Abstract
Land snails are subject to daily and seasonal variations in temperature and in water availability and depend on a range of behavioral and physiological adaptations for coping with problems of maintaining water, ionic, and thermal balance. Heat shock proteins (HSPs) are a multigene family of proteins whose expression is induced by a variety of stress agents. We used experimental desiccation to test whether adaptation to different habitats affects HSP expression in two closely related Sphincterochila snail species, a desiccation-resistant, desert species Sphincterochila zonata, and a Mediterranean-type, desiccation-sensitive species Sphincterochila cariosa. We examined the HSP response in the foot, hepatopancreas, and kidney tissues of snails exposed to normothermic desiccation. Our findings show variations in the HSP response in both timing and magnitude between the two species. The levels of endogenous Hsp72 in S. cariosa were higher in all the examined tissues, and the induction of Hsp72, Hsp74, and Hsp90 developed earlier than in S. zonata. In contrary, the induction of sHSPs (Hsp25 and Hsp30) was more pronounced in S. zonata compared to S. cariosa. Our results suggest that land snails use HSPs as part of their survival strategy during desiccation and as important components of the aestivation mechanism in the transition from activity to dormancy. Our study underscores the distinct strategy of HSP expression in response to desiccation, namely the delayed induction of Hsp70 and Hsp90 together with enhanced induction of sHSPs in the desert-dwelling species, and suggests that evolution in harsh environments will result in selection for reduced Hsp70 expression.
Keywords: HSP, Land snails, Desiccation, Environmental stress
Introduction
Although Mollusca originally evolved from a marine environment, the order Pulmonata is abundant on land, including arid and semiarid zones, where conditions of high ambient temperature and low humidity prevail. Therefore, the ability of land snails to colonize terrestrial habitats is a result of their development of a range of structural, behavioral, and physiological adaptations for coping with problems of maintaining water, ionic, and thermal balance (Riddle 1983) that will ensure their survival under their specific microhabitat conditions. Aestivation during the dry and hot seasons can represent such adaptations. Land snails are subject to annual cycles of activity in relation to seasonal changes in temperature, humidity, and water availability. In general, during aestivation, there is a marked decrease in metabolic activity (Storey and Storey 1990; Pakay et al. 2002; Ramnanan et al. 2009). This metabolic slowdown is especially evident in land snails and allows them to survive even in extreme arid conditions (Schmidt-Nielsen et al. 1971). The onset of activity occurs with the beginning of the rainy season, when snails replenish their water reserves. Comparative studies in land snails have revealed that, in general, resistance to heat and aridity is correlated with distribution patterns and with abiotic environmental variation (Machin 1967). A series of studies on water relations and resistance to experimental desiccation of Israeli land snails (Arad et al. 1989, 1992, 1993; Arad 2001) have demonstrated that Mediterranean snails are less resistant than desert species and populations and that the distribution pattern of each species and its microhabitat are related to its ability to cope with desiccating conditions. In addition, interspecific differences were found that stem from a variety of morphological, behavioral, and physiological adaptations such as snail size, epiphragm thickness, site selection and lifestyle, and osmoregulatory capacities.
Environmental stressors can cause the induction of a selected group of proteins known as stress proteins or heat shock proteins (HSPs) in diverse organisms (Lindquist and Craig 1988; Feder and Hofmann 1999; Sorensen et al. 2003). Although initially named because their production is induced by heat, there are many other inducers of HSPs including various chemicals, osmotic stress, and desiccation. Generally, HSPs have been categorized into four major families on the basis of their molecular weight and degrees of homology: Hsp90 (83–99 kDa), Hsp70 (68–80 kDa), Hsp60, and a family of small HSPs (15–40 kDa). Among them, the highly conserved families of Hsp70 and Hsp90 are the most studied ones. The 70-kDa family is considered the most prominent eukaryotic family of stress proteins, and several isoforms exist including the constitutively expressed heat shock cognate protein 70 (Hsc70) and the heat-inducible Hsp70, whereas Hsp90 is one of the most abundant cytosolic proteins in eukaryotes. Under nonstress conditions, Hsp70 and Hsp90 display essential roles in the cell, chaperoning proteins during folding, assembly, intracellular trafficking, and degradation and are involved in cell regulatory pathways (Csermely et al. 1998; Mayer and Bukau 2005).
It is generally accepted that HSPs protect organisms from the detrimental effects of heat, and possibly other stressors (Lindquist 1986; Somero 1995). Overall, it appears that thermotolerance depends on the synthesis of HSPs and that intracellular levels of Hsp70 are good indicators of thermotolerance (Moseley 1997; Nollen et al. 1999; King et al. 2002). It should be noted, however, that there are several studies suggesting that thermotolerance can be induced with a delayed HSP response (Shabtay and Arad 2005) or without the induction of HSP synthesis (Widelitz et al. 1986; Boon-Niermeijer et al. 1987) or that induction of HSPs may be uncoupled from acquired thermotolerance (Easton et al. 1987; Smith and Yaffe 1991). Boon-Niermeijer et al. (1986, 1988), for example, characterized two states of thermotolerance in snail larvae, a rapidly appearing transient state that depends on constitutively present HSPs, and a longer, more stable thermotolerant condition that requires the synthesis of new HSPs. The stress needed to induce HSPs is strongly related to the niche of the organism in question (Feder and Hofmann 1999). For example, Edgerly et al. (2005) found that two Australian embiid insect species upregulated Hsp70 at higher temperatures than a rainforest species, implying adaptation to higher habitat temperatures in the former. HSP levels may change over microevolutionary timescales, and in such cases, environmental temperature is a potentially important selective agent (Dietz and Somero 1992; Norris et al. 1995; Feder et al. 2002).
Yet, if stress proteins in ectothermic species are involved in adaptation to extreme environmental conditions, we might expect an extensive stress protein response in species that naturally inhabit such environments, and by studying closely related species that differ in the HSP response and in thermotolerance we may identify the underlying adaptive strategies. The land snail Sphincterochila (Sphincterochilidae) is represented in Israel by five broadly parapatric species that replace one another along a climatic gradient that ranges from rainy Mediterranean environment to the arid desert environment and were previously found to differ in their susceptibility to desiccation stress (Arad et al. 1989). Stress conditions associated with lack of water were previously found to induce the expression of HSPs in different organisms. Brooks and Storey (1995) found induction of stress-related proteins (members of the 30-, 50-, and 70-kDa families) in the land snail Otala lactea during aestivation and suggested that these de novo produced stress proteins are important components of the overall aestivation strategy. In the Antarctic midge Belgica antarctica, the genes of sHSPs, Hsp70, and Hsp90 were upregulated in response to dehydration (Lopez-Martinez et al. 2009). Induction of Hsp70 mRNA was also found in the eutardigrade Milnesium tardigradum in the transitional stage between activity and desiccation (cryptobiosis; Schill et al. 2004) and of Hsp70 and Hsp23 in the flesh fly (Sarcophaga crassipalpis) pupae following desiccation exposure (Tammariello et al. 1999; Hayward et al. 2004). However, our knowledge of how species that differ in desiccation tolerance vary in expression patterns of HSPs with changing humidity is still limited. In the current study, we examined whether experimental desiccation causes the induction of stress-related proteins in the land snail Sphincterochila and whether there are differences in the HSP response patterns to desiccation between genetically desiccation-resistant desert snails Sphincterochila zonata and the more desiccation-sensitive Mediterranean-type Sphincterochila cariosa snails. Our approach was to test the effect of desiccation on the snails by analyzing the foot, kidney, and hepatopancreas tissues for the presence and quantity of various HSPs.
Materials and methods
Adult S. zonata (Bourguignat 1853) and S. cariosa (Olivier 1804) were collected in the Negev desert near Sde-Boqer and in the northern Mediterranean coast of Israel near Atlit, respectively. The snails were collected during winter months (November–December), brought to the laboratory, and maintained in aquaria within a temperature-controlled room at 25 ± 0.3°C (a temperature within the natural range of both species) and a 12L:12D photoperiod.
