Abstract
Antimicrobial photodynamic therapy (PDT) has recently emerged as an effective modality for the selective destruction of bacteria and other pathogenic microorganisms. We investigated whether PDT induced protective responses such as heat shock proteins in bacteria. Using the photosensitizer Toluidine Blue O (TBO) at sub-lethal PDT conditions, a 7-fold increase in bacterial heat shock protein GroEL and a 3-fold increase in heat shock protein DnaK were observed in Escherichia coli post PDT. Pretreatment with 50o C heat for 30 minutes reduced PDT killing in both E. coli and in Enterococcus faecalis, with the most pronounced inhibition occurring at 50-μM TBO with 5-J/cm2 635 nm light, where E. coli killing was reduced by 2- log10 and E. faecalis killing was reduced by 4-log10. Finally, inhibition of the highly conserved chaperone DnaK using a small molecule benzylidene lactam heat shock protein inhibitor potentiated (but not significantly) the effect of PDT at a TBO concentration of 2.5 μM in E. faecalis; however, this effect was not observed in E. coli presumably because inhibitor could not gain access due to Gram-negative permeability barrier. Induction of heat shock proteins may be a mechanism whereby bacteria could become resistant to PDT and warrants the need for further study in the application of dual PDT-heat shock protein-inhibition therapies.
Introduction
The need for novel antimicrobial techniques has become critical for a number of reasons. Excessive prescription and misuse of antibiotics accelerates the emergence of resistant strains and existing antimicrobials work poorly in chronic infections even when susceptibility is tested and confirmed in vitro (1, 2). The issue of resistance is best underscored by the recent and formidable emergence of vancomycin-intermediate and vancomycin-resistant, methicillin-resistant Staphylococcus aureus, that are considered highly virulent and lethal strains (3). Additionally, antibiotics are incapable of eradicating bacterial biofilms and spores, such as those found in Staphylococcus spp. and soil Bacillus spp., respectively (4, 5). As the end of the antibiotic age approaches, it becomes vital to develop novel solutions for the treatment of infections.
Photodynamic therapy (PDT) was accidentally discovered in 1900 when Raab et al. noted the antimicrobial action of acridine and light on Paramecium spp. (6). In the latter part of the twentieth-century, PDT emerged as a therapeutic clinical modality, receiving regulatory approval for the treatment of neoplastic and ophthalmological conditions, and has demonstrated efficient eradication of bacteria both in vitro and in vivo (7–9). During PDT, hyperproliferating cells take up non-toxic, light-sensitive dyes known as photosensitizers (PS). Cells are then irradiated with the appropriate visible wavelength, causing the PS to transition to an excited singlet state. Through intersystem crossing, the PS then reaches a triplet state with a sufficiently long lifetime to react with molecular oxygen. Such interactions result in the formation of reactive oxygen species (ROS) through either a Type I or Type II photochemical pathway (10).
The Type I pathway involves electron-transfer reactions from the triplet state photosensitizer, generating toxic ROS, including superoxide, hydroxyl radicals, and hydrogen peroxide. The Type II pathway involves an energy transfer from the triplet state photosensitizer to ground state molecular oxygen, yielding the highly reactive and transient singlet oxygen (1O2). 1O2 and ROS are capable of oxidizing nucleic acids, lipids, and proteins, ultimately causing cellular inactivation and death (10).
Heat shock proteins (HSPs) are a group of ubiquitous chaperone proteins responsible for the refolding, repair and recycling of damaged proteins and stabilization of lipid membranes during cellular stress (11–13). In microbial cells, the heat shock proteome has best been characterized in Escherichia coli, and it has been found that two major HSP families– GroEL/GroES and DnaK/DnaJ/GrpE – are chiefly responsible for protecting against stress in both Gram-negative and Gram-positive bacteria (14–18). Upregulation of HSPs in E. coli occurs several minutes after stress, increasing up to 50-fold the original concentration, only to subsequently stabilize until a given stress diminishes (19). Moreover, upregulation of HSPs during oxidative, antibiotic, osmotic, and acid stress is associated with resistance to these stresses, and upregulation of HSPs prior to subsequent stress enables bacterial cells to acquire “tolerance” to the particular stress (18).
DnaK/DnaJ/GrpE and GroEL/GroES protein repair works in an ATP-dependent process whereby DnaK and GroEL bind stretches of exposed hydrophobic residues on partially denatured proteins. GroEL folding occurs when a non-native polypeptide is encapsulated in a GroEL-GroES complex, driving the peptide into a cavity where folding occurs (20). By an unknown mechanism in the DnaK system, refolding occurs in concert with co-chaperones GrpE and DnaJ in conjunction with DnaK-mediated ATP hydrolysis (21). Moreover, HSPs are recognized as major targets during oxidative stress and it has been suggested that the chaperone DnaK acts as a “shield,” protecting proteins against oxidative stress (22). Previously, it has been demonstrated that the DnaK and GroEL families confer resistance to a wide array of stresses including antibiotic stress (23, 18). A study by Ziegelhoffer et al. showed that in Rhodobacter sphaeroides, a Gram-negative “purple bacterium,” synthesis of the alternative sigma factor E (σE) increased post 1O2 stress; σE is one member of the major HSP transcription factors and initiates sigma factor 32 (σ32), the transcription factor of DnaK and GroEL in E. coli and other Gram-negative bacteria (15, 24, 25). HSP genes, specifically dnaK, are associated with carotenoid biosynthesis in S. aureus, where carotenoids are pigments capable of quenching ROS and 1O2 (18). It has also been demonstrated that increased HSP expression of eukaryotic homologs of GroEL and DnaK, the 60 kDa heat shock protein HSP60 and the 70 kDa heat shock protein HSP70, respectively, occurs in murine and human cancer cells post PDT-mediated oxidative stress (26–28, 22).
