Abstract
Lipocalin-2 (Lcn2, NGAL) is a member of the lipocalin super family with diverse function such as the induction of apoptosis, the suppression of bacterial growth, and modulation of inflammatory response. Much interest has recently been focused on the physiological/pathological role of the lipocalin-2 that is considered to be a novel protective factor against oxidative stress. However, its precise biological roles in this protection are not fully understood. In this report we intended to test the effect of lipocalin-2 on the expression of heme oxygenase (1, 2) and superoxide dismutase (1, 2) which are two strong antioxidants. NGAL was cloned to pcDNA3.1 plasmid by using genetic engineering method. The recombinant vector was transfected to CHO and HEK293T to establish stable cell expressing NGAL and the expression of HO-1, 2 and SOD1, 2 were compared with appropriate controls by RT-PCR and western blot. On the other hand, expression of NGAL was suppressed by siRNA transfection in order to study the effect of lipocalin-2 on mentioned genes/proteins. The results showed that the expression of HO-1 and SOD1, 2 enzymes were higher in cells expressing recombinant lipocalin-2 compared with the control cells. Although the expression of HO-1 was lower in NGAL silencing cells, the expression of SOD1 and SOD2 were higher. Our data suggest that NGAL is a potent inducer of HO-1 and somewhat SOD1 and SOD2 and it appears that part of antioxidant property of NGAL could be attributed to the induction of HO-1and SOD1, 2.
Keywords: NGAL/Lcn2; HO-1, 2; SOD1, 2; Antioxidant enzymes; Oxidative stress
Introduction
The neutrophil gelatinase-associated lipocalin (NGAL), or lipocalin-2/24p3, belongs to the super family of structurally related small extracellular lipocalin proteins with great functional diversity (Flower 1996, Kjeldsen et al. 2000, Ziegler et al. 2007). NGAL is a 25-kDa glycoprotein that has initially been discovered in granules of human neutrophils and was later shown to be expressed by certain epithelial cells as well, especially during inflammation (Kubben et al. 2007). In addition, NGAL is thought to be an acute phase protein whose expression is induced under harmful conditions such as intoxication, renal injury, burn injury, human cancers, inflammatory bowel disease, infection, and other forms of cellular stresses (Nielsen et al. 1996, Seth et al. 2002, Mishra et al. 2003, Flo et al. 2004, Mishra et al. 2004, Vemula et al. 2004, Tong et al. 2005, Zhang et al. 2007, Bauer et al. 2008, Cho and Kim 2009, Katano et al. 2009).
Moreover, it has been reported that NGAL is induced in the mouse liver when exposed to alpha particles and X-rays (Roudkenar et al. 2007). Also, it was shown that NGAL acts as a cytoprotective factor against H2O2 and cisplatin toxicity (Roudkenar et al. 2008a, Roudkenar et al. 2008b).
While it has been reported that NGAL might protect cells from ROS (Roudkenar et al. 2007, Roudkenar et al. 2008a, Roudkenar et al. 2008b), its precise biological roles in this protection is not fully understood. Oxidative damage to macromolecules (nucleic acids, proteins, carbohydrates, and lipids) is mediated by reactive oxygen species (ROS) of intracellular origin such as hydrogen peroxide, oxygen free radicals, singlet oxygen, hypochlorite anion, nitric oxide, and peroxynitrite anion (Lavrovsky et al. 2000, Limón-Pacheco and Gonsebatt 2009). Protective mechanisms including antioxidant enzymes play an important role for removal of these toxic oxygen byproducts. Two major antioxidant enzymes are heme oxygenases (HO) and superoxide dismutases (SOD). Heme oxygenase not only regulates the cellular content of the pro-oxidant heme but also produces catabolites with regulatory and protective functions (Immenschuh and Ramadori 2000).
It has been shown that HO-1 gene expression is induced by stimuli that increase the cellular generation of ROS, but scavengers of ROS, such as N-acetyl cycteine, inhibit the HO-1 up-regulation by oxidative stress. In contrast to the inducible expression of HO-1, HO-2 is a constitutive isozyme and its expression is not changeable (Immenschuh and Ramadori 2000, Lavrovsky et al. 2000).
SODs act as the first line antioxidant enzyme defense system against ROS and particularly superoxide anion radicals (Zelko et al. 2002). SODs mRNA level increases following induction by a wide range of mechanical, chemical, and biological messengers such as UVB and X-irradiation, ozone, and lipopolysaccharide (LPS) that increase ROS (Zelko et al. 2002). In fact, the SODs and HO-1 gene expression are considered to be an adaptive cellular defense mechanism against oxidative stress. In regard to these notions and to clarify the function of NGAL in protection against oxidative stress, this study was performed to examine whether NGAL can induce the expression of SOD1, 2 and HO-1, 2.