Experimental design
The experimental groups of snails were transferred to a damp substrate and allowed to hydrate for 48 h to a stable mass. Active snails were blotted dry and weighed on an analytical balance to the nearest 0.1 mg, and each of the species was divided into two groups of approximately the same body mass. A group of control snails was sacrificed in the fully hydrated state. The second group of each species was placed in small individual plastic vials with perforated caps and exposed to normothermic desiccation (complete dryness) for up to 21 days at an ambient temperature of 25°C, within the temperature-controlled room (see details in Arad et al. 1989). For protein analysis, samples of three to five snails were sacrificed on days 1, 2, 3, 4, 7, and 10 of desiccation. The tissues (hepatopancreas, foot, and kidney) were dissected out and immediately frozen in liquid nitrogen. In a separate experiment that lasted 21 days, the extrapallial fluid was collected and analyzed for total osmolality (as an index of dehydration).
Sample processing for stress protein analysis
Frozen tissues were homogenized in ice-cold buffer containing 0.1 M NaCl, 20 mM Tris pH 7.4, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Igepal, 1 mM dithiothreitol (DTT), Protease Inhibitor Cocktail (Sigma P-8340), and 1 mM phenylmethylsulfonyl fluoride (PMSF). The homogenate was centrifuged (10 min, 17,000×g at 4°C), and total protein concentration in each supernatant was determined by a standard method (Bradford). The remaining pellet (the detergent-insoluble fraction that contains cytoskeletal components, nuclei, and aggregated proteins) was further processed to obtain the cytoskeletal–nuclear proteins. The pellet was solubilized in extraction solution (6 M urea, 0.46 M NaCl, 20 mM Tris pH 7.4, 1 mM EDTA, 1% Igepal, 1 mM DTT, and 1 mM PMSF), vortexed for 15 s, and incubated on ice for 10 min. The procedure was repeated three times; the homogenate was centrifuged (10 min, 17,000×g at 4°C), and the total protein concentration in the supernatant was determined by a standard method (Bradford).
Western blotting
Equivalent amounts of protein (35 μg) from tissue lysates prepared from individual snails were boiled in sample buffer containing DTT and loaded into each lane. Proteins were separated using sodium dodecyl sulfate–polyacrylamide gel with a 10% or 12% acrylamide gel and transferred onto nitrocellulose membrane. The membranes were probed with mouse monoclonal antibody against bovine brain Hsp70 (Sigma H-5147), recognizing both the constitutive (Hsc70, 73 kDa) and inducible (Hsp70, 72 kDa) forms of mammalian Hsp70, mouse monoclonal antibody against Hsp90 (Sigma H-1775), mouse monoclonal antibody against actin (Sigma A-4700), and rabbit polyclonal antibody against chicken Hsp25 (a gift from Professor Geiger, Weizmann Institute, Israel). Hsp70 antigen (72 kDa, 250 ng; StressMarq SPR-115A) and Hsp90 antigen (200 ng; Stressgen SR-P770) were simultaneously run to serve as standards for analysis of Hsp70 and Hsp90. The secondary antibodies were goat antimouse immunoglobulin G-horse radish peroxidase (IgG-HRP; Sigma A-2554) or goat antirabbit IgG-HRP (Santa Cruz Biotechnology sc-2004).
Protein quantification
The proteins were visualized by enhanced chemiluminescence, and the intensity of the bands was quantified using densitometry software (ImageJ). In the cases where two consecutive gels were combined, the intensity of the bands was related to the internal Hsp70 or Hsp90 standard to normalize the bands on different gels. In addition, three samples were simultaneously run on both gels in order to confirm the correctness of normalization. The obtained value of Hsp70, Hsp90, or Hsp25 in the cytosolic fraction was normalized to actin for the foot and kidney tissues. In the hepatopancreas, the level of actin significantly increased during desiccation in both species, so the obtained values of different HSPs were not normalized to actin. Relative endogenous levels of Hsp70 and Hsp90 in fully hydrated control snails were calculated by expressing the intensity of HSP bands in control snails relative to the intensity of the internal HSP standards.
Statistics
The results are reported as summation of three to four independent experiments and expressed as percentage change from control (mean values ± SE). Once data were confirmed to fit the validation criteria, the statistical analysis software program SPSS 16.0 was used to perform a one-way analysis of variance (ANOVA) with Bonferroni post hoc test for multiple comparisons. In case of changes in the expression of cytoskeletal–nuclear proteins and in case of mass and osmolality changes during normothermic desiccation experiments, the significance was verified by unpaired t test. In all the cases, p < 0.05 was considered significant.
Results
Normothermic desiccation
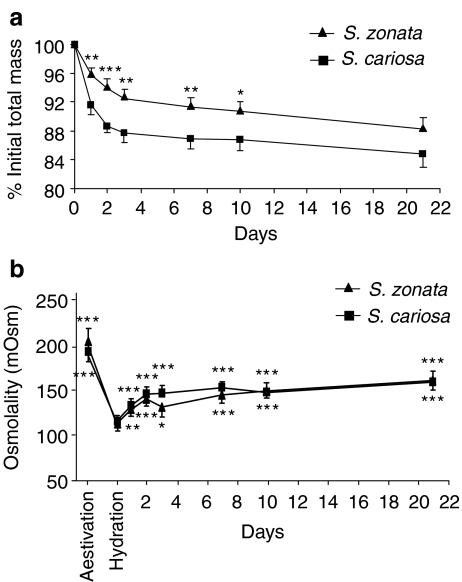
During 21 days of normothermic desiccation, S. zonata and S. cariosa lost 11.8 ± 1.1% and 15.2 ± 1.7%, respectively, of total mass (Fig. 1a). A major difference was established in the response during the first 2 days where S. zonata lost significantly less water than S. cariosa, 4.3 ± 0.3% vs. 8.4 ± 1.1% (p < 0.01), respectively, on day 1 and 6.1 ± 0.7% vs. 11.3 ± 0.7% (p < 0.001), respectively, on day 2. Thereafter, rate of change was moderated and after day 4 body mass practically stabilized for the rest of the period. The extrapallial fluid was collected and analyzed for total osmolality, as an index of dehydration (Fig. 1b). In both species, osmolality dropped significantly from its peak values during aestivation upon arousal on damp substrate (p < 0.0001). The osmolality significantly increased from day 1 of desiccation onward (p < 0.001 for S. cariosa and p < 0.01 for S. zonata). The high level reached at the end of 21 days of desiccation was still significantly lower than that in the aestivated snails (p < 0.05 for S. cariosa and p < 0.01 for S. zonata). There were no statistical differences between species at any time point.
Fig. 1.
Changes in mass and in extrapallial fluid osmolality during 21 days of normothermic desiccation in S. cariosa and S. zonata. a Changes in mass. The results are summation of two to three independent experiments (n = 21 snails for S. zonata and 32 snails for S. cariosa). All values are expressed as percentage of initial fully hydrated mass (mean ± SE). Asterisks denote significant differences between the two species (*p < 0.05; **p < 0.01; ***p < 0.001). b Changes in extrapallial fluid osmolality. All values are expressed as mean ± SE (n = 6 snails, except for aestivation group where n = 9–14 snails and rehydration group n = 4–6 snails). Asterisks denote significant differences from hydration group (*p < 0.05; **p < 0.01; ***p < 0.001)
Expression of Hsp70
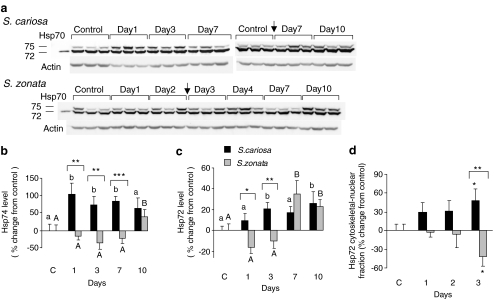
In the foot of both species, the monoclonal antibody to Hsp70 detected two bands of approximately 72 and 74 kDa. Both isoforms of Hsp70 were upregulated in S. cariosa foot during normothermic desiccation (Fig. 2a–c). Hsp74 was induced already on the first day (increase of 104 ± 32.4%, p < 0.01), while the 72-kDa band seems to be induced later, from day 3 onwards (20.2 ± 6.3%, p < 0.05 on day 3). In contrast, there was no induction of both isoforms in S. zonata foot in the first 3 days of desiccation. Only on day 7 (Hsp72, 34.8 ± 12.7%, p < 0.001) and day 10 (both isoforms, 22.7 ± 6.8% p < 0.01 for Hsp72; 38.8 ± 20% p < 0.05 for Hsp74) was there a significant upregulation of Hsp70 isoforms. Our results show a significant elevation in Hsp72 level in the cytoskeletal–nuclear fraction in S. cariosa but not in S. zonata foot in the first 3 days of desiccation (48.7 ± 17.8%, p < 0.05 on day 3; Fig. 2d). ANOVA tests between the two species revealed significant differences in the expression of Hsp70 isoforms in both the cytosolic and cytoskeletal–nuclear fraction (p < 0.001).