To date, the relationship between bacterial HSPs and PDT-mediated stress in bacteria has yet to be extensively elucidated. There is only one report by Bolean and Paulino et al. who demonstrated that GroEL levels increased following Rose Bengal-mediated PDT of the Gram-positive Streptococcus mutans (29). Consequently, the goals of this study were threefold: to determine if expression of DnaK and GroEL increases post PDT; to determine if heat pretreatment provides increased stress tolerance to PDT; and to analyze the efficacy of dual HSP-inhibitor, antimicrobial PDT.
Materials and Methods
Bacterial strains
The microorganisms used in this study were Escherichia coli ATCC 33780 and Enterococcus faecalis ATCC 29212, chosen as representatives of Gram-negative and Gram-positive bacteria, respectively. Samples were routinely suspended in brain heart infusion (BHI) (Becton, Dickinson, and Company, Franklin Lakes, NJ) broth and grown overnight, aerobically in a 37o C shaker incubator (New Brunswick Scientific, Edison, NJ). Cells were removed at an optical density (OD) of 1.0 at 600 nm, approximately equivalent to 108–9 colony forming units (CFU)/mL as determined by spectrophotometry (Thermo Scientific, Waltham, MA)
Photosensitizer
A stock solution of 1 mM Toluidine Blue O (TBO) (Sigma-Aldrich, St. Louis, MO) was prepared in distilled water and stored at 4o C in the dark for no more than two weeks prior to use. For photodynamic inactivation, TBO was diluted to the desired concentrations in phosphate buffered saline (PBS).
Photodynamic inactivation minimum inhibitory concentration (MIC) determination
To determine the effects of sub-lethal PDT stress on bacterial HSP expression, the minimal inhibitory concentration (MIC) of TBO in the light and dark was first established. MIC is defined as the lowest concentration of a drug that will inhibit visible growth after overnight incubation, where growth is defined as the presence of turbidity in wells (30). 100 μL of diluted TBO (1000–0.50 μM) was added to 100 μL E. coli samples of 108–9 CFU/mL suspended in BHI broth. PBS was used for control. Samples were placed in 96-well plates (Becton, Dickinson, and Company) and light samples were exposed to a custom built white LED illuminator device designed to homogenously illuminate a 96-well plate. Cells were irradiated for 30 min at a power density of 15.0 mW/cm2 as measured by a power meter (Thorlabs, Newton, NJ) to deliver a total fluence of 13 J /cm2. Spectrophotometry was then performed using the SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) where optical density of bacterial samples in BHI broth with varying TBO concentrations was compared to broth with varying TBO concentrations alone. The point at which the treated culture OD600 was greater than the OD600 of broth and TBO alone was considered the highest concentration that did not induce lethal effects.
Photodynamic stress conditions
Results of the MIC test demonstrated that whereas 62.5 μM TBO in concert with 13 J/cm2 light did not permit visible growth, 31.25 μM TBO in concert with 13 J/cm2 white light allowed for visible growth. Accordingly, for sub-lethal PDT, 100 μL of 31.25 μM TBO was added to 100 μL PBS cell suspensions (108–9 CFU/mL) of E. coli. Samples were irradiated for 30 min as described above. Following stress, cells were prepared for SDS-PAGE.
For lethal PDT (killing studies), cells were illuminated with 5 J/cm2 of 635-nm light using a Lumacare LC-122 lamp (Lumacare, Newport Beach, CA) at concentrations of 50, 25, 5, and 2.5 μM TBO. The TBO absorption peak is 633 nm, such that excitation of this PS at this wavelength resulted in greater ROS production as opposed to broad-band white light (31). Samples were incubated with PS in the dark for 5 min and PDT was performed. Samples were then plated following methods by Jett et al. (32).
SDS-PAGE
Following PDT, cell lysis was performed using the CelLytic™ B Plus kit (Sigma-Aldrich). To ensure equal loading of protein concentration, each sample was harvested at an OD600 ≈ 1.0 (concentration of 108–9 CFU/mL) and the bichinchoninic acid (BCA) protein quantification method was used. Cell lysates were then boiled at 90o C and treated with reducing Laemmli buffer containing 10% SDS (w/v) and 5% β-mercaptoethanol (w/v). 11–15 μL of cell lysates (corresponding to a protein concentration ≈ 7 μg) and 6 μL prestained SDS-PAGE standard solution weight ladder (Sigma-Aldrich), were loaded and separated via electrophoresis using the XCell II Surelock Mini-Cell (Invitrogen, Calsbad, CA). A NuPAGER 4–12% Bis-Tris (pH 8.5) Gel (Invitrogen, Carlsbrad, CA) was used and SDS-PAGE was performed at 100 V for 2 hours. The gel was then stained with 0.1 % CoomassieR stain (Thermo Scientific) to check loading, destained in distilled water, and prepared for immunoblotting.
Western blot
Protein samples were transferred onto a 0.2 μm nitrocellulose membrane (Invitrogen) using the XCell II Surelock Blot Module (Invitrogen) for 1.5 h and membranes were blocked with Tris-buffered saline containing 5% (w/v) skim milk and washed three times in TBS containing 0.1% (v/v) Tween (TBS-T). Washing was performed for 5 min and membranes were incubated with mouse IgG mAb against E. coli HSPs GroEL and DnaK (Enzo Life Sciences, Farmingdale, NY) overnight at 4° C at dilutions of 1:2,000 and 1:10,000, respectively, following the manufacturer’s recommendations. After rinsing in TBS-Tween, membranes were then incubated with horseradish peroxidase-conjugated secondary antibody goat anti-mouse IgG (Fab) mAb (Enzo Life Sciences, Inc.), diluted at 1:5,000 as recommended by the manufacturer. Finally, membranes were washed twice with TBS-T and once with TBS and developed using NovexR ECL Chemiluminescent Substrate Kit (Invitrogen) which was imaged using the Kodak Image Station 4000R (Carestream Health, Rochester, NY). Note that no loading control was used, consistent with previous bacterial HSP studies that incorporate immunoblotting (33, 34). Accordingly, for accurate comparison of experimental and control groups, all cells were harvested at the same time at an OD600 of 1.0, and the BCA assay was used to ensure equal protein loading, similar to procedures of other works that did not incorporate loading controls (35, 36).
