ/+ littermate control and compound mutant embryos. (e) Developing thymic primordia (asterisks) contain an outer layer of Pax3-lineage-labeled cells in controls. (f) Compound mutant thymic primordia are drastically reduced in size (asterisks) and devoid of Pax3-lineage-labeled cells. The trachea and esophagus from mutant embryos have a significant loss of surrounding labeled cells, and morphology is abnormal; the two structures are not as distinctly separated and appear less compact. A, atria; AA, ascending aorta; AoA, aortic arch artery; E, esophagus; PT, pulmonary trunk; PTA, persistent truncus arteriosus; T, trachea; V, ventricle.
FIG. 3.
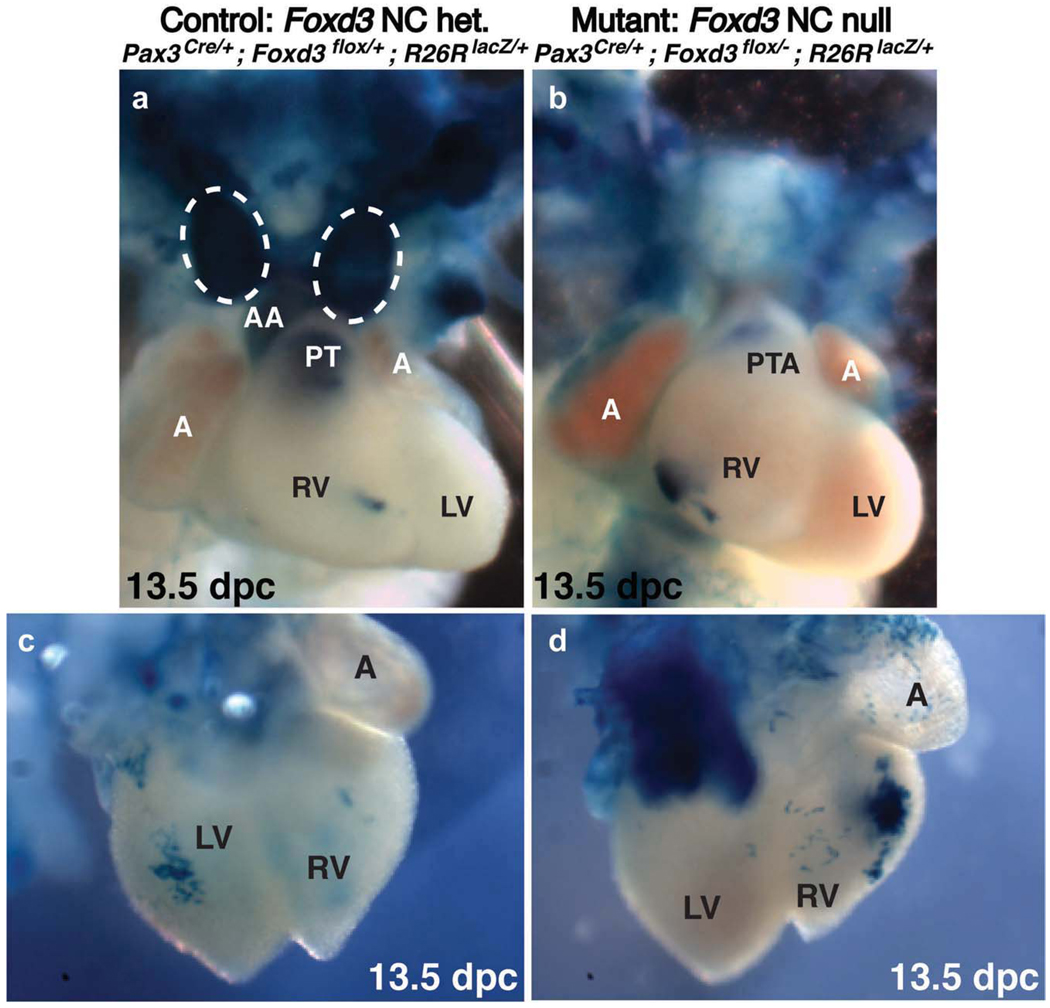
Whole-mount lineage-labeling analysis reveals disruption of cardiac NCCs caused by lack of Foxd3 and reduced Pax3 gene dosage. Whole-mount X-gal labeling of 13.5 dpc control (a,c) and compound mutant (b,d) littermate embryos. (a,b) Ventral views of dissected and stained hearts. (a) Labeled NCCs are present in the pulmonary trunk and ascending aorta of Pax3Cre/+; Foxd3flox/+ control hearts. The thymus (circled with a dashed line) is present in controls and robustly labeled. (b) In hearts from compound mutant embryos, the number of X-gal-positive cells in the truncus arteriosus is drastically reduced compared with the septated truncus of control hearts, and a large mass of labeled cells is present in the right ventricle. A thymus is not present in mutant embryos at 13.5 dpc. (c,d) Dorsal views of dissected and stained hearts. (d) The same mass of cells seen in (b) can be seen from the dorsal side of the heart. (Note: the large mass of blue labeled cells in the top left corner of (d) is the descending aorta, projecting out perpendicularly from the plane of the image. This structure is labeled with X-gal staining from Pax3-lineage cells that are not of NC origin in both mutants and controls; in the control embryo shown in (c), the aorta had been dissected away.) A, atrium; AA, ascending aorta; LV, left ventricle; PT, pulmonary trunk; PTA, persistent truncus arteriosus; RV, right ventricle.
Ink Fills, Resin Casts, and Histological Analysis Highlight the Cardiac NC Phenotype
Ink injections and resin casts of control (n = 6 and 2, respectively) and mutant (n = 6 and 2, respectively) littermate hearts confirmed PTA in mutants and revealed truncated pulmonary arteries at 13.5 dpc (Fig. 1e compared with 1f). It is unclear whether this was a defect in the pulmonary arteries or a filling artifact because of obstructed fluid flow through the abnormal OFT morphology and lack of a ductus arteriosus. Otherwise, the patterning of the aortic arch arteries was generally unperturbed. Morphological and histological analyses and whole-mount immunohistochemistry for CD31/PECAM to examine endothelial cells revealed no differences (Supplemental Fig. 4).
