Abstract
Heat shock protein 27 (Hsp27) is over-expressed when cells are exposed to stressful conditions that include oxidative stress. Oxidative stress has been implicated in the pathogenesis of cardiovascular disease (CVD), diabetes and insulin resistance. We have investigated the concentrations of serum Hsp27 antigen and antibodies in subjects from different glycaemic categories, who either did or did not have established CVD. Serum Hsp27 antigen and antibody levels (immunoglobulins M and G (IgM and IgG)) were determined by enzyme-linked immunosorbent assays (ELISAs) in 68 individuals: 26 with normal glucose tolerance (NGT), 10 with (+) and 16 without (−) a history of CVD and 42 individuals with varying degrees of glucose intolerance (GI; 21 with and 21 without a history of CVD). Insulin sensitivity was determined in each subject using indices derived from the homeostasis model assessment of sensitivity and the insulin sensitivity index for glycaemia. Serum Hsp27 concentrations were significantly higher in GI (+CVD) subjects compared to GI (−CVD) subjects (p = 0.03), NGT (−CVD) subjects (p = 0.02) and NGT (+CVD) subjects (p = 0.04) and were positively correlated to fasting plasma glucose for all subjects (r = 0.28, p = 0.03). IgM antibody levels were significantly higher in GI (+CVD) subjects compared to NGT (−CVD) group (p = 0.02) and were inversely related to fasting insulin concentrations (r = −0.27, p = 0.04) and the 2-h insulin concentrations (r = −0.29, p = 0.03) for all subjects. Serum IgG antibody levels were higher in GI (+CVD) group compared to GI (−CVD) group (p = 0.06). In conclusion, Hsp27 and its antibody concentrations appear to relate to the presence of cardiovascular complications in patients with GI.
Keywords: Hsp27, Antibodies, Glucose tolerance, Cardiovascular disease
Introduction
Insulin resistance is characterized by an inadequate response of insulin sensitive tissues (liver, adipose tissue and skeletal muscle) to circulating insulin, resulting in an impaired handling of a glucose load. Initially insulin resistance is compensated for by increased insulin secretion allowing for the preservation of a normal glucose tolerance (Ceriello and Motz 2004; Kahn 2003). Deterioration to impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) occurs when insulin resistance increases further and/or the compensatory insulin secretory response decreases (Carnevale Schianca et al. 2003; Ceriello and Motz 2004). These latter conditions are associated with a substantially increased risk of type 2 diabetes mellitus (Unwin et al. 2002). The prevalence of insulin resistance is increasing globally and is a consistent feature of metabolic syndrome (Zimmet et al. 2005) and obesity (Kahn and Flier 2000). Furthermore metabolic syndrome and glucose intolerance are both important risk factors for cardiovascular disease (Kannel and McGee 1979; Lakka et al. 2002; Tominaga et al. 1999).
It has been proposed that oxidative stress is one pathogenic factor underlying the onset and progression of insulin resistance and diabetes and consequently on vascular complications (Brownlee 2005; Ceriello and Motz 2004; Evans et al. 2003). Following exposure to one of a number of environmental stressors, that include heat and oxidative stress, cells over-express a group of highly conserved proteins known as the heat shock proteins (Hsps). Whilst many of these proteins function as molecular chaperones, facilitating the correct folding of nascent peptides and the refolding of denatured or mis-folded proteins (Georgopoulos and Welch 1993), they may also have other vital roles. Furthermore, insulin resistance and type 2 diabetes are associated with impaired expression of heat shock proteins by insulin sensitive tissues and thus may leave them vulnerable to oxidative damage (Hooper and Hooper 2009). Hsp27 is a member of the small HSP family that is over-expressed when cells are exposed to oxidative stress (Mehlen et al. 1995). Potential mechanisms by which Hsp27 may enable cells to adapt to exposure to oxidative stress include the upregulation of glucose-6-phosphate dehydrogenase and glutathione peroxidase and by decreasing intracellular levels of iron (Preville et al. 1999; Arrigo et al. 2005).
In vitro studies have shown that Hsps are released from cells exposed to stress (Child et al. 1995; Liao et al. 2000), which would explain their presence in serum in vivo and why they may stimulate an autoimmune response (Xu 2002). Studies have reported that antibody titres to some Hsps, such as Hsp60, are related to circulating antigen concentrations (Xu et al. 2000) and that there are elevated concentrations of autoantibodies to Hsps in patients with atherosclerosis (Xu et al. 1993), and furthermore, elevated concentrations of serum Hsp60 are associated with higher risk of coronary heart disease (Zhang et al. 2008).