Dot blots
To allow for rapid determination of relative HSP expression values, a BCA assay was used and lysates were boiled at 90o C and treated with reducing Laemmli buffer containing equal protein concentration (7 μg) were manually loaded on nitrocellulose membranes. Membranes were allowed to dry and blocking, antibody incubation, and detection procedures were performed as described above. Densitometry was performed on the Kodak Image Station 4000R to compare relative mean net luminescence intensities, given in arbitrary units. Blotting was performed in triplicate and the positive and negative error bars are given by the standard deviation of samples from the mean value.
Heat pretreatment and tolerance studies
To determine if pretreatment with 50o C heat (a known upregulator of HSPs) would reduce cell killing by PDT, cells were first harvested at an OD600 of 1.0. Samples were then centrifuged at 12,000 rpm for 3 min and resuspended in PBS and incubated with desired TBO concentrations for 5 min. Lethal PDT was performed as described earlier.
HSP-inhibitor and PDT combination therapy
To determine if inhibiting bacterial heat shock protein DnaK would enable photodynamic effects at low concentrations of TBO and light, 100 mM stock solution of heat shock protein inhibitor I (KNK437, N-Formyl-3,4-methylenedioxy-benzylidene-γ-butyrolactam) (Calbiochem, San Diego, CA) was dissolved in dimethyl sulfoxide (DMSO, (CH3)2SO) (Sigma). First, a 100 mM KNK437 solution was dissolved in DMSO, and the MIC was determined, as stated earlier. The final concentration of DMSO in cells was < 0.5% (v/v). Once the MIC was determined, samples were exposed to 100 μL 500 μM KNK437 in conjunction with 2.5 μM TBO and 5 J/cm2 635 nm light. Samples were then serially diluted on agar and colonies were counted.
Statistical Analysis
One-way Analysis of Variance (1-way ANOVA) was used to compare survival fractions of bacterial samples following combination therapy. Analysis was performed using Microsoft Excel Statistical Package (Microsoft, Redmond, Washington) and results were considered significant when p < 0.05.
Results and Discussion
Upregulation of GroEL and DnaK in E. coli following PDT
GroEL expression was analyzed immediately after 30 minutes of sub-lethal photodynamic inactivation. Compared to cells grown in BHI and cells exposed to 31.25 μM TBO without light, there was an observed approximate 3-fold increase in GroEL expression as determined by dot blot (Fig 2). To confirm the specificity of antibody binding and to demonstrate positive upregulation of GroEL to 50o C heat, a conventional western blot was performed. Western blot revealed approximate kDa weights of GroEL and DnaK to be 65 and 75, respectively (Fig 3A and 3B). These results are consistent with previous findings (37). It is not possible to compare intensities of bands between Figs 3A or 3B and those in Fig 3C because the images were captured from different gels using different magnifications and exposure times.
Fig 2.
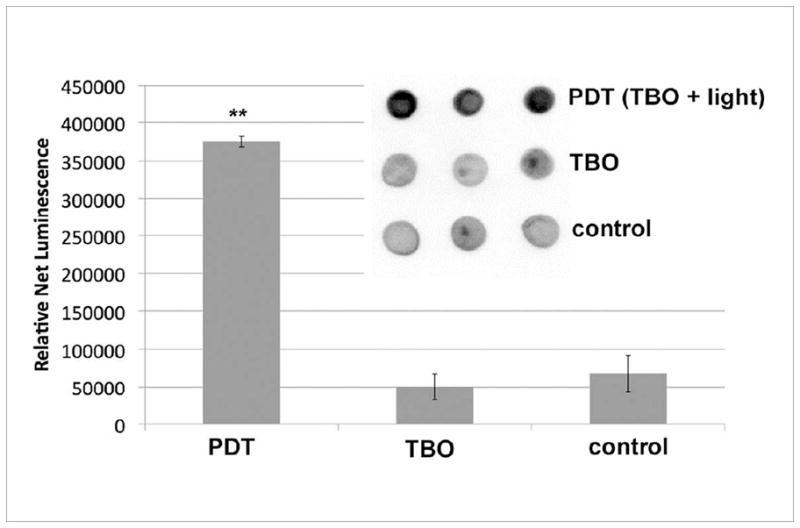
GroEL expression in E. coli 30 min after PDT. PDT consisted of 31.5 μM TBO and 13 J/cm2 of white light. 1) Results from triplicate dot blots of cell lysate with standard immunoblotting technique collected from a single experiment. 7 μg protein per dot, as determined by BCA assay. (2) Mean relative expression values as determined by densitometry; bars are SD; ** P <0.01 vs TBO and vs control (ANOVA).
Fig 3.