Lineage Labeling Shows Reduced NC Contribution to the OFT of the Heart
Pax3 and Foxd3 are both expressed in premigratory and early migrating cardiac NC (Goulding et al., 1991; Serbedzija and McMahon, 1997). However, as migration continues and cardiac NCCs enter the OFT, expression of these genes is lost (Epstein et al., 2000; Labosky and Kaestner, 1998; Mundell and Labosky, manuscript submitted). To follow the fate of NCCs and progeny in control and mutant embryos, the R−6RlacZ reporter allele was used with Pax3Cre (Engleka et al., 2005; Jiang et al., 2002). This combination indelibly marks the entire lineage of Pax3-expressing cells, regardless of current Pax3 expression status. We used this approach to lineage map Pax3-expressing NC in embryos with a Pax3Cre - driven deletion of Foxd3 compared directly with embryos with one wild-type Foxd3 allele. Pax3Cre/+; Foxd3flox/+; R26RlacZ/+ control and Pax3Cre/+; Foxd3-flox/−; R26RlacZ/+ compound mutant embryos were compared to assess defects in mutant NC migration and expansion. In control embryonic OFTs at 12.5 and 13.5 dpc, we observed lineage-labeled X-gal-positive cells in sections (arrowhead in Fig. 2a, n = 3) and in whole-mount embryos (Fig. 3a) as a wedge of cells in the cardiac OFT cushions of the conotruncus. Embryos without either the Pax3Cre or R26RlacZ alleles had no X-gal-positive cells (Supplemental Fig. 5). Lineage-labeled cells were present in compound mutant embryonic OFTs throughout the anterior-to-posterior axial levels of the conotruncus, but the population of lineage-labeled NCCs was drastically reduced along the entire axis (Fig. 2a compared with 2b, and data not shown). Whole-mount lineage labeling of 13.5 dpc compound mutant hearts also revealed mislocalized Pax3-lineage marked cells. A few labeled cells were seen in the right ventricle of control embryos (Fig. 3a), but we observed a larger cluster of cells in compound mutants (Fig. 3b) that extended to the dorsal side of the heart (Fig. 3d). The size of this patch of X-gal-positive cells was variable but typically within the same location, and fell within a spectrum of largest (Fig. 3b) to smallest (Supplemental Fig. 3b), but was always observed (n = 5, three of these were large and two were small). In contrast, the smaller patch of cells in controls was not always seen (Fig. 3a, two of five samples). In sections of compound mutant embryos, aberrantly localized labeled cells were observed near or in myocardial walls (arrows in Fig. 2d). Experimental pursuit of this observation was beyond the scope of our work here, but it is possible that appearance of these cells could be an artifact of ectopic Cre expression or related to non-NC Pax3 expression.
Pax3Cre/+; Foxd3flox/− Mutant Embryos Also Have Thymus Defects
NCCs contribute to mesenchyme surrounding the developing thymus (Bockman and Kirby, 1989; Le Lievre and Le Douarin, 1975). Wnt1-Cre; Fox3flox/− mutant embryos had severe defects in most of the NC, with the exception of the cardiac NC; they also had only subtle thymic defects with occasional undescended and asymmetric thymic lobes (Teng et al., 2008; unpublished data). We observed no thymic defects in Pax3Sp/+ or Pax3Cre/+ heterozygous embryos, consistent with previous studies (Conway et al., 1997; Griffith et al., 2009). However, in Pax3Cre/+; Foxd3flox/− embryos, the thymus was severely reduced in size compared with controls and lacked the outer layer of surrounding NCCs at 12.5 dpc (n = 4, Fig. 2e,f). Serial sections were examined to exclude the possibility of an undescended thymus. The remaining mutant thymic rudiments at 12.5 dpc did not always descend as far as control thymus, but there was never evidence of a normal undescended thymus (data not shown). By 13.5 dpc, the thymus was clearly visible in whole-mount dissections of controls (Fig. 3a; see also Supplemental Fig. 3a), but was not detected in whole-mount dissections (n = 5) of mutant embryos (Fig. 3a,b; see also Supplemental Fig. 3). Pax3Cre/+; Foxd3flox/− compound mutants, in addition to marked loss of the thymus, appear to have an irregular morphology of the trachea and esophagus, areas normally invested with NC (Fig. 2e,f).
Pax3Cre-Mediated Deletion of Foxd3 From the NC Results in an Early Loss of NC Progenitors Caudal to the First Pharyngeal Arch
To determine whether the reduced populations of Pax3-lineage presumptive NCCs in cardiac NC-derived structures were a result of an earlier loss of progenitor NCCs, we used the same fate-mapping strategy to examine embryos at earlier developmental stages. Although Pax3 expression begins by 8.5 dpc, we observed no detectable levels of β-galactosidase activity in either control or mutant embryos at 8.5 dpc (data not shown), most likely because of a delay in Cre activation of the R26R reporter gene. However, at 9.5 dpc, we saw robust X-gal staining, indicating Cre activity from the Pax3 locus in control embryos (Fig. 4a,c, n = 4). In these control embryos, presumptive NCCs filled the first three pharyngeal arches and contributed to the presumptive cranial skeleton. In Pax3; Foxd3 compound mutant embryos (Fig. 4b,d), we observed a decrease in lineage-labeled NCCs at 9.5 dpc (n = 2). The extent of migration of Pax3-lineage cells into the first pharyngeal arch was comparable with controls, but the intensity of X-gal staining was reduced, suggesting fewer NCCs were present. Strikingly, there was no detectable X-gal staining caudal to the first pharyngeal arch of mutant embryos. This was in contrast to the reduction of NCCs seen in the Wnt1-Cre-driven deletion of Foxd3, where reduced numbers of presumptive NC lineage-positive cells were located in all pharyngeal arches at 9.5 dpc (Teng et al., 2008). The apparent lack of Pax3-lineage-positive cells in the second and third pharyngeal arches, despite a significant population within the first pharyngeal arch, suggests that Pax3 and Foxd3 are required together to confer migratory competence to NC progenitors or preferentially control the initial expansion or survival of progenitors that later enter the second and third pharyngeal arches.
