Abstract
Objective
The subventricular zone (SVZ) of the brain constitutes a niche for neural stem and progenitor cells that can initiate repair after central nervous system (CNS) injury. In a relapsingremitting model of experimental autoimmune encephalomyelitis (EAE), the neural stem cells (NSCs) become activated and initiate regeneration during acute disease, but lose this ability during the chronic phases of disease. We hypothesized that chronic microglia activation contributes to the failure of the NSC repair potential in the SVZ.
Methods
Using BrdU injections at different time points during EAE, we quantified the number of proliferating and differentiating progenitors, and evaluated the structure of the SVZ by electron microscopy. In vivo minocycline treatment during EAE was used to address the effect of microglia inactivation on SVZ dysfunction
Results
In vivo treatment with minocycline, an inhibitor of microglia activation, increases stem cell proliferation in both naïve and EAE animals. Minocycline treatment decreases cortical and periventricular pathology in the chronic phase of EAE, improving the proliferation of Sox2 stem cells and NG2 oligodendrocyte precursors cells originating in the SVZ and their differentiation into mature oligodendrocytes.
Interpretation
These data suggest that failure of repair observed during chronic EAE correlates with microglia activation and that treatments targeting chronic microglial activation have the potential for enhancing repair in the CNS.
INTRODUCTION
Multipotent neural stem/precursor cells (NSCs) reside within specialized compartments or niches in the adult mammalian central nervous system (CNS)2. One of these niches is in the subventricular zone (SVZ) of the lateral ventricles, directly underlying the ependymal cell layer. The SVZ is an important reservoir of progenitors in the adult brain, harboring cell populations that could be used for neuroregenerative therapy and contains three major cell types. The CNS stem cells (type B cells) display an astrocyte-like phenotype, express the glial-fibrillary acidic protein (GFAP) and give rise to intermediate transit amplifying progenitors (type C cells) that lose the GFAP immunoreactivity and acquire the expression of the distal-less homeobox (Dlx)-2. These type C cells can, in turn, differentiate into neuroblasts (type A cells) that express the polysialylated form of neural cell adhesion molecule (PSA-NCAM) in addition to doublecortin (Dcx) and migrate to the olfactory bulb. A small subpopulation of actively dividing type C cells express oligodendrocyte lineage transcription factor 2 (Olig2)3 and become oligodendrocytes. The cell lineage differentiation pathway proceed from type B, through type C to type A cells, with the type B cell believed to be the self-renewing CNS stem cell4.
Multiple sclerosis (MS) is an autoimmune disease that causes demyelination and axonal damage in the central nervous system (CNS)5. The majority of the patients experience relapsing-remitting symptoms followed by a secondary progressive phase leading to permanent disability once chronic disease has set in6. Experimental autoimmune encephalomyelitis (EAE), an animal model of MS, provides a powerful tool for investigating the pathogenesis of MS. Although current evidence shows that remyelination and regeneration occur spontaneously to some extent, they are not sufficient in MS7. Remyelinating oligodendrocytes arise from differentiation of neural progenitors8 and lack of progenitor function has been proposed to play a role in remyelination failure in MS7, 9. Stem cell niches may respond to damage in the vicinity of the subventricular zone (SVZ)10, 11. In EAE there are reports of activation of the SVZ8, 12 including our recent work13 in the C57BL6 myelin oligodendrocyte glycoprotein-induced model that has minimal pathological alterations in the forebrain8. Recently, we have characterized the cortical and periventricular pathology in the relapsing-remitting SJL model and found that this model closely resembles the patterns of pathology observed in many MS patients14, this model also mimics the clinico-radiological paradox observed in human disease15. In contrast to the C57BL6 MOG-induced EAE, the SJL model shows persistent activation of microglia in the forebrain14 similar to what was observed in the cortex of MS patients14, 16, making it a more suitable MS model to study the SVZ.
Microglia belong to the monocyte lineage and are considered to be precursors17. These cells may have a positive or negative influence in neurodegenerative diseases. For example, in vitro data using microglia cell lines co-cultured with NSCs suggest that depending on their state of activation, microglia can enhance or impair neural progenitor function18–20. In vivo, in a cranial radiation model activation of microglia was associated with a decrease in neurogenic potential, which was reversed with anti-inflammatory compounds21. However, there are contradictory data on the outcome of microglia inactivation22–26.
Here we performed a detailed analysis of the SVZ niche during EAE and show that microglia cells are an integral part of the SVZ niche, and the responses of stem cells in the SVZ during EAE correlate with different states of microglia activation. We show as a proof of concept that minocycline, a second-generation semi-synthetic tetracycline27 is beneficial in ameliorating the impact of microglia mediated inflammation on the intrinsic properties of the SVZ NSCs in EAE, suggesting that long term preservation of stem cell niches should be a goal for translational therapy in chronic neurodegenerative diseases including MS.
