Abstract
Purpose
We used computer assisted sperm selection (MSOME) during cycles of intracytoplasmic sperm injection to test whether this technique improves results over traditional ICSI protocols. We also used the TUNEL assay to test whether MSOME could deselect physiologically abnormal spermatozoa.
Methods
Individual spermatozoa were examined with MSOME. Normal and abnormal spermatozoa were tested for the level of DNA fragmentation using TUNEL assay. In a prospective, randomized trial, patients were selected for standard ICSI, or IMSI techniques. We tested the two groups for biological and clinical parameters.
Results
64.8% of spermatozoa, otherwise selectable for ICSI, were characterized by abnormalities after computer-assisted sperm analysis. These sperm were also characterized by an increase in the level of DNA fragmentation. We noted an increase in embryo quality, pregnancy and implantation rates after computerized sperm selection during ICSI procedures.
Conclusions
Computerised selection of spermatozoa during ICSI procedures deselects physiological abnormal spermatozoa and improves clinical results.
Keywords: MSOME, IMSI, TUNEL assay, ICSI, Human embryo
Introduction
The introduction of intracytoplasmic sperm injection procedures into the techniques of assisted reproduction marked an important milestone in the development of the field [1]. With this procedure, patients whose semen samples were insufficient for intrauterine insemination or in vitro fertilisation techniques could achieve pregnancy through the use of micromanipulation techniques [2]. Although oocyte quality has been suggested to be a strong determinant for success during assisted reproduction [3–5], it is generally thought that the quality of ejaculated spermatozoa has little effect on outcome [6–8]. However, one potential problem with micromanipulation is the fact that the operator selects the spermatozoon for microinjection and therefore the natural selection barrier to fertilisation is bypassed which may contribute to the creation of embryos with low implantation potential. The development of optical techniques such as Hoffman differential interference contrast optics has increased the resolution of the optics employed during micromanipulation. This has enabled the visualisation of morphological features in oocytes and spermatozoa even when these were manipulated in 1 mm thickness plastic ICSI dishes such as the Falcon 1006. However, the 40× Hoffman objective still has a relatively limited optical resolution. Small cells—such as spermatozoa—have morphological features not observable with these optics (such as vacuoles, midpiece abnormalities etc). In order to reveal morphological features in cells such as spermatozoa, a greater resolution (such as that provided by a 60× or 100× DIC objective) is more indicated.
The introduction of computer-enhanced digital microscopy has enabled the analysis and quantification of features in small cells such as spermatozoa. This was originally applied to motile sperm cells and the analysis ‘Motile Sperm Organelle Morphology Examination’ (MSOME) developed to examine sperm cells potentially selectable for ICSI [9–12]. With MSOME, subtle morphological features such as abnormal proportions of sperm head size, midpiece abnormalities and the presence of vacuoles in the sperm head have been characterised [11, 13]. MSOME is stricter than Tygerberg morphology assessment and deselects spermatozoa that would otherwise have been used for ICSI techniques [14]. MSOME has been applied to the selection of sperm for ICSI [9, 10, 12, 15]. This technique, termed intracytoplasmic morphology selected sperm injection (IMSI, [16, 17]) has been reported to increase pregnancy rates and reduce spontaneous pregnancy loss in ICSI cycles [10, 17–20]. In particular, data from IMSI cycles has suggested that the presence of vacuoles in the nuclei of motile spermatozoa negatively affects reproductive outcome [10], although it has been suggested that these are derived from the acrosome region and are simply indicative of the presence of the acrosome [21]. The fact that the selection of spermatozoa with MSOME improves results in ICSI cycles suggests that the technique deselects a physiological abnormality in spermatozoa. One physiological abnormality shown to have a correlation with pregnancy outcome after ICSI is the level of DNA fragmentation [22–24].
In this work, we apply the techniques MSOME and IMSI to a series of patients to test whether digital examination of sperm morphology prior to ICSI can improve clinical outcome. We first examined the morphological features of spermatozoa that would have been selected for ICSI under normal conditions, and confirm previous findings demonstrating that spermatozoa that would have been selected for ICSI under standard laboratory conditions often contain morphological defects. We characterise these defects and determine the proportion of spermatozoa with defects in defined conditions. We further characterise the relationship between morphological defects and physiological defects (in this case DNA fragmentation) in individual spermatozoa. The data from a clinical trial indicates that MSOME prior to ICSI can improve clinical results in cycles of assisted reproduction.