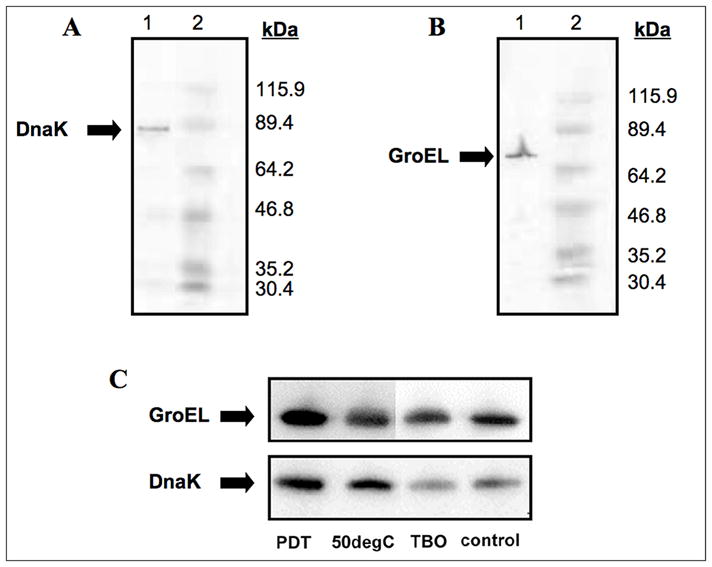
Western blot analyses of major bacterial HSP family members. (A) Western analysis of DnaK. Lane 1, E. coli cell extract, samples were detected with mouse mAb anti-E. coli DnaK; lane 2 molecular weight ladder. (B) Western analysis of GroEL. Lane 2, E. coli cell extract; lane 2 molecular weight ladder, samples were detected with mouse mAb anti-E. coli GroEL. For both experiments, E. coli cells were harvested after growth overnight and lysed. Lysates were then subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and subsequently detected. Molecular weight ladders are as follows: -galactosidase from E. coli (115,900), lactoferrin from human milk (89,400), pyruvate kinase from chicken muscle (64,200), ovalbumin from chicken egg (46,800), lactic dehydrogenase from rabbit muscle (35,200), triosephosphate isomerase from rabbit muscle (30,400). (C) Comparison was made between PDT (31.25 μM TBO + 13 J/cm2 of white light, 50° C heat shock, 31.25 μM TBO in the dark, and control E. coli cells. For all blots, 7 μg protein was loaded in each well. To ensure equal loading of protein, a BCA assay was performed.
This PS concentration and light dose corresponded to less than 1 log10 loss in cell viability (data not shown), indicating that sub-lethal PDT stress was, indeed, occurring inside cells. Moreover, if increased HSP expression is used as a marker for internal stress, then TBO in the dark at a concentration of 31.25 μM was not sufficient enough to induce toxic conditions that warrant upregulation of GroEL (Fig 2 and Fig 3C).
Immediately after PDT, there was an observed 3-fold increase in intracellular DnaK concentrations (Fig 4). Similar to GroEL, DnaK may act as a marker for intracellular stress, such that the slight increase in DnaK observed in the dark may indicate stress from TBO localization and subsequent irregularities in the E. coli cell wall. Heat at 50o C demonstrated a significant increase in DnaK concentrations, as determined by the western blot (Fig 3C).
Fig 4.
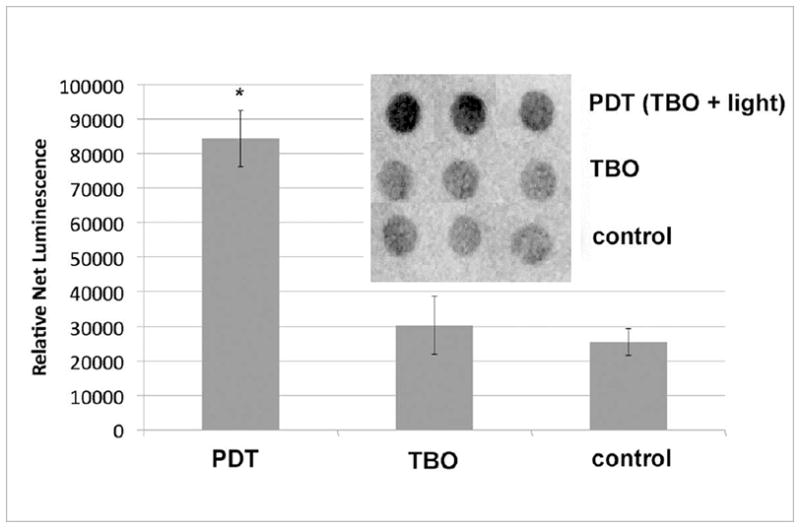
DnaK expression in E. coli 30 min after PDT. PDT consisted of 31.5 μM TBO and 13 J/cm2 of white light. (1) Results from triplicate dot blots of cell lysate with standard immunoblotting technique collected from a single experiment. 7 μg protein per dot, as determined by BCA assay. (2) Mean relative expression values as determined by densitometry; bars are SD; * P <0.05 vs TBO and vs control (ANOVA).
It was surprising that GroEL had a greater increase after PDT than was found for DnaK as detected by immunoblotting. Although DnaK is generally considered more responsive to heat stress, this may, perhaps, indicate that GroEL is predominately involved in stabilizing membranes and participating in post-ribosomal synthesis folding of proteins, a ubiquitous feature of chaperonin protein family members (38). Augmentation of internal GroEL levels is, thus, consistent with reports of TBO localization in bacterial cells: TBO, a cationic phenothiazinium dye (Fig 1), of the same chemical class as methylene blue, is known to directly bind to the negatively charged lipopolysaccharide (LPS) present on the outer cell envelope of Gram-negative bacteria and to the anionic teichuronic acid residues of the Gram-positive peptidoglycan cell walls, such that when TBO is excited, the predominate form of bacterial destruction is via membrane stress (39, 31, 40). Furthermore, a report by Fredriksson et al. demonstrated that in E. coli, the GroEL/GroES chaperone complex was more important than the DnaK/DnaJ/GrpE in protecting against protein carbonylation; it is thought that protein carbonylation is a major oxidative byproduct of PDT (16, 41)
Fig 1.
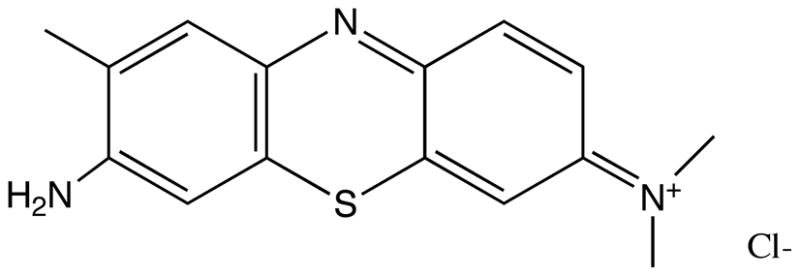
Molecular structure of Toluidine blue O (TBO).