It has been proposed that oxidative stress associated with hyperglycaemia may be involved in the vascular complications of type 1 diabetes (Evans et al. 2003), and in a recent study, Hsp27 antigen concentrations were found to be independently associated with the presence of distal symmetrical polyneuropathy in these patients (Gruden et al. 2008), though Hsp27 antibody levels did not correlate with the presence of the antigens in the same group of patients (Burt et al. 2009).
We hypothesized that serum Hsp27 concentrations may be a marker of macrovascular complications in patients with insulin resistance and that Hsp27 antibody levels may reflect the presence of circulating antigen. The aim of this present study was to investigate the relationship between the levels of serum Hsp27 and its antibody levels [immunoglobulins M and G (IgM and IgG)] in individuals from different glycaemic categories either with or without a concomitant history of CVD.
Materials and methods
Subjects
Subjects from different glycaemic categories were included in this study. The criteria for inclusion were age 20–65 years old and body mass index (BMI) <35 kg/m2; diabetic subjects were either newly diagnosed or achieving good glycaemic control by dietary means alone. Exclusion criteria included a known history of other chronic diseases such as cancer or treatment with insulin, oral hypoglycaemic agents, thyroxine or other medication that might modify insulin sensitivity, such as steroids. A history of cardiovascular disease (CVD) was defined as the presence of established coronary artery disease, stroke or peripheral artery disease as determined from their medical notes. Glycaemic status was determined by a standard oral glucose tolerance test using WHO criteria (Alberti and Zimmet 1998). The study population consisted of 16 subjects who had a normal glucose tolerance without CVD [NGT (−CVD)], 10 with normal glucose tolerance and CVD [NGT (+CVD)], 21 with glucose intolerance without CVD, [GI (−CVD)], and 21 with glucose intolerance and a history of CVD [GI (+CVD)]. Subjects with glucose intolerance were pooled from impaired fasting glucose, impaired glucose tolerance and type 2 diabetes.
The study was approved by the South West Surrey Local Research Ethics Committee (Guildford, UK). All patients signed a consent form prior to participation.
Blood sampling protocol
Subjects attended for a fasting blood sample after which 75 g glucose was given orally. They rested until the next blood sample 2 h later. Specimens were centrifuged promptly, and samples for routine analysis (glucose and HbA1c) were sent to the pathology laboratory. Samples for other assays were stored at −80°C until analysis.
Routine blood analyses, plasma insulin and insulin sensitivity determination
Glucose was assayed by the hexokinase method using a Bayer Advia 1650 analyser, while glycated haemoglobin, HbA1c, was analysed by high-pressure liquid chromatography technique on a Biorad Variant II instrument. Plasma insulin concentrations were determined using a solid phase two-site enzyme immunoassay kit (Mercodia). The assay had a sensitivity of 7.0 pmol/l and a maximum analytical CV of 4.9%. Insulin sensitivity was assessed using indices derived from the homeostasis model assessment of sensitivity (HOMA-S), which calculates insulin sensitivity using fasting glucose (mU/l) and insulin concentrations (mmol/l) using the formula 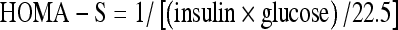 and the insulin sensitivity index for glycaemia (ISI-gly), which calculates the sum of fasting and 2-h insulin (insulinp) in μU/ml and fasting and 2-h glucose (glucosep) in units of mg/dl using the formula
and the insulin sensitivity index for glycaemia (ISI-gly), which calculates the sum of fasting and 2-h insulin (insulinp) in μU/ml and fasting and 2-h glucose (glucosep) in units of mg/dl using the formula 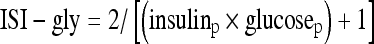 . HOMA-S reflects hepatic insulin sensitivity since only fasting values are used in the calculation, while ISI-gly provides an estimate of whole-body insulin sensitivity since the 2-h component, additionally, gives information on peripheral insulin resistance (Borai et al. 2007).
. HOMA-S reflects hepatic insulin sensitivity since only fasting values are used in the calculation, while ISI-gly provides an estimate of whole-body insulin sensitivity since the 2-h component, additionally, gives information on peripheral insulin resistance (Borai et al. 2007).