Materials and methods
Part 1: Correlation between sperm morphology and DNA fragmentation
Preparation and morphological selection of spermatozoa
Sperm samples were collected by masturbation and examined after liquefaction. All samples were washed using a silicon-based gradient of 40% overlaid over an 80% silicon solution (COOK Sperm Gradient, Ireland). The sample was centrifuged for 20 min at 1000 G, followed by a wash in Hams F-10. The final precipitate was resuspended to a final concentration of 1 × 106 sperm/ml and conserved in an atmosphere of 37°C and 6% CO2 until required.
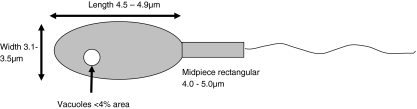
Morphological selection of spermatozoa was performed with a Nikon Eclipse Ti-U inverted microscope (Nikon, Florence, Italy) equipped with Narishige micromanipulators (Narishige, London, UK). Sperm samples were placed into a 5 μl drop of equilibrated PVP in a WillCo Glass Bottom dish (WillCo Wells, Amsterdam, Netherlands) and individual spermatozoa captured with ICSI pipettes and positioned in groups of 10 to facilitate analysis. Sperm was analysed under differential interference contrast optics using a 100× oil-immersion objective. Computer-enhanced analysis and measurement of individual spermatozoa was achieved through digital recording of individual cells with a Nikon Digital Sight DS-2MBW camera followed by image analysis and measurement using the Nikon NIS-Elements image enhancement package (Nikon NIS-Elements, Florence, Italy). Spermatozoa were considered morphologically suitable for ICSI procedures where the head of the sperm was a regular oval shape, 4.5–4.9 μm in length and 3.1–3.5 μm in width, and was characterised by a maximum of a single vacuole not more than 4% the total area of the sperm head (8, Fig. 1). The midpiece was also required to be a regular, rectangular shape of between 4.0 and 5.0 μm in length (Fig. 1).
Fig. 1.
Characteristics of normal morphology spermatozoa with MSOME. Standard measurements of length (4.5–4.9 μm) and width (3.1–3.5 μm) of the sperm head are shown. The midpiece should also have a standard length of between 4.0–5.0 μm. The size of vacuoles is calculated as percentage of the area of the sperm head. Measurements are based on ref 9
DNA Fragmentation assay
Spermatozoa were captured for analysis as for standard ICSI procedures and placed individually into 1 μl drops of PVP on a WillCo dish for morphological analysis. Spermatozoa considered morphologically normal after MSOME were then passed through drops of Hams F-10 medium to remove the PVP residue and attached on to a slide in which a grid had been incised. The position of the spermatozoa was recorded for retrieval of the individual cell post analysis. DNA fragmentation was measured with the TUNEL assay. For the TUNEL assay, we used the In situ Cell Death Detection Kit according to manufacturer’s protocol (Roche Diagnostics, Mannheim, Germany), with slight modifications. Slides were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 1 h at room temperature. Slides were then washed in PBS supplemented with 1% BSA. Slides were rinsed twice in PBS before being permeabilized with 0.1% Triton X100 in 0.1 % sodium citrate for 2 min on ice. Slides were then washed twice with PBS and incubated in a humidified chamber at 37°C for 60 min with terminal deoxyribonucleotidyl transferase-mediated dUTP nick-end labelling in order to allow DNA elongation. Slides were then rinsed twice in PBS and counterstained with 1 mg/ml 4,6 diamidoino-2-phenylindole (DAPI). Negative (omitting TdT from the reaction mixture) and positive (using only DNAse I, 1 mg/ml for 30 min at room temperature) controls were tested for each sample. Individual spermatozoa were traced by the grid marking, and by DAPI staining of the nuclei. The presence of DNA fragmentation was detected if fluorescein fluorescence (green) was present (TUNEL positive).
