Abstract
Heat stress prior to diving has been shown to confer protection against endothelial damage due to decompression sickness. Several lines of evidence indicate a relation between such protection and the heat shock protein (HSP)70 and HSP90 and the major cellular red-ox determinant, glutathione (GSH). The present study has used human endothelial cells as a model system to investigate how heat stress and simulated diving affect these central cellular defense molecules. The results demonstrated for the first time that a simulated dive at 2.6 MPa (26 bar) had a potentiating effect on the heat-induced expression of HSP70, increasing the HSP70 concentration on average 54 times above control level. In contrast, a simulated dive had no significant potentiating effect on the HSP90 level, which might be due to the higher baseline level of HSP90. Both 2 and 24-h dive had similar effects on the HSP70 and HSP90, suggesting that the observed effects were independent of duration of the dive. The rapid HSP response following a 2-h dive with a decompression time of 5 min might suggest that the effects were due to compression or pressure per se rather than decompression and may involve posttranslational processing of HSP. The exposure order seemed to be critical for the HSP70 response supporting the suggestion that the potentiating effect of dive was not due to de novo synthesis of HSP70. Neither heat shock nor a simulated dive had any significant effect on the intracellular GSH level while a heat shock and a subsequent dive increased the total GSH level approximately 62%. Neither of these conditions seemed to have any effect on the GSH red-ox status.
Keywords: Endothelial cells, Diving, Decompression, Heat shock protein, Glutathione
Introduction
Several lines of evidence point to the endothelium as a main target for damage caused by vascular gas bubbles that are formed during decompression (Brubakk et al. 2007). The mechanisms behind these effects are still unclear. Investigations in both animals and man have demonstrated that physical exercise 20–24 h prior to diving significantly reduced the bubble formation and conferred protection against decompression sickness (DCS). Furthermore, exposing rats to heat stress (42°C) for 1 h 24 h prior to a dive did not affect bubble formation but protected the animals against injury due to decompression. Notably, the protection was accompanied by an increased production of heat shock protein 70 (HSP70; Brubakk et al. 2007). Similar results have previously been demonstrated in rats that were subjected to rapid decompression. Occurrence of DCS was associated with increased HSP70 expression and heat shock prior to diving reduced air bubble-induced lung injury (Huang et al. 2003). Recent experiments with rats exposed to rapid decompression indicated an association between occurrence of DCS and increased gene expression of small heat shock proteins in brain and lung, but not in liver. Remarkably, HSP70 was not significantly affected in any of the three organs (Montcalm-Smith et al. 2007).
HSPs are one of several cellular defense mechanisms. They have several important cytoprotective functions; one of the most important is to function as molecular chaperones in the folding of proteins and maintenance of the native conformation to ensure biological activity. HSPs (also termed stress proteins) are induced by a number of stimuli including heat stress, exercise, and oxidative stress (Kregel 2002). Recent observations also indicate that HSPs may play an important role in the cellular defense against several toxic chemicals (Nishida et al. 2006; Tolson et al. 2006).
Another critical factor related to bubble formation and endothelial dysfunction seems to be nitric oxide (NO). The molecule is synthesized endogenously by nitric oxide synthase (NOS) and functions both as a signaling molecule and a vasorelaxation factor (Alp and Channon 2004). Recent investigations have pointed to a possible role of NO in bubble formation and/or endothelial injury resulting from such bubbles. Reducing NO availability by inhibiting its synthesis increased bubble formation and reduced survival of rats exposed to rapid decompression (Wisløff et al. 2003). Conversely, administration of NO by a NO-releasing chemical reduced bubble formation and increased survival in rats after rapid decompression (Wisløff et al. 2004). NOS, the enzyme responsible for the endogenous production of NO, requires several cofactors, reduced form of nicotinamide adenine dinucleotide phosphate, flavin adenine dinucleotide, flavin mononucleotide, Fe, and tetrahydrobiopterin (BH4). In addition, the molecular chaperone HSP90 has been identified as a regulator of NOS activity (Alderton et al. 2001). Regulation of NOS activity also seems to be mediated by phosphorylation of specific amino acid residues in the enzyme (Chen et al. 2008; Förstermann 2006), and recent investigations have demonstrated that phosphorylation of a specific serine residue increased the association of NOS and HSP90 concurrent with an increased NO synthesis (Davis et al. 2006). Notably, NOS seems to have two catalytic endpoints, i.e., normally it produces NO, but under certain conditions, it will produce superoxide, which interact with NO to form peroxynitrite. The latter one will rapidly oxidize BH4 and lead to NOS uncoupling and resulting endothelial dysfunction. Current research suggests that maintenance of adequate levels of BH4 in the endothelium is a critical factor in regulating the balance between NO and superoxide synthesis by NOS in vascular disease (Alp and Channon 2004). In this context, antioxidants may play important roles, although the literature so far shows conflicting results. Ascorbic acid has been shown to increase both glutathione (GSH) and NOS activity in human endothelial cells (Smith et al. 2002), and the effect seems to be due to stabilization of the BH4 molecule (Werner et al. 2003). Glutathione is a major cellular red-ox determinant, but some investigators have indicated that glutathione does not seem to have significant impact on the NOS (Huang et al. 2001), while others have indicated a correlation between GSH level and NOS activity (Laursen et al. 2001; Smith et al. 2002). Novel findings have suggested a regulatory role of glutathione in glutathiolating specific proteins at specific residues; the reversible glutathiolation may thus protect these otherwise reactive sites against irreversible oxidation (West et al. 2006). Moreover, NO has also been shown to stimulate glutathiolation of a specific protein which results in cyclic guanosine monophosphate-independent arterial relaxation (Cohen and Adachi 2006). Recent studies suggest that the biopterin red-ox status is more important to the activity of NOS than the total amount of biopterin and that GSH contributes to maintain this ratio (Crabtree et al. 2008, 2009).