FIG. 4.
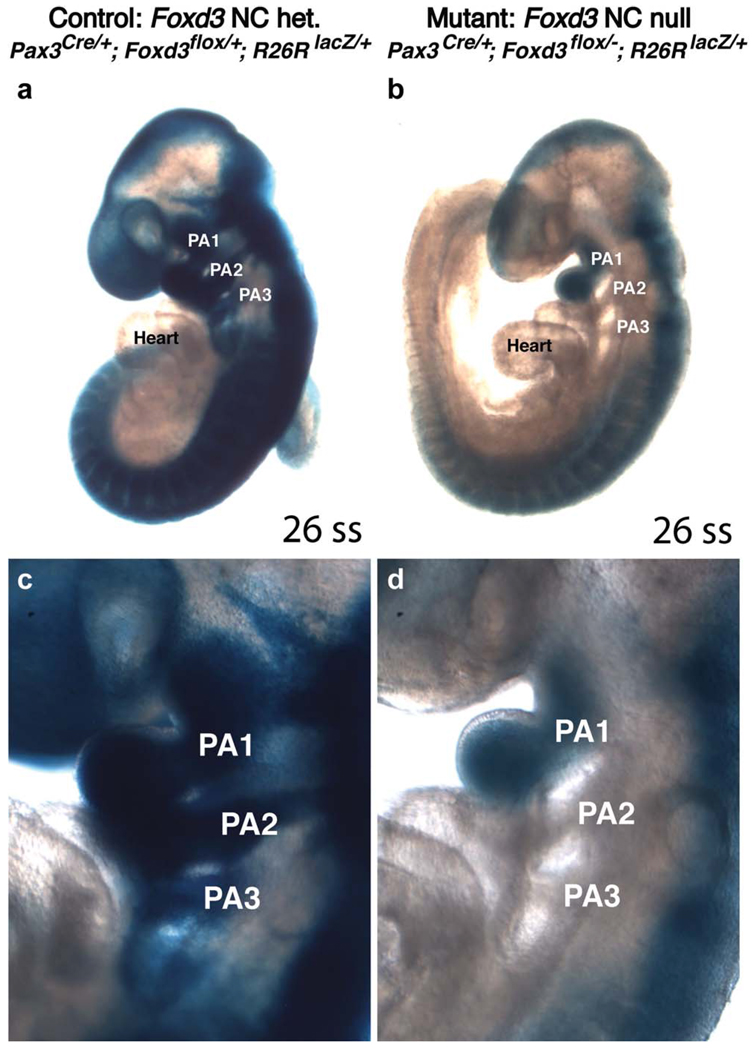
Compound mutant embryos have a drastic early reduction in NCCs. Littermate control (a,c) and compound mutant (b,d) whole-mount embryos at 9.5 dpc labeled by X-gal staining to trace Pax3-lineage cells. In controls, blue Pax3-lineage cells fill the first three pharyngeal arches (PA1, PA2, and PA3) and more of the developing skull. In littermate compound mutant embryos, only PA1 contains Pax3-lineage cells; PA2 and PA3 appear devoid of Pax3-lineage cells. Both embryos are at identical developmental stages, each with 26 somites. (c) and (d) are enlarged regions from (a) and (b), respectively. PA, pharyngeal arch; ss, somite stage.
Cell Death Within the Early, Premigratory NC Progenitor Pool Is Increased in Compound Mutants
To determine whether the drastic reduction in NCCs was the result of cell death of premigratory or migratory NC progenitors, we performed a LysoTracker assay to visualize dying cells. At 8.5 dpc, mutant embryos (n = 4) had a distinct stripe of LysoTracker-positive cells along the lateral edge of the neural folds (corresponding to the origin of premigratory NCCs) that was not present in controls (n = 3) (Fig. 5a–f). After 9.0 dpc, we detected no discernable differences in pattern or intensity of LysoTracker staining (Fig. 5g–i).
FIG. 5.