MATERIALS AND METHODS
EAE Induction
All experiments were conducted under the approval of the Harvard Medical School Animal Care committee and the Animal Care and Research Ethics Committee at the Príncipe Felipe Research Center. SJL/J mice were immunized subcutaneously in two sites (left and right flank) with 150 μg of PLP139–151 (New England Peptide LLC., Gardner, MA) emulsified in complete Freund's adjuvant (CFA, Sigma Aldrich, Saint Louis, MO) containing 200 μg Mycobacterium Tuberculosis (Difco Laboratories, Detroit, MI). Mice received 200 ng pertussis toxin (PT, List Biological Laboratories Inc., Campbell, CA) in 0.2 ml PBS (Lonza, Walkersville, MD) intraperitoneally (ip) at the time of immunization and 48 h later. Control mice were immunized with CFA followed by PT (12). The mice were sacrificed at different time points and divided into four groups depending on the course of disease and clinical score. Defining the time points was not based on the number of days but was individualized according to disease.
In vivo analysis of SVZ stem cells proliferation and quantification
Mice were injected ip (intraperitoneally) with BrdU (5-bromo-2'-deoxyuridine, 120 mg/kg body weight, Sigma Aldrich) once a day for seven consecutive days and were killed 2 hrs after the last injection as previously described (9). For determination of differentiation into mature neurons and oligodendrocytes, the mice were killed 1 and 4 weeks after the last injection of BrdU. The BrdU injections were started at various time points depending on the course of disease and clinical score. Additional stereology information is provided in the Supplementary Methods section
Electron microscopy processing and quantification
At the appropriate time points mice were deeply anesthetized in a CO2 chamber and transcardially perfused with 0.9% PBS containing 1% heparin followed by 2% paraformaldehyde/2.5% glutaraldehyde solution (PFA/GA, Electron Micoscopy Sciences, Hatfield, PA) in PBS. Brains were removed, post-fixed in PFA/GA overnight and rinsed in cold PBS (5×10 min). After fixation, brains were cut into 50 μm sections, cut on a vibratome (Leica VT-1000), rinsed, dehydrated and embedded in araldite (Durcupan, Fluka). Semi-thin sections (1.5 μm) were cut with a diamond knife and stained lightly with 1% toluidine blue. The sections were re-embedded in an araldite block and detached from the glass slide by repeated freezing (liquid nitrogen) and thawing. The block with semi-thin sections was cut in ultra-thin (0.05 μm) sections with a diamond knife, stained with lead citrate and examined under a Fei Tecnai spirit electron microscopy. Pictures were taken with Soft Image System (Morada) camera. For quantification purposes, 3 mice for each group were used and serial sections corresponding to the same SVZ region for all mice (separated by 250 μm) were chosen and examined under a FEI Tecnai Spirit electron microscopy using established protocols and criteria28.
Immunohistochemistry
Brains were placed in 30% sucrose for at least 24 h for cryoprotection. Coronal blocks of brain tissue from bregma −2 to +2 mm were frozen in cryo-protective O.C.T.-solution (Sakura Finetek, Torrance, CA) at −80°C. The tissue was cut into floating sections of 40 μm thickness on a freezing microtome. Floating sections were incubated in a 2% solution of sodium borohydride for 20 min to reduce autofluorescence and then denatured by incubation in 2M hydrochloric acid for 2 hrs at room temperature before staining. Sections were blocked with 8% horse serum for 1 hr and incubated overnight with rat antibody against BrdU (1:250, Accurate Chemical, Westbury, NY) and neural antibodies. Further information is provided in the Supplementary Methods section.
Confocal analysis, reconstruction and pixel intensity analysis
Regions of interest around the lateral ventricle were analyzed with a confocal microscope (LSM 510 Laser Scanning Microscope and LSM 3D analysis software, Linux, Ogdensburg, NY). Cells labeled with Sox-2, Dcx, NG2, NeuN, CNPase and/or BrdU were quantified using a using a 63× water-immersion objective lens. The number of cells was averaged from four coronal sections per animal 120 μm apart starting at rostracaudal level +1.10 mm from bregma (Paxinos and Franklin, 2001) with four mice per experimental group. The number of BrdU+ cells in the corpus callosum was quantified from a 20μm thick z-stack from 200×200μm square right below the genu of the corpus callosum, while the number of BrdU+ cells and the labeling index for Sox2 in the lateral wall were quantified from a 20μm thick z-stack over a distance of 200μm extending down from the corner of the SVZ.
In vivo minocycline regimen
Animals were treated ip daily with minocycline or vehicle (PBS), beginning on day 20 post-immunization, when clinical disease reached a maximum. Ten animals per group were used. Control mice were injected with 200 μl saline daily, while minocycline-treated mice received 50 mg/kg twice a day for the first two days, once daily for the next 5 days, followed by 25 mg/kg for the subsequent days until the animals entered a relapse on day 65 post-immunization. Mice were injected ip with BrdU (5-bromo-2'-deoxyuridine, 120 mg/kg body weight, Sigma Aldrich) once a day for seven consecutive days. The minocycline treatment was continued during the BrdU administration and the mice were sacrificed 2 hrs after the last injection. For determination of differentiation into mature neurons and oligodendrocytes, three mice per group were sacrificed 4 weeks after the last injection of BrdU.