Taken together, these findings indicate that there is a close relation between several heat shock proteins, endothelial nitric oxide production and the cofactors that influence this activity, and the possible endothelial injury that may follow decompression. In this respect, glutathione may play an important role either by providing reducing power for the tetrahydrobiopterin or by glutathiolation of vital proteins as indicated above.
An important aspect of saturation diving is that it is performed with an elevated level of oxygen, usually from 35–80 kPa (0.35–0.80 bar) depending on the different phases of a dive. The hyperoxic conditions impose a significant oxidative stress on the divers and are counteracted by several defense mechanisms. One of these is the glutathione system which represents a first-line defense against reactive oxygen species that are formed, e.g., by exposure to hyperoxic conditions. We have previously shown that the glutathione system is markedly affected under diving conditions (Djurhuus et al. 1998, 1999) and that the glutathione level in blood cells of divers was markedly reduced after a simulated dive corresponding to a depth of 250 m sea water (msw; Djurhuus et al. 2006).
This might be interpreted as if this defense system is compromised; this may be important during decompression where elevated NO production may be a prerequisite for protection against endothelial damage. The present investigation was therefore undertaken as a first step in elucidation of the relationship between HSP70 (HSPA1A/HSPA1B), HSP90 (HSPC1; Kampinga et al. 2009), and glutathione in their potential role in protection against endothelial damage due to decompression stress. Human endothelial cells were used as a model system to study how both heat stress and simulated diving affect these central cellular defense molecules.
Materials and methods
Chemicals and gas
N-Ethylmorpholine, heparin (Na salt, porcine intestinal mucosa), and 2% gelatin solution (bovine skin) were obtained from Sigma-Aldrich, Inc. (St. Louis, MO, USA). l-Glutamine, hydrogen bromide, 5-sulfosalicylic acid, perchloric acid, acetic acid, orthophosphoric acid, methanol, acetonitrile, and dimethyl sulfoxide were obtained from Merck (Darmstadt, Germany). Sodium borohydride, dithioerythritol (DTE), reduced (GSH) and oxidized (GSSG) glutathione were obtained from Fluka Chemie AG (Switzerland), and monobromobimane was from Molecular Probes (Eugene, OR, USA). Trypsin (2.5%) was obtained from Gibco, Invitrogen Ltd. (Paisley, UK). ELISA kits for HSP70 (EKS-700B; recognizes human HSPA1A/HSPA1B) and HSP90α (EKS-895; recognizes human HSPC1) were obtained from Assay Designs, Inc. (Ann Arbor, MI, USA). Premixed gas consisting of 75% He, 20% O2, and 5% CO2 (mixed from 6.0, 5.5, and 5.0 qualities, respectively) was obtained from Yara Industrial AS (Bergen, Norway), and pure He (6.0) was from Gardner Cryogenics A/S (Sandnes, Norway).
Cells and culture conditions
Human umbilical vein endothelial cells (HUVEC; American Type Culture Collection (ATCC) no. CRL-1730) were obtained from ATCC (Manassas, VA, USA). The cells were grown without addition of antibiotics in MCDB-131 medium (Gibco, Invitrogen Ltd., Paisley, UK) supplemented with heparin (50 µg/ml), endothelial cell growth supplement (50 μg/ml; BD Biosciences, Bedford, MA, USA), and 20% heat-inactivated fetal calf serum (Gibco, Invitrogen Ltd., Paisley, UK). Cells were seeded on standard cell culture plastic flasks or dishes, all pretreated with a solution of 0.2% gelatin in Dulbecco’s phosphate-buffered saline (PBS) for 30 min at room temperature. Stock cultures of the cells were maintained at 37°C in an atmosphere of 5% CO2 in air and a relative humidity of 95% (standard conditions). For experiments, the cells were exposed in a pressure chamber as indicated below.
Exposure design 1
Unless otherwise indicated, the experiments consisted of four groups:
Control—incubated in the cell culture incubator at standard conditions (see above) throughout the experiment
HS—exposed to 45°C for 1 h and further incubated at standard conditions (48 h) until harvesting
Dive—exposed to simulated dive at 2.6 MPa (26 bar; corresponding to a depth of 250 msw) for 24 h at 37°C until harvesting
HS + dive—exposed to 45°C for 1 h, further incubated at standard conditions for 24 h, and then exposed to simulated dive at 2.6 MPa (26 bar) for 24 h at 37°C until harvesting
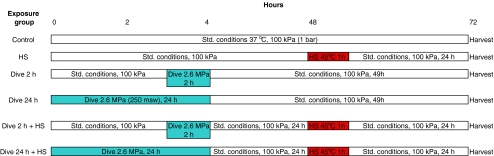
An overview of the different exposure conditions is shown in Fig. 1.
Fig. 1.