Determination of serum Hsp27 antigen
Serum Hsp27 antigen concentrations were determined using a sandwich ELISA developed in-house. One hundred microlitres of a 2.5 μg/ml solution of monoclonal Hsp27 antibody (SPA-800, Stressgen Bioreagents) in PBS was used to coat a 96-well microtitre plate. After an overnight incubation, the plate was washed three times with 0.05% Tween-20 in PBS and then blocked with 4% goat serum for 1.5 h. Fifty microlitres of standards, 0.94, 1.875, 3.75, 7.5 and 15 ng/ml of recombinant Hsp27 (SPP-715, Stressgen Bioreagents), and 1:3 diluted samples were then added into duplicate wells. After 30 min and washing, 50 μl of 1:6,000 dilution of rabbit anti-human Hsp27 polyclonal antibody (SPA-803, Stressgen Bioreagents) was added. After 30 min incubation and three cycles of washing, 50 μl of 1:6,000 dilution of goat anti-rabbit horseradish peroxidase-conjugated antibody (A 0545, Sigma) was then added, and incubation was done for 30 min. After a final wash of four cycles, 50 μl of the substrate solution tetramethylbenzidine dihydrochloride (TMB) was added. The reaction was stopped after 20 min with 2 M HCl and the absorbance was read at 450 nm. The sensitivity of the assay was 0.94 ng/ml, and the inter- and intra-assay coefficients of variation were 3.7% and 5.8%, respectively.
Determination of serum IgM and IgG levels of Hsp27
IgG and IgM levels were determined by indirect ELISAs developed in-house. Fifty microlitres of 300 ng recombinant Hsp27 (SPP-715, Stressgen Bioreagents) in carbonate coating buffer pH 9.6 was coated onto a microtitre plate and left overnight. The plate was then blocked with 200 μl of 3% bovine serum albumin in PBS at 37°C for 1.5 h at room temperature. The plate was washed three times and 50 μl of samples diluted 1:100 in blocking buffer was added and incubated for 30 min. After three wash cycles, 50 μl of anti-human IgG (A6029, Sigma) or anti-human IgM (A6907, Sigma) diluted 1:2,000 was then added for the determination of IgG and IgM, respectively, and incubation done for 1 h. After a final wash of four cycles, 50 μl of TMB substrate solution was then added and incubation was done for 15 min. Reaction was stopped with 50 μl of 2 M HCl and absorbance was read at 450 nm. The sensitivity of the assay was 0.007 absorbance units for both IgM and IgG. The within assay coefficients of variation were 4.3% for the IgM and 4.7% for the IgG assay, respectively, and the between assay coefficients of variation were 7.7% for IgM and 6.1% for IgG.
Statistical analysis
Uni- and bivariate statistical analysis was undertaken using Graphpad Prism version 5 for Windows (Graphpad Software Inc, USA), while multivariate analysis was done using SPSS software version 16 (SPSS Inc, Chicago, IL, USA). Comparison between groups for skewed data was performed using the Mann–Whitney test, and data were reported as median and inter-quartile range about the median, except for tabulated clinical and biochemical data, which uses mean and standard deviation throughout. Comparison between groups for normally distributed data was analysed using the t test, and data were reported as mean with standard deviation. Fisher’s exact and the Chi-square tests were used to compare categorical variables. Spearman’s rank correlation analysis was used to investigate the relationship between variables and stepwise multiple linear regression analysis used to investigate the influence of variables on Hsp27 concentration. Results were considered significant with p < 0.05.
Results
Demographic and clinical characteristics of the study groups
The groups were matched for all variables except for age and fasting plasma glucose (FPG; Table 1). Glucose intolerance groups comprised of individuals with isolated IFG/IGT, combined IFG with IGT and type 2 diabetes mellitus. There was no significant difference in the distribution of the different categories of glucose intolerance between the –CVD and +CVD groups. For most of the clinical characteristics, there was no significant difference between the –CVD and +CVD groups for both NGT and GI subjects, except for weight and age (Table 1). The mean BMI for both NGT groups was in the overweight category, while the mean BMI for the glucose-intolerant groups was just above the cutoff value for overweight in the obese category (WHO 2004).
Table 1.