In vitro Effect of Minocycline
Microglia were isolated from the CNS of adult SJL/J mice. Brains were minced with a razor blade and the tissue was digestion using the Neural Tissue Dissociation Kit (P) (Miltenyi Biotec.) according to the manufactorer's instructions. The cell suspension was subject to percoll gradient seperation (37% / 70%) where mononuclear cells were harvested from the interphase. Microglia cells were isolated with magnetic beads coated with anti-CD11b (Microglia beads, Miltenyi Biotec.), washed in serum-free HL-1 medium (HL-1 with, L-glutamine (1mM), (1mM), 1× Antibiotic Antimycotic Solution (Sigma)). Microglia cells (1×105 cells/ml) were cultured in HL-1 medium in the presence of increasing doses of Minocycline (μg/ml) and increasing doses of LPS (ng/ml) or IFNγ (ng/ml) respectively at 37°C / 5% CO2. After 24 hours, microglia conditioned medium and control medium were transferred to neural stem cells (NSC) cultures (5× 104/ml). After 3 days of culture, differentiated NSCs were stained for Topro3 and Map2 or NG2. The differentiation/survival index of neural precursor cells and oligodendrocyte precursor cells respectively, is represented by the delta of the percentage of differentiated cells in conditioned and control medium.
Statistical Analysis
All data is presented as mean±SEM. Statistical analysis was performed using the unpaired, two-sided t-test comparison between EAE and control or by using one-way analysis of variance (ANOVA) with Turkey's Multiple Comparison Test. Significant differences were assumed at the 5% level and represented as P-values (p<0.05).
RESULTS
NSCs in the SVZ exhibit repair potential in the acute phase that is lost in the chronic phase of relapsing-remitting EAE
We have previously shown that in contrast with other EAE models, relapsing-remitting EAE exhibits dysfunction of cortical projecting neurons in specific layers of the forebrain14 associated with microglia activation, resembling the callosal and cortical pathology present in MS brains16. In this model we found lesions near the SVZ with demyelination and astrogliosis (Figure 1 A and supplementary fig 1 e). We also observed an increased number of resident SVZ cells during the acute phase that returned to control level with relapse. These cells display dark cytoplasm and elongated nuclei when observed in semithin sections corresponding with type A and Type C cells28 (Figure 1 B,C).
Figure 1. Loss of architecture of SVZ niche during EAE.
(A) Luxol fast blue histology images from the SVZ during acute disease. CC: corpus callosum LV: lateral ventricle. The arrow shows an inflammatory infiltrate. The black box indicates the SVZ. (B) High magnification of semi-thin section of the lateral ventricle wall. (C) EM of SVZ from control (left panel), acute disease (middle panel) and relapse (right panel) showing migratory neuroblast (type A cells), and labeled type C and B cells. Mit: mitosis of B and C cells, E: ependymal cells, dashed lines surround proliferating neuroblasts, arrow in right panel point to a capillary.
Sox2 is a transcription factor expressed by NSCs in the SVZ29, 30. We quantified the labeling index of BrdU/Sox2+ cells 7 days after BrdU injection and found an increase in the number of NSCs during acute disease compared with control (acute 62.67%±3.038 versus control 36.75%±3.038, p<0.001, n=4 mice/group). However with subsequent relapses the proliferation returned to control level (Figure 2 A). Since NSCs have a long cell cycle length31, we performed the same quantification after a 28-day wash out period (Figure 3 and Supplementary Table 1) to determine the number of the long term retaining (LTR) BrdU/Sox2+ cells during the different phases of EAE (n=28 mice). We observed an increase in the numbers of LTR-BrdU/Sox2+ putative stem cells during the acute phase followed by a decrease of the number of LTR-BrdU/Sox2+ cells in the periventricular areas at the late time points (n=4 mice/group) (Figure 3 d). Furthermore, we confirmed these observations using electron microscopy (EM) analysis of the type B, C and A cells according to established ultrastructural criteria32 (supplementary fig 1). The decrease in Sox2+ during chronic disease is not due to apoptosis of these cells, since we did not detect any sox2+TUNEL+ cells during any phases of the disease. In the control mice we observed the normal organization of B, C and A cells in the niche with occasional mitosis (Figure 1 C). During the acute phase of EAE we found an increase in the number of type A and type C cells (Figure 1 B,1C), and a significant increase in the number of type B cells that were in contact with the ventricle compared to control (acute 3±0.9 cells/mm2 versus control 0.2±0.14 cells/mm2, p=0.0153, n=2 mice/group). In contrast, during the relapse and chronic phase, there was a decrease in the number of progenitors cells (Figure 1 C), without changes in the ultrastructural features of the type B, C or A cells. Thus, our EM data confirm the BrdU labeling experiments showing a transient increase in proliferation of stem cells during acute EAE that is lost during the chronic phase of the disease despite persistent injury.
Figure 2. Proliferative response of NSCs during relapsing EAE.
(A) Labeling index for Sox2+ cells in the lateral wall in the different phases of EAE (n=4 mice/group *p<0.001), showing an increase in proliferating Sox2+ cells during acute disease. (B) Numbers of neurosphere colonies from cells isolated from SVZ of animals at different stages of EAE (*p<0.0001, **p<0.0001 and #p<0.0001 vs. control), showing increased number of colonies by cells from acute disease and decreased colonies thereafter.
Figure 3. Immunization with PLP results in transient increase in the SVZ cell proliferation.