Exposure design 1. Overview of exposure to heat shock (HS; 45°C, 1 h) and subsequent simulated diving at 37°C. Standard conditions were in an incubator with 5% CO2 in air, 100% humidity, and 37°C. Both heat shock and simulated diving were in an atmosphere of 20 kPa (0.20 bar) O2 and 5 kPa (0.05 bar) CO2 in He
Exposure design 2
Reversed order is the dive exposure prior to heat shock—including both 2- and 24 h dive—otherwise similar conditions as design 1 (see Fig. 2). All groups were seeded simultaneously at the same cell density and grown to confluence or near confluence before initiating the different exposures. Each group consisted of nine to ten parallel dishes, three to four dishes were used for analysis of heat shock proteins, three to four dishes used for analysis of glutathione, and three dishes used for determination of cell number as described below.
Fig. 2.
Exposure design 2. Overview of exposure to simulated diving at 37°C and subsequent heat shock (HS; 45°C, 1 h). Standard conditions, simulated diving, and heat shock conditions were as in exposure design 1
Exposure of cells to heat and simulated dive
Exposure to heat and simulated dive was performed in a cylindrical, 15-l stainless steel pressure chamber. The chamber was equipped with instruments for control and continuous monitoring of temperature, oxygen concentration, humidity, and pressure.
Heat shock The cell cultures were placed in the chamber, which was flushed with He/O2/CO2 mixture (75/20/5) to remove the air and isolated at atmospheric pressure and 100% relative humidity at a temperature of 45°C for 1 h. The partial pressures during the exposure thus were 20 kPa O2 (0.20 bar; 20%), 5 kPa CO2 (0.05 bar; 5%), and 75 kPa He (0.75 bar; 75%). After end of exposure, the chamber was opened and the culture dishes moved to the cell culture incubator and further incubated for 24 h at standard conditions.
Simulated dive The cell cultures were placed in the chamber, which was flushed with He/O2/CO2 mixture (75/20/5) to remove the air. The chamber was then pressurized with pure helium to an absolute pressure of 2.6 MPa (26 bar; corresponding to 250 msw) and isolated at this pressure and 100% relative humidity at 37°C for 24 h. The partial pressures during the dive thus were 20 kPa O2 (0.20 bar; equivalent to 20% at surface), 5 kPa CO2 (0.05 bar; equivalent to 5% at surface), and the rest was He making up the total pressure to 2.6 MPa. Both oxygen partial pressure as well as the total pressure was monitored continuously during the dive, and the pO2 was 20.0 ± 0.5 kPa (0.200 ± 0.005 bar) when all experimental dives are included (data not shown). At the end of the dive, the chamber was rapidly decompressed (10 kPa/s), opened, and the cells immediately harvested for analysis as described below. Visual inspection of the cultures did not indicate any immediate adverse effects of the dive/decompression, and no change of pH was observed as judged by the color of the pH indicator in the medium.
Determination of intracellular HSP70 and HSP90
Harvesting of cells The medium was removed and the cells washed with 5 ml calcium- and magnesium-free PBS (PBS-CMF) and detached by incubation for 10 min at 37°C with 0.25% trypsin–0.5 mM ethylenediaminetetraacetic acid. The trypsin was inactivated by addition of basal Eagle’s medium containing 10% fetal calf serum and the cells harvested by centrifugation. The cells were then washed twice by addition of 5-ml ice-cold PBS-CMF, gently resuspended, and centrifuged. The cells were then resuspended in 1.5-ml ice-cold PBS-CMF, transferred to a microtube, and centrifuged. After removal of PBS-CMF, the cell pellet was frozen at −20°C for later analysis.
Analysis of HSP Frozen cell pellets were thawed and lysed in lysis buffer according to the manufacturer’s instructions for the HSP70 and HSP90α ELISA kits, respectively. Both ELISA kits are quantitative sandwich immunoassays that determine a colored product formed by a horseradish peroxidase conjugated to a secondary antibody. Color formation was determined in a microplate reader at 450 nm, and HSP was quantitated from a standard curve generated with HSP standard protein provided in the kits. The concentrations were calculated in nanograms per 106 cells and the results presented as fold change relative to the control group.
Determination of intra- and extracellular glutathione
The analysis of glutathione was performed as described previously (Djurhuus et al. 1991). Briefly, after removal from chamber or incubator, the culture dishes were immediately placed on ice.
Extracellular glutathione A 0.9-ml medium from each dish was added to 0.1-ml ice-cold sulfosalicylic acid (50%, containing 500 μM DTE), mixed, and frozen at −20°C. For analysis, the samples were thawed and centrifuged, and the supernatant was used for determination of total glutathione as described below.
Intracellular glutathione The rest of the medium was removed from the dish and the cells gently washed twice with 5-ml ice-cold balanced salt solution. The cells were then immediately extracted with 300-μl 5% ice-cold sulfosalicylic acid containing 50 μM DTE; the cells scraped off the dish with a rubber policeman, transferred to a microtube, and frozen at −20°C. For analysis, the samples were thawed and centrifuged, and the supernatant was used for determination of reduced and total glutathione as described below.Total free glutathione, which represents the sum of GSH, GSSG, and soluble mixed disulfides (GSSR), and reduced glutathione (GSH) were determined in the acid extracts according to a modification (Mansoor et al. 1992) of a chromatographic procedure described previously (Svardal et al. 1990). The results are expressed as equivalents of GSH in nanomoles per 106 cells.
Determination of cell number
All experimental groups contained three parallel dishes for determination of cell number. The cells were trypsinized and counted in an electronic cell counter.
Statistical analysis
All results are calculated as the average ± standard deviation of all individual dishes seeded from the same stock culture and undergoing the same exposure. The results were evaluated by analysis of variance (ANOVA) with the Tukey–Kramer procedure for multiple comparisons. Effects were considered significant when P < 0.05.