Demographic, clinical and biochemical characteristics of study subjects
| NGT | GI | p values | ||||||
|---|---|---|---|---|---|---|---|---|
| (i) −CVD | (ii) +CVD | (iii) −CVD | (iv) +CVD | (i) vs (ii) | (i) vs (iv) | (ii) vs (iv) | (iii) vs (iv) | |
| n | 16 | 10 | 21 | 21 | ||||
| Age (years) | 36.3 (13.3) | 58.2 (5.7) | 47.8 (9.7) | 57.0 (6.5) | <0.001 | <0.001 | 0.62 | <0.001 |
| Weight (kg) | 72.5 (14.9) | 85.9 (16.2) | 83.8 (10.5) | 89.6 (14.8) | 0.04 | 0.001 | 0.53 | 0.15 |
| Body mass index (kg/m2) | 25.1 (4.1) | 28.0 (4.6) | 30.7 (3.6) | 30.5 (3.9) | 0.11 | <0.001 | 0.12 | 0.82 |
| Systolic blood pressure (mmHg) | 115 (10) | 119 (20) | 132 (16) | 130 (16) | 0.48 | 0.002 | 0.11 | 0.68 |
| Fasting plasma glucose (mmol/l) | 5.2 (0.4) | 5.5 (0.7) | 6.7 (1.6) | 6.5 (1.2) | 0.21 | <0.001 | 0.004 | 0.74 |
| 2-h plasma glucose (mmol/l) | 5.5 (1.1) | 5.5 (1.6) | 10.1 (3.8) | 10.4 (3.0) | 0.92 | <0.001 | <0.001 | 0.78 |
| HbA1c (%) | 5.3 (0.4) | 5.7 (0.5) | 6.5 (1.3) | 6.0 (0.7) | 0.01 | 0.001 | 0.05 | 0.14 |
| Fasting plasma insulin (pmol/l) | 70 (33) | 120 (53) | 99 (39) | 142 (57) | 0.01 | <0.001 | 0.42 | 0.009 |
| 2-h plasma insulin (pmol/l) | 284 (200) | 352 (239) | 601 (227) | 711 (182) | 0.69 | <0.001 | 0.002 | 0.09 |
| HOMA-S | 0.54 (0.36) | 0.25 (0.10) | 0.28 (0.17) | 0.26 (0.32) | 0.02 | <0.001 | 0.07 | 0.01 |
| ISI-Gly | 1.00 (0.32) | 0.88 (0.35) | 0.68 (0.22) | 0.56 (0.20) | 0.47 | <0.001 | 0.04 | 0.03 |
Data are presented as mean (SD)
CVD cardiovascular disease, HOMA-S homeostasis model assessment of sensitivity, ISI-gly insulin sensitivity index for glycaemia
Subjects in the control group, with neither CVD nor glucose intolerance, NGT (−CVD), differed significantly from the group which has both CVD and glucose intolerant, GI (+CVD), for weight, BMI, systolic BP, HbA1c and measures of insulin resistance (p ≤ 0.002 for all).
Glucose, HbA1c, insulin levels and indices of insulin sensitivity
There was no significant difference for mean FPG, 2-h post 75 g glucose (2hPG) and the 2-h serum insulin levels between the groups with and those without CVD (Table 1). In contrast, the fasting plasma insulin (FPI) was significantly different between the groups with and without CVD for subjects with NGT and GI. Though the mean HbA1c values between the glucose-intolerant groups with and without CVD did not differ significantly, the mean HbA1c value for the NGT (+CVD) group was significantly higher than the NGT (−CVD) group (p = 0.01) and was within the range that identifies individuals at increased risk of developing CVD (Jesudason et al. 2003). For the glucose-intolerant groups, the mean FPI was significantly higher and the insulin sensitivity indices significantly lower for the +CVD group than the –CVD group. Among the groups, the ISI-gly insulin sensitivity index was lowest for the glucose-intolerant group with CVD and highest for the control group, NGT (−CVD).
Serum Hsp27 antigen and IgM and IgG levels
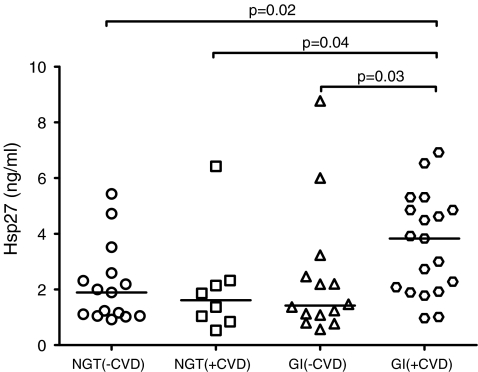
Glucose-intolerant subjects with CVD had the highest level of serum Hsp27 antigen concentrations, significantly higher than the GI (−CVD) group [3.83 (1.92–4.85) vs 1.42 (1.01–2.65), p = 0.03], the NGT (−CVD) group [3.59 (1.80) vs 2.15 (1.41), p = 0.02] and the NGT (+CVD) group [3.83 (1.92–4.85) vs 1.62 (0.90–2.28), p = 0.04] (Fig. 1).
Fig. 1.