SJL/J mice immunized with PLP 139–151 developed relapsing-remitting EAE. (a) Representative graph of the clinical course and the timing of BrdU injection and timing of sacrifice (indicated by down arrows). (b) BrdU+ cells in the forebrain during different phases of disease. The figure shows the corner of the lateral wall (LV), the SVZ and corpus callosum (CC). (c) Number of BrdU+ positive cells/mm2 in the corpus callosum in different phases of disease with no washout (*p<0.0005, **p=0.0055 versus control); 8 days wash out (*p<0.0005 versus control) and 28 days wash out (*p=0.0129, **p=0.0002 versus control) n=4 mice/ group. (d) Number of BrdU+ positive cells/mm in the lateral wall of the lateral ventricle with no washout (*p<0.002 vs. control); 8 days wash out (*p=0.0009 vs. control); 28 days wash out (*p=0.0314 versus control) n=4 mice/group. (e) Electron microscopy image from the corpus callosum (CC) of a normal control (left panel) and EAE (right panel). Astrocytic fibers indicated by arrows are enlarged during relapse. (scale bar in b represents 5 μm).
Neural progenitors cells generate multipotent neurospheres in vitro. We thus performed the neurosphere assay at different time points during SJL relapsing remitting EAE, to confirm the change in proliferative capacity of NSCs from the SVZ during the different phases of the disease. In agreement with our in vivo observations, we observed an increase in the numbers of neurospheres during the acute phase that decreased below the level observed in controls during the chronic phase (Figure 2 B).
Altered migration and maturation of neural progenitors in EAE
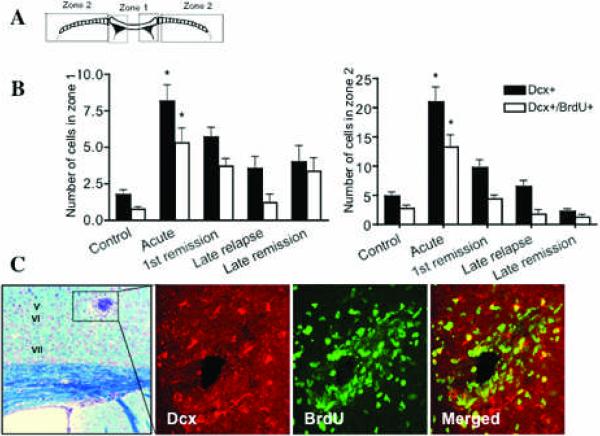
During normal physiological states, migration of SVZ cells is primarily targeted to the olfactory bulb with radial migration to the corpus callosum10. For this analysis, the corpus callosum was divided into two zones, zone 1 being the medial part of the corpus callosum representing local migration from the SVZ and zone 2 being the para-medial part (Figure 4 A), representing distant migration form the SVZ toward the distal corpus callosum or cortex (n=42 mice). We used doublecortin (Dcx) to identify migrating (Dcx+) and proliferating (Dcx/BrdU+) type A cells28, 33 in the SVZ. Dcx is a marker of migratory postnatal neuroblasts, which is not expressed by activated immune cells (supplementary fig 2). Neuroblasts show active migration as demonstrated by a leading edge that expresses Dcx10. We observed an increase in the numbers of both migrating and proliferating type A cells in the corpus callosum especially in zone 2 (distant migration) during the acute phase, which decreased during the chronic phase of disease, while these cells are not usually found in the control animals (n=6 mice/group) (Figure 4 B). During the acute phase, many Dcx/BrdU+ cells were found migrating long distances from the SVZ, towards infiltrates in the cortex and around perivenular lesions (Figure 4 C). Some of these cells were found near inflammatory infiltrates, with substantial changes in their typical morphology with deranged leading process and cell bodies (supplementary fig 3). Given previous reports of neurogenesis in the spinal cord in EAE34, we wished to examine whether terminal differentiation of neurons occurred in the forebrain during EAE. We perfused the animals 28 days after the last BrdU injection to allow for maturation of neuroblasts to NeuN+ neurons. We did not find BrdU/NeuN+ cells in the cortex in any of the 12 EAE mice examined despite the high numbers of migratory neuroblasts that differentiate into NeuN+ cells in the olfactory bulb (supplementary fig 3). Furthermore, we found no apoptotic neural progenitor cells or mature neurons, but could observe several apoptotic T cells (data not shown). We then wondered whether these cells might be able to differentiate into oligodendrocytes.
Figure 4. Neuroblast migration during EAE.
(A) Schematic diagram showing Zones 1 and 2 of he Corpus callosum. (B) Left panel: Intracortical lesion in cortical layer V (day 20 dpi), LFB staining with cresyl violet. High magnification of the lesion area stained with Dcx (red), BrDU (green) and the merged picture (right panel). (B) Quantification of migrating neuroblasts in zones 1 and 2 of the corpus callosum during EAE (*p<0.001 vs. control, n=6 mice/group), showing an increase in neuroblasts during the acute phase that is lost thereafter.