Results
Heat shock protein expression
Exposure design 1 (Fig. 1)
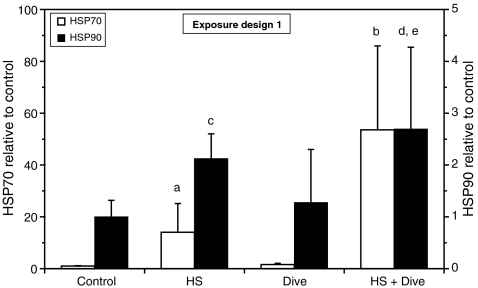
Heat shock (45°C, 1 h) increased HSP70 in HUVEC several fold (P < 0.05; data from five independent experiments) as shown on Fig. 3. A simulated dive for 24 h had no significant effect, while a dive performed after a heat shock had a potentiating effect on the HSP70 expression increasing the HSP70 concentration on average 54 times compared to control (P < 0.001; data from five independent experiments).
Fig. 3.
Expression of HSP70 and HSP90 in HUVEC after exposure to heat shock (HS) and simulated diving according to exposure design 1. The cells were harvested immediately after end of decompression and analyzed for immunodetectable HSP70. The results are shown as fold change relative to control. Data are from five separate experiments, and the results are given as the average ± SD of all the dishes within each treatment. HSP70: n = 19 (control, HS); n = 20 (dive); n = 12 (HS + dive). HSP90: n = 14 (control, HS); n = 9 (dive); n = 6 (HS + dive). a P < 0.05 vs. control, dive; b P < 0.001 vs. control, HS, dive; c P < 0.01 vs. control; d P < 0.001 vs. control; e P < 0.01 vs. dive
The HUVEC had a higher baseline level of HSP90 than HSP70 (on average eight times higher, data not shown), and heat shock increased this level only on average 2.1 times compared to control (P < 0.01; data from four independent experiments) as shown on Fig. 3. A simulated dive for 24 h had only minor, nonsignificant effects on the HSP90 level, but in contrast to HSP70, a simulated dive after a heat shock only slightly increased the HSP90 up to 2.7 times above the control level (P < 0.001; data from four independent experiments). The additional effect of a simulated dive after a heat shock compared to heat shock alone was, however, not significant.
Exposing the cells to a 2-h simulated dive at identical conditions according to exposure design 1 (Fig. 1) and harvesting immediately after end of decompression resulted in approximately similar effects on the HSP70 and HSP90 expression as the 24-h dive, suggesting that the observed effects was independent of duration of the dive (data not shown).
Exposure design 2 (Fig. 2)
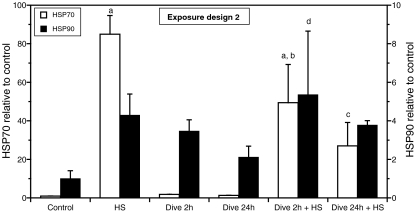
To investigate if the order of events was critical for the outcome of the exposures, the exposure order was reversed. Comparing the outcome from these two experimental designs might provide information about possible mechanisms behind the effects observed after exposure design 1. The cells were first exposed to a simulated dive and then subsequently to heat shock as indicated on Fig. 2. It should be emphasized that reversal of the exposure order also implies that the time elapsed between dive exposure and harvesting and between heat exposure and harvesting also was different compared to exposure design 1. Again, the results shown in Fig. 4 demonstrated that heat shock increased HSP70 many fold (P < 0.001), while a dive performed prior to heat shock decreased the HSP70 response (P < 0.05). Although not significant, the data suggested that this time the effect of the dive seemed to be at least partly dependent on the duration of the dive, since a dive for 24 h decreased the HSP70 response to heat shock more than a 2-h dive.
Fig. 4.
Expression of HSP70 and HSP90 in HUVEC after exposure to simulated diving and heat shock according to exposure design 2. The cells were harvested 24 h after heat shock and analyzed for immunodetectable HSP70 and HSP90. The results are shown as fold change relative to control. The results are given as the average ± SD of three separate dishes. a P < 0.001 vs. control; b P = 0.012 vs. HS; c P < 0.001 vs. HS; d P < 0.05 vs. control
In contrast to HSP70, the effects on the HSP90 level were much less pronounced. Although the data could indicate that all treatments increased the HSP90 level (Fig. 4), only the group exposed to a 2-h dive followed by a heat shock showed a significant increase compared to control when analyzed by ANOVA (P < 0.05). However, comparing all groups that were exposed to heat shock with all groups exposed to dive only, ANOVA indicated that heat shock significantly increased the HSP90 level compared to control (P < 0.01) but that the effect of dive was not significant.