Part 2: Cycle-matched trial
Patients
Patients were attending the ‘Centro Fecondazione Assistita’, Naples, Italy for intracytoplasmic sperm injection procedures. Patients were prepared using standard controlled ovarian hyperstimulation protocols including downregulation of the pituitary gland with a GnRH agonist (Decapeptyl, Ipsen, Italy) followed by ovarian stimulation with exogenous FSH (Gonal-F, Serono, Italy). A single member of the medical staff co-ordinated all stimulation protocols, ensuring standardisation. Oocyte retrieval was performed 36 h after the administration of 10,000 IU hCG when 2–3 follicles of 18–20 mm diameter were observed by ultrasound examination, and blood 17 β-oestradiol levels reached 150–200 pg/ml/follicle over 18 mm. Luteal phase supplementation was achieved with intramuscular injections of prontogest 50 mg/day. All oocytes in the present project were treated with ICSI 3 h after oocyte retrieval (60 min after removal of the cumulus complex). A single team of biologists co-ordinated all biological work, ensuring that both culture protocols and embryo assessment were standardised. Oocytes were processed for ICSI using commercial IVF medium (COOK, Limerick, Ireland), pre-equilibrated to 37°C and 6% CO2. In standard ICSI procedures, spermatozoa were placed in a drop of PVP in a Falcon 1006 ICSI dish (Beckton Dickinson, New Jersey, USA), selected with a 40x objective equipped with Nomarski optics and injected into oocytes using Narishige micromanipulators and COOK microtools (ICSI and holding pipette, COOK, Ireland). Zygote quality was scored 16–17 h after ICSI. Embryo quality on day 3 was assessed 64–65 h after insemination. Zygote and embryo evaluation was performed according to previous data [25]. Three embryos were transferred in all cases on the third day after oocyte retrieval. The establishment of a pregnancy was considered as a positive β-hCG test of over 60 IU/l 14 days after embryo transfer. The implantation rate was calculated by the observation of foetal heart beats after ultrasound analysis, 8 weeks after the establishment of pregnancy.
A group of patients not achieving pregnancy in the initial cycle of ICSI were offered, and accepted IMSI for a second cycle. Patients were prepared for the second IVF cycle using identical protocols to the initial cycle. Laboratory techniques were identical to the initial cycle apart from the insertion of the MSOME protocol for the selection of spermatozoa. In this technique, an excess number of spermatozoa were selected and placed into a drop of PVP as for standard ICSI techniques. Spermatozoa were placed in groups of 10 and the pattern drawn in order to assist the retrieval of the single spermatozoa after analysis. Individual spermatozoa were analysed by MSOME, and morphologically normal spermatozoa marked on the pattern for later retrieval. Upon selection of a sufficient number of spermatozoa to complete the technique, selected spermatozoa were replaced into the PVP drop of a standard prepared Falcon 1006 ICSI dish and subsequently injected into oocytes through standard ICSI procedures. Biological procedures were equivalent to the initial cycle and again, three embryos were transferred in all cases.
Part 3: Prospective, randomised trial
Trial design
The study is a prospective, randomised trial to test the effect of morphological selection of spermatozoa prior to ICSI in 232 patients treated in Italy between May 2009 and September 2009. All couples went through a gynecological and andrological work-up. Couples were accepted into the trial after informed consent. Couples with 1–3 years of infertility, female menstrual cycle ranged 24–35 days (intra-individual variability ± 3 days), if the karyotype of both subjects of the couple was normal and if biochemical assessments demonstrated the absence of metabolic, autoimmune and infectious disorders. Couples were also included in the trial if semen characteristics were between 1 × 106/ml and 20 × 106/ml since above this level, standard IVF was performed. Patients were excluded from the program if the female basal FSH was >10 IU/l, body mass index (BMI = weight (kg)/height (m)2) > 29, if biochemical and/or USG evidence suggested polycystic ovarian syndrome, if the female partner had stage III–IV endometriosis, if autoimmune, thyroid or chromosomal abnormalities were present, or if only one ovary was present. Couples in which the semen sample was derived from either a cryopreserved sample or surgical retrieval techniques were also excluded to prevent a male-factor bias. The registration number of the patients was inserted into a computer and selected randomly to assign the couple to the control or trial groups. In the 122 patients selected for sperm morphological assessment, all oocytes were injected with morphologically analysed spermatozoa. In the 110 patients where no sperm morphological selection was performed, normal ICSI procedures were performed. Biological procedures were equivalent in all cycles, apart from the insertion of the MSOME protocol into the cycles in which IMSI was performed. A maximum of 4 embryos were transferred in all cases.
Statistics
All data were plotted as mean±standard deviation unless stated. Regression lines were calculated by the method of least squares and the significance of the regression lines was tested with the Pearson product-moment test. The z-test with Yates correction was used to test the significance of proportions where necessary.