Alteration of oligodendrogenesis from the SVZ in EAE
We investigated the generation of new myelinating oligodendrocytes and their precursors in the periventricular area of the SVZ. We first stained for NG2, a proteoglycan expressed by oligodendrocyte precursors35, quantified the labeling index of NG2/BrdU+ cells that show typical enlongated process of OPCs in the area around the lateral ventricle in 28 mice and found a significant increase during acute disease compared to control (Figure 5 A). However, with each subsequent relapse the labeling index decreased and was no longer significant compared to controls (Figure 5 A). We also determined whether the oligodendrocyte progenitor cells would mature and become CNPase+ oligodendrocytes after BrdU injection and 8-day washout. We found numerous CNPase/BrdU+ positive cells during the acute phase expressing the typical morphology of mature oligodendrocytes (n=4 mice) (Figure 5 B, C). However during the chronic stage of the disease and especially during the late relapse there was a significant decrease in the number of newly generated oligodendrocytes compared to controls. These results suggest that during the chronic phase, maturation of oligodendrocytes generated from SVZ progenitor cells is impaired. Since there are OPCs scattered in the cortex and corpus callosum, it is likely that the maturation of these cells is also impaired during the chronic phase.
Figure 5. Alterations in oligodendrogenesis around the SVZ in EAE demonstrating the increase in oligodendrocyte progenitors and mature cells during acute disease.
(A) Labeling index of NG2+ progenitor cells in the SVZ as percentage out of total NG2+ cells (*p<0.001, n=4 mice/group). (B) Number of newly generated mature CNPase/BrdU+ oligodendrocytes during disease (*p<0.001, n=4 mice/group). (C) Confocal images of CNPase+/BrdU+ cells (arrows) around the SVZ in control animals (top panels) and animals with acute disease.
Changes in repair potential during EAE correlate with microglia activation in SVZ and RMS
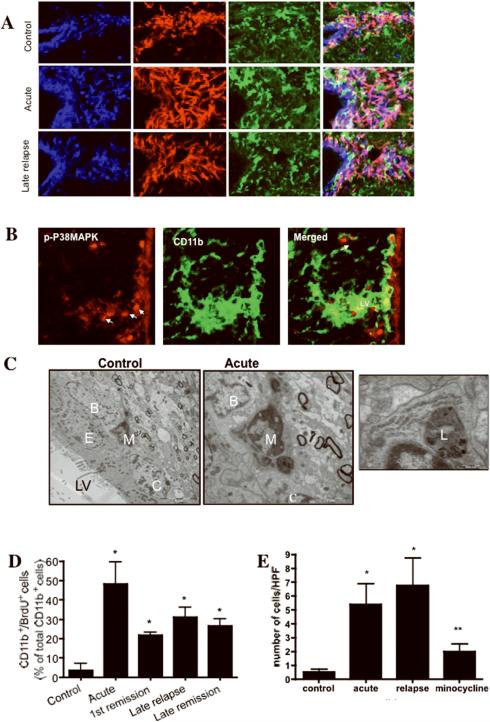
Microglia are present in the normal adult SVZ and closely interact with Sox2/GFAP+ bona fide stem cells in the SVZ (Figure 6 A). Consistent with a potential role of microglia as a niche cell, we found that cultured microglia cells express noggin, a known stem cell and neurogenesis regulator after treatment with IFN-γ (supplementary fig 4). We have previously shown that relapsing-remitting EAE results in persistent activation of cortical and callosal periventricular microglia14. Here we show that in the acute and chronic stages an increased number of CD11b+ cells were closely associated with Sox2+ stem cells (Figure 6 A) and Dcx+ progenitor cells (data not shown). We observed increased GFAP expression in Sox2+ cells and upregulated expression of Sox2 in the cytoplasm, instead of the normal nuclear expression (Figure 6 A). Similar alterations of the nucleocytoplasmic expression of other transcription factors such as Olig2 have been observed during preferential astrocytic differentiation of adult progenitor cells in response to injury36, 37. In addition, the SVZ microglia had an activated morphology with an enlarged cell body and retraction of their thin processes. We confirmed the activation of microglia during acute and late relapse by demonstrating the expression of high levels of phosphorylated p38MAPK (Figure 6 B), a specific activation marker of microglia27 in EAE. Furthermore, EM of the SVZ showed the presence of secondary lysosomes in the SVZ microglia during the acute and chronic phases of EAE, consistent with activation (Figure 6 C), and these microglia were closely associated with B and C cells (Figure 6 C). We also found significantly increased numbers of proliferating microglia (BrdU+) throughout disease compared with control (Figure 6 D).
Figure 6. Microglia are closely associated with B and A cells in the SVZ niche.
(A) CD11b+ microglia and Sox-2/GFAP+ stem cells in the SVZ in control animals (top panels), and in acute disease (middle panels), and in chronic disease (lower panels). LV: lateral ventricle. (B) Confocal images of p-p38MAPK (red) labeled cells (arrows) during late relapse in EAE in CD11b+ microglia (green). (Scale bar 10μm). (C) EM of a representative microglia (M) in the SVZ from control animal (left panel) and an activated microglia from acute EAE (middle panel) showing secondary lysosomes interacting with B and C cells. Magnification of secondary lysozome (right panel). LV: lateral ventricle, E: ependymal cell. (D) Labeling index of CD11b+ microglia during the different phases of EAE (*p=0.0117, **p=0.0088, #p=0.004 vs. control, n=3 mice/group), demonstrating continued proliferation of microglia throughout the disease duration. (E) Number of CD11b+ microglia expressing p-p38MAPK during the different phases of disease and in the relapse phase of minocycline treated animals (*p<0.005 vs. control, **p<0.05 vs. acute or relapse).