Furthermore, the promoter region of SOD1, 2 and HO-1 contain putative NF-КB transcription regulatory element and much of the gene regulatory effect of oxidative stress is mediated by the redox-sensitive transcription factor, NF-КB (Lavrovsky et al. 1994, Lavrovsky et al. 2000, Zelko et al. 2002). We were very interested to study the effect of NGAL on expression of HO-1 and SOD1, 2 through NF-КB pathway. In order to elucidate this hypothesis, stable clones ectopically expressing NGAL were generated and the expression of HO-1, HO-2, SOD1, SOD2, and NF-КB were compared with appropriate controls. On the other hand, expression of NGAL was suppressed by siRNA transfection in order to study the effect of lipocalin-2 on HO-1, 2, SOD1, 2, and NF-КB.
Materials and methods
Cell culture
Human hepatoma cell line (HepG2) Chinese hamster ovary cell line (CHO), lung carcinoma cell line (A549), and human embryonic kidney cell line (HEK293T) were obtained from national cell bank of Pasteur Institute of Iran. These cell lines were grown in RPMI-1640 medium (Gibco-BRL, Germany) with 10% fetal bovine serum (Gibco-BRL, Germany). Cells were cultured at 37°C in an atmosphere of 5% CO2.
Generation of stable NGAL-expressing cell lines
To construct the NGAL expression plasmid, total RNA was isolated and full length human NGAL cDNA was synthesized by reverse transcriptase polymerase chain reaction (RT-PCR) using the previously described primers for NGAL gene (Roudkenar et al. 2008a, b). The amplified cDNA containing EcoRI and NotI restriction enzyme sites was cloned into mammalian expression vector pcDNA3.1(+) in the sense orientation. The identity and orientation of this construct were confirmed by DNA sequencing. CHO and HEK293T cells were transfected with 1 μg of linearized DNA of pcDNA3.1-NGAL, using the FuGENE 6 (Roche, Mannheim, Germany) according to the manufacturer’s protocol. The CHO and HEK293T cells with stably expressing human NGAL were selected in a medium containing 600 μg/ml Geneticin (Roche) for at least 14 days. After transfection, the expression level of NGAL was examined by RT-PCR and western blot analysis.
Gene expression analysis by reverse transcriptase polymerase chain reaction
Initially, total RNA was isolated from CHO and HEK293T cells by Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Reverse transcription was performed by superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) with 1,000 ng of total RNA followed by DNase I (Invitrogen, Carlsbad, CA, USA) treatment and heat inactivation.
PCR was performed using Taq DNA polymerase (Takara, Shiga, Japan) in GeneAmp PCR system 9600 (PerkinElmer life and Analytical sciences, Inc., Wellesley, MA, USA) and expression of β-actin gene was examined for normalization. Primer sets for antioxidant genes, NF-КB and β-actin are shown in Table 1. Seven microliters of amplified PCR products were mixed with 1 μl of loading buffer and then run on a 2% agarose gel. Finally, the expression pattern of genes was analyzed by UVIdoc Gel Documentation System (Avebury House 36a Union Lane Cambridge CB4 1QB-uk).
Table 1.
Primer sets for NGAL, HO-1, HO-2, SOD1, SOD2, NF-КB, and β-actin
| Genes | Primer sequences (5′–3′) | Annealing temperature (°C) | Size (bp) |
|---|---|---|---|
| NGAL | Forward: 5′-TCA CCT CCG TCC TGT TTA GG-3′ | 60 | 240 |
| Reverse: 5′-CGA AGT CAG CTC CTT GGT TC-3′ | |||
| Βactin | Forward: 5′-TTC TAC AAT GAG CTG CGT GTG G-3′ | 59 | 119 |
| Reverse: 5′-GTG TTG AAG GTC TCA AAC ATG AT-3′ | |||
| HO-1 | Forward: 5′-ATG ACA CCA AGG ACC AGA GC-3′ | 55 | 153 |
| Reverse: 5΄-GTG TAA GGA CCC ATC GGA GA-3΄ | |||
| HO-2 | Forward: 5′-GGA AAC CTC AGA GGG GGT AG-3′ | 55 | 198 |
| Reverse: 5′-GTG GCC AGC TTA AAC AGC TC-3΄ | |||
| SOD1 | Forward: 5′-AGG GCA TCA TCA ATT TCG AGC-3′ | 55 | 217 |
| Reverse: 5′-ACA TTG CCC AAG TCT CCA AC-3′ | |||
| SOD2 | Forward: 5′-GGA AGC CAT CAA ACG TGA CT-3′ | 55 | 162 |
| Reverse: 5′-CCT TGC AGT GGA TCC TGA TT-3′ | |||
| NF-КB | Forward: 5′-TGG GAA TGG TGA GGT CAC TC-3′ | 55 | 197 |
| Reverse: 5′-TCT CAT CCT GCA CAG CAG TG-3′ |
Western blot analysis
For detection of antioxidant proteins, total proteins were extracted by Complete Lysis-M reagent (Roche, Germany) according to the manufacture’s instruction. Cell culture medium was for detection of NGAL. Total protein was boiled in loading buffer containing 4% sodium dodecyl sulfate (SDS), 20% glycerol, and bromophenol blue for 5 min. Proteins were resolved on 12% SDS-PAGE and transblotted onto PVDF membrane (Roche, Germany). Then proteins were detected with specific polyclonal antibodies (Santa Cruz biotechnology, Santa Cruz, CA, USA).