Although DnaK levels increased 3-fold, it is entirely possible that some DnaK was not detected. This may be explained by considering that HSPs, including DnaK and the homologous HSP70 of eukaryotes, are significantly upregulated in an intracellular manner in response to oxidative stress (26, 22). Several studies of bacteria exposed to H2O2 stress have shown that the DnaK molecule itself is significantly oxidized, as determined by 2,4-dinitrophenylhydrazine (DNPH) immunoblotting for protein carbonyl groups on DnaK, a marker for oxidative stress (42). After oxidation of amino acids, protein aggregation may occur, preventing effective detection via immunoblotting methods.
Prior heat pretreatment confers resistance to PDT in E. coli and E. faecalis
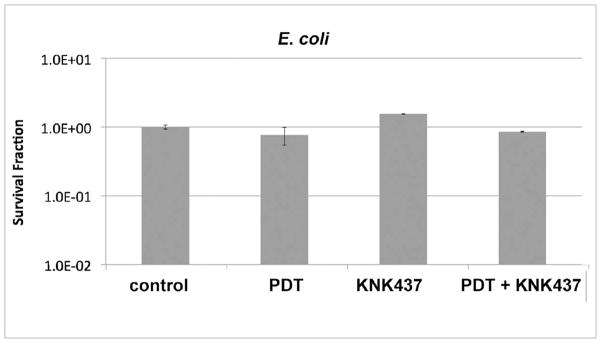
To determine if upregulation of HSPs may limit the effectiveness of PDT for the inactivation of microbes, cell samples were preheated for 30 min at 50o C, a known positive inducer of HSPs (Fig 3). We found that previous heat treatment led to ≈ 2 log10 less killing than of E. coli at a TBO concentration of 50 μM and a reduction in killing of about 0.5 log10 at a TBO concentration of 5 μM as compared to killing without heat pretreatment (Fig 5).
Fig 5.
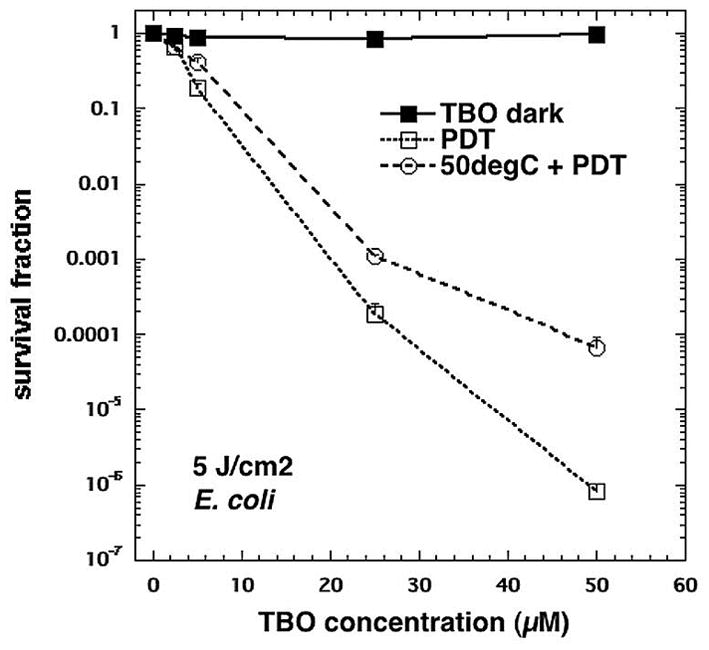
The effect of heat pretreatment on E. coli susceptibility to TBO-PDT lethal stress. Following pretreatment at 50° C or 37° C (control) for 30 min, samples were immediately incubated with various TBO concentrations and subjected or not to 5 J/cm2 of 635-nm light. TBO dark = cells exposed to varying TBO concentrations without light, PDT = TBO + 5J/cm2, 50degC + PDT = 30 min 50° C followed by immediate PDT with varying concentrations of TBO + 5 J/cm2. Cell death is expressed in terms of survival fraction relative to absolutecontrol; bars are SD; * P <0.05 vs PDT alone (ANOVA).
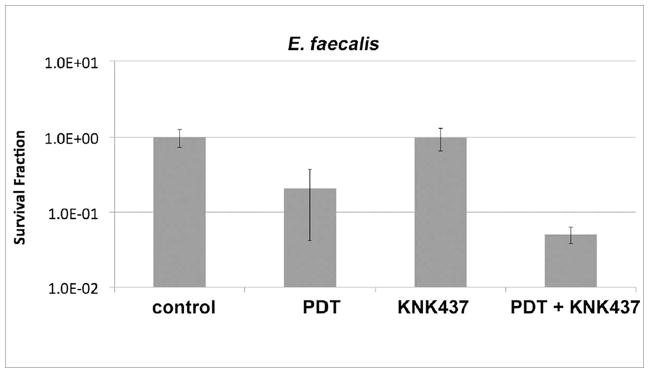
Unlike E. coli, E. faecalis had an extremely pronounced reduction in cell death when preheated before PDT. Whereas 2 log10 less killing was observed in E. coli post PDT at 50 μM, heat pretreatment-induced more resistance (approaching 4 log10 less killing) in E. faecalis (Fig 6).
Fig 6.