Results
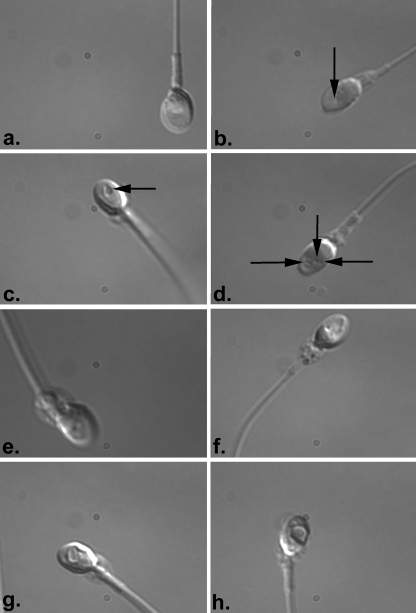
To determine the proportion of spermatozoa, otherwise selected for ICSI, which were characterised by morphological abnormalities, we performed a mock ICSI trial in which individual spermatozoa derived from gradient-selected purified sperm samples were captured and then analysed with digital techniques to examine morphology. In our trial, a total of 182 spermatozoa derived from 5 normospermic semen samples were analysed. Interestingly, 118 of these spermatozoa (64.8%) were deselected after digital analysis. Reasons for rejection of spermatozoa included poor morphology (26 spermatozoa, 14.3%, Fig. 2), presence of multiple vacuoles (22 spermatozoa, 12.1%, Fig. 2), presence of vacuoles over 4% of area (38 spermatozoa, 20.8%, Fig. 2) and poor morphology of midpiece (5 spermatozoa, 2.7%, Fig. 2). Amorphous morphologies were present in 27 spermatozoa (14.8%, Fig. 2). These data suggest that computer-enhanced selection of spermatozoa reveals morphological features not visible in normal ICSI procedures and deselects spermatozoa otherwise selected for ICSI procedures in normospermic patients.
Fig. 2.
MSOME of spermatozoa selected for ICSI. 182 spermatozoa otherwise considered suitable for ICSI were analysed with MSOME. The technique reveals several morphological defects not noted in standard ICSI procedures. a and b Normal morphology and presence of a single vacuole not greater than 4% of the surface area (arrow in b.). These examples represent 64 spermatozoa analysed in the trial c–h. Abnormal morphologies. c Presence of a single vacuole greater than 4% surface area. The arrow delineates the vacuole. Similar examples were observed in 38 spermatozoa. d Spermatozoa with multiple vacuoles (delineated by arrows). This example is representative of 22 spermatozoa analysed with MSOME. e Spermatozoa with a midpiece defect and a single vacuole over 4% of surface area. f Spermatozoa with a midpiece defect and multiple vacuoles in the sperm head (representative of 5 spermatozoa analysed). g and h Amorphous spermatozoa. Such examples were found in 27 spermatozoa otherwise considered normal for ICSI
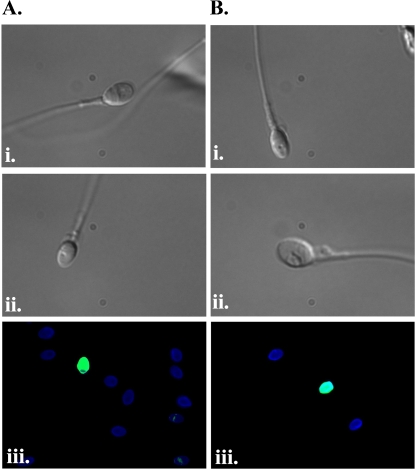
We examined whether the morphological analysis of spermatozoa revealed features that correlated with physiological abnormalities by testing individual spermatozoa for DNA fragmentation after MSOME. Individual spermatozoa were selected as for ICSI, examined with MSOME and then fixed and analysed for DNA fragmentation using TUNEL assay. In the same sample, spermatozoa considered suitable for IMSI were separated from those found to be morphologically abnormal, enabling the comparison of gametes from the same ejaculate. A total of 860 spermatozoa derived from 8 separate analyses were analysed with MSOME. Three hundred and thirty-one (37.6 ± 13.0%, n = 8) of these spermatozoa were considered morphologically normal after MSOME. Of these, 14 spermatozoa (4.2%, mean 6.1 ± 7.2%, n = 8) were characterised with fragmented DNA after TUNEL assay (Fig. 3 and Table 1). Vacuoles in spermatozoa could indicate the initial stages of apoptosis, although they have also been suggested to indicate non-acrosome reacted spermatozoa [21]. Seventy-six of the 529 sperm characterised by vacuoles after MSOME (14.4%, mean 14.7% ± 7.2%, n = 8) were found to contain fragmented DNA (Fig. 3 and Table 1), a significantly higher proportion of spermatozoa than MSOME-normal spermatozoa (p = 0.031, Students t-test). The data therefore suggests a link between abnormal morphology after MSOME and the presence of fragmented DNA.