Small interfering RNA (siRNA) gene silencing
NGAL mRNA was down-regulated in A549 cells with HS-Lcn2-6-HP validated siRNA (Qiagen, Hilden, Germany). Sequences of control siRNA (Qiagen, Hilden, Germany) were sense: UUC UCC GAA CGU GUC ACG U dT dT and antisense: ACG UGA CAC GUU CGG AGA A dT dT.
Five nanomolars of the synthetic double-stranded siRNA oligo nucleotide was then delivered into A549 cells using different doses of HiPerFect transfection reagent (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Silencing of NGAL gene expression was measured by both RT-PCR, 72 h after transfection, and assessment of the amount of NGAL protein secreted into the medium.
Cell proliferation assay
The cells were exposed to different concentration of H2O2 and hemin (Sigma, USA) at different time intervals. For MTT assay, 2 × 104 cells/well were seeded in a 96-well plate and after 12 h, the cells were exposed to H2O2 and hemin for different time courses. At appropriate time points, cells were incubated with 10 µl of a 5 mg/ml solution of 3-(4,5-dimethlthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, Dusseldorf, Germany) at 37°C in a 5% CO2 atmosphere for 4 h. Finally the reaction was stopped by addition of 10% SDS and 0.01 M HCl. When the insoluble crystals were completely dissolved in DMSO; the absorbance at 570 nm was measured using a microplate reader.
Reagents and antibodies
Following antibodies were used for detection of target proteins: anti-human NGAL rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti β-actin antibody (Sigma), polyclonal anti–HO-1 antibody (Stressgen Biotechnologies, Victoria, BC, Canada), anti-human SOD1, 2 rabbit polyclonal antibody (Acris Antibodies GmbH ,Germany), and anti-human NF-КB rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). All secondary antibodies were from Sigma Chemical Company.
Statistical analysis
Cell proliferation results are expressed as mean ± SD of three independent experiments. Differences were determined using ANOVA with the Tukey–Kramer Multiple Comparison Test.
Results
Expression of NGAL in CHO and HEK 293T cell lines
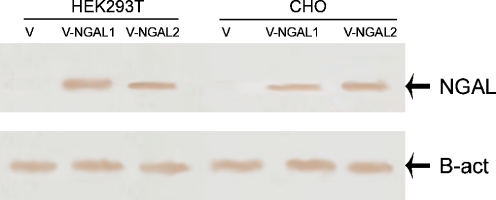
To investigate whether NGAL-transfected cells express human NGAL, RNA was extracted from CHO and HEK293T cells transfected with construct and empty vector. cDNA was synthesized and RT-PCR was performed. CHO and HEK293T cells transfected with the construct expressed NGAL mRNA, but there were no expression in the CHO and HEK293T cells transfected with pcDNA3.1 vector (Fig. 1a and b). Similar results were also observed when the cell culture media of human NGAL stable transfected cells were used for detection of NGAL protein by Western blot analysis (Fig. 2). Two stables clones of CHO and HEK293T cells expressing NGAL were established.
Fig. 1.
Expression of NGAL in stable clones of CHO and HEK293T. To assess the effect of lipocalin-2 on the expression of antioxidants, two NGAL recombinant cells were established and expression of NGAL was assessed by RT-PCR. a HEK293T cells transfected with recombinant pcDNA3.1 showed a 240-bp band but no expression was observed in HEK293T cells transfected with pcDNA3.1. b CHO cells transfected with recombinant pcDNA3.1 showed a 240-bp band but no expression was observed in CHO cells transfected with pcDNA3.1. V-NGAL 1 and V-NGAL 2 denote two stable clones. Lower figure the expression was normalized by β-actin gene
Fig. 2.