Fig. 2.
Expression of Hsp70 isoforms (Hsp72 and Hsp74) in the foot tissue of S. cariosa and S. zonata. Total protein was extracted from fully hydrated control and desiccated snails during 10 days of desiccation and subjected to Western blotting. a A representative blot for tissue lysate of three independent experiments is shown. Arrows denote the point of connection, where two consecutive gels were combined. b and c are graphical presentations of the relative levels of Hsp74 (b) and Hsp72 (c) in tissue lysates. n = 6–15 snails. Different letters above the bars denote significant differences within each species (lower case—S. cariosa; upper case—S. zonata). Asterisks denote significant differences between the two species (*p < 0.05; **p < 0.01; ***p < 0.001). d Relative levels of Hsp72 in the foot cytoskeletal–nuclear fraction. n = 7–8 snails. Asterisks denote significant differences within each species and also between the two species (*p < 0.05; **p < 0.01)
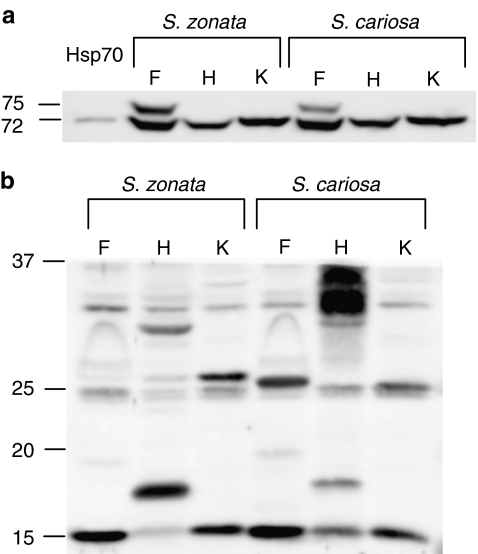
In the kidney and in the hepatopancreas, only the lower 72-kDa band appeared (Fig. 3a). In the kidney tissue, Hsp72 expression remained unchanged during the first 10 days of desiccation in both species. In the hepatopancreas tissue, Hsp72 expression was significantly increased in S. cariosa on day 10 (30.6 ± 11.8%, p < 0.05), whereas in S. zonata the level of Hsp72 remained unchanged (data not shown).
Fig. 3.
Expression of Hsp70 and sHSPs in the foot (F), kidney (K), and hepatopancreas (H) tissues of S. cariosa and S. zonata. Total protein was extracted from snail tissues and subjected to Western blotting using an anti-Hsp70 (a) and anti-Hsp25 (b) antibodies. Lanes contain equivalent amounts of protein from tissue extracts prepared from individual snails
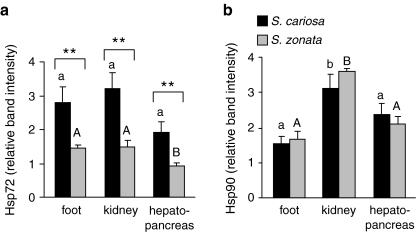
We also measured the relative endogenous levels of Hsp72 in the foot, kidney, and hepatopancreas tissues of fully hydrated control S. cariosa and S. zonata snails. As shown in Fig. 4a, the relative levels of Hsp72 synthesis were significantly higher in S. cariosa than in S. zonata in all the examined tissues (p < 0.001).
Fig. 4.
Relative endogenous levels of Hsp72 and Hsp90 in the foot, kidney, and hepatopancreas tissues of S. cariosa and S. zonata. Total protein was extracted from fully hydrated, control snails and subjected to Western blotting. Levels of Hsp72 (a) and Hsp90 (b) are expressed relative to an internal Hsp70 protein (250 ng) or Hsp90 protein (200 ng) standards. n = 8–14 snails. Different letters above the bars denote significant differences within each species (lower case—S. cariosa; upper case—S. zonata). Asterisks denote significant differences between the two species (**p < 0.01)
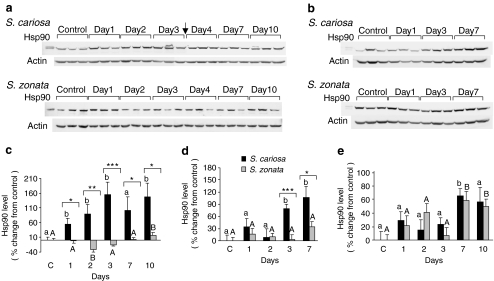
Expression of Hsp90
The expression of Hsp90 in the foot and kidney was significantly upregulated during desiccation in S. cariosa but remained unchanged or was only mildly induced in S. zonata (Fig. 5). In S. cariosa foot, Hsp90 was significantly upregulated already on the first day (55.7 ± 19.9%, p < 0.05; Fig. 5a, c). In contrast, in S. zonata foot, Hsp90 was mildly increased only on day 10 (16.7 ± 10.8%, p < 0.01). In the kidney, the expression of Hsp90 in S. cariosa was significantly upregulated from day 3 on (79.8 ± 10.2%, p < 0.01 on day 3) but remained unchanged in S. zonata (Fig. 5b, d). The two species differed significantly in the expression of Hsp90 in both tissues (p < 0.001).
Fig. 5.
Expression of Hsp90 in the foot, kidney, and hepatopancreas tissues of S. cariosa and S. zonata. Total protein was extracted from fully hydrated control and desiccated snails during 10 days of desiccation and subjected to Western blotting. A representative blot of three independent experiments in the foot (a) and kidney (b) is shown. Arrows denote the point of connection where two consecutive gels were combined. Relative levels of Hsp90 in the foot (c), kidney (d), and hepatopancreas (e) are shown. n = 6–15 snails. Different letters above the bars denote significant differences within each species (lower case—S. cariosa; upper case—S. zonata). Asterisks denote significant differences between the two species (*p < 0.05; **p < 0.01; ***p < 0.001)
In the hepatopancreas, Hsp90 expression was significantly increased during desiccation in both species on day 7 (65.2 ± 10.3%, p < 0.05 for S. cariosa; 58.1 ± 13.8%, p < 0.01 for S. zonata) and in S. zonata also on day 10 (49.6 ± 10.4%, p < 0.05; Fig. 5e).
In both species, relative levels of Hsp90 in the kidney of fully hydrated snails were significantly higher than in the foot (p < 0.01) and also significantly higher than in the hepatopancreas in S. zonata (p < 0.001; Fig. 4b).
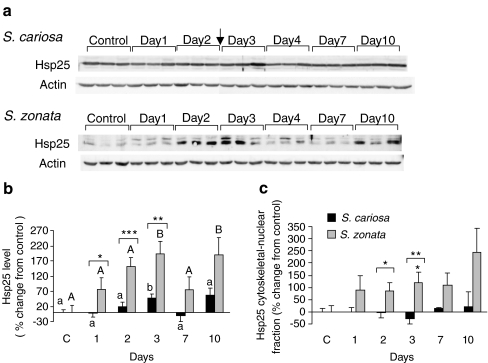
Expression of sHSPs
Western blots incubated with the polyclonal anti-Hsp25 antibody demonstrated the presence of four bands of approximately 15, 17, 25, and 30 kDa (Fig. 3b). In the foot tissue, normothermic desiccation affected the expression of Hsp25 in both the cytosolic and the cytoskeletal–nuclear fractions. In S. zonata, the expression of Hsp25 in both fractions was significantly enhanced (192.7 ± 41.0%, p < 0.01, on day 3 in the cytosolic fraction; 120.8 ± 43.1%, p < 0.05, on day 3 in the cytoskeletal–nuclear fraction), while in S. cariosa, the expression of Hsp25 in the cytosolic fraction was only mildly induced (49.7 ± 11.6%, p < 0.05 on day 3), and in the cytoskeletal–nuclear fraction it remained unchanged (Fig. 6). The two species demonstrated a significant difference in the expression of Hsp25 in both the cytosolic and cytoskeletal–nuclear fractions (p < 0.001). The expression of Hsp30 in the cytosolic fraction remained unchanged in both species (data not shown).