Scatter plot of the distribution of serum Hsp27 antigen concentrations in individuals with normal glucose tolerance (NGT) and glucose intolerance (GI) with and without established cardiovascular disease (CVD). Horizontal lines represent the median of the distribution
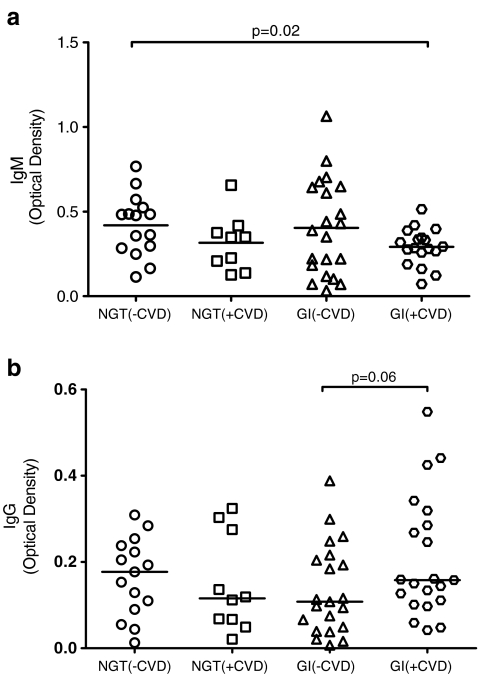
There was a trend to a lower serum Hsp27 IgM antibody concentration for both CVD groups compared to corresponding groups without CVD, though statistical significance was not attained. Statistical significance, however, was reached when comparing the GI (+CVD), with the NGT (−CVD) group [0.292 (0.109) vs 0.419 (0.181), p = 0.02] (Fig. 2a).
Fig. 2.
Scatter plots of the distribution of serum (a) IgM and (b) IgG in individuals with normal glucose tolerance (NGT) and glucose intolerance (GI) with and without established cardiovascular disease (CVD). Horizontal lines represent the median for the distribution
No obvious trend or statistical significance was found with regards to serum Hsp27 IgG antibody levels among the groups. Only borderline significance was achieved in the GI group, with GI (+CVD) subjects having higher antibody levels compared to those in the GI (−CVD) group [0.158 (0.106-0.302) vs 0.108 (0.044-0.210), p = 0.06] (Fig. 2b).
Univariate analysis
Correlation analysis was undertaken between serum Hsp27 antigen with its antibody titres (IgM and IgG) and other biochemical variables and the results reported in Table 2. It was found that Hsp27 was positively associated with the FPG values for the groups combined (r = 0.28, p = 0.03). In the CVD groups, serum Hsp27 concentrations were positively correlated with the 2hPG values (r = 0.42, p = 0.04). For the groups combined, the IgM concentrations were inversely related to fasting insulin concentrations (r = −0.30, p = 0.02) and the 2-h insulin level (r = −0.29, p = 0.03). The IgM level was also associated with the insulin sensitivity indices HOMA-S (r = 0.28, p = 0.03) and ISI-gly (r = 0.27, p = 0.04), but these associations were lost when fasting insulin was the control variable in a partial correlation analysis. For the CVD groups, the IgG level was positively associated with the FPG values (r = 0.37, p = 0.04). Though CVD subjects were significantly older than their non-CVD counterparts, there was no correlation between age and Hsp27, its IgM and IgG antibodies. Neither was there a relationship between BMI and Hsp27 or its IgM and IgG antibodies.
Table 2.
Summary of Spearman’s rank correlation analysis between Hsp27, IgM, IgG and associated variables
| All groups combined | CVD groups only | |||
|---|---|---|---|---|
| Correlation coefficient | p value | Correlation coefficient | p value | |
| Hsp27 antigen | ||||
| FPG | 0.28 | 0.03 | ||
| 2hPG | 0.42 | 0.04 | ||
| Hsp27 IgM | ||||
| Fasting insulin | −0.30 | 0.02 | ||
| 2-h insulin | −0.29 | 0.03 | ||
| HOMA-S | 0.28 | 0.03 | ||
| ISI-Gly | 0.27 | 0.04 | ||
| Hsp27 IgG | ||||
| FPG | 0.37 | 0.04 | ||
Multivariate analysis
For multivariate analysis all variables were included in the analysis. Categorical variables such as gender, presence of CVD and glucose intolerance were coded dichotomously. Using a stepwise method, for all groups combined, it was found that the association between FPG and Hsp27 concentrations remained the only significant relationship among the variables entered into the analysis (Table 3). FPG was found to explain 8.1% of the variation in Hsp27 concentration. Mean FPG values for NGT and GI non-CVD and +CVD groups were not significantly different, and therefore it is unlikely that FPG is a confounding factor in the comparison of means analysis between both groups. After adjusting for the FPG values, the GI (+CVD) group Hsp27 concentration remained significantly higher than that of the NGT (−CVD) group (p = 0.02) and the NGT (+CVD) group (p = 0.04).