NGAL protein expression in NGAL transfected cells. NGAL protein expression was analyzed in HEK293T and CHO cells transfected with the construct and empty vector by western blot analysis. V-NGAL 1 and V-NGAL 2 denote two stable clones. Lower figure the expression was normalized by β-actin protein
Up-expression of HO-1, SOD1, and SOD2 in ectopic expression NGAL cells
The effect of lipocalin-2 on HO-1, SOD1, and SOD2 expression was examined by using RT-PCR and western blot analysis. Compared to control cells, HO-1, SOD1, 2 mRNA were increased in the stable cells, CHO-NGAL and HEK293T-NGAL. Data presented in Fig. 3 show that HO-1 Expression level in cells transfected with recombinant plasmid were much higher than control cells. But, as HO-2 is a constitutively expressed isozyme, its expression level, in contrast to HO-1, was similar in both recombinant and control cells (Fig. 3b). Furthermore, recombinant cells expressing lipocalin-2 show higher level of SOD1, 2 when compared with controls (Fig. 3c, d). Accordingly, HO-1 and SOD1, 2 protein levels, detected by western blotting, were increased in cells expressing NGAL compared to control cells (Fig. 4) whereas no change in expression level of HO-2 was observed .
Fig. 3.
Up-expression of antioxidants and NF-КB in recombinant cells expressing NGAL. a NGAL recombinant cells [HEK293T-V-NGAL (1, 2), CHO-V-NGAL (1, 2)] show higher mRNA level of HO-1 compared with control cells (HEK293T-V, CHO-V). b In contrast to HO-1, HO-2 is constitutively expressed, and its expression in recombinant and control cells was the same. c, d SOD1, 2 expressions in stable clones of CHO and HEK293T, transfected with the vector-NGAL is higher than stable clones of CHO and HEK293T, transfected with the empty vector. e To determine the effect of lipocalin-2 on NF-КB, RT-PCR test was performed for NF-КB as well, results show that the expression of NF-КB in cells expressing lipocalin-2 [HEK293T-V-NGAL (1, 2), CHO-V-NGAL (1, 2)] is higher than control cells (HEK293T-V, CHO-V). f The expression of β-actin in both recombinant and control cells. V-NGAL 1 and V-NGAL 2 denote two stable clones. M, 100-bp ladder marker
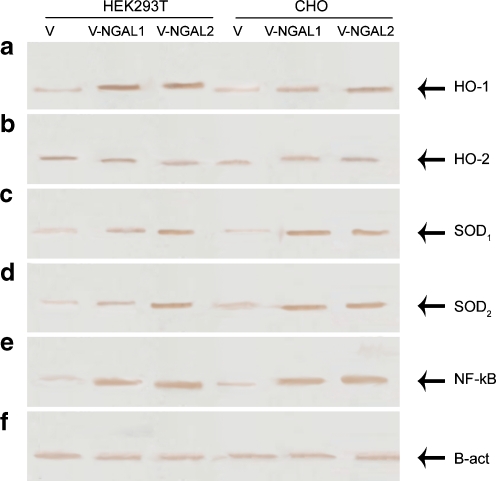
Fig. 4.
Up-regulation of HO-1, SOD1, 2, and NF-КB were measured by assessment of the amount of antioxidants and NF-КB protein secreted into the medium by Western blot. a CHO and HEK293T cells transfected with the construct express higher HO-1 protein than CHO and HEK293T cells transfected with pcDNA3.1. b Protein expression of HO-2 in recombinant and control cells was the same. c, d Protein expression of SOD1, 2 was up-regulated in cells transfected with vector-NGAL [HEK293T-V-NGAL (1, 2), CHO-V-NGAL (1, 2)] compared with the controls (HEK293T-V, CHO-V). e Protein expression of NF-КB was higher in cells transfected with construct compared with control cells. V-NGAL 1 and V-NGAL 2 denote two stable clones. f Results were normalized by β-actin
Induction of NF-КB in cells expressing NGAL
To test the involvement of the NGAL in NF-КB expression, expression of NF-КB was also assessed by RT-PCR and western blot analysis. NF-КB (p50) was studied in this project because of its role in modulation of response to oxidative stress (Beinke et al. 2004, Zhou et al. 2001).
RT-PCR results showed that the expression of NF-КB in the CHO and HEK293T cells expressing NGAL was higher than empty vector-transfected cells, which indicate the role of NGAL in NF-KB regulation (Fig. 3e). Data presented in Fig. 4d show that protein level of NF-КB in ectopically expressing NGAL cells is higher than control cells. These data suggest that lipocalin-2 induces the expression of NF-КB.