Glutathione levels
Exposure design 1 (Fig. 1)
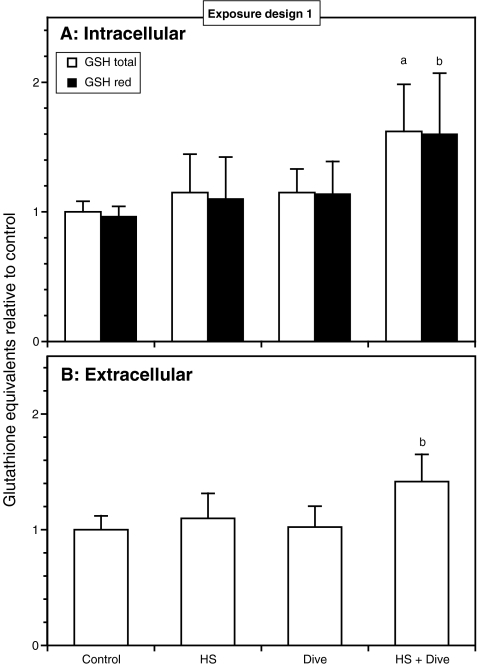
Neither heat shock nor a simulated dive had any significant effect on the intracellular glutathione level as shown in Fig. 5A. However, as for the HSPs, the combination of a heat shock followed by a simulated dive increased the total glutathione level significantly (P < 0.001; data from four independent experiments), although to a much lower extent (average 62%, four independent experiments). The extracellular total glutathione level (Fig. 5B) seemed to reflect the intracellular level showing a similar increase after heat shock and dive, although the increase after heat shock and simulated dive was slightly less pronounced yielding an average increase of 42% compared to control (P < 0.003; data from four independent experiments). The increase in intracellular glutathione was approximately the same for both reduced and total glutathione, and statistical analysis on data from four independent experiments showed that the different exposures had no effect on the glutathione red-ox status (Fig. 5A).
Fig. 5.
Effects of heat shock and simulated diving on intra- and extracellular glutathione level of HUVEC. The cells were exposed according to exposure design 1. The cells were harvested immediately after end of decompression and analyzed for reduced glutathione (GSH red) and total glutathione (GSH total). The results are expressed as equivalents of GSH relative to control. Data are from four separate experiments, and the results are given as the average ± SD of all the dishes within each treatment. A Intracellular glutathione content, n = 13. B Extracellular glutathione content determined as total glutathione (GSH total), n = 13 (control, HS); n = 10 (dive, HS + dive). a P < 0.001 vs. control, HS, dive; b P < 0.003 vs. control, HS, dive
Exposure design 2 (Fig. 2)
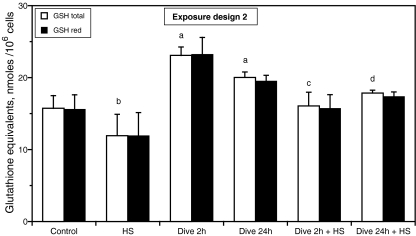
Reversal of the exposure order (ref. Fig. 2) demonstrated a 47% and a 27% increase in intracellular GSH levels after a 2-h dive and a 24-h dive, respectively (total glutathione, P < 0.001), while a heat shock decreased the glutathione levels approximately 24% (total glutathione, P = 0.003) as shown on Fig. 6. Moreover, a subsequent heat shock attenuated the GSH response to a dive (total glutathione, P < 0.001; ANOVA, Tukey–Kramer). Again, the ratio of reduced to total glutathione did not change indicating that neither a dive nor a heat shock had any effect on the red-ox status of glutathione (Fig. 6).
Fig. 6.
Effects of simulated diving and heat shock on intracellular glutathione level of HUVEC. The cells were exposed according to exposure design 2. The cells were harvested 24 h after heat shock and analyzed for reduced (GSH red) and total glutathione (GSH total). The results are expressed as equivalents of GSH per 106 cells and represent the average of three separate dishes ± SD. a P < 0.001 vs. control; b P = 0.003 vs. control; c P < 0.001 vs. dive 2 h; d P < 0.001 vs. dive 24 h
Discussion
The elevation of HSP70 after a heat shock is comparable to other investigations using endothelial cells (Bernardini et al. 2005; Harris et al. 2003). The present investigation is to our knowledge the first to demonstrate a potentiating effect of heat shock and a simulated dive on HSP expression and glutathione level. The largest increase in HSP70 and HSP90 after heat shock was noted after exposure design 2 (Figs. 3 and 4). According to the exposure design 2 (Fig. 2), the cells were harvested for HSP analysis 24 h after heat shock, while in exposure design 1 (Fig. 1), the cells were harvested 48 h after heat shock, suggesting that the HSP response declines from 24 to 48 h after exposure. A transient induction of HSP70 has been demonstrated in porcine aortic endothelial cells with a maximal level at 15 h after a heat shock. At 24 h, the level had declined but was still elevated (Bernardini et al. 2005).
Interestingly, the present study demonstrated similar elevation of HSP70 both after a 24-h dive (Fig. 3) and a 2-h dive following a heat shock (exposure design 1, data not shown). Since the decompression in both dives lasted only for approximately 5 min and the cells were harvested immediately afterward, it is tempting to suggest that the decompression itself is not the trigger of the HSP70 elevation, but rather the compression or elevated pressure per se. If so, 2 h is still a short time for protein expression and might suggest activation or posttranslational protein processing as a more plausible mechanism than gene expression and subsequent protein synthesis. A time course study of HSP70 induction after heat shock in human endothelial cells showed that after 30 min, detectable levels of HSP70 mRNA were observed increasing up to a peak level at 2 h after heat shock. Analysis of HSP70 immunodetectable protein showed a considerable delay with detectable levels appearing between 60 and 90 min and increasing for at least 6 h (Wagner et al. 1999). The immunodetection technique used may not differentiate between an inactive, preprocessed HSP70 and a fully active protein. The involvement of posttranslational mechanisms in regulation of heat shock proteins has been suggested by several authors and may include oxidation of cysteine residues (Kregel 2002; Winter and Jakob 2004). A possible mechanism for the results in the present paper might therefore be that the heat shock induces gene expression with a subsequent translation of the HSP70 mRNA, but only part of it is processed to biologically active proteins. A subsequent dive might then induce the posttranslational processing of the inactive HSP70 pool resulting in the high level of HSP70 observed.