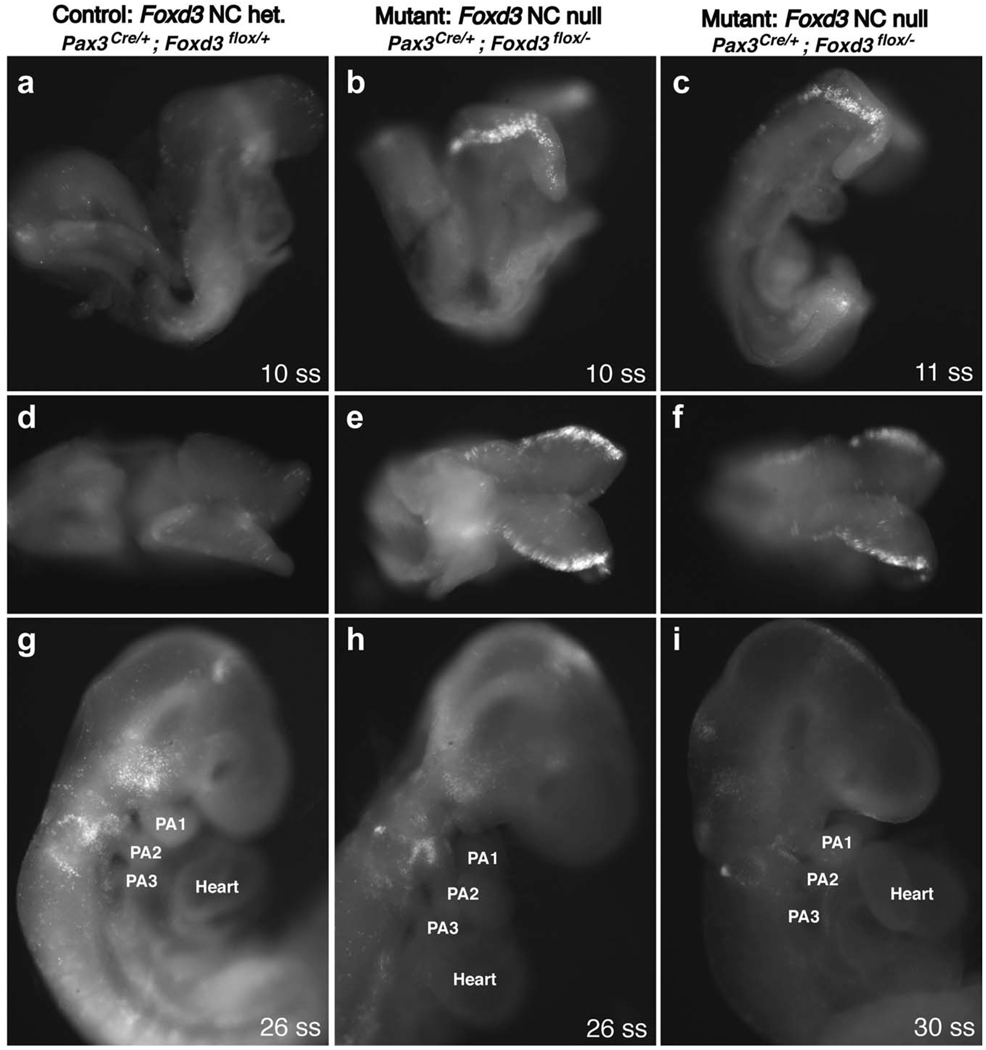
Compound mutant Pax3; Foxd3 embryos have greatly increased cell death in the neural folds at 8.5 dpc. Littermate control and compound mutant whole-mount embryos at 8.5 dpc (a–f), 9.5 dpc (g,h), and 10.0 dpc (i), treated with LysoTracker to monitor cell death. (a– c) and (g–i) are lateral views, and (d–f) are dorsal views of the same embryos from (a–c). The embryos in (a,d) and (b,e) each have 10 somites, whereas (c,f) has 11 somites. (g) and (h) are both ~ 26 somites, (i) is ~ 30 somites. PA, pharyngeal arch; ss, somite stage.
Loss of One Functional Allele of Pax3 Combined With NC Deletion of Foxd3 Is Responsible for PTA and Lethality
At least two plausible hypotheses exist for the marked difference in phenotype between Wnt1-Cre; Foxd3flox/− embryos and Pax3Cre/+; Foxd3flox/− embryos. One hypothesis is that subtle differences in the spatiotemporal expression of Cre when driven by Wnt1 versus Pax3 regulatory sequences or differences in Cre efficiency cause differential deletion of Foxd3.
This could result in a population of compensatory Foxd3-expressing cells in Wnt1-Cre-mediated Foxd3 mutants not present when Pax3Cre/+ is used. An alternative hypothesis is that the dosage of Pax3 in a Foxd3-conditional null background is critical for NC survival and, therefore, OFT septation, and loss of one allele of Pax3 combined with NC-specific loss of Foxd3 causes the more severe phenotype.
To distinguish between these hypotheses, mice were bred to introduce the Pax3Sp or Pax3Sp-d mutant alleles into the Wnt1-Cre; Foxd3flox/− background (Supplementary Fig. 1). Pax3Sp is a null allele encoding a truncated Pax3 protein (Chalepakis et al., 1994). Homozygous Pax3Sp/Sp mutants die at midgestation, with a reduced cardiac NC progenitor pool and PTA (Conway et al., 2000; Epstein et al., 2000). Pax3Sp-d is a hypomorphic allele encoding a missense mutation in the Pax3 DNA binding domain (Underhill et al., 1995; Vogan et al., 1993). Homozygous Pax3Sp-d/Sp-d mutants have mild NC defects compared with Pax3Sp/Sp embryos and no PTA (Franz, 1993), surviving until birth when they die from neural tube defects. Neither allele causes discernable cardiac defects when heterozygous. We compared control Wnt1-Cre; Foxd3flox/− embryos with Wnt1-Cre-driven Foxd3 mutant embryos containing either of these mutant Pax3 alleles (Pax3Sp/+; Wnt1-Cre; Foxd3flox/− and Pax3Sp-d/+; Wnt1-Cre; Foxd3flox/− embryos). When the Wnt1-Cre transgene was used to delete Foxd3 in NCCs, additional loss of one functional allele of Pax3 resulted in midgestational embryonic lethality and 100% penetrant PTA, mimicking the Pax3-Cre/+; Foxd3flox/− compound mutant phenotype (Table 3). Both Splotch and Splotch-delayed alleles gave the same phenotype. These results supported the second hypothesis above: reductions in Pax3 gene dosage are responsible for midgestation lethality and PTA in conjunction with loss of Foxd3 in the NC. This experiment also eliminated the possibility of differences in genetic background between Wnt1-Cre and Pax3Cre strains being responsible for phenotypic differences: Pax3Sp/+; Wnt1-Cre; Foxd3flox/− compound mutant embryos with PTA and early lethality were on identical genetic backgrounds with littermate controls (Wnt1-Cre; Foxd3flox/−) that did not display early lethality or PTA.
Table 3.