These results suggest that microglia are bona fide niche cells that may play a role in the repair potential of NSCs.
Inactivation of chronically activated microglia by minocycline ameliorates the SVZ niche dysfunction and improves repair potential
Inactivation of microglia during acute injury was reported to alter the regenerative response of neural progenitors22 and worsen the outcome of CNS injury23. We hypothesized that modulation of the chronic activation of microglia may improve repair in EAE and potentially in MS. Minocycline modulates microglia in vivo by specific inactivation of the phosphorylated form of p38MAPK27. Administration of minocycline has previously been shown to protect from EAE26 and reduce gadolinium-enhancing lesions in MS26. We used minocycline starting on day 20 after immunizaion to target microglia activation in vivo27, 38, and observed a significant decrease in clinical disease severity (with decreased disease score) after administration of minocycline (Figure 7 A) and a reduction in number of forebrain lesions by immunohistology during the chronic phase of disease (Figure 7 B). Notably, we found a reduction in microglia activation in the SVZ with a decrease in number of CD11b+p-p38MAPK+ cells in the SVZ of minocycline treated animals (p<0.05 vs. acute or chronic EAE, Figure 6 E) as well as a decrease in mean fluorescent intensity of the p-p38MAPK staining (p<0.005 vs. acute or chronic EAE, not shown). At the ultrastructural level, the SVZ microglia in minocycline treated mice exhibited a decrease in secondary lysosomes (not shown), associated with an improvement in the number of proliferating Sox2/BrdU+ neural stem cells in the minocycline treated animals compared to PBS treated animals (n=10 mice/group) (Figure 7 C). Consistent with the improvement in the number of B cells, we found an increase in the number of proliferating NG2+ oligodendrocyte precursors and subsequently more mature oligodendrocytes expressing CNPase compared to PBS treated animals (Figure 7 C). Furthermore, minocycline decreased the GFAP expression of reactive astrocytes in the corpus callosum and SVZ (Figure 7 C).
Figure 7. Minocycline treatment ameliorates EAE and improves repair.
(A) Clinical course of animals treated with Minocycline starting on day 20 post-immunization. Disease grade is significantly lower in minocycline treated (circles) versus PBS treated (black squares) between days 20 and 55. (B) Number of forebrain lesions *p=0.0069, n=4 animals/group. (C) Labeling index of Sox-2+ cells in the SVZ after treatment with minocycline compared with PBS (*p=0.0423), labeling index of NG2+ cells (*p<0.0001), number of CNPase/BrdU+ cells (*p=0.0078), and mean pixel intensity of GFAP in the corpus callosum (*p=0.0077) (n=4 mice/group). (D) High-resolution 2.5-dimensional image of the MBP intensity in the corpus callosum (left panels). Quantification of the pixel intensity of MBP in control (black line) versus acute EAE (red line) and in late relapse after treatment with minocycline (blue line) compared to PBS treated mice (black line) *p<0.0001 (right panels).
Consistent with the increase in oligodendrogenesis and the number of mature oligodendrocytes, minocycline treatment preserved the integrity of myelin in the corpus callosum compared to PBS treated animals (Figure 7 D, supplementary fig 5, and Supplementary Table 3). Interestingly, minocycline treatment restored the normal architecture in the SVZ niche and improved the architecture of the neuroblast chains in the SVZ compared to PBS treated mice (supplementary fig 6). While we have focused on the effect of microglia on SVZ NPCs, it is likely that activated microglia probably have similar effects on OPCs that are scattered in the cortex and corpus callosum, although we have not formally shown this.
To investigate whether microglia have a direct effect on neurogenesis, we measured the number of neuroblasts generated from NSCs that were plated as single cells and exposed to supernatants of microglia. Compared to NSCs cultured in HL-1 medium, cultures exposed to resting microglia supernatants had increased number of neuroblasts, while cultures exposed to supernatants of IFN-γ activated microglia had decreased neurogenesis (Figure 8). Similar results were obtained if microglia were stimulated with LPS (supplementary fig 7). This was not due to direct toxicity of LPS since direct addition of LPS to the NSCs had no effect. In contrast, supernantants of IFN-γ or LPS activated microglia treated with minocycline showed a dose-response increase in neurogenesis (Figure 8 and supplementary fig 7), while minocycline itself did not have an effect on NSCs in vitro (data not shown).
Figure 8. Minocycline reduces toxicity of activated microglia to neural and oligodendrocyte progenitors.
A) MAP+ cells counted after 5 days of NSC differentiation in the presence of microglia supernatants from unstimulated microglia (HL-1 medium), IFN-γ treated microglia dose response showing a dose-response decrease in MAP+ cells. B) minocycline treatment of microglia reverses the deleterious effect of IFN-g stimulation on MAP+ cell survival. C) NG2+ cell survival after exposure to microglia supernatants stimulated with IFN-γ, showing a dose response decrease in NG2+ cells. D) minocycline treatment of microglia reverses the deleterious effect of IFN-γ stimulation on NG2+ cell survival. (*p<0.05, ***p<0.0001)
In order to determine whether minocycline affects microglia directly, or only works through suppression of infiltrating inflammatory macrophages, we treated naive SJL mice with increasing doses of minocycline (10 and 50 mg/kg) for 15 days. We found a significant decrease in the number of microglia in the SVZ with a parallel increase in the number of SVZ stem cells concurrent with the increased doses of minocycline (Figure 9). These data suggest that SVZ microglia may directly affect NSC proliferation in the SVZ.