As indicated above, we have previously demonstrated an association between protection against endothelial injury due to decompression stress and production of HSP70 (Medby et al. 2008). If the endothelial damage involves rapid biochemical reactions at the cellular surface, a protection might require the presence of defense systems at the time the decompression stress occurs. A mechanism that involves generation of defense molecules like HSPs prior to the decompression thus seems attractive, supporting the assumption that it is the compression or the pressure per se that induces the HSP70 response. A previous investigation on rabbits, however, may argue against such a mechanism. In these experiments, rabbits that had undergone rapid decompression followed by hyperbaric oxygen treatment were less susceptible to DCS following later decompression. Remarkably, a significant increased expression of HSP70 was noted only in the animals suffering from DCS indicating that presence of HSP70 was not sufficient to protect the animals against DCS (Su et al. 2004).
The results from exposure design 2 indicated that the order of events was critical for the potentiating effect of HSP70. In fact, exposure of the cells to a dive prior to heat shock seemed to decrease the heat shock induction of HSP70. This observation provides further information regarding possible mechanisms behind this effect and adds to the suggestion that a dive itself did not induce synthesis of HSP70 but might induce posttranslational processing of inactive molecules induced by heat shock. Another possibility might be that the dive (as in exposure design 1) inhibited degradation of HSP70 and thus prolonged the HSP70 elevation after a heat shock. In both cases, one might expect that a dive performed prior to a heat shock had no potentiating effect on the heat shock induction of HSP70, corresponding with the results observed.
It is interesting to note that a human study on a simulated saturation dive to 4.1 MPa (41 bar; corresponding to 400 msw) demonstrated a slight decrease in the HSP70 content of blood mononuclear cells during the dive and with an increase after decompression up to approximately 1.8 times above the predive level. However, the dive was performed in a He–O2 atmosphere with 42–50 kPa O2 (0.42–0.50 bar), and it is therefore difficult to differentiate the effect of compression/pressure and elevated oxygen (Matsuo et al. 2000).
HSP90 seemed to be regulated differently from HSP70 since a simulated dive had no significant potentiating effect on the HSP90 level; only heat shock resulted in a modest increase either with or without subsequent dive exposure (Fig. 3). Again, the reversed exposure order (exposure design 2) displayed a slightly different picture from exposure design 1, but the difference may be attributed to the difference in time between exposure and harvesting as noted for HSP70. The results thus add to those from exposure design 1 demonstrating significant effects of heat shock but not of simulated diving. The effect of heat shock is comparable to that reported from a study demonstrating a 2.5 times increase in HSP90 levels in aorta of rats 24 h after a heat shock to 42°C, while similar experiments on bovine endothelial cells exposed to 45°C for 1 h failed to demonstrate significant increases in HSP90 (Harris et al. 2003). In contrast, a recent study on rats could not demonstrate any effects on HSP90 in aorta and left ventricle after a dive to 700 kPa (7 bar) with or without a prior heat shock to 42°C (Medby et al. 2008). It should also be noted that the rather small effect on HSP90 compared to HSP70 in the present study might be due to the fact that the basal level of HSP90 was eight times higher (on average) than the HSP70 level and that changes similar to HSP70 on a molecular scale would be less noticeable as a fold change.
The present results did not show significant effects on GSH after a simulated dive alone (Fig. 5, exposure design 1). This is in accordance with previous studies by us exposing both mouse cells and human lung cells for simulated diving corresponding to 490 msw (5 MPa; 50 bar) for 24 h in a He/O2 atmosphere containing 20 kPa O2 (0.20 bar) with no effects on intracellular GSH. However, when the pO2 was raised to 40 kPa (0.40 bar), there was a 40–60% increase in GSH (Djurhuus et al. 1998, 1999, 2006). One might speculate if the elevated oxygen triggers the GSH system in a similar way as a prior heat shock does in the present study. This argument fails, however, since the referred studies also showed that 40 kPa (0.40 bar) oxygen alone inferred a similar increase in GSH.
A human study performed on divers after a simulated dive to 250 msw (2.6 MPa; 26 bar) showed a decrease in GSH content in peripheral blood cells isolated immediately after end of decompression (Djurhuus et al. 2006). However, these results are difficult to compare with the present results since the former were obtained after the divers had been exposed to high pressure and 35–70 kPa (0.35–0.70 bar) O2 for 7–8 days.
In contrast to the GSH increase that was observed only after a heat shock followed by a dive (exposure design 1), exposing the cells in a reverse order (exposure design 2) demonstrated clearly different results with an increase in GSH after a simulated dive alone. The reason for this controversy most probably is that the previous design determined the GSH immediately after decompression, while the design 2 allowed for expression of altered GSH level for 48 h after decompression. Notably, a heat shock following the dive seemed to reduce the GSH increase (Fig. 6), concordant with the effect of heat shock on HSP70 (Fig. 4). These results may add to recent investigations indicating a marked decrease in the expression of HSP70 in mice depleted of glutathione (Park et al. 2007). Overexpression of HSP70 in canine kidney cells has recently been shown to increase the cellular glutathione red-ox status, presumably by increasing the activities of glutathione peroxidase and glutathione reductase (Guo et al. 2007). However, several other investigations have shown conflicting results, and oxidative stress reduced the intracellular levels in bovine endothelial cells with a concordant increase in HSP70 gene expression (Aucoin et al. 1995), and a protein involved in Parkinson's disease have been shown to upregulate both GSH and HSP70 but by two independent mechanisms (Zhou and Freed 2005).