Fig. 6.
Expression of Hsp25 in the foot tissue of S. cariosa and S. zonata. Total protein was extracted from fully hydrated control and desiccated snails during 10 days of desiccation and subjected to Western blotting. a A representative blot for tissue lysate of three independent experiments is shown. Arrows denote the point of connection where two consecutive gels were combined. b Relative levels of Hsp25 protein in tissue lysate. n = 6–15 snails. Different letters above the bars denote significant differences within each species (lower case—S. cariosa; upper case—S. zonata). Asterisks denote significant differences between the two species (*p < 0.05; **p < 0.01; ***p < 0.001). c Relative levels of Hsp25 protein in the cytoskeletal–nuclear fraction. n = 3–8 snails. Asterisks denote significant differences within each species and also between the two species (*p < 0.05; **p < 0.01)
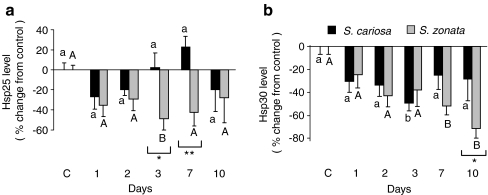
In the kidney tissue, both Hsp25 and Hsp30 expression significantly decreased in S. zonata during desiccation (−48.4 ± 11.4%, p < 0.05, on day 3 for HSP25; −52 ± 7.6%, p < 0.05, on day 7 for Hsp30) whereas in S. cariosa kidney Hsp30 expression decreased (−49.4 ± 6.9%, p < 0.01, on day 3), but Hsp25 expression was not affected (Fig. 7). The two species demonstrated a significant difference in the expression of Hsp25 (p < 0.01) and Hsp30 (p < 0.05).
Fig. 7.
Expression of sHSPs in the kidney tissue of S. cariosa and S. zonata. Total protein was extracted from fully hydrated control and desiccated snails during 10 days of desiccation and subjected to Western blotting. a Relative levels of Hsp25. b Relative levels of Hsp30. n = 6–15 snails. Different letters above the bars denote significant differences within each species (lower case—S. cariosa; upper case—S. zonata). Asterisks denote significant differences between the two species (*p < 0.05; **p < 0.01)
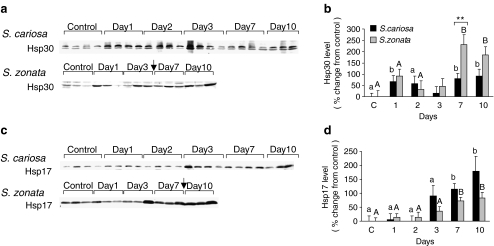
In the hepatopancreas, Hsp25 expression was not affected by desiccation (data not shown). The expression of Hsp30 in S. cariosa and in S. zonata was upregulated on day 1 (67.3 ± 26.2%, p < 0.05 and 91.9 ± 30.7%, ns, respectively) and from day 7 on (79.7 ± 23.7%, p < 0.05 and 230.3 ± 45.2%, p < 0.001, respectively; Fig. 8a, b). The two species demonstrated a significant difference in the expression of Hsp30 (p < 0.05). In both species, a band of approximately 17 kDa was significantly increased during desiccation from day 7 on (114.3 ± 20.5%, p < 0.05, in S. cariosa; 71.5 ± 14.4%, p < 0.05 in S. zonata; Fig. 8c, d).
Fig. 8.
Expression of sHSPs in the hepatopancreas tissue of S. cariosa and S. zonata. Total protein was extracted from fully hydrated control and desiccated snails during 10 days of desiccation and subjected to Western blotting. a, c A representative blot of three independent experiments is shown. Arrows denote the point of connection where two consecutive gels were combined. b Levels of Hsp30 protein. d Levels of Hsp17 protein. n = 6–15 snails of both species. Different letters above the bars denote significant differences within each species (lower case—S. cariosa; upper case—S. zonata). Asterisks denote significant differences between the two species (**p < 0.01)
Discussion
The desert-inhabiting S. zonata seems to have developed effective regulatory mechanisms to withstand extreme environmental conditions (Machin 1967; Schmidt-Nielsen et al. 1972). In an interspecific study of Sphincterochila, Arad et al. (1989) showed that S. zonata was the most resistant species to desiccation, characterized by the lowest rates of water loss, thickest epiphragm, lowest epiphragm-area-specific water vapor conductance, and the most favorable surface-to-volume ratio, compared to all other species, including the Mediterranean-type S. cariosa. The present study was designed to examine the cellular stress response machinery of Sphincterochila snails that allow them to cope with desiccation. Specifically, we aimed to find whether the difference in desiccation resistance between S. cariosa and S. zonata is correlated with differential upregulation of the HSP response. Our study suggests that species that differ in their resistance to desiccation developed distinct strategies of HSP expression in response to desiccation that reflect the difference in aridity encountered in their natural habitats.
In the present study, the relative endogenous levels of Hsp72 in all the tissues examined were significantly higher in S. cariosa than in S. zonata. These results suggest that the desiccation-sensitive S. cariosa snails maintain larger pools of Hsp72 compared to the desiccation-resistant S. zonata and are in line with other interspecific studies demonstrating higher endogenous levels of Hsp70 in the more thermally sensitive species compared to the heat-resistant ones. Thus, Tomanek and Somero (2002) studied three congeneric marine snails of the marine subtidal to intertidal gastropod Tegula differing in their heat tolerance and found higher endogenous levels of Hsp74 (yet lower levels of Hsp72) in the heat-sensitive species compared to the heat-tolerant ones in field-acclimatized specimens. Likewise, studies in northeastern pacific Nucella species revealed lower levels of Hsp70 in the more thermotolerant Nucella emarginata than in the less thermotolerant species (Sorte and Hofmann 2005). It is important to note, however, that other interspecific studies revealed correlations between a greater heat tolerance and higher constitutive levels of HSPs (Evgen’ev et al. 2007). Zatsepina et al (2000), for example, found higher levels of both Hsp70 mRNA and protein in desert-dwelling lizard species than in nondesert species. Similarly, in study in four limpets of the genus Lottia, Dong et al. (2008) found higher constitutive levels of Hsp70 in the two high-intertidal species compared to the low and mid-intertidal species. We are currently performing field experiments in order to determine if Sphincterochila snails in the natural habitat reveal similar HSP responses as those held under laboratory conditions. Preliminary results (unpublished) in snails collected after the first rains indicate that also in nature S. cariosa is characterized by higher pools of Hsp70 in both the foot and kidney tissues.
Interspecific comparisons of ectothermic species from different latitudes have also shown that, typically, species adapted to higher temperature niche were more heat tolerant, induced synthesis of HSPs such as Hsp70 and Hsp90 at higher temperatures, and had higher upper thermal limits of protein synthesis (Hofmann and Somero 1996; Tomanek and Somero 1999; Nakano and Iwama 2002; Evgen’ev et al. 2007). In this context, Shabtay and Arad (2005) demonstrated that heat-resistant fowl strains are characterized by a delayed induction of the HSP machinery (including activation of heat shock transcription factor (HSF), transcription and translation of HSP) compared to heat-sensitive strains. Thus, the observed delay in the Hsp70 and Hsp90 response to desiccation in the foot tissue of S. zonata compared to S. cariosa resembles the variation in the HSP response to heat between species that differ in their resistance to heat. The finding that in the kidney tissue Hsp90 was induced only in S. cariosa further supports this assumption. In contrast to S. zonata foot, Hsp70 and Hsp90 were already upregulated in S. cariosa foot during the first days of desiccation. The first 3 days of aestivation in land snails are critical to the transition from active state to dormancy. At this time window, changes in the degree of enzyme phosphorylation occur (Storey 2002; Ramnanan et al. 2007), respiration falls, and the metabolic rate decreases. Our study shows that the major difference in water loss between the two species was established also during the first 3 days of desiccation. Thus, the rapid increase in Hsp70 and Hsp90 synthesis in S. cariosa during the early stages of desiccation suggests that these proteins play a role not only in protecting proteins from desiccation stress but might also be important components of the aestivation mechanism. Further support for this assumption came from the finding that the level of Hsp72 in the cytoskeletal–nuclear fraction in S. cariosa foot increased during the first 3 days of desiccation, due to either de novo synthesis and/or translocation of Hsp72 from the cytoplasm. Previous studies demonstrated translocation of Hsp70 into the nucleus during heat shock and oxidative stress (Dastoor and Dreyer 2000; Adhikari et al. 2004). Alastalo et al. (2003) proposed that this regulated subcellular distribution of Hsp70 is an important regulatory mechanism of HSF-1-mediated heat shock response. We suggest that in resemblance to heat stress, in desiccation stress, Hsp70 proteins may play important roles in cellular processes that take place in both the cytoplasmic and nuclear compartments. Another observation concerning Hsp70 expression in S. cariosa foot was that although both isoforms of Hsp70 were upregulated in response to desiccation, Hsp74 was induced earlier and to a higher magnitude than Hsp72. These results, together with the finding that Hsp72 had higher constitutive level than Hsp74 in the foot of control snails, suggest that, in Sphincterochila snails, Hsp74 is the inducible form of Hsp70 and Hsp72 is the constitutive form.