Figure 9. In vivo minocycline reduces the number of total microglia and increases proliferation in the SVZ.
(A) Proliferation of SVZ cells increases with minocycline treatment (* =p<0.01). (B) Number of Iba+ microglia cells in the SVZ decreases with minocycline treatment (* =p<0.01). Data represent the mean ± SD.
DISCUSSION
Endogenous neural stem cells are able to engage in reparative functions10, 11. Consistent with this role, we found that NSCs proliferate and engage in repair during the acute phase of EAE, but this capacity is lost during the chronic phase of the disease. The enhanced NSC proliferation seen in this model during acute disease is in contrast with our previous report in the B6 mouse EAE model13, and in keeping with the prominent forebrain and periventricular pathology in SJL as well as the relapsing nature of the disease. Proliferation of progenitors and enlargement of the SVZ have been reported in lysolecithin-induced demyelination3 and TNF injected EAE animals12. Other studies in mice with EAE have reported transiently-increased proliferation of PSA-NCAM+ and NG2+ cells in the corpus callosum, which the authors inferred as indirect evidence of enhanced proliferation of stem and precursor cells in the SVZ8, 39, 40. However, none of these papers has evaluated the behavior of NSCs during the course of relapsing disease, or its relationship to microglia in the niche.
After acute CNS injury, surviving cells produce mediators that enhance stem cell function as reported in stroke models41–45. Thus, our finding of enhanced NSC proliferation in acute disease is consistent with other models of CNS injury. The interesting finding of decreased proliferative response in the SVZ during the chronic phase of EAE prompted our hypothesis that chronic activation of glial cells induces a transition from a permissive to a non-permissive microenvironment that can affect stem cells. Although we do not discount a role for astrocytes46, we focused our attention on microglia given our observation of activated microglia during the chronic phase of EAE14 and as reported in MS16.
There are conflicting reports on the effect of microglia on NSC function with some reports suggesting a deleterious role21 while others suggest a beneficial role20, 47. Our data suggest that microglia have both beneficial and deleterious roles at different phases of disease, and that manipulation of microglia activation may impact the repair potential in the CNS. In addition to SVZ progenitors, there are widespread OPC progenitors in the CNS and our data is consistent with the idea that chronic microglia activation may affect OPCs outside of the SVZ impairing oligodendrocyte production.
Future studies are warranted to establish the molecular networks that govern the regenerative vs. degenerative phenotype of microglia during EAE.
Interestingly, we found that modulation of microglia activation even in naïve animals is associated with an increase in the numbers of proliferative SVZ cells (Figure 8). Our data suggest an inverse relationship between the number of microglia and proliferation in the SVZ, and demonstrate that minocycline can decrease microglia numbers and their activation state in the SVZ, while enhancing proliferation in the SVZ neurogenic niche. These findings suggest that microglia in the SVZ may have a tonic inhibitory role on adult NSC proliferation. A similar effect has been suggested for macrophages/microglia during development48. In addition chronic microglia activation leads to a non-permissive environment for repair, while specific inactivation of microglia with minocycline ameliorates the loss of reparative potential of endogenous stem cells.
Minocycline has also inhibitory effects on macrophage activation and has been reported to protect animals from EAE26 presumably by inhibiting peripheral immune cell activation. It can also inhibit matrix metalloproteinases and decrease T cell transmigration to the CNS49. We have used minocycline after the acute phase of the disease to minimize the impact of therapy on peripheral immune activation, although we cannot discount a contribution of macrophage inactivation in our model. Inactivation of microglia may also downregulate expression of MHC class II, which could result in reduced T cell reactivation within the CNS, but we think this is unlikely to contribute to the observed benefits of minocycline here, since the number of T cells in the lesions is minimal during the chronic EAE14.
In contrast to a previous report34 we did not find terminal differentiation of neurons in the SVZ. Difference in models and strains may explain this discrepancy. But this raises the question of the fate of progenitors that are generated during acute disease. Our data suggest that the cells remain in a state of altered differentiation and morphology but we cannot rule out the possibility of Dcx+ cell apoptosis since removal of dead cells in the CNS occurs rapidly. Our data show similarity to a recent report in MS tissue that demonstrates a limited expansion of progenitors cells and thickening of the SVZ1.
Our findings have implications for therapeutic strategies targeting NSC mobilization from germinal niches by inhibiting microglia activation, but also suggest that caution should be exercised so as not to inhibit beneficial glial activation.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grants (AI46374 and AI043496 to SJK), NMSS RG 3945 grant to SJK, and Fidelity Foundation grant to SJK, and PP1236 from the National Multiple Sclerosis Society to JI, a faculty PhD scholarship from the University of Southern Denmark and a grant from Hørslev Fonden in Denmark to SR and the Red Terapia Regenerativa, Spain to AAS and JMGV.