The present investigations also clearly showed that neither heat shock nor dive did change the ratio of reduced to total glutathione, indicating that even if there was a relation between GSH level/synthesis and HSP70 expression, the latter did not modulate the red-ox status of GSH.
Recent studies have indicated significant correlations between HSP90 and expression of NOS (Sud et al. 2007), and interestingly, a synergistic induction of NOS expression by HSP70 and HSP90 has recently been demonstrated in human endothelial cells (Uchiyama et al. 2007). A further step in the current investigations is to determine the effect of simulated diving on NOS, the enzyme responsible for the production of NO, and the direct relation to the HSP70, HSP90, and glutathione. An important task for future studies will be an experimental design that allow for separation of the effects of different elements of a dive-like compression, pressure per se, oxygen level, and decompression.
Conclusion
In conclusion, the present results demonstrated that a simulated dive had notable effects on cellular defense mechanisms in human endothelial cells after a prior heat shock, conditions that have previously been shown to confer protection against endothelial damage due to decompression sickness. In particular, the study demonstrated for the first time that a simulated dive had a potentiating effect on the heat-induced expression of HSP70, which might involve posttranslational mechanisms. Notably, exposure to a dive prior to heat shock did not potentiate the heat-induced expression of HSP70 indicating that the order of events was critical and thus supporting the suggestion that the potentiating effect of dive was not due to de novo synthesis of HSP70. Moreover, the results suggested that the effects on HSP70 were due to compression or the pressure exposure per se, rather than the decompression. The HSP70 induction after a heat shock and subsequent dive was followed by an increase in intracellular glutathione level, while a dive did not seem to have significant effects on the HSP90 level. More detailed studies of the relationship between the heat shock proteins, NOS, and glutathione are currently in progress.
Acknowledgment
The authors would like to express their appreciation to Mrs. Torill Sage and Mr. Harald A. Sundland, both NUI AS, for excellent technical assistance in performing the cell culture work and exposure in pressure chambers. The authors are grateful for the careful analysis of glutathione performed by Mrs. Torunn Eide at Institute of Medicine, University of Bergen. This work was supported by StatoilHydro (formerly Statoil and Norsk Hydro) and Exxon Mobil, Norway.
References
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- Aucoin MM, Barhoumi R, Kochevar DT, Granger HJ, Burghardt RC. Oxidative injury of coronary venular endothelial cells depletes intracellular glutathione and induces HSP 70 mRNA. Am J Physiol. 1995;268:H1651–H1658. doi: 10.1152/ajpheart.1995.268.4.H1651. [DOI] [PubMed] [Google Scholar]
- Bernardini C, Zannoni A, Turba ME, Fantinati P, Tamanini C, Bacci ML, Forni M. Heat shock protein 70, heat shock protein 32, and vascular endothelial growth factor production and their effects on lipopolysaccharide-induced apoptosis in porcine aortic endothelial cells. Cell Stress Chaperones. 2005;10:340–348. doi: 10.1379/CSC-98R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubakk AO, Eftedal OS, Wisløff U. Endothelium and diving. In: Aird WC, editor. Endothelial biomedicine. Cambridge: Cambridge University Press; 2007. pp. 497–505. [Google Scholar]
- Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem. 2008;283:27038–27047. doi: 10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Adachi T. Nitric-oxide-induced vasodilatation: regulation by physiologic s-glutathiolation and pathologic oxidation of the sarcoplasmic endoplasmic reticulum calcium ATPase. Trends Cardiovasc Med. 2006;16:109–114. doi: 10.1016/j.tcm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5, 6, 7, 8-tetrahydrobiopterin to 7, 8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol. 2008;294:H1530–H1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: Insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- Djurhuus R, Svardal AM, Mansoor MA, Ueland PM. Modulation of glutathione content and the effect on methionine auxotrophy and cellular distribution of homocysteine and cysteine in mouse cell lines. Carcinogenesis. 1991;12:241–247. doi: 10.1093/carcin/12.2.241. [DOI] [PubMed] [Google Scholar]
- Djurhuus R, Svardal AM, Thorsen E. Toxicity of hyperoxia and high pressure on C3H/10T1/2 cells and effects on cellular glutathione. Undersea Hyperb Med. 1998;25:33–41. [PubMed] [Google Scholar]
- Djurhuus R, Svardal AM, Thorsen E. Glutathione in the cellular defense of human lung cells exposed to hyperoxia and high pressure. Undersea Hyperb Med. 1999;26:75–85. [PubMed] [Google Scholar]
- Djurhuus R, Segadal K, Svardal AM. Glutathione in blood cells decreases without DNA breaks after a simulated saturation dive to 250 msw. Aviat Space Environ Med. 2006;77:597–604. [PubMed] [Google Scholar]
- Förstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem. 2006;387:1521–1533. doi: 10.1515/BC.2006.190. [DOI] [PubMed] [Google Scholar]