Table 3.
Fasting plasma glucose as a predictor of Hsp27 concentration from stepwise multiple linear regression analysis
| Model | B | Standard error of B | Βeta | p |
|---|---|---|---|---|
| Constant | 0.095 | 1.270 | ||
| Fasting plasma glucose | 0.423 | 0.203 | 0.285 | 0.04 |
Discussion
In this study we proposed that Hsp27 concentrations may also be associated with the presence of macrovascular complications in patients with insulin resistance, and we wished to investigate the relationship between the levels of serum Hsp27 and its antibody levels in individuals with different degrees of insulin resistance either with or without a concomitant history of CVD. We found that serum Hsp27 antigen concentrations were significantly higher, and Hsp27 IgM antibody titres were lower in individuals with a combination of glucose intolerance and CVD, compared to subjects with either condition separately or who had neither condition. Whilst it is possible that some individuals with glucose intolerance had subclinical cardiovascular disease, these data suggest that Hsp27 antigen concentrations are increased in individuals whose glucose intolerance is associated with an increased risk of CVD. However, it is not possible to be certain whether Hsp27 and its antibody concentrations are markers of disease or whether they are aetiological factors in atherogenesis perhaps through the formation of immune complexes or by other mechanisms as reviewed recently (Bielecka-Dabrowa et al. 2009).
The positive association of serum Hsp27 with FPG suggests that hyperglycaemia may be related to an increased cellular expression of Hsp27 leading to the higher serum concentration. Insulin has been shown to increase the expression of heat shock proteins or Hsp27 phosphorylation (Franklin et al. 2008; Li et al. 2008); however we did not find a significant relationship between serum insulin and Hsp27 concentrations in this study, and therefore our data do not indicate that insulin is a major determinant of serum Hsp27 in these groups of patients, although it may be a determinant of tissue levels of Hsp27. Hsp27 is constitutively expressed in many normal adult human tissues and different cell types including endothelial cells of the vasculature (Ciocca et al. 1993; Portig et al. 1996). In endothelial cells, hyperglycaemia leads to increased intracellular glucose concentration since the glucose transport into these cells takes place by insulin-independent facilitated diffusion (Mueckler 1994), and in vitro studies show that a high ambient glucose concentration leads to increased intracellular reactive oxygen species (ROS) formation in endothelial cells (Giardino et al. 1996). When cells are unable to scavenge the ROS formed, the excess free radicals result in oxidative stress. In addition, it has been shown that when endothelial cells are exposed to oxidative stress in vitro, an increase in the expression of Hsp27 occurs (Dreher et al. 1995). The generation of free radicals has also been shown to cause endothelial dysfunction in vitro (Tesfamariam and Cohen 1992). The positive correlation between serum Hsp27 and 2-h post-glucose for the CVD groups is in line with the concept that the 2-h post-glucose concentration is an independent risk factor of CVD (Ceriello 2006), which has been suggested to be due to oxidative stress generated after a meal.
In glucose-intolerant subjects with a history of CVD, hyperglycaemia may result in oxidative stress in the cells leading to the release of Hsp27 into the extracellular fluid and causing higher serum Hsp27 in these individuals (Liao et al. 2000; Vega et al. 2008). Since both groups of GI subjects had comparable fasting and 2-h plasma glucose levels, one possible reason for the difference in serum Hsp27 concentration is that endothelial cells of GI subjects without CVD may not be exposed to oxidative stress due to the possible differences in antioxidant status (Bhatia et al. 2003; Atli et al. 2004; Ozdemir et al. 2005; Ceriello et al. 2000).
Once released into the circulation, Hsp27 may be recognized by the immune system and elicit an autoimmune response. We have previously observed that the IgM class of antibodies reacts earlier to the released Hsp27 compared to the IgG antibodies, in patients with acute coronary syndromes. Serum IgM concentrations fell as did those of serum Hsp27, in samples taken 12 h post-event compared to samples taken at an earlier time point (unpublished work). This may be due to the formation of immune complexes that may facilitate the clearance of the released Hsp27. Over time, with repeated release of Hsp27, this rapid response would lead to consumption and fall in the level of these IgM antibodies in the circulation. In GI subjects with history of CVD who had higher serum Hsp27 compared to those without history of CVD, though significance was not reached, the lower level of IgM antibodies in the former group seems to support this scenario. The same trend of lower IgM was also observed in CVD patients who are normoglycaemic. With higher insulin levels at normal glucose level (compared to NGT without CVD) and insulin sensitivity indices similar to the glucose-intolerant subjects, NGT (+CVD) subjects probably have reduced hepatic insulin sensitivity but are normoglycaemic due to compensatory hyperinsulinaemia. Also, with significantly higher HbA1c values but comparable FPGs and 2hPGs, individuals with NGT (+CVD) may be exposed to periods of acute hyperglycaemia leading to oxidative stress (Marfella et al. 2001), release of Hsp27 and consumption of IgM antibodies.