Pax3Sp/+; Wnt1-Cre; Foxd3flox/− Compound Mutant Embryos Die at Midgestation
| Mutant | ||||||
|---|---|---|---|---|---|---|
| Control Wnt1-Cre; Foxd3flox/− |
Wnt1-Cre; Pax3Sp/+; Foxd3flox/− |
Wnt1-Cre; Pax3Sp-d/+; Foxd3flox/− |
||||
| Alive/total | % Alive |
Alive/total | % Alive |
Alive/total | % Alive |
|
| 11.5 dpc | 8/8 | 100 | 2/3 | 67 | 2/2 | 100 |
| 12.5 dpc | 10/10 | 100 | 3/5 | 60 | 3/6 | 50 |
| 13.5 dpc | 7/7 | 100 | 1/7 | 14 | 1/6 | 17 |
| ≥ 14.5 dpc | 19/19 | 100 | 0/13 | 0 | 0/18 | 0 |
Deletion of Foxd3 from the NC in two independent Pax3 heterozygous mutant backgrounds resulted in midgestational lethality and a 100% penetrant persistent truncus arteriosus, independent of Cre line. All mutant embryos examined had a PTA.
DISCUSSION
Our data show that Foxd3 and Pax3 interact genetically during development of the NC, specifically affecting the cardiac NC and the thymus beyond defects seen in either mutant alone, revealing previously unappreciated roles for these two proteins. Foxd3 NC homozygous mutants do not have severe heart or thymus defects, and Pax3 heterozygous or homozygous null mutants do not have severe thymus hypoplasia, but compound mutants had both severe heart defects and thymus hypoplasia, and at greater penetrance. Although these defects approximate those in human VCFS, Foxd3 and Pax3 are not initiators of VCFS (neither gene is located on mouse chromosome 16, syntenic to human chromosome 22 (Galili et al., 1997)), but our work here suggests that Foxd3 and Pax3 may serve as effectors in the same gene regulatory networks. Alternatively, with respect to the cardiac NC, the Foxd3/Pax3 compound mutants may more closely approximate a NC ablation model, suggesting that these two genes may be involved in the master control of earlier steps in pathways affecting cardiac NC development, including expansion, survival, or maintenance of a cardiac NC progenitor pool, and in fact, our lineage-labeling experiments (Figs. 2–4) support this notion.
In the process of OFT septation, the combined loss of both alleles of Foxd3 and one allele of Pax3 is similar to loss of both alleles of Pax3. These mutants do not display neural tube defects characteristic of Pax3−/− mutants, but the expression domain of Foxd3 does not overlap with Pax3 in the non-NC dorsal neural tube. This suggests that in the NC, Foxd3 and Pax3 play a similar role in mediating progenitor fate, or that Pax3 may be acting through Foxd3. Indeed, a reduction in Foxd3 expression in Pax3Sp/Sp mutants suggests that the latter is true (Dottori et al., 2001): Foxd3 is genetically downstream of Pax3. Work in other vertebrates demonstrated a similar relationship between Foxd3 and Pax3; in Xenopus laevis NC induction, Pax3, together with Zic1, induces early NC genes, including Foxd3 (Sato et al., 2005).
This genetic interaction may reflect a physical interaction, and if the interaction were direct, the two transcription factors could be acting at shared targets or at the protein–protein level; ectopic expression of Foxd3 in an avian melanoma cell line results in Foxd3 repressing melanin synthesis by directly binding Pax3 protein and sequestering it, preventing Pax3 binding and activation of the Mitf promoter (Thomas and Erickson, 2009). However, Pax3 and Foxd3 could just as likely be acting through separate, parallel pathways. There are a number of possibilities for the molecular mechanism of this genetic interaction that remain to be addressed in future studies. Regardless, the function of Pax3 on transcription figures to be important for the genetic interaction we observe, considering that there was no difference in the genetic interaction we defined here between the null Pax3Sp and the DNA-binding domain mutant Pax3Sp-d alleles.
Use of the classic Pax3Sp and Pax3Sp-d alleles confirms that we have uncovered a true genetic interaction rather than differences between Wnt1-Cre and Pax3Cre expression domains, despite the fact that there are known differences in Cre expression in these two mouse lines (Engleka et al., 2005; Jiang et al., 2000). Pax3 is expressed before Wnt1 in the NC (Fenby et al., 2008; Osorio et al., 2009), and Pax3 binds directly to the Wnt1 promoter to activate Wnt1 transcription (Fenby et al., 2008), suggesting it is upstream. Wnt1-Cre is more commonly used for NC-specific deletion, but will act on cells within the developing roof plate and midbrain and does not label NCCs from the posterior-most levels of the NT (Jiang et al., 2000). Pax3Cre has a broader expression domain than Wnt1-Cre, active in presomitic mesoderm and the NC, but also in cells originating from the non-NC dorsal NT. To our knowledge, no direct comparison between Wnt1-Cre and Pax3Cre in the NC has been published, but a few studies have used both with no differences. A conditional knockout of Myocardin using Wnt1-Cre or Pax3Cre separately resulted in no significant phenotypic differences (Huang et al., 2008), and a conditional deletion of Connexin43 with Wnt1-Cre versus Pax3Cre resulted in a more severe phenotype with Pax3Cre, but these defects were related to Pax3 expression in the non-NC dorsal neural tube (Kretz et al., 2006; Liu et al., 2006). Therefore, our work represents the first example of a genetic interaction discovered with this approach.