While desiccation stress induced Hsp72 and Hsp74 in the foot tissue, in the kidney and in the hepatopancreas the level of Hsp72 remained unchanged or only mildly induced. Tissue-specific variation in the expression of Hsp70 proteins is known to occur in both vertebrates and invertebrates. In the land snail O. lactea, Hsp70 protein was induced by aestivation in the foot tissue but not in the hepatopancreas (Brooks and Storey 1995). Scott et al. (2003) found a tissue-specific pattern of Hsp70 isoforms expression in the western painted turtle during both unstressed and stressed conditions. Similarly, the levels of Hsp70, Hsp72, and Hsp78 in the gill, mantle, and adductor tissues in Mytilus edulis showed variation in both the magnitude of induction by heat stress and in their basal level in unstressed tissues (Chapple et al. 1997). Other studies suggested that the Hsp70 response is specific to those organs undergoing stress (Flanagan et al. 1995; Krebs and Feder 1997b). One reason for the presence of two members of the Hsp70 family in the foot tissue and for the observed upregulation of Hsp70 proteins in the foot and not in the kidney and hepatopancreas during the first 7 days of desiccation may be related to its being “in the front,” directly facing environmental stress. Thus, the synthesis of both members of the Hsp70 family in the foot is necessary for survival under environmental stress conditions, while the increased synthesis of only one Hsp70 protein is sufficient for a stress response in the inner organs maintained at a rather constant environment. Similarly, Ulmasov et al. (1993) found that the lymphocytes of the desert camel Camelus dromedarius are able to respond to an increased temperature by the increased synthesis of only a constitutive member of the Hsp70 family, whereas the skin fibroblasts which are exposed to the environment can also synthesize the inducible form. However, the observed tissue-specific variability in Hsp70 induction during desiccation may also reflect a differential involvement of Hsp70 in the transition processes from active to dormant states in the different tissues.
In both species, the relative endogenous levels of hsp90 in the kidney were significantly higher than in the foot and in the hepatopancreas, suggesting that Hsp90 may have important roles in processes that take place in the kidney. In addition to participating in the cell stress response in refolding denatured proteins, Hsp90 proteins have specific roles in signal transduction pathways such as cell growth, differentiation, and survival. Hsp90 client proteins include, for example, steroid hormone receptors and several protein kinases (Pratt and Toft 2003). In previous studies, Hsp90 was found to be induced in the kidney by stressors such as nephrotoxic agents and heat (Beck et al. 2000) and in the liver and hepatopancreas by heat and desiccation stress (Brooks and Storey 1995; Jiang et al. 2009). The dual involvement of Hsp90 in stress response and in signal transduction raises the possibility that Hsp90 may participate in several specific processes during desiccation stress. For example, the involvement of Hsp90 in regulation of protein phosphorylation and in regulation of sodium (Ramirez et al. 2004) is consistent with the changes in enzyme phosphorylation and the increase in extrapallial fluid osmolality observed in aestivating snails and further supports this assumption.
Our study also highlights the importance of sHSPs in processes accompanying desiccation. sHSPs comprise the most widespread but also the most poorly conserved family of molecular chaperones (Haslbeck et al. 2005). Their variability in sequence and size was demonstrated in our study, as we found considerable variation in the size of proteins detected by the polyclonal antibody to Hsp25, not only between the different tissues but also between the two species. Most sHSPs share characteristic features, including a conserved α-crystallin domain, formation of large oligomers, induction by stress conditions, and chaperone activity in suppressing protein aggregation (Jakob et al. 1993; Morrow et al. 2006). Hsp27 and Hsp30 were found to be involved in development, cell cycle, and cell differentiation (Heikkila 2004; Parcellier et al. 2005), and their expression was induced by different stressors including heat shock (Mirkes et al. 1996; Norris et al. 1997; Mulligan-Tuttle and Heikkila 2007) and hypertonic stress (Dasgupta et al. 1992; Neuhofer et al. 1998). Overexpression of sHSPs including Hsp27 and α-crystallin has been shown to confer resistance to heat-shock as well as to oxidative stress in a number of organisms and cell types (Rollet et al. 1992; Mehlen et al. 1995).
As discussed earlier in the text, previous studies demonstrated upregulation of sHSPs during stress conditions associated with lack of water in different organisms. The observed increase in sHSPs level during desiccation in our study is in line with these studies and further emphasizes the importance of sHSPs during organismal stress response. One of the most intriguing observations in the present study is the finding that the response of both Hsp25 and Hsp30 during desiccation was more pronounced in S. zonata compared to S. cariosa, in opposite to the more prominent response of both Hsp70 and Hsp90 in S. cariosa. In the foot of S. zonata, Hsp25 was induced during the first days of desiccation and to a greater extent compared to the mild induction detected in S. cariosa. Furthermore, desiccation stress also induced the expression of Hsp25 in the cytoskeletal–nuclear fraction in S. zonata foot. Previous studies found that heat shock, ischemia, and other stressors induce the translocation of Hsp27 and Hsp25 (rodent homolog of human Hsp27) to the nucleus and to the cytoskeletal fraction (Lavoie et al. 1993; Bryantsev et al. 2002; Adhikari et al. 2004). Hsp25/27 were found to interact with and stabilize certain components of the cytoskeleton such as actin, thus preventing disruption of the cytoskeleton resulting from stresses. Taken together, these findings suggest a biological function of Hsp25 in the foot tissue during desiccation stress, either in the mechanism regulating cytoskeleton stabilization and/or in chaperoning activity in the cytoplasm and in the nucleus. Previous study in the crustacean Artemia supports this assumption. Clegg et al. (1994) detected massive amounts (about 15% of the total nonyolk embryo protein) of the sHSP p26 in the stress-resistant encysted embryos of Artemia. They also found that p26 undergoes translocation to the nucleus during anoxia, heat shock, and in diapause embryos and suggested that this protein may play the role of a metabolic regulator and/or a protective molecular chaperone.
The observed decrease in cytosolic Hsp25 and Hsp30 levels in the kidney during desiccation is in line with other studies demonstrating reduction of soluble Hsp25 and the migration of Hsp25 into the cytoskeletal–nuclear fraction during renal ischemia (Schober et al. 1997; Aufricht et al. 1998). The involvement of Hsp25/27 in cytoskeleton stabilization following stresses such as renal ischemia and high concentration of NaCl (Aufricht et al. 1998; Neuhofer et al. 1998; Beck et al. 2000) and in protecting medullary cells against the deleterious effects of high urea concentrations (Ohno et al. 1997; Neuhofer et al. 2005) suggests possible roles for sHSPs in protecting kidney tissue from the deleterious effects of high urea concentration and osmolality accompanying desiccation in land snails (Arad 2001).