The association of IgM and insulin level may be due to the acute response of both, whereby once insulin is secreted to lower the serum glucose concentration, the period of acute oxidative stress is over, Hsp27 is released into the extracellular fluid and consumption of IgM occurs. Higher insulin levels would be required to facilitate glucose disposal in insulin-resistant subjects, while the inverse is the case for insulin sensitive individuals and thus less consumption of antibodies and higher IgM level.
Sustained hyperglycaemia as in the glucose-intolerant subjects and release of Hsp27 may lead to further production of IgG antibodies, which are involved in the secondary adaptive immune response. Repeated exposure to released Hsp27 would lead to frequent production of the IgG antibodies and thus higher level of this antibody class in the circulation. Although only borderline significance was achieved, this trend was observed in glucose-intolerant subjects with CVD. The IgG class of antibodies can promote inflammation through the formation of immune complexes, which can activate Fc receptors on mononuclear cells (Ravetch and Clynes 1998), and since atherosclerosis is a chronic inflammatory disease (Ross 1999), the higher IgG levels may also be involved in the accelerated development of macrovascular complications in GI subjects.
In conclusion, this study further supports the concept of oxidative stress with hyperglycaemia and insulin resistance and the development of macrovascular complications associated with these conditions. Further studies using larger numbers of subjects are required to confirm these findings.
Acknowledgements
DFPB is a recipient of a PhD studentship from the Brunei Government. We thank colleagues in the Clinical Investigation Unit at the Royal Surrey County Hospital and Peter Williams from the Statistics Department, University of Surrey.
References
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, az-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal. 2005;7:414–422. doi: 10.1089/ars.2005.7.414. [DOI] [PubMed] [Google Scholar]
- Atli T, Keven K, Avci A, Kutlay S, Turkcapar N, Varli M, Aras S, Ertug E, Canbolat O. Oxidative stress and antioxidant status in elderly diabetes mellitus and glucose intolerance patients. Arch Gerontol Geriatr. 2004;39:269–275. doi: 10.1016/j.archger.2004.04.065. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Shukla R, Venkata MS, Kaur GJ, Madhava PK. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin Biochem. 2003;36:557–562. doi: 10.1016/S0009-9120(03)00094-8. [DOI] [PubMed] [Google Scholar]
- Bielecka-Dabrowa A, Barylski M, Mikhailidis DP, Rysz J, Banach M. HSP 70 and atherosclerosis—protector or activator? Expert Opin Ther Targets. 2009;13:307–317. doi: 10.1517/14728220902725149. [DOI] [PubMed] [Google Scholar]
- Borai A, Livingstone C, Ferns GA. The biochemical assessment of insulin resistance. Ann Clin Biochem. 2007;44:324–342. doi: 10.1258/000456307780945778. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Burt D, Bruno G, Chaturvedi N, Schalkwijk C, Stehouwer CD, Witte DR, Fuller JH, Pinach S, Cavallo PP, Gruden G. Anti-heat shock protein 27 antibody levels and diabetic complications in the EURODIAB study. Diabetes Care. 2009;32:1269–1271. doi: 10.2337/dc08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale Schianca GP, Rossi A, Sainaghi PP, Maduli E, Bartoli E. The significance of impaired fasting glucose versus impaired glucose tolerance: importance of insulin secretion and resistance. Diabetes Care. 2003;26:1333–1337. doi: 10.2337/diacare.26.5.1333. [DOI] [PubMed] [Google Scholar]
- Ceriello A (2006) Postprandial hyperglycemia and cardiovascular complications of diabetes. Journ Annu Diabetol Hotel Dieu 75–78 [PubMed]
- Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Morocutti A, Mercuri F, Quagliaro L, Moro M, Damante G, Viberti GC. Defective intracellular antioxidant enzyme production in type 1 diabetic patients with nephropathy. Diabetes. 2000;49:2170–2177. doi: 10.2337/diabetes.49.12.2170. [DOI] [PubMed] [Google Scholar]