Down-regulation of HO-1 and NF-KB in A549 cells transfected with NGAL siRNA
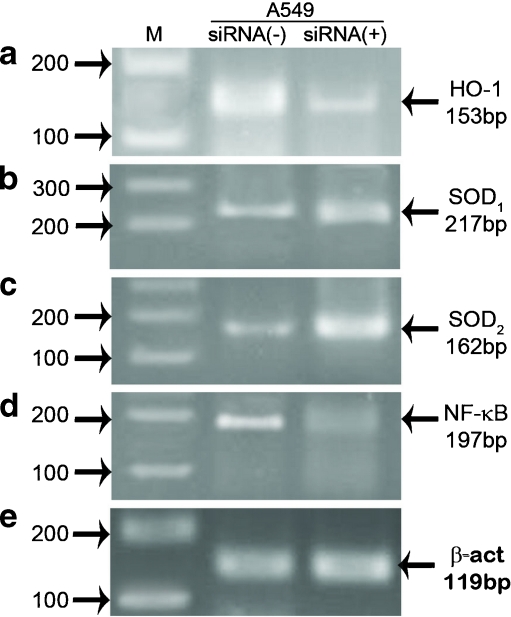
To investigate, whether down-regulation of NGAL affects expression of SOD1, SOD2, HO-1, and NF-KB, NGAL gene was down-regulated in one of the cell lines that basically express high levels of NGAL, i.e., A549 by siRNA technology followed by assessment of expression of the aforementioned genes/proteins. Down-regulation of NGAL gene was confirmed by RT-PCR (Fig. 5). Expressions of other genes were also assessed by RT-PCR analysis. Interestingly, results showed that the expression of HO-1 and NF-КB were decreased in A549 cells after treatment with NGAL siRNA, while the expression of SOD1, 2 were increased in comparison with control cells (Fig. 6). Similar results were also observed when SOD1, SOD2, HO-1, and NF-KB protein levels, detected by western blot analysis (Fig. 7).
Fig. 5.
Down-regulation of NGAL gene expression of lipocalin-2 in A549 cells was suppressed effectively by NGAL siRNA, and NGAL silencing was analyzed by RT-PCR. NGAL expression in the cells transfected with siRNA (A549 siRNA+) is lower than A549 cells transfected with control siRNA (A549 siRNA−). M, 100-bp ladder marker
Fig. 6.
Analysis of antioxidants and NF-КB expression in cells transfected with NGAL siRNA. a Cells transfected with siRNA (A549 siRNA+) show lower expression of HO-1 compared with the control cells (A549 siRNA−). b, c Transfection of NGAL siRNA-induced SOD1, 2 mRNA in A549 cells. d lipocalin-2 silencing caused down-regulation of NF-КB in A549 siRNA+ cells. e Results were normalized by β-actin. M 100-bp ladder marker
Fig. 7.
NGAL silencing affects on HO-1, SOD1, 2, and NF-КB protein expression in A549 cells. Total proteins were extracted and expression of HO-1, SOD1, 2, and NF-КB were determined by Western blot analysis. a, d Cells transfected with siRNA (A549 siRNA+) show lower protein expression of HO-1 and NF-КB compared with the control cells (A549 siRNA−). b, c SOD1, SOD2 show higher levels of SOD1, 2 in cells transfected with siRNA (A549 siRNA+) compared with the control cells (A549 siRNA−). e β-actin was used for normalization
NGAL-expressing cells decreased toxicity of H2O2 and hemin
In our previous study, cytoprotective effect of NGAL in CHO cells against H2O2 toxicity was observed (Roudkenar et al. 2008b). To determine whether the same effects would be observed in HEK293T cells or against hemin toxicity, the stable cells expressing NGAL and a control transfected clone were treated with different doses of H2O2 and hemin at different time intervals followed by cytotoxicity and proliferation assays. Cell proliferation was higher in HEK293T cells expressing NGAL in doses of 4 and 6 mM H2O2 after 2 h compared to the control (p < 0.001; Fig. 8a). Cell proliferation was also higher in CHO cells expressing NGAL in doses of 100 and 150 μM hemin and for HEK293T cells expressing NGAL in doses of 50 and 100 μM hemin after 24 h compared to the control (Fig. 8b, c) suggesting NGAL as a protective factor against H2O2 and hemin.
Fig. 8.
Cytotoxicity effects of different concentrations of H2O2, and Hemin on the stable cells transfected with pcDNA3.1-NGAL or pcDNA3.1: MTT assay. a The levels of cell proliferation in HEK293Tcells transfected with construct were higher than control at concentrations of 4, 6, and 8 mM H2O2, whereas HEK293T cells transfected with pcDNA3.1 were susceptible to H2O2 at these concentrations. N1 and N2 are two different stable clones expressing NGAL. b, c The levels of cell proliferation in CHO cells expressing NGAL were higher than the control at concentrations of 100 and 150 μM of hemin. Same results were also observed for HEK293T cells expressing NGAL but at concentration of 50 and 100 μM of hemin (mean ± SD; p < 0.001; number of replicates, 3)
Discussion
To clarify the functions of NGAL in protection against oxidative stress, the current study was designed to determine whether NGAL can induce the expression of HO-1, 2 and SOD1, 2 mRNA which are two strong antioxidants. The recombinant PcDNA3.1-NGAL was constructed and transfected to CHO and HEK293T cell lines. These cell lines do not express NGAL normally. Thus, after transfection, the expression of HO-1, HO-2, SOD1, and SOD2 could be attributed to NGAL.