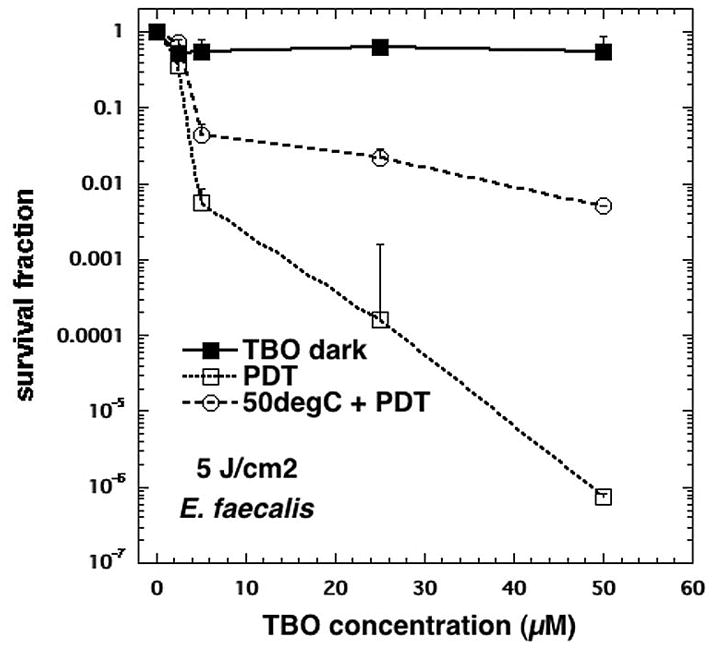
The effect of heat pretreatment on E. faecalis susceptibility to TBO-PDT lethal stress. Following pretreatment or not at 50° C at or 37° C (control) 30 min, samples were immediately incubated with various TBO concentrations and subjected or not to 5 J/cm2 of 635-nm light. TBO dark = cells exposed to varying TBO concentrations without light, PDT = TBO + 5J/cm2, 50degC + PDT = 30 min 50° C followed by immediate PDT with varying concentrations of TBO + 5 J/cm2. Cell death is expressed in terms of survival fraction relative to absolute control; bars are SD; ** P <0.01 vs PDT alone (ANOVA).
It is interesting that E. faecalis acquired more tolerance to PDT stress, compared to E. coli, considering Gram-positive bacteria are known to be significantly more susceptible to the effects of PDT (7, 8). But this is not unexpected, as E. faecalis, and most Enterococci are known to be able to express intense stress resistance (43, 44, 17). Most notably, a study by Boutibonnes et al. demonstrated that pretreatment with 50o C heat for 30 min conferred 95% survival of bacterial cultures in otherwise lethal, 60o C heat (43). It is also known that HSPs do not discriminate between the types of stress that leads to their upregulation, and that there may be cross-induction of stress protection.
TBO-PDT, KNK437 combination therapy
After demonstrating that pretreatment with 50o C heat conferred stress tolerance to PDT, inhibition of DnaK by a HSP-inhibitor, a benzylidene lactam called KNK437, in conjunction with PDT was explored. After incubating cells with 500 μM KNK437 and subjecting them to PDT at a TBO concentration of 2.5 μM, it was found that KNK437 did indeed result in an enhancement of TBO-PDT killing of E. faecalis by ≈ 53%, although not statistically significant, p =0.299 (Fig 7). On the contrary, combination therapy of HSP-inhibitor and TBO-PDT had no noticeable effect on E. coli susceptibility compared to low-level PDT (Fig 8). It is important to note that incubation with 500 μM KNK437 had no effect on E. faecalis or E. coli viability (Fig 7 & Fig 8).
Fig 7.
The effect of pretreatment with KNK437 on E. faecalis susceptibility to 2.5 μM TBO + 5 J/cm2 of 635-nm light. After a 1 h incubation with 500 μM KNK437, samples were resuspended in fresh PBS and incubated with 2.5 μM TBO for 5 min. Irradiation reached 5 J/cm2.
Fig 8.
The effect of pretreatment with KNK437 on E. coli susceptibility to 2.5 μM TBO + 5 J/cm2 of 635-nm light. After a 1 h incubation with 500 μM KNK437, samples were resuspended in fresh PBS and incubated with 2.5 μM TBO for 5 min. Irradiation reached 5 J/cm2.
The difference in KNK437-mediated combination therapy amongst the two species tested may relate to the composition of Gram-negative and Gram-positive cell structures. It is well accepted that the presence of anionic LPS and the Gram-negative double membrane reduces binding and subsequent uptake of hydrophobic compounds (45). Considering KNK437 is hydrophobic and requires dissolution in DMSO, it is possible that the KNK437 molecules bound less to E. coli, as the LPS would repel the hydrophobic molecule. This hypothesis is consistent with the observation whereby Gram-negative bacteria are inherently resistant to hydrophobic antibiotics (46). Moreover, hydrophobic molecules have been employed for the destruction of Gram-positive bacteria, including Bacillus subtilis, explaining the efficacy of dual therapy in E. faecalis (47).
Thus, future research is needed to evaluate the efficacy of combination therapy consisting of PDT and HSP inhibitors. Currently, the supply of HSP inhibitors is limited, and thus there are obvious limitations with this aspect of the work. An HSP inhibitor that selectively targets bacterial DnaK would be ideal and has been investigated by Credito et al., where application of CHP-105, a synthetic peptide, allowed for levofloxacin-mediated killing of otherwise levofloxacin-resistant Gram-negative bacilli (23). At the time this study was conducted, CHP-105 was not commercially available.
Conclusion
This study effectively demonstrated that the expression of two major bacterial HSPs, GroEL and DnaK, increased 7-fold and 3-fold in E. coli, respectively, as determined by immunoblotting. Heat pretreatment allowed for a reduction of E. coli killing by 2 log10 and E. faecalis killing by 4 log10 at a TBO concentration of 50 μM. Finally, combination therapy demonstrated some potentiation of killing in E. faecalis and no noticeable potentiation in E. coli. It has been suggested that microbes are incapable of developing resistance to PDT due to the non- site-specific killing of bacteria by photosensitizers (48). Previous studies have attempted to induce resistance to PDT, by repeatedly killing and re-growing bacterial samples (49). However it is well accepted that it is more likely that bacteria will develop resistance by growing in the presence of a low level stressor, best exemplified by the emergence of resistant organisms in antibiotic-fed livestock (50). PDT is usually designed to be a relatively short procedure that would last somewhere around 5 min (51). In our study, sub-lethal PDT was performed using white light over an extended period of time (30 min).
As PDT emerges as a potent approach for the inactivation of localized microbial infections and sterilization of wounds and surgical sites, it is crucial to evaluate PDT efficacy impartially. This study has demonstrated that following sub-lethal PDT, there is a significant upregulation of bacterial HSPs, and that upregulation prior to PDT may confer stress tolerance. Although combination therapy did not prove to be statistically significant, this study underscores a need for novel combination therapies, thus warranting the exploration of HSP inhibition in concert with PDT.