- Guo S, Wharton W, Moseley P, Shi H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones. 2007;12:245–254. doi: 10.1379/CSC-265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MB, Blackstone MA, Ju H, Venema VJ, Venema RC. Heat-induced increases in endothelial NO synthase expression and activity and endothelial NO release. Am J Physiol Heart Circ Physiol. 2003;285:H333–H340. doi: 10.1152/ajpheart.00726.2002. [DOI] [PubMed] [Google Scholar]
- Huang A, Xiao H, Samii JM, Vita JA, Keaney JF., Jr Contrasting effects of thiol-modulating agents on endothelial NO bioactivity. Am J Physiol Cell Physiol. 2001;281:C719–C725. doi: 10.1152/ajpcell.2001.281.2.C719. [DOI] [PubMed] [Google Scholar]
- Huang KL, Wu CP, Chen YL, Kang BH, Lin YC. Heat stress attenuates air bubble-induced acute lung injury: a novel mechanism of diving acclimatization. J Appl Physiol. 2003;94:1485–1490. doi: 10.1063/1.1586981. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Boesgaard S, Trautner S, Rubin I, Poulsen HE, Aldershvile J. Endothelium-dependent vasorelaxation in inhibited by in vivo depletion of vascular thiol levels: role of endothelial nitric oxide synthase. Free Radic Res. 2001;35:387–394. doi: 10.1080/10715760100300901. [DOI] [PubMed] [Google Scholar]
- Mansoor MA, Svardal AM, Ueland PM. Determination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine, and glutathione in human plasma. Anal Biochem. 1992;200:218–229. doi: 10.1016/0003-2697(92)90456-H. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Shinomiya N, Suzuki S. Hyperbaric stress during saturation diving induces lymphocyte subset changes and heat shock protein expression. Undersea Hyperb Med. 2000;27:37–41. [PubMed] [Google Scholar]
- Medby C, Bye A, Wisløff U, Brubakk AO. Heat shock increases survival in rats exposed to hyperbaric pressure. Diving Hyperbar Med. 2008;38:189–193. [PubMed] [Google Scholar]
- Montcalm-Smith E, Caviness J, Chen Y, McCarron RM. Stress biomarkers in a rat model of decompression sickness. Aviat Space Environ Med. 2007;78:87–93. [PubMed] [Google Scholar]
- Nishida T, Matsura T, Nakada J, et al. Geranylgeranylacetone protects against acetaminophen-induced hepatotoxicity by inducing heat shock protein 70. Toxicology. 2006;219:187–196. doi: 10.1016/j.tox.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Park JW, Moon C, Yun S, Kim SY, Bae YC, Chun MH, Moon JI. Differential expression of heat shock protein mRNAs under in vivo glutathione depletion in the mouse retina. Neurosci Lett. 2007;413:260–264. doi: 10.1016/j.neulet.2006.11.052. [DOI] [PubMed] [Google Scholar]
- Smith AR, Visioli F, Hagen TM. Vitamin C matters: increased oxidative stress in cultured human aortic endothelial cells without supplemental ascorbic acid. FASEB J. 2002;16:1102–1104. doi: 10.1096/fj.01-0825fje. [DOI] [PubMed] [Google Scholar]
- Su CL, Wu CP, Chen SY, Kang BH, Huang KL, Lin YC. Acclimatization to neurological decompression sickness in rabbits. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1214–R1218. doi: 10.1152/ajpregu.00260.2004. [DOI] [PubMed] [Google Scholar]
- Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1444–L1453. doi: 10.1152/ajplung.00175.2007. [DOI] [PubMed] [Google Scholar]
- Svardal AM, Mansoor MA, Ueland PM. Determination of reduced, oxidized, and protein-bound glutathione in human plasma with precolumn derivatization with monobromobimane and liquid chromatography. Anal Biochem. 1990;184:338–346. doi: 10.1016/0003-2697(90)90691-2. [DOI] [PubMed] [Google Scholar]
- Tolson JK, Dix DJ, Voellmy RW, Roberts SM. Increased hepatotoxicity of acetaminophen in Hsp70i knockout mice. Toxicol Appl Pharmacol. 2006;210:157–162. doi: 10.1016/j.taap.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Uchiyama T, Atsuta H, Utsugi T, et al. HSF1 and constitutively active HSF1 improve vascular endothelial function (heat shock proteins improve vascular endothelial function) Atherosclerosis. 2007;190:321–329. doi: 10.1016/j.atherosclerosis.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Wagner M, Hermanns I, Bittinger F, Kirkpatrick CJ. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am J Physiol. 1999;277:L1026–L1033. doi: 10.1152/ajplung.1999.277.5.L1026. [DOI] [PubMed] [Google Scholar]
- Werner ER, Gorren AC, Heller R, Werner-Felmayer G, Mayer B. Tetrahydrobiopterin and nitric oxide: mechanistic and pharmacological aspects. Exp Biol Med (Maywood) 2003;228:1291–1302. doi: 10.1177/153537020322801108. [DOI] [PubMed] [Google Scholar]
- West MB, Hill BG, Xuan YT, Bhatnagar A. Protein glutathiolation by nitric oxide: an intracellular mechanism regulating redox protein modification. FASEB J. 2006;20:1715–1717. doi: 10.1096/fj.06-5843fje. [DOI] [PubMed] [Google Scholar]
- Winter J, Jakob U. Beyond transcription—new mechanisms for the regulation of molecular chaperones. Crit Rev Biochem Mol Biol. 2004;39:297–317. doi: 10.1080/10409230490900658. [DOI] [PubMed] [Google Scholar]
- Wisløff U, Richardson RS, Brubakk AO. NOS inhibition increases bubble formation and reduces survival in sedentary but not exercised rats. J Physiol. 2003;546:577–582. doi: 10.1113/jphysiol.2002.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisløff U, Richardson RS, Brubakk AO. Exercise and nitric oxide prevent bubble formation: a novel approach to the prevention of decompression sickness? J Physiol. 2004;555:825–829. doi: 10.1113/jphysiol.2003.055467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]