In the hepatopancreas, in addition to Hsp30, desiccation stress induced also the expression of a protein of approximately 17 kDa. sHSPs in the size range of 15–18 kDa were previously shown to be induced by heat shock in fungi extracts (Pisolithus sp.; Ferreira et al. 2005) and implicated not only in chaperoning of proteins but also in the development of thermotolerance and in stabilization of lipid membranes (Lee et al. 2000; Torok et al. 2001). Although the question whether the observed 17 kDa protein in the present study belongs to the HSP family is still open, its tissue-specific expression and significant induction in the hepatopancreas of both species suggest an important role of this protein in cellular processes following desiccation. In the case that this protein belongs to the HSP family, we suggest that it is needed in the hepatopancreas not only as chaperone but also in stabilizing the cell membrane.
The findings that, in all the tissues examined, sHSP responses were more pronounced in S. zonata compared to S. cariosa suggest a distinct strategy of activating sHSPs in the desert snail. The suggestion that organisms use sHSPs to adapt to extreme environmental conditions is supported by other interspecific studies. Sanders et al. (1991 ) studied two limpets that occupy different intertidal habitats and found in the heat-tolerant species, but not in the more temperature-sensitive one, an induction of a complex group of low molecular weight stress proteins in response to heat shock. Similarly, a study in three Drosophila species showed that the most thermotolerant species surpassed thermosensitive species in the level of sHSPs throughout the temperature range tested (Shilova et al. 2006), and study in the blue mussel Mytilus showed induction of a 30-kDa stress protein in response to heat stress in the heat-resistant Mytilus galloprovincialis but not in the more thermally-sensitive Mytilus trossulus (Hofmann and Somero 1996). Furthermore, not only resistance to heat but also resistance to dehydration was correlated with the presence of sHSPs (Ravindran et al. 2005).
In summary, this study has demonstrated differences in the HSP response to experimental desiccation stress in nonmodel species of land snails that differ in their resistance to desiccation. The desert-inhabiting S. zonata, which is naturally exposed to extreme environmental conditions, exhibits a delayed response of Hsp72, Hsp74, and Hsp90 to desiccation stress, together with an enhanced response of sHSPs. In contrast, the Mediterranean-type, desiccation-sensitive species S. cariosa maintains larger pools of Hsp72 and has high inducible synthesis of Hsp72, Hsp74, and Hsp90, but only a moderate response of sHSPs. Upregulation of HSPs may enhance survival under stress exposure by rescuing critical proteins and reducing the energetic cost associated with protein damage but can also be deleterious for growth and development. For example, Krebs and Feder (1997a) found deleterious effects of Hsp70 overexpression in Drosophila melanogaster larvae. Thus, HSP expression may incur fitness costs on individuals that regularly experience environmental stress, suggesting that evolution in harsh environments will result in selection for reduced HSP expression. Indeed, other studies found reduced expression and slower kinetics of Hsp70 in strain of D. melanogaster adapted to constant comparatively high temperature than in a standard wild-type strain and suggested that the reduction in Hsp70 expression may stem from insertion of transposable elements in the hsp70 gene promoter (Bettencourt et al. 1999; Zatsepina et al. 2001; Lerman et al. 2003). Feder (1999) summarizes that the deleterious consequences of overexpression of HSPs “are clear and apparently trade off against the benefits in the evolutionary sense, either constraining directional selection for increased HSP expression or necessitating especially effective autoregulation of Hsp expression.” Our results support this position, showing a lower expression of Hsp70 and a limited use of Hsp70 and Hsp90 in desert snails in response to desiccation stress. Our study also indicates that these energy costs vary for different tissues and under different physiological conditions. We suggest that this differential HSP response reflects the relative vulnerability and the physiological function of different tissues, with likely consequences for the ecology and distribution of different land snails.
Acknowledgements
We thank Dr. Ariel Shabtay for his comments. This work was supported by the Israel Science Foundation grant no. 1125/07.
References
- Adhikari AS, Sridhar Rao K, Rangaraj N, Parnaik VK, Mohan Rao C. Heat stress-induced localization of small heat shock proteins in mouse myoblasts: intranuclear lamin A/C speckles as target for alpha B-crystallin and Hsp25. Exp Cell Res. 2004;299:393–403. doi: 10.1016/j.yexcr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Alastalo TP, Hellesuo M, Sandqvist A, Hietakangas V, Kallio M, Sistonen L. Formation of nuclear stress granules involves HSF2 and coincides with the nucleolar localization of Hsp70. J Cell Sci. 2003;116:3557–3570. doi: 10.1242/jcs.00671. [DOI] [PubMed] [Google Scholar]
- Arad Z. Desiccation and rehydration in land snails—a test for distinct set points in Theba pisana. Isr J Zool. 2001;47:41–53. [Google Scholar]
- Arad Z, Goldenberg S, Heller J. Comparative water economy in five species of Sphincterochila. J Zool (Lond) 1989;218:353–364. doi: 10.1111/j.1469-7998.1989.tb02549.x. [DOI] [Google Scholar]
- Arad Z, Goldenberg S, Heller J. Intraspecific variation in resistance to desiccation and climatic gradients in the distribution of the land snail Xeropicta vestalis. J Zool (Lond) 1992;226:643–656. doi: 10.1111/j.1469-7998.1992.tb07507.x. [DOI] [Google Scholar]
- Arad Z, Goldenberg S, Heller J. Intraspecific variation in resistance to desiccation and climatic gradients in the distribution of the bush-dwelling land snail Trochoidea simulata. J Zool (Lond) 1993;229:249–265. doi: 10.1111/j.1469-7998.1993.tb02634.x. [DOI] [Google Scholar]
- Aufricht C, Ardito T, Thulin G, Kashgarian M, Siegel NJ, Why SK. Heat-shock protein 25 induction and redistribution during actin reorganization after renal ischemia. Am J Physiol. 1998;274:F215–F222. doi: 10.1152/ajprenal.1998.274.1.F215. [DOI] [PubMed] [Google Scholar]
- Beck FX, Neuhofer W, Muller E. Molecular chaperones in the kidney: distribution, putative roles, and regulation. Am J Physiol Renal Physiol. 2000;279:F203–F215. doi: 10.1152/ajprenal.2000.279.2.F203. [DOI] [PubMed] [Google Scholar]
- Bettencourt BR, Feder ME, Cavicchi S. Experimental evolution of HSP70 expression and thermotolerance in Drosophila melanogaster. Evolution. 1999;53:484–492. doi: 10.2307/2640784. [DOI] [PubMed] [Google Scholar]
- Boon-Niermeijer EK, Tuyl M, Scheur H. Evidence for two states of thermotolerance. Int J Hyperthermia. 1986;2:93–105. doi: 10.3109/02656738609019998. [DOI] [PubMed] [Google Scholar]
- Boon-Niermeijer EK, Souren JE, Wijk R. Thermotolerance induced by 2, 4-dinitrophenol. Int J Hyperthermia. 1987;3:133–141. doi: 10.3109/02656738709140381. [DOI] [PubMed] [Google Scholar]
- Boon-Niermeijer EK, Souren JE, Waal AM, Wijk R. Thermotolerance induced by heat and ethanol. Int J Hyperthermia. 1988;4:211–222. doi: 10.3109/02656738809029311. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Storey KB. Evidence for aestivation specific proteins in Otala lactea. Mol Cell Biochem. 1995;143:15–20. doi: 10.1007/BF00925922. [DOI] [PubMed] [Google Scholar]
- Bryantsev AL, Loktionova SA, Ilyinskaya OP, Tararak EM, Kampinga HH, Kabakov AE. Distribution, phosphorylation, and activities of Hsp25 in heat-stressed H9c2 myoblasts: a functional link to cytoprotection. Cell Stress Chaperones. 2002;7:146–155. doi: 10.1379/1466-1268(2002)007<0146:DPAAOH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple JP, Smerdon GR, Hawkins AJS. Stress-70 protein induction in Mytilus edulis: tissue-specific responses to elevated temperature reflect relative vulnerability and physiological function. J Exp Mar Biol Ecol. 1997;217:225–235. doi: 10.1016/S0022-0981(97)00057-9. [DOI] [Google Scholar]