Acknowledgments
The authors would like to thank Ursula Jakob and the Jakob Lab of the Department of Molecular, Cellular, and Developmental Biology at the University of Michigan for advice on immunoblotting techniques. The authors would also like to thank Gitika Kharkwal and Sulbha K. Sharma of the Wellman Center for Photomedicine for assistance with SDS-PAGE. Research in the Hamblin laboratory is supported by the US-NIH (grants R01AI50875, R01CA/AI838801 to MRH and R01CA137108 to Long Y Chiang), the Center for Integration of Medicine and Innovative Technology (DAMD17-02-2-0006), the CDMRP Program in TBI (W81XWH-09-1-0514) and the Air Force Office of Scientific Research (F9950-04-1-0079).
References
- 1.Lee S, Hinz A, Bauerle E, Angermeyer A, Juhaszova K, Kaneko Y, Singh PK, Manoil C. Targeting a bacterial stress response to enhance antibiotic action. Proc Natl Acad Sci U S A. 2009;106:14570–14575. doi: 10.1073/pnas.0903619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norrby SR, Nord CE, Finch R. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis. 2005;5:115–119. doi: 10.1016/S1473-3099(05)01283-1. [DOI] [PubMed] [Google Scholar]
- 3.Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 4.Prince AS. Biofilms, antimicrobial resistance, and airway infection. N Engl J Med. 2002;347:1110–1111. doi: 10.1056/NEJMcibr021776. [DOI] [PubMed] [Google Scholar]
- 5.Russell AD. Bacterial spores and chemical sporicidal agents. Clin Microbiol Rev. 1990;3:99–119. doi: 10.1128/cmr.3.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raab O. Ueber diewirkung fluoreszierender stoffe auf infusoria. Z Biol. 1900;39:524–526. [Google Scholar]
- 7.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections--state of the art. Photodiagnosis Photodyn Ther. 2009;6:170–188. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J Photochem Photobiol B. 2005;81:15–25. doi: 10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanin IC, Goncalves RB, Junior AB, Hope CK, Pratten J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J Antimicrob Chemother. 2005;56:324–330. doi: 10.1093/jac/dki232. [DOI] [PubMed] [Google Scholar]
- 10.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-Photosensitizers, photochemistry and cellular localization. Photodiag Photodyn Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath I, Multhoff G, Sonnleitner A, Vigh L. Membrane-associated stress proteins: more than simply chaperones. Biochim Biophys Acta. 2008;1778:1653–1664. doi: 10.1016/j.bbamem.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Schlesinger MJ. Heat shock proteins. J Biol Chem. 1990;265:12111–12114. [PubMed] [Google Scholar]
- 13.Torok Z, Horvath I, Goloubinoff P, Kovacs E, Glatz A, Balogh G, Vigh L. Evidence for a lipochaperonin: association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc Natl Acad Sci U S A. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad M, Smith DG, Mahboob S. Determination of the Heat shock Response in Enterococcus faecium and Enterococcus faecalis. Int J Agricult Biol. 2002;4:231–233. [Google Scholar]
- 15.Arsene F, Tomoyasu T, Bukau B. The heat shock response of Escherichia coli. Int J Food Microbiol. 2000;55:3–9. doi: 10.1016/s0168-1605(00)00206-3. [DOI] [PubMed] [Google Scholar]
- 16.Fredriksson A, Ballesteros M, Dukan S, Nystrom T. Defense against protein carbonylation by DnaK/DnaJ and proteases of the heat shock regulon. J Bacteriol. 2005;187:4207–4213. doi: 10.1128/JB.187.12.4207-4213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laport MS, Lemos JA, Bastos Md Mdo C, Burne RA, Giambiagi-De Marval M. Transcriptional analysis of the groE and dnaK heat-shock operons of Enterococcus faecalis. Res Microbiol. 2004;155:252–258. doi: 10.1016/j.resmic.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Singh VK, Utaida S, Jackson LS, Jayaswal RK, Wilkinson BJ, Chamberlain NR. Role for dnaK locus in tolerance of multiple stresses in Staphylococcus aureus. Microbiology. 2007;153:3162–3173. doi: 10.1099/mic.0.2007/009506-0. [DOI] [PubMed] [Google Scholar]
- 19.Krueger JH, Walker GC. groEL and dnaK genes of Escherichia coli are induced by UV irradiation and nalidixic acid in an htpR+-dependent fashion. Proc Natl Acad Sci U S A. 1984;81:1499–1503. doi: 10.1073/pnas.81.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigler PB, Xu Z, Rye HS, Burston SG, Fenton WA, Horwich AL. Structure and function in GroEL-mediated protein folding. Annu Rev Biochem. 1998;67:581–608. doi: 10.1146/annurev.biochem.67.1.581. [DOI] [PubMed] [Google Scholar]
- 21.Chesnokova LS, Slepenkov SV, Witt SN. The insect antimicrobial peptide, L-pyrrhocoricin, binds to and stimulates the ATPase activity of both wild-type and lidless DnaK. FEBS Lett. 2004;565:65–69. doi: 10.1016/j.febslet.2004.03.075. [DOI] [PubMed] [Google Scholar]
- 22.Luna MC, Ferrario A, Wong S, Fisher AM, Gomer CJ. Photodynamic therapy-mediated oxidative stress as a molecular switch for the temporal expression of genes ligated to the human heat shock promoter. Cancer Res. 2000;60:1637–1644. [PubMed] [Google Scholar]
- 23.Credito K, Lin G, Koeth L, Sturgess MA, Appelbaum PC. Activity of levofloxacin alone and in combination with a DnaK inhibitor against gram-negative rods, including levofloxacin-resistant strains. Antimicrob Agents Chemother. 2009;53:814–817. doi: 10.1128/AAC.01132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagai H, Yano R, Erickson JW, Yura T. Transcriptional regulation of the heat shock regulatory gene rpoH in Escherichia coli: involvement of a novel catabolite-sensitive promoter. J Bacteriol. 1990;172:2710–2715. doi: 10.1128/jb.172.5.2710-2715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziegelhoffer EC, Donohue TJ. Bacterial responses to photo-oxidative stress. Nat Rev Microbiol. 2009;7:856–863. doi: 10.1038/nrmicro2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curry PM, Levy JG. Stress protein expression in murine tumor cells following photodynamic therapy with benzoporphyrin derivative. Photochem Photobiol. 1993;58:374–379. doi: 10.1111/j.1751-1097.1993.tb09577.x. [DOI] [PubMed] [Google Scholar]