Another hypothesis is that Pax3 and Foxd3 pathways are not intertwined, and mutations in both are merely two separate NC disruptions converging on the same readout. However, other than a reduction in melanocytes resulting in a belly spot, there is no evidence of functional defects in heterozygous Pax3 mutants or double-heterozygous Pax3+/−; Foxd3+/− mice. In a Wnt1-Cre; PUΔTK mouse NC ablation model, severity of ablation was controlled by the number of gancyclovir injections (Porras and Brown, 2008), and the greater the extent of NC ablation, the higher the percentage of embryos with PTA. The 100% penetrant PTA we observed, coupled with the drastic reduction of lineage-labeled NCCs, suggests that with respect to the cardiac NC, the compound mutants here approximate an NC ablation model. This may represent a difference from single homozygous mutations in Pax3 or Foxd3 resulting in NC deficiency but with enough NCCs remaining to facilitate at least partial thymic and/or cardiovascular development. Whether the genetic interaction we describe represents the action of Foxd3 and Pax3 in the same pathway, converging pathways, or whether they are completely separate insults that together reduce the population of NCCs below levels critical for OFT and thymus development, our results indicate Foxd3 and Pax3 together may be master control genes for the early cardiac NC.
METHODS
Mouse Strains and Breeding
Mus musculus were used for all experiments; these mice were of a mixed genetic background derived from C57BL6, SvEv129S6, and CD-1 strains. Genetically manipulated mice described here are compared with control littermates. Because of the need for several different alleles in each mouse line generated, the intermediate mouse strains are generally on an outbred, mixed genetic background. All mouse strains (Table 1) used are previously described and listed here: Foxd3− refers to Foxd3tm2Lby (Hanna et al., 2002) in all experiments except those using the R26RYFP reporter allele Gt(ROSA) 26Sortm1(EYFP)Cos (Srinivas et al., 2001), in which case Foxd3− refers to Foxd3tm1Lby (Hanna et al., 2002). Foxd3flox is Foxd3tm3Lby (Teng et al., 2008), Pax3Cre is Pax3tm1(Cre)Joe (Engleka et al., 2005), and Wnt1-Cre is Tg(Wnt1-Cre)11Rth (Danielian et al., 1998). Pax3Sp and Pax3Sp-d refer to the Pax3 alleles Splotch and Splotch-delayed (Dickie, 1964), and R26RlacZ is Gt(ROSA)26Sortm1Sor (Soriano, 1999); these last three lines were obtained from The Jackson Laboratory (Bar Harbor, ME). Breeding details are shown in Supplemental Figure 1. Pax3Cre/+; Foxd3flox/− compound mutant embryos were generated from Foxd3flox/flox crossed to Pax3Cre/+; Foxd3+/− mice. Matings were also carried out with Foxd3flox/flox; R26RlacZ/lacZ dams for lineage mapping of Cre-expressing cells. To obtain Pax3xSplotch/+; Wnt1-Cre; Foxd3flox/− embryos and littermate controls, Pax3Splotch/+; Wnt1-Cre; Foxd3 −/+ male mice were mated to Foxd3flox/flox dams. The same breeding scheme was used with Pax3Splotch-delayed in place of Pax3Splotch to generate the equivalent littermate mutants and controls. Genotyping of mice and embryos was performed with polymerase chain reaction (PCR) as previously described (Danielian et al., 1998; Engleka et al., 2005; Hanna et al., 2002; Teng et al., 2008). All mice were maintained and handled in accordance with Association for Assessment and Accreditation of Laboratory Animal Care standards as approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee (IACUC).
Embryo Dissections
For timed matings, females were checked daily for presence of a vaginal plug; noon on the day of an observed plug was considered 0.5 dpc. Embryos were harvested around noon of the day of dissection, so that these embryos would correspond to x.5 dpc (x = number of days after plug observed). Embryos were dissected from the uterus and surrounding deciduum into phosphate buffered saline (PBS) plus 0.1% bovine serum albumin (BSA) and immediately examined for signs of early lethality, including pale tissue, blood pooling, lack of heartbeat, decreased size, and in cases of embryonic lethality occurring presumably more than a day before dissection, gross tissue death and readsorption of the embryo.
Visualization of Whole-Mount Cardiac Morphology
For cardiovascular analysis, embryos from 10.5 to 13.5 dpc were dissected in PBS, and the chest cavities opened to reveal the embryonic heart. For ink injections, a 1:10 dilution of Super Black India Ink (Speed-ball) in PBS was injected into the ventricles using a 1.0 mm outer diameter/0.78 mm inner diameter borosilicate glass pipette needle (pulled with a Kopf Vertical Pipette Puller). For resin casts, Mercox II resin containing methyl methacrylate was mixed with red dye and catalyst (Ladd Research Industries, Williston, VT) and immediately injected using needles as above until resin was visualized in the heart, OFT, and great vessels. The resin was hardened overnight at 4°C, samples macerated at 50°C with maceration solution containing potassium hydroxide (Polysciences, Inc., Warrington, PA) and washed in water for visualization and storage.