- Clegg JS, Jackson SA, Warner AH. Extensive intracellular translocations of a major protein accompany anoxia in embryos of Artemia franciscana. Exp Cell Res. 1994;212:77–83. doi: 10.1006/excr.1994.1120. [DOI] [PubMed] [Google Scholar]
- Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/S0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Hohman TC, Carper D. Hypertonic stress induces alpha B-crystallin expression. Exp Eye Res. 1992;54:461–470. doi: 10.1016/0014-4835(92)90058-Z. [DOI] [PubMed] [Google Scholar]
- Dastoor Z, Dreyer J. Nuclear translocation and aggregate formation of heat shock cognate protein 70 (Hsc70) in oxidative stress and apoptosis. J Cell Sci. 2000;113(Pt 16):2845–2854. doi: 10.1242/jcs.113.16.2845. [DOI] [PubMed] [Google Scholar]
- Dietz TJ, Somero GN. The threshold induction temperature of the 90-kDa heat shock protein is subject to acclimatization in eurythermal goby fishes (genus Gillichthys) Proc Natl Acad Sci U S A. 1992;89:3389–3393. doi: 10.1073/pnas.89.8.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Miller LP, Sanders JG, Somero GN. Heat-shock protein 70 (Hsp70) expression in four limpets of the genus Lottia: interspecific variation in constitutive and inducible synthesis correlates with in situ exposure to heat stress. Biol Bull. 2008;215:173–181. doi: 10.2307/25470698. [DOI] [PubMed] [Google Scholar]
- Easton DP, Rutledge PS, Spotila JR. Heat shock protein induction and induced thermal tolerance are independent in adult salamanders. J Exp Zool. 1987;241:263–267. doi: 10.1002/jez.1402410214. [DOI] [PubMed] [Google Scholar]
- Edgerly JS, Tadimalla A, Dahlhoff EP. Adaptation to thermal stress in lichen-eating web spinners (Embioptera): habitat choice, domicile construction and the potential role of heat shock proteins. Funct Ecol. 2005;19:255–262. doi: 10.1111/j.1365-2435.2005.00957.x. [DOI] [Google Scholar]
- Evgen’ev MB, Garbuz DG, Shilova VY, Zatsepina OG. Molecular mechanisms underlying thermal adaptation of xeric animals. J Biosci. 2007;32:489–499. doi: 10.1007/s12038-007-0048-6. [DOI] [PubMed] [Google Scholar]
- Feder ME. Organismal, ecological, and evolutionary aspects of heat-shock proteins and the stress response: established conclusions and unresolved issues. Am Zool. 1999;39:857–864. [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Feder ME, Bedford TB, Albright DR, Michalak P. Evolvability of Hsp70 expression under artificial election for inducible thermotolerance in independent populations of Drosophila melanogaster. Physiol Biochem Zool. 2002;75:325–334. doi: 10.1086/342350. [DOI] [PubMed] [Google Scholar]
- Ferreira AS, Totola MR, Kasuya MCM, Araujo EF, Borges AC. Small heat shock proteins in the development of thermotolerance in Pisolithus sp. J Therm Biol. 2005;30:595–602. doi: 10.1016/j.jtherbio.2005.08.004. [DOI] [Google Scholar]
- Flanagan SW, Ryan AJ, Gisolfi CV, Moseley PL. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Physiol. 1995;268:R28–R32. doi: 10.1152/ajpregu.1995.268.1.R28. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Hayward SA, Rinehart JP, Denlinger DL. Desiccation and rehydration elicit distinct heat shock protein transcript responses in flesh fly pupae. J Exp Biol. 2004;207:963–971. doi: 10.1242/jeb.00842. [DOI] [PubMed] [Google Scholar]
- Heikkila JJ. Regulation and function of small heat shock protein genes during amphibian development. J Cell Biochem. 2004;93:672–680. doi: 10.1002/jcb.20237. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Somero GN. Interspecific variation in thermal denaturation of proteins in the congeneric mussels Mytilus trossulus and M. galloprovincialis: evidence from the heat-shock response and protein ubiquitination. Mar Biol. 1996;126:65–75. doi: 10.1007/BF00571378. [DOI] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Jiang S, Qiu L, Zhou F, Huang J, Guo Y, Yang K. Molecular cloning and expression analysis of a heat shock protein (Hsp90) gene from black tiger shrimp (Penaeus monodon) Mol Biol Rep. 2009;36:127–134. doi: 10.1007/s11033-007-9160-9. [DOI] [PubMed] [Google Scholar]
- King YT, Lin CS, Lin JH, Lee WC. Whole-body hyperthermia-induced thermotolerance is associated with the induction of heat shock protein 70 in mice. J Exp Biol. 2002;205:273–278. doi: 10.1242/jeb.205.2.273. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Deleterious consequences of Hsp70 overexpression in Drosophila melanogaster larvae. Cell Stress Chaperones. 1997;2:60–71. doi: 10.1379/1466-1268(1997)002<0060:DCOHOI>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Tissue-specific variation in Hsp70 expression and thermal damage in Drosophila melanogaster larvae. J Exp Biol. 1997;200:2007–2015. doi: 10.1242/jeb.200.14.2007. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- Lee S, Owen HA, Prochaska DJ, Barnum SR. HSP16.6 is involved in the development of thermotolerance and thylakoid stability in the unicellular cyanobacterium, Synechocystis sp. PCC 6803. Curr Microbiol. 2000;40:283–287. doi: 10.1007/s002849910056. [DOI] [PubMed] [Google Scholar]
- Lerman DN, Michalak P, Helin AB, Bettencourt BR, Feder ME. Modification of heat-shock gene expression in Drosophila melanogaster populations via transposable elements. Mol Biol Evol. 2003;20:135–144. doi: 10.1093/molbev/msg015. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lopez-Martinez G, Benoit JB, Rinehart JP, Elnitsky MA, Lee RE, Jr, Denlinger DL. Dehydration, rehydration, and overhydration alter patterns of gene expression in the Antarctic midge, Belgica antarctica. J Comp Physiol B. 2009;179:481–491. doi: 10.1007/s00360-008-0334-0. [DOI] [PubMed] [Google Scholar]
- Machin J. Structural adaptation for reducing water-loss in three species of terrestrial snails. J Zool Lond. 1967;152:55–65. doi: 10.1111/j.1469-7998.1967.tb01638.x. [DOI] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo AP. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:363–374. [PubMed] [Google Scholar]
- Mirkes PE, Little SA, Cornel L, Welsh MJ, Laney TN, Wright FH. Induction of heat shock protein 27 in rat embryos exposed to hyperthermia. Mol Reprod Dev. 1996;45:276–284. doi: 10.1002/(SICI)1098-2795(199611)45:3<276::AID-MRD3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Morrow G, Heikkila JJ, Tanguay RM. Differences in the chaperone-like activities of the four main small heat shock proteins of Drosophila melanogaster. Cell Stress Chaperones. 2006;11:51–60. doi: 10.1379/CSC-166.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol. 1997;83:1413–1417. doi: 10.1152/jappl.1997.83.5.1413. [DOI] [PubMed] [Google Scholar]
- Mulligan-Tuttle A, Heikkila JJ. Expression of the small heat shock protein gene, hsp30, in Rana catesbeiana fibroblasts. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:308–316. doi: 10.1016/j.cbpa.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Nakano K, Iwama G. The 70-kDa heat shock protein response in two intertidal sculpins, Oligocottus maculosus and O. snyderi: relationship of hsp70 and thermal tolerance. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:79–94. doi: 10.1016/S1095-6433(02)00115-0. [DOI] [PubMed] [Google Scholar]
- Neuhofer W, Muller E, Burger-Kentischer A, Beck FX. Hypertonicity affects heat shock protein 27 and F-actin localization in Madin-Darby canine kidney cells. Kidney Int Suppl. 1998;67:S165–S167. doi: 10.1046/j.1523-1755.1998.06735.x. [DOI] [PubMed] [Google Scholar]