- 27.Hanlon JG, Adams K, Rainbow AJ, Gupta RS, Singh G. Induction of Hsp60 by Photofrin-mediated photodynamic therapy. J Photochem Photobiol B. 2001;64:55–61. doi: 10.1016/s1011-1344(01)00189-0. [DOI] [PubMed] [Google Scholar]
- 28.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–1026. [PubMed] [Google Scholar]
- 29.Bolean M, Paulino Tde P, Thedei G, Jr, Ciancaglini P. Photodynamic therapy with rose bengal induces GroEL expression in Streptococcus mutans. Photomed Laser Surg. 2010;28(Suppl 1):S79–84. doi: 10.1089/pho.2009.2635. [DOI] [PubMed] [Google Scholar]
- 30.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(Suppl 1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 31.Usacheva MN, Teichert MC, Biel MA. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg Med. 2001;29:165–173. doi: 10.1002/lsm.1105. [DOI] [PubMed] [Google Scholar]
- 32.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 33.Fayet O, Ziegelhoffer T, Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989;171:1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krska J, Elthon T, Blum P. Monoclonal antibody recognition and function of a DnaK (HSP70) epitope found in gram-negative bacteria. J Bacteriol. 1993;175:6433–6440. doi: 10.1128/jb.175.20.6433-6440.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 36.Sinha J, Chen F, Miloh T, Burns RC, Yu Z, Shneider BL. beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2008;295:G996–G1003. doi: 10.1152/ajpgi.90343.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman DI, Olson ER, Georgopoulos C, Tilly K, Herskowitz I, Banuett F. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage lambda. Microbiol Rev. 1984;48:299–325. doi: 10.1128/mr.48.4.299-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ying BW, Taguchi H, Kondo M, Ueda T. Co-translational involvement of the chaperonin GroEL in the folding of newly translated polypeptides. J Biol Chem. 2005;280:12035–12040. doi: 10.1074/jbc.M500364200. [DOI] [PubMed] [Google Scholar]
- 39.Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38:468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 40.Usacheva MN, Teichert MC, Biel MA. The role of the methylene blue and toluidine blue monomers and dimers in the photoinactivation of bacteria. J Photochem Photobiol B. 2003;71:87–98. doi: 10.1016/j.jphotobiol.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Magi B, Ettorre A, Liberatori S, Bini L, Andreassi M, Frosali S, Neri P, Pallini V, Di Stefano A. Selectivity of protein carbonylation in the apoptotic response to oxidative stress associated with photodynamic therapy: a cell biochemical and proteomic investigation. Cell Death Differ. 2004;11:842–852. doi: 10.1038/sj.cdd.4401427. [DOI] [PubMed] [Google Scholar]
- 42.Tamarit J, Cabiscol E, Ros J. Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J Biol Chem. 1998;273:3027–3032. doi: 10.1074/jbc.273.5.3027. [DOI] [PubMed] [Google Scholar]
- 43.Boutibonnes P, Giard JC, Hartke A, Thammavongs B, Auffray Y. Characterization of the heat shock response in Enterococcus faecalis. Antonie van Leeuwenhoek. 1993;64:47–55. doi: 10.1007/BF00870921. [DOI] [PubMed] [Google Scholar]
- 44.Giard JC, Laplace JM, Rince A, Pichereau V, Benachour A, Leboeuf C, Flahaut S, Auffray Y, Hartke A. The stress proteome of Enterococcus faecalis. Electophoresis. 2001;22:2947–2954. doi: 10.1002/1522-2683(200108)22:14<2947::AID-ELPS2947>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 45.Peterson AA, Hancock RE, McGroarty EJ. Binding of polycationic antibiotics and polyamines to lipopolysaccharides of Pseudomonas aeruginosa. J Bacteriol. 1985;164:1256–1261. doi: 10.1128/jb.164.3.1256-1261.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974;235:109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda-Yasaki Y, Namiki-Kanie S, Hachisuka Y. Inhibition of Bacillus subtilis spore germination by various hydrophobic compounds: demonstration of hydrophobic character of the L-alanine receptor site. J Bacteriol. 1978;136:484–490. doi: 10.1128/jb.136.2.484-490.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassidy CM, Donnelly RF, Tunney MM. Effect of sub-lethal challenge with Photodynamic Antimicrobial Chemotherapy (PACT) on the antibiotic susceptibility of clinical bacterial isolates. J Photochem Photobiol B. 2010;99:62–66. doi: 10.1016/j.jphotobiol.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Tavares A, Carvalho CM, Faustino MA, Neves MG, Tome JP, Tome AC, Cavaleiro JA, Cunha A, Gomes NC, Alves E, Almeida A. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Mar Drugs. 2010;8:91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmberg SD, Osterholm MT, Senger KA, Cohen ML. Drug-resistant Salmonella from animals fed antimicrobials. N Engl J Med. 1984;311:617–622. doi: 10.1056/NEJM198409063111001. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Young MJ, Battaglino RA, Morse LR, Fontana CR, Pagonis TC, Kent R, Soukos NS. Endodontic antimicrobial photodynamic therapy: safety assessment in mammalian cell cultures. J Endod. 2009;35:1567–1572. doi: 10.1016/j.joen.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]