Our results showed the higher expression of HO-1 and SOD1, 2 in CHO- and HEK293T-expressing recombinant NGAL compared with the control cells and it suggested the role of NGAL on the expression of SOD1, 2 and notably HO-1.
It has been reported that hypoxic stress and acute infraction increase Lcn2 expression in mouse macrophages (Hemdahl et al. 2006, Jiang et al. 2008). This supports the idea that expression of lipocalin-2 is a response that these cells show against hypoxic stress. Furthermore, a complementary DNA microarray analysis in acute lung injury showed the induction of lipocalin-2 by LPS and diesel exhaust particles (DEP). DEP contain a variety of heavy metals such as iron and copper and generate hydroxyl radicals in the murine lungs through an iron catalyzed reaction of superoxide and H2O2 (Meheus et al. 1993, Yanagisawa et al. 2004). Thus, it seems that induction of NGAL in acute lung injury is related to generation of free radicals by DEP and LPS.
Oxidative stress in cells is associated with an increased expression and activity of antioxidant enzymes (Ding et al. 2002) and these defense systems are critical for ROS detoxification (Limón-Pacheco and Gonsebatt 2009). There are mounting evidence indicating that HO-1 gene expression is induced by stimulates that increase the cellular production of ROS, including heme, heavy metals, UV light, hydrogen peroxide, and lipopolysaccharide (Immenschuh and Ramadori 2000, Hayashi et al. 2001, Bussolati et al. 2004, Kikuchi et al. 2005, Bach 2006).
In previous studies, we have shown the protective role of NGAL against ROS (Roudkenar et al. 2007, Roudkenar et al. 2008a, Roudkenar et al. 2008b) and in this study we report that NGAL induces HO-1 expression. To support this hypothesis, Kiayoshi Mori et al. found that ischemia-reperfusion enhances the expression of HO-1. But, when mice were treated with NGAL, the enzyme was further up regulates five to ten fold. Moreover, while NGAL protects the kidney from ischemia-reperfusion damage, injection of the HO inhibitor blocked this effect (Mori et al. 2005). Hence, HO-1 expression may be required for NGAL-mediated protection.
Furthermore, in some diseases in which ROS is involved, the expression of both HO-1 and NGAL genes is induced and it seems that same stimuli can induce expression of NGAL and HO-1. Taken together, NGAL may, through up-regulation of HO-1, exert this effect. Our results indicate that NGAL does not affect the expression of HO-2. This observation supports the notion that in contrast to the inducible isozyme HO-1, HO-2 is a constitutive enzyme that contributes to the basal HO activity (Ryter et al. 1998). Also, our results show that lipocalin-2 induces SOD1, 2. This is in line with the observation that enhancement of transcription of SOD genes is a part of cellular stress response (Mruk et al. 2002, Hartog et al. 2003, Johnson and Giulivi 2005, Liochev and Fridovich 2007) and it has been shown that such stressful conditions up-regulate both lipocalin-2 and SOD genes (Lavrovsky et al. 1994, Immenschuh and Ramadori 2000, Lavrovsky et al. 2000, Zelko et al. 2002, Rivera et al. 2006, Limón-Pacheco and Gonsebatt 2009). Therefore, our data can indicate that lipocalin-2 by induction of SOD gene adds to the cellular ability to counteract the oxidant challenge. We further tested our hypothesis by down-regulation of NGAL expression in A549 cell lines by gene silencing technology. Our results showed that the expression of HO-1 was lower in siRNA-transfected cells compared to control A549 cells. However, the expression of SOD1, 2 were higher in siRNA-transfected cells.
These data suggest that down-regulation of lipocalin-2 and following HO-1 down-regulation is accompanied by compensatory induction of SOD1 and SOD2 for protection against oxidative stress. In other words, SOD1 and SOD2 can compensate for the loss of HO-1 and lipocalin-2 expression.