- Neuhofer W, Fraek ML, Ouyang N, Beck FX. Differential expression of heat shock protein 27 and 70 in renal papillary collecting duct and interstitial cells—implications for urea resistance. J Physiol. 2005;564:715–722. doi: 10.1113/jphysiol.2004.081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CE, diIorio PJ, Schultz RJ, Hightower LE. Variation in heat shock proteins within tropical and desert species of poeciliid fishes. Mol Biol Evol. 1995;12:1048–1062. doi: 10.1093/oxfordjournals.molbev.a040280. [DOI] [PubMed] [Google Scholar]
- Norris CE, Brown MA, Hickey E, Weber LA, Hightower LE. Low-molecular-weight heat shock proteins in a desert fish (Poeciliopsis lucida): homologs of human Hsp27 and Xenopus Hsp30. Mol Biol Evol. 1997;14:1050–1061. doi: 10.1093/oxfordjournals.molbev.a025711. [DOI] [PubMed] [Google Scholar]
- Ohno A, Muller E, Fraek ML, Thurau K, Beck F. Solute composition and heat shock proteins in rat renal medulla. Pflugers Arch. 1997;434:117–122. doi: 10.1007/s004240050371. [DOI] [PubMed] [Google Scholar]
- Pakay JL, Withers PC, Hobbs AA, Guppy M. In vivo downregulation of protein synthesis in the snail Helix aspersa during estivation. Am J Physiol Regul Integr Comp Physiol. 2002;283:R197–204. doi: 10.1152/ajpregu.00636.2001. [DOI] [PubMed] [Google Scholar]
- Parcellier A, Schmitt E, Brunet M, Hammann A, Solary E, Garrido C. Small heat shock proteins HSP27 and alpha B-crystallin: cytoprotective and oncogenic functions. Antioxid Redox Signal. 2005;7:404–413. doi: 10.1089/ars.2005.7.404. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Ramirez V, Uribe N, Garcia-Torres R, Castro C, Rubio J, Gamba G, Bobadilla NA. Upregulation and intrarenal redistribution of heat shock proteins 90alpha and 90beta by low-sodium diet in the rat. Cell Stress Chaperones. 2004;9:198–206. doi: 10.1379/CSC-22R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnanan CJ, Groom AG, Storey KB. Akt and its downstream targets play key roles in mediating dormancy in land snails. Comp Biochem Physiol B Biochem Mol Biol. 2007;148:245–255. doi: 10.1016/j.cbpb.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Ramnanan CJ, Allan ME, Groom AG, Storey KB. Regulation of global protein translation and protein degradation in aerobic dormancy. Mol Cell Biochem. 2009;323:9–20. doi: 10.1007/s11010-008-9959-2. [DOI] [PubMed] [Google Scholar]
- Ravindran RK, Tablin F, Crowe JH, Oliver AE. Resistance to dehydration damage in hela cells correlates with the presence of endogenous heat shock proteins. Cell Preserv Technol. 2005;3:155–164. doi: 10.1089/cpt.2005.3.155. [DOI] [Google Scholar]
- Riddle WA. Physiological ecology of land snails and slugs. In: Russell-Hunter WD, editor. The Mollusca. London: Academic; 1983. pp. 431–461. [Google Scholar]
- Rollet E, Lavoie JN, Landry J, Tanguay RM. Expression of Drosophila’s 27 kDa heat shock protein into rodent cells confers thermal resistance. Biochem Biophys Res Commun. 1992;185:116–120. doi: 10.1016/S0006-291X(05)80963-5. [DOI] [PubMed] [Google Scholar]
- Sanders BM, Hope C, Pascoe VM, Martin LS. Characterization of the stress protein response in two species of Collisella limpets with different temperature tolerances. Physiol Zool. 1991;64:1471–1489. [Google Scholar]
- Schill RO, Steinbruck GH, Kohler HR. Stress gene (hsp70) sequences and quantitative expression in Milnesium tardigradum (Tardigrada) during active and cryptobiotic stages. J Exp Biol. 2004;207:1607–1613. doi: 10.1242/jeb.00935. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K, Taylor CR, Shkolnik A. Desert snails: problems of heat, water and food. J Exp Biol. 1971;55:385–398. doi: 10.1242/jeb.55.2.385. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K, Taylor CR, Shkolnik A. Desert snail: problems of survival. Symp Zool Soc Lond. 1972;31:1–13. [Google Scholar]
- Schober A, Muller E, Thurau K, Beck FX. The response of heat shock proteins 25 and 72 to ischaemia in different kidney zones. Pflugers Arch. 1997;434:292–299. doi: 10.1007/s004240050399. [DOI] [PubMed] [Google Scholar]
- Scott MA, Locke M, Buck LT. Tissue-specific expression of inducible and constitutive Hsp70 isoforms in the western painted turtle. J Exp Biol. 2003;206:303–311. doi: 10.1242/jeb.00107. [DOI] [PubMed] [Google Scholar]
- Shabtay A, Arad Z. Ectothermy and endothermy: evolutionary perspectives of thermoprotection by HSPs. J Exp Biol. 2005;208:2773–2781. doi: 10.1242/jeb.01705. [DOI] [PubMed] [Google Scholar]
- Shilova VY, Garbuz DG, Evgen’ev MB, Zatsepina OG. Small heat shock proteins and adaptation of various Drosophila species to hyperthermia. Mol Biol. 2006;40:235–239. doi: 10.1134/S0026893306020087. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Yaffe MP. Uncoupling thermotolerance from the induction of heat shock proteins. Proc Natl Acad Sci U S A. 1991;88:11091–11094. doi: 10.1073/pnas.88.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero GN. Proteins and temperature. Annu Rev Physiol. 1995;57:43–68. doi: 10.1146/annurev.ph.57.030195.000355. [DOI] [PubMed] [Google Scholar]
- Sorensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003;6:1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [DOI] [Google Scholar]
- Sorte CJB, Hofmann GE. Thermotolerance and heat-shock protein expression in Northeastern Pacific Nucella species with different biogeographical ranges. Mar Biol. 2005;146:985–993. doi: 10.1007/s00227-004-1508-2. [DOI] [Google Scholar]
- Storey KB. Life in the slow lane: molecular mechanisms of estivation. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:733–754. doi: 10.1016/S1095-6433(02)00206-4. [DOI] [PubMed] [Google Scholar]
- Storey KB, Storey JM. Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q Rev Biol. 1990;65:145–174. doi: 10.1086/416717. [DOI] [PubMed] [Google Scholar]
- Tammariello SP, Rinehart JP, Denlinger DL. Desiccation elicits heat shock protein transcription in the flesh fly, Sarcophaga crassipalpis, but does not enhance tolerance to high or low temperatures. J Insect Physiol. 1999;45:933–938. doi: 10.1016/S0022-1910(99)00073-6. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Somero GN. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J Exp Biol. 1999;202:2925–2936. doi: 10.1242/jeb.202.21.2925. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Somero GN. Interspecific- and acclimation-induced variation in levels of heat-shock proteins 70 (hsp70) and 90 (hsp90) and heat-shock transcription factor-1 (HSF1) in congeneric marine snails (genus Tegula): implications for regulation of hsp gene expression. J Exp Biol. 2002;205:677–685. doi: 10.1242/jeb.205.5.677. [DOI] [PubMed] [Google Scholar]
- Torok Z, Goloubinoff P, Horvath I, Tsvetkova NM, Glatz A, Balogh G, Varvasovszki V, Los DA, Vierling E, Crowe JH, Vigh L. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc Natl Acad Sci U S A. 2001;98:3098–3103. doi: 10.1073/pnas.051619498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov HA, Karaev KK, Lyashko VN, Evgen’ev MB. Heat-shock response in camel (Camelus dromedarius) blood cells and adaptation to hyperthermia. Comp Biochem Physiol B. 1993;106:867–872. doi: 10.1016/0305-0491(93)90043-5. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Magun BE, Gerner EW. Effects of cycloheximide on thermotolerance expression, heat shock protein synthesis, and heat shock protein mRNA accumulation in rat fibroblasts. Mol Cell Biol. 1986;6:1088–1094. doi: 10.1128/mcb.6.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatsepina OG, Ulmasov KA, Beresten SF, Molodtsov VB, Rybtsov SA, Evgen’ev MB. Thermotolerant desert lizards characteristically differ in terms of heat-shock system regulation. J Exp Biol. 2000;203:1017–1025. doi: 10.1242/jeb.203.6.1017. [DOI] [PubMed] [Google Scholar]
- Zatsepina OG, Velikodvorskaia VV, Molodtsov VB, Garbuz D, Lerman DN, Bettencourt BR, Feder ME, Evgenev MB. A Drosophila melanogaster strain from sub-equatorial Africa has exceptional thermotolerance but decreased Hsp70 expression. J Exp Biol. 2001;204:1869–1881. doi: 10.1242/jeb.204.11.1869. [DOI] [PubMed] [Google Scholar]