- Child DF, Williams CP, Jones RP, Hudson PR, Jones M, Smith CJ. Heat shock protein studies in type 1 and type 2 diabetes and human islet cell culture. Diabet Med. 1995;12:595–599. doi: 10.1111/j.1464-5491.1995.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Oesterreich S, Chamness GC, McGuire WL, Fuqua SA. Biological and clinical implications of heat shock protein 27, 000 (Hsp27): a review. J Natl Cancer Inst. 1993;85:1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- Dreher D, Vargas JR, Hochstrasser DF, Junod AF. Effects of oxidative stress and Ca2+ agonists on molecular chaperones in human umbilical vein endothelial cells. Electrophoresis. 1995;16:1205–1214. doi: 10.1002/elps.11501601201. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- Franklin JL, Keeton AB, Bortoff KD, Messina JL. Insulin dependant gene expression of heat shock protein 60 in H4IIE hepatoma cells. Int J Clin Exp Med. 2008;1:89–97. [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Giardino I, Edelstein D, Brownlee M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J Clin Invest. 1996;97:1422–1428. doi: 10.1172/JCI118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden G, Bruno G, Chaturvedi N, Burt D, Schalkwijk C, Pinach S, Stehouwer CD, Witte DR, Fuller JH, Perin PC. Serum heat shock protein 27 and diabetes complications in the EURODIAB prospective complications study: a novel circulating marker for diabetic neuropathy. Diabetes. 2008;57:1966–1970. doi: 10.2337/db08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14:113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesudason DR, Dunstan K, Leong D, Wittert GA. Macrovascular risk and diagnostic criteria for type 2 diabetes: implications for the use of FPG and HbA(1c) for cost-effective screening. Diabetes Care. 2003;26:485–490. doi: 10.2337/diacare.26.2.485. [DOI] [PubMed] [Google Scholar]
- Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-003-1190-9. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2:120–126. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Li G, Ali IS, Currie RW. Insulin-induced myocardial protection in isolated ischemic rat hearts requires p38 MAPK phosphorylation of Hsp27. Am J Physiol Heart Circ Physiol. 2008;294:H74–H87. doi: 10.1152/ajpheart.00675.2007. [DOI] [PubMed] [Google Scholar]
- Liao DF, Jin ZG, Baas AS, Daum G, Gygi SP, Aebersold R, Berk BC. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–196. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- Marfella R, Quagliaro L, Nappo F, Ceriello A, Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J Clin Invest. 2001;108:635–636. doi: 10.1172/JCI13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo AP. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:363–374. [PubMed] [Google Scholar]
- Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- Ozdemir G, Ozden M, Maral H, Kuskay S, Cetinalp P, Tarkun I. Malondialdehyde, glutathione, glutathione peroxidase and homocysteine levels in type 2 diabetic patients with and without microalbuminuria. Ann Clin Biochem. 2005;42:99–104. doi: 10.1258/0004563053492838. [DOI] [PubMed] [Google Scholar]
- Portig I, Pankuweit S, Lottspeich F, Maisch B. Identification of stress proteins in endothelial cells. Electrophoresis. 1996;17:803–808. doi: 10.1002/elps.1150170431. [DOI] [PubMed] [Google Scholar]
- Preville X, Salvemini F, Giraud S, Chaufour S, Paul C, Stepien G, Ursini MV, Arrigo AP. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp Cell Res. 1999;247:61–78. doi: 10.1006/excr.1998.4347. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu Rev Immunol. 1998;16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–S420. doi: 10.1016/S0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992;263:H321–H326. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata diabetes study. Diabetes Care. 1999;22:920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De MA. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180:4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- WHO Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–1559. doi: 10.1161/01.ATV.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- Xu Q, Willeit J, Marosi M, Kleindienst R, Oberhollenzer F, Kiechl S, Stulnig T, Luef G, Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–259. doi: 10.1016/0140-6736(93)92613-X. [DOI] [PubMed] [Google Scholar]
- Xu Q, Schett G, Perschinka H, Mayr M, Egger G, Oberhollenzer F, Willeit J, Kiechl S, Wick G. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.cir.102.1.14. [DOI] [PubMed] [Google Scholar]
- Zhang X, He M, Cheng L, Chen Y, Zhou L, Zeng H, Pockley AG, Hu FB, Wu T. Elevated heat shock protein 60 levels are associated with higher risk of coronary heart disease in Chinese. Circulation. 2008;118:2687–2693. doi: 10.1161/CIRCULATIONAHA.108.781856. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]