It is increasingly becoming apparent that much of the gene regulatory effect of oxidative stress is mediated by the redox-sensitive transcription factor NF-KB (Lavrovsky et al. 1994, Lavrovsky et al. 2000). Additionally, the promoter region of the human, mouse and chicken SOD1, SOD2, and HO-1 have a putative binding site for NF-KB (Lavrovsky et al. 1994, Immenschuh and Ramadori 2000, Lavrovsky et al. 2000, Zelko et al. 2002). It prompted us to clarify whether NGAL can affect the expression of HO-1 and SOD1, 2 by NF-KB pathway. To elucidate this hypothesis, we have performed the aforementioned experiment for NF-KB as well. We found that although the expression of NF-KB and HO-1 were lower in NGAL silencing cells, the expression of SOD1 and SOD2 were higher. Based on this result, we propose that lipocalin-2 induces the expression of HO-1 by NF-KB. Although, NF-KB is known to regulate SOD1, 2 gene expression, it is unlikely that this transcriptional factor is involved in the mechanism of SOD1, 2 inductions by lipocalin-2. Overall, our data suggest that NGAL is a potent inducer of HO-1 and somewhat SOD1 and SOD2. This study also proposes that the effect of NGAL on the expression of HO-1 is apparently mediated by NF-KB. In view of the results presented here, it is tempting to speculate that some beneficial effects of NGAL on protection against oxidative stress might take place because of the intrinsic ability of this protein to increase HO-1 and SOD1, 2 expressions. However, further studies are required to clarify the mechanisms underlying NGAL regulation of the mentioned genes/proteins.
References
- Bach FH. Heme oxygenase-1 and transplantation tolerance. Hum Immunol. 2006;67:430–432. doi: 10.1016/j.humimm.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat. 2008;108:389–397. doi: 10.1007/s10549-007-9619-3. [DOI] [PubMed] [Google Scholar]
- Beinke S, Ley SC. Functions of NF-κB1 and NF-κB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati B, Ahmed A, Pemberton H, Landis RC, Carlo F, Haskard DO, Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- Cho H, Kim J-H. Lipocalin2 expressions correlate significantly with tumor differentiation in epithelial ovarian cancer. J Histochem Cytochem. 2009;57:513–521. doi: 10.1369/jhc.2009.953257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HQ, Zhou BJ, Liu L, Cheng S. Oxidative stress and metallothionein expression in the liver of rats with severe thermal injury. Burns. 2002;28:215–221. doi: 10.1016/S0305-4179(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318(Pt 1):1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartog GJMd, Haenen GRMM, Vegt E, WJFvd V, Bast A. Superoxide dismutase: the balance between prevention and induction of oxidative damage. Chem Biol Interact. 2003;145:33–39. doi: 10.1016/S0009-2797(02)00160-6. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Haneda M, Koya D, Maeda S, Isshiki K, Kikkawa R. Enhancement of glomerular heme oxygenase-1 expression in diabetic rats. Diabetes Res Clin Pract. 2001;52:85–96. doi: 10.1016/S0168-8227(01)00218-2. [DOI] [PubMed] [Google Scholar]
- Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, Thoren P, Hansson GK. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26:136–142. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000;60:1121–1128. doi: 10.1016/S0006-2952(00)00443-3. [DOI] [PubMed] [Google Scholar]
- Jiang W, Constantea M, Santos MM. Anemia upregulates lipocalin 2 in the liver and serum. Blood Cell Mol Dis. 2008;41:169–174. doi: 10.1016/j.bcmd.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med. 2005;26:340–352. doi: 10.1016/j.mam.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Katano M, Okamoto K, Arito M, Kawakami Y, Kurokawa MS, Suematsu N, Shimada S, Nakamura H, Xiang Y, Masuko K, Nishioka K, Yudoh K, Kato T. Implication of granulocyte-macrophage colony-stimulating factor induced neutrophil gelatinase-associated lipocalin in pathogenesis of rheumatoid arthritis revealed by proteome analysis. Arthritis Res Ther. 2009;11:R3. doi: 10.1186/ar2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi G, Yoshida T, Noguchi M. Heme oxygenase and heme degradation. Biochem Biophys Res Commun. 2005;338:558–567. doi: 10.1016/j.bbrc.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochimica et Biophysica Acta (BBA) 2000;1482:272–283. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- Kubben FJGM, Sier CFM, Hawinkels LJAC, Tschesche H, Wv D, Zuidwijk K, JJvd R, Hanemaaijer R, Griffioen G, Lamers CBHW, Verspaget HW. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur J Cancer. 