Histological and Whole-Mount Embryo Labeling
Embryos were prepared for histology, and histology was performed by standard procedures (Nagy et al., 2003; Presnell and Schreibman, 1997) and carried out on 7-µM paraffin sections on SuperFrost-Plus slides (Fisher, Fair Lawn, NJ). 5-Bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal) reactions were performed following standard techniques (Nagy et al., 2003). For histological sections of X-gal-labeled embryos, samples were processed using Histoclear in place of Xylenes and sections counterstained with eosin Y. Antibodies used on histological sections were rabbit anti-Foxd3 (Washington Biotechnology, Simpsonville, MD) at 1:500 and chicken anti-GFP (Abcam, Cambridge, MA) at 1:500. Secondary antibodies used were donkey anti-rabbit Cy3 (1:500; Jackson ImmunoResearch, West Grove, PA) and donkey anti-chicken biotin (1:500; Jackson ImmunoResearch) amplified with horse radish peroxidase (HRP)-conjugated streptavidin (Vector RTU Kit) plus tyramide-Alexa488 (1:400; Invitrogen, Carlsbad, CA), respectively. Antibodies used for whole-mount PECAM staining were rat anti-PECAM-1/CD31 (3:500; BD Pharmingen, Sparks, MD) and a biotinylated donkey anti-rat secondary (1:1,000, Jackson Immunoresearch) in combination with VectaStain ABC (Vector Laboratories, Burlingame, CA). Immunohistochemistry for Foxd3 on sections from 9.5 dpc mutant and control embryos was performed as previously described (Tompers et al., 2005). Whole embryo images were captured with a Q-Imaging Micropublisher camera and a Nikon SMZ-U stereomicroscope; sections were imaged with a Q-Imaging Retiga 2000r camera on a Zeiss Axioskop2 Plus or a Nikon Eclipse E600 microscope. For whole-mount cell death visualization, embryos were dissected in Hanks balanced salt solution and incubated in 2-µM LysoTracker (Molecular Probes) for either 1 h (9.5 dpc and above) or 15–20 min (8.5 dpc) at 37°C with gentle agitation. Embryos were then washed and fixed overnight in 4% paraformaldehyde (PFA) in PBS, washed in PBS, partially dehydrated in 50% methanol in PBS, and imaged with a Leica MZ16 FA stereoscope equipped with fluorescence and a Q-Imaging Retiga 4000r camera.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful for assistance from Alison LeGrone in maintaining, weaning, and genotyping mice, and from Audrey Frist with histology and genotyping embryos. They thank the past and current members of the Labosky Lab and the laboratories of Drs. Mark Magnuson and Stacey Huppert for thoughtful discussion and use of equipment. They also thank Dr. Lu Teng for preliminary data on this project, and Dr. Christopher Brown and Nathan Mundell for guidance on ink injections, resin casting, and discussion. They also thank Dr. Jonathan Epstein for Pax3Cre/+ mice and Dr. H. Scott Baldwin and Jennifer Plank for critical reading and discussion of the manuscript.
Contract grant sponsor: NIH, Contract grant numbers: R01 HD036720 (to P.A.L.), T32 HD007043 (to B.L.N.), and T32 HD007502 (to E.R.P.), Contract grant sponsor: American Heart Association, Contract grant number: Postdoctoral Fellowship 0725615B (to B.L.N)
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Bando K, Turrentine MW, Sharp TG, Sekine Y, Aufiero TX, Sun K, Sekine E, Brown JW. Pulmonary hypertension after operations for congenital heart disease: Analysis of risk factors and management. J Thorac Cardiovasc Surg. 1996;112:1600–1607. doi: 10.1016/S0022-5223(96)70019-3. discussion 1607–1609. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Kirby ML. Neural crest function in thymus development. Immunol Ser. 1989;45:451–467. [PubMed] [Google Scholar]
- Chalepakis G, Goulding M, Read A, Strachan T, Gruss P. Molecular basis of Splotch and Waardenburg Pax-3 mutations. Proc Natl Acad Sci U S A. 1994;91:3685–3689. doi: 10.1073/pnas.91.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Cheung CS, Yung KM, Copp AJ. Cardiac neural crest of the mouse embryo: Axial level of origin, migratory pathway and cell autonomy of the Splotch (Sp2H) mutant effect. Development. 2004;131:3367–3379. doi: 10.1242/dev.01197. [DOI] [PubMed] [Google Scholar]
- Choi M, Klingensmith J. Chordin is a modifier of tbx1 for the craniofacial malformations of 22q11 deletion syndrome phenotypes in mouse. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000395. e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary B, Ito Y, Makita T, Sasaki T, Chai Y, Sucov HM. Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFbeta receptor (Tgfbr2) mutant mice. Dev Biol. 2006;289:420–429. doi: 10.1016/j.ydbio.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Bundy J, Chen J, Dickman E, Rogers R, Will BM. Decreased neural crest stem cell expansion is responsible for the conotruncal heart defects within the Splotch (Sp(2H))/Pax3 mouse mutant. Cardiovasc Res. 2000;47:314–328. doi: 10.1016/s0008-6363(00)00098-5. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Henderson DJ, Copp AJ. Pax3 is required for cardiac neural crest migration in the mouse: Evidence from the Splotch (Sp2H) mutant. Development. 1997;124:505–514. doi: 10.1242/dev.124.2.505. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Dickie MM. New Splotch alleles in the mouse. J Hered. 1964;55:97–101. doi: 10.1093/oxfordjournals.jhered.a107317. [DOI] [PubMed] [Google Scholar]
- Dottori M, Gross MK, Labosky P, Goulding M. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol. 2005;280:396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Li J, Lang D, Chen F, Brown CB, Jin F, Lu MM, Thomas M, Liu E, Wessels A, Lo CW. Migration of cardiac neural crest cells in Splotch embryos. Development. 2000;127:1869–1878. doi: 10.1242/dev.127.9.1869. [DOI] [PubMed] [Google Scholar]
- Fenby BT, Fotaki V, Mason JO. Pax3 regulates Wnt1 expression via a conserved binding site in the 5′ proximal promoter. Biochim Biophys Acta. 2008;1779:115–121. doi: 10.1016/j.bbagrm.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Franz T. The Splotch (Sp1H) and Splotch-delayed (Spd) alleles: Differential phenotypic effects on neural crest and limb musculature. Anat Embryol (Berl) 1993;187:371–377. doi: 10.1007/BF00185895. [DOI] [PubMed] [Google Scholar]