REFERENCES
- 1.Nait-Oumesmar B, Picard-Riera N, Kerninon C, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A. 2007;104:4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 3.Menn B, Garcia-Verdugo JM, Yaschine C, et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imitola J, Chitnis T, Khoury SJ. Insights into the molecular pathogenesis of progression in multiple sclerosis: potential implications for future therapies. Arch Neurol. 2006;63:25–33. doi: 10.1001/archneur.63.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 7.Imitola J, Snyder ES, Khoury SJ. Genetic programs and responses of neural stem/progenitor cells during demyelination: potential insights into repair mechanisms in multiple sclerosis. Physiol Genomics. 2003;14:171–197. doi: 10.1152/physiolgenomics.00021.2002. [DOI] [PubMed] [Google Scholar]
- 8.Picard-Riera Nea. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. PNAS. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 11.Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 12.Magalon K, Cantarella C, Monti G, et al. Enriched environment promotes adult neural progenitor cell mobilization in mouse demyelination models. Eur J Neurosci. 2007;25:761–771. doi: 10.1111/j.1460-9568.2007.05335.x. [DOI] [PubMed] [Google Scholar]
- 13.Pluchino S, Muzio L, Imitola J, et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008 doi: 10.1093/brain/awn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen S, Wang Y, Kivisakk P, et al. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing--remitting experimental autoimmune encephalomyelitis. Brain. 2007;130:2816–2829. doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- 15.Wuerfel J, Tysiak E, Prozorovski T, et al. Mouse model mimics multiple sclerosis in the clinico-radiological paradox. Eur J Neurosci. 2007;26:190–198. doi: 10.1111/j.1460-9568.2007.05644.x. [DOI] [PubMed] [Google Scholar]
- 16.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 17.Santambrogio L, Belyanskaya SL, Fischer FR, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci U S A. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- 20.Butovsky O, Landa G, Kunis G, et al. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J Clin Invest. 2006;116:905–915. doi: 10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 22.Li WW, Setzu A, Zhao C, Franklin RJ. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J Neuroimmunol. 2005;158:58–66. doi: 10.1016/j.jneuroim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Fan Y, Won SJ, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- 24.Maier K, Merkler D, Gerber J, et al. Multiple neuroprotective mechanisms of minocycline in autoimmune CNS inflammation. Neurobiol Dis. 2007;25:514–525. doi: 10.1016/j.nbd.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Nikodemova M, Watters JJ, Jackson SJ, et al. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282:15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- 26.Popovic N, Schubart A, Goetz BD, et al. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol. 2002;51:215–223. doi: 10.1002/ana.10092. [DOI] [PubMed] [Google Scholar]
- 27.Yune TY, Lee JY, Jung GY, et al. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27:7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis P, Fagan BM, Magness ST, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 30.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Verdugo JM, Doetsch F, Wichterle H, et al. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 33.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 34.Danilov AI, Covacu R, Moe MC, et al. Neurogenesis in the adult spinal cord in an experimental model of multiple sclerosis. Eur J Neurosci. 2006;23:394–400. doi: 10.1111/j.1460-9568.2005.04563.x. [DOI] [PubMed] [Google Scholar]
- 35.Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- 36.Raponi E, Agenes F, Delphin C, et al. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55:165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magnus T, Coksaygan T, Korn T, et al. Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes. J Neurosci Res. 2007;85:2126–2137. doi: 10.1002/jnr.21368. [DOI] [PubMed] [Google Scholar]
- 38.Zhang SC, Goetz BD, Duncan ID. Suppression of activated microglia promotes survival and function of transplanted oligodendroglial progenitors. Glia. 2003;41:191–198. doi: 10.1002/glia.10172. [DOI] [PubMed] [Google Scholar]
- 39.Calza L, Giardino L, Pozza M, et al. Proliferation and phenotype regulation in the subventricular zone during experimental allergic encephalomyelitis: in vivo evidence of a role for nerve growth factor. Proc Natl Acad Sci U S A. 1998;95:3209–3214. doi: 10.1073/pnas.95.6.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nait-Oumesmar B, Decker L, Lachapelle F, et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 41.Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. PNAS. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corti S, Locatelli F, Papadimitriou D, et al. Multipotentiality, homing properties, and pyramidal neurogenesis of CNS-derived LeX(ssea-1)+/CXCR4+ stem cells. Faseb J. 2005;19:1860–1862. doi: 10.1096/fj.05-4170fje. [DOI] [PubMed] [Google Scholar]
- 43.Miller JT, Bartley JH, Wimborne HJ, et al. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robin AM, Zhang ZG, Wang L, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- 46.Collin T, Arvidsson A, Kokaia Z, Lindvall O. Quantitative analysis of the generation of different striatal neuronal subtypes in the adult brain following excitotoxic injury. Exp Neurol. 2005;195:71–80. doi: 10.1016/j.expneurol.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Thored P, Heldmann U, Gomes-Leal W, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 48.Li MO, Sarkisian MR, Mehal WZ, et al. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 2003;302:1560–1563. doi: 10.1126/science.1087621. [DOI] [PubMed] [Google Scholar]
- 49.Brundula V, Rewcastle NB, Metz LM, et al. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125:1297–1308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.