2007;43:1869–1876. doi: 10.1016/j.ejca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Schwartzman M, Levere R, Kappas A, Abraham N. Identification of binding sites for transcription factors NFkB and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci U S A. 1994;91:5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrovsky Y, Song CS, Chatterjee B, Roy AK. Age-dependent increase of heme oxygenase-1 gene expression in the liver mediated by NFkB. Mech Ageing Dev. 2000;114:49–60. doi: 10.1016/S0047-6374(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Limón-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res. 2009;674:137–147. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Liochev SI, Fridovich I. The effects of superoxide dismutase on H2O2 formation. Free Radical Bio Med. 2007;42:1465–1469. doi: 10.1016/j.freeradbiomed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Meheus LA, Fransen LM, Raymackers JG, Blockx HA, Beeumen JJV, Bun SMV, AVd V. Identification by microsequencing of lipopolysaccharide-induced proteins secreted by mouse macrophages. J Immunol. 1993;151:1535–1547. [PubMed] [Google Scholar]
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- Mishra J, Morib K, Maa Q, Kellya C, Baraschb J, Devarajana P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin-siderophoreiron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Silvestrinib B, M-y M, Cheng CY. Antioxidant superoxide dismutase—a review: its function, regulation in the testis, and role in male fertility contraception. Contraception. 2002;65:305–311. doi: 10.1016/S0010-7824(01)00320-1. [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera E, Flores I, Rivera E, Appleyard CB. Validation of known inflammatory genes and identification of novel disease-associated targets. Inflamm Bowel Dis. 2006;12:950–966. doi: 10.1097/01.mib.0000231575.11678.8c. [DOI] [PubMed] [Google Scholar]
- Roudkenar MH, Kuwahara Y, Baba T, Roushandeh AM, Ebishima S, Abe S, Ohkubo Y, Fukumoto M. Oxidative stress induced lipocalin 2 gene expression: addressing its expression under the harmful conditions. J Radiat Res (Tokyo) 2007;48:39–44. doi: 10.1269/jrr.06057. [DOI] [PubMed] [Google Scholar]
- Roudkenar MH, Ghasemipour Z, Halabian R, Roushandeh AM, Yaghmai P, Gharehbaghian A, Oodi A, Massrori N, Amirizadeh N, Ma S. Lipocalin 2 acts as a cytoprotective factor against cisplatin toxicity, an in vitro study. Daru. 2008;16:106–111. [Google Scholar]
- Roudkenar MH, Halabian R, Ghasemipour Z, Roushandeh AM, Rouhbakhsh M, Nekogoftar M, Kuwahara Y, Fukumoto M, Shokrgozar MA. Neutrophil gelatinase-associated lipocalin acts as a protective factor against H(2)O(2) toxicity. Arch Med Res. 2008;39:560–566. doi: 10.1016/j.arcmed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Ryter S, Kvam E, Richman L, Hartmann F, Tyrrell R. A chromatographic assay for heme oxygenase activity in cultured human cells: application to artificial heme oxygenase overexpression. Free Radical Bio Med. 1998;24:959–971. doi: 10.1016/S0891-5849(97)00380-8. [DOI] [PubMed] [Google Scholar]
- Seth P, Porter D, Lahti-Domenici J, Geng Y, Richardson A, Polyak K. Cellular and molecular targets of estrogen in normal human breast tissue. Cancer Res. 2002;62:4540–4544. [PubMed] [Google Scholar]
- Tong Z, Wu X, Ovcharenko D, Zhu J, Chen C-S, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391:441–448. doi: 10.1042/BJ20051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula M, Berthiaume F, Jayaraman A, Yarmush ML. Expression profiling analysis of the metabolic and inflammatory changes following burn injury in rats. Physiol Genomics. 2004;18:87–98. doi: 10.1152/physiolgenomics.00189.2003. [DOI] [PubMed] [Google Scholar]
- Yanagisawa R, Takano H, Inoue K-I, Ichinose T, Yoshida S-I, Sadakane K, Takeda K, Yoshino S, Yamaki K, Kumagai Y, Yoshikawa T. Complementary DNA microarray analysis in acute lung injury induced by lipopolysaccharide and diesel exhaust particles. Exp Biol Med. 2004;229:1081–1087. doi: 10.1177/153537020422901013. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Bio Med. 2002;33:337–349. doi: 10.1016/S0891-5849(02)00905-X. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xu L, Xiao D, Xie J, Zeng H, Wang Z, Zhang X, Niu Y, Shen Z, Shen J, Wu X, Li E. Upregulation of neutrophil gelatinase-associated lipocalin in oesophageal squamous cell carcinoma:significant correlation with cell differentiation and tumour invasion. J Clin Pathol. 2007;60:555–561. doi: 10.1136/jcp.2006.039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S, Röhrs S, Tickenbrock L, Langerak A, Chu S-T, Feldmann I, Jakubowski N, Müller O. Lipocalin 24p3 is regulated by the Wnt pathway independent of regulation by iron. Cancer Genet Cytogen. 2007;174:16–23. doi: 10.1016/j.cancergencyto.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Zhou LZ-H, Johnson AP, Rando TA. NFkB and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radical Bio Med. 2001;31:1405–1416. doi: 10.1016/S0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]