- Galili N, Baldwin HS, Lund J, Reeves R, Gong W, Wang Z, Roe BA, Emanuel BS, Nayak S, Mickanin C, Budarf ML, Buck CA. A region of mouse chromosome 16 is syntenic to the DiGeorge, velocardiofacial syndrome minimal critical region. Genome Res. 1997;7:17–26. doi: 10.1101/gr.7.1.17. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith AV, Cardenas K, Carter C, Gordon J, Iberg A, Engleka K, Epstein JA, Manley NR, Richie ER. Increased thymus- and decreased parathyroid-fated organ domains in Splotch mutant embryos. Dev Biol. 2009;327:216–227. doi: 10.1016/j.ydbio.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Gene Dev. 2002;16:2650–2661. doi: 10.1101/gad.1020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Cheng L, Li J, Chen M, Zhou D, Lu MM, Proweller A, Epstein JA, Parmacek MS. Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest. 2008;118:515–525. doi: 10.1172/JCI33304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Choudhary B, Merki E, Chien KR, Maxson RE, Sucov HM. Normal fate and altered function of the cardiac neural crest cell lineage in retinoic acid receptor mutant embryos. Mech Dev. 2002;117:115–122. doi: 10.1016/s0925-4773(02)00206-x. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kirby ML. Alteration of cardiogenesis after neural crest ablation. Ann N Y Acad Sci. 1990;588:289–295. doi: 10.1111/j.1749-6632.1990.tb13218.x. [DOI] [PubMed] [Google Scholar]
- Kretz M, Eckardt D, Kruger O, Kim JS, Maurer J, Theis M, van Rijen HV, Schorle H, Willecke K. Normal embryonic development and cardiac morphogenesis in mice with Wnt1-Cre-mediated deletion of connexin43. Genesis. 2006;44:269–276. doi: 10.1002/dvg.20204. [DOI] [PubMed] [Google Scholar]
- Labosky PA, Kaestner KH. The winged helix transcription factor Hfh2 is expressed in neural crest and spinal cord during mouse development. Mech Dev. 1998;76:185–190. doi: 10.1016/s0925-4773(98)00105-1. [DOI] [PubMed] [Google Scholar]
- Lang D, Lu MM, Huang L, Engleka KA, Zhang M, Chu EY, Lipner S, Skoultchi A, Millar SE, Epstein JA. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: Analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Liu S, Liu F, Schneider AE, St Amand T, Epstein JA, Gutstein DE. Distinct cardiac malformations caused by absence of connexin 43 in the neural crest and in the non-crest neural tube. Development. 2006;133:2063–2073. doi: 10.1242/dev.02374. [DOI] [PubMed] [Google Scholar]
- Liu Y, Labosky PA. Regulation of embryonic stem cell self-renewal and pluripotency by Foxd3. Stem Cells. 2008;26:2475–2484. doi: 10.1634/stemcells.2008-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SC, Lee HY, Relaix F, Sandell LL, Levorse JM, Loeken MR. Cardiac outflow tract septation failure in Pax3-deficient embryos is due to p53-dependent regulation of migrating cardiac neural crest. Mech Dev. 2008;125:757–767. doi: 10.1016/j.mod.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Ventersten K, Behringer R. Manipulating the mouse embryo: A laboratory manual. 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- Osorio L, Teillet MA, Palmeirim I, Catala M. Neural crest ontogeny during secondary neurulation: A gene expression pattern study in the chick embryo. Int J Dev Biol. 2009;53:641–648. doi: 10.1387/ijdb.072517lo. [DOI] [PubMed] [Google Scholar]
- Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: Current knowledge: A scientific statement from the American Heart Association Congenital Cardiac Defects Committee. Council on Cardiovascular Disease in the Young: Endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- Porras D, Brown CB. Temporal-spatial ablation of neural crest in the mouse results in cardiovascular defects. Dev Dyn. 2008;237:153–162. doi: 10.1002/dvdy.21382. [DOI] [PubMed] [Google Scholar]
- Presnell JK, Schreibman MP. Humason’s animal tissue techniques. Baltimore, MD: The Johns Hopkins University Press; 1997. [Google Scholar]
- Richarte AM, Mead HB, Tallquist MD. Cooperation between the PDGF receptors in cardiac neural crest cell migration. Dev Biol. 2007;306:785–796. doi: 10.1016/j.ydbio.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Sasai N, Sasai Y. Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development. 2005;132:2355–2363. doi: 10.1242/dev.01823. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, McMahon AP. Analysis of neural crest cell migration in Splotch mice using a neural crest-specific LacZ reporter. Dev Biol. 1997;185:139–147. doi: 10.1006/dbio.1997.8551. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: 30 Years of study. Dev Disabil Res Rev. 2008;14:3–10. doi: 10.1002/ddrr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider P, Olaopa M, Firulli AB, Conway SJ. Cardiovascular development and the colonizing cardiac neural crest lineage. Sci World J. 2007;7:1090–1113. doi: 10.1100/tsw.2007.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankunas K, Shang C, Twu KY, Kao SC, Jenkins NA, Copeland NG, Sanyal M, Selleri L, Cleary ML, Chang CP. Pbx/Meis deficiencies demonstrate multigenetic origins of congenital heart disease. Circ Res. 2008;103:702–709. doi: 10.1161/CIRCRESAHA.108.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng L, Mundell NA, Frist AY, Wang Q, Labosky PA. Requirement for Foxd3 in the maintenance of neural crest progenitors. Development. 2008;135:1615–1624. doi: 10.1242/dev.012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Erickson CA. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development. 2009;136:1849–1858. doi: 10.1242/dev.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompers DM, Foreman RK, Wang Q, Kumanova M, Labosky PA. Foxd3 is required in the trophoblast progenitor cell lineage of the mouse embryo. Dev Biol. 2005;285:126–137. doi: 10.1016/j.ydbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Underhill DA, Vogan KJ, Gros P. Analysis of the mouse Splotch-delayed mutation indicates that the Pax-3 paired domain can influence homeodomain DNA-binding activity. Proc Natl Acad Sci U S A. 1995;92:3692–3696. doi: 10.1073/pnas.92.9.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogan KJ, Epstein DJ, Trasler DG, Gros P. The Splotch-delayed (Spd) mouse mutant carries a point mutation within the paired box of the Pax-3 gene. Genomics. 1993;17:364–369. doi: 10.1006/geno.1993.1333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.