Fig. 3.
TUNEL assay on MSOME-analysed spermatozoa. a Normal morphology spermatozoa. Images i. and ii. are spermatozoa with normal morphology after MSOME. iii. The image represents TUNEL assay on spermatozoa considered normal after MSOME. Blue images are DAPI-stained TUNEL negative sperm heads (i.e. not containing fragmented DNA). Green-stained sperm heads are TUNEL-positive (i.e. containing fragmented DNA). b Abnormal morphology spermatozoa after MSOME. Images i. and ii. are representative examples of spermatozoa with abnormal morphology after MSOME iii. The image represents TUNEL assay on spermatozoa considered abnormal after MSOME. Blue images are DAPI-stained TUNEL negative sperm heads (i.e. not containing fragmented DNA). Green-stained sperm heads are TUNEL-positive (i.e. containing fragmented DNA)
Table 1.
Correlation between sperm vacuoles and DNA fragmentation in spermatozoa
| Sperm sample | Number sperm analysed | TUNEL positive/MSOME normal (%) | TUNEL positive/MSOME abnormal (%) | Significance (p-value) |
|---|---|---|---|---|
| 1 | 102 | 1/64 (1.5%) | 6/38 (15.8%) | 0.02 |
| 2 | 114 | 0/37 (0%) | 12/77 (15.6%) | 0.03 |
| 3 | 86 | 3/16 (18.8%) | 3/70 (4.36%) | 0.86 |
| 4 | 100 | 5/31 (16.1%) | 4/69 (5.8%) | 0.80 |
| 5 | 158 | 1/75 (1.3%) | 9/83 (10.8%) | 0.04 |
| 6 | 88 | 1/34 (2.9%) | 12/54 (22.2%) | 0.03 |
| 7 | 112 | 2/41 (4.9%) | 16/71 (22.5%) | 0.03 |
| 8 | 100 | 1/33 (3.0%) | 14/67 (20.9%) | 0.04 |
Spermatozoa from 8 diverse semen samples were analysed and separated as MSOME normal and abnormal. TUNEL assay was performed on these same spermatozoa. The number of TUNEL assay positive (i.e. with fragmented DNA) spermatozoa is shown in each sample. Proportions are shown in parentheses. The two-tailed z-test was used to calculate the significance, p < 0.05 is considered significant
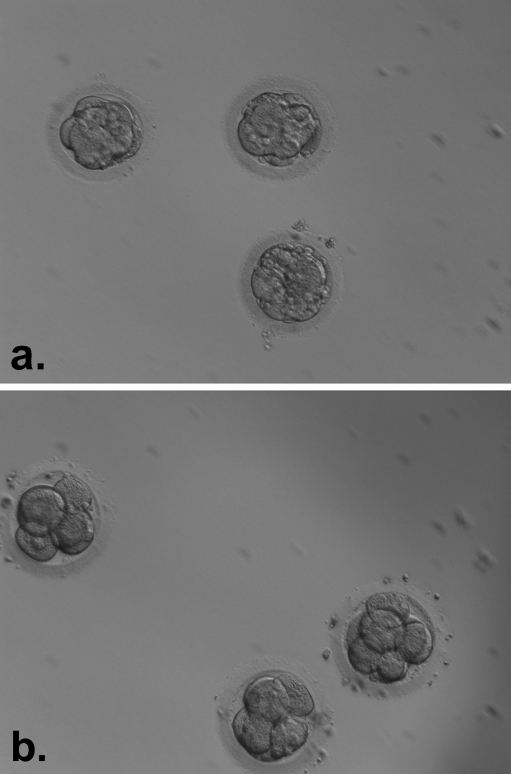
In the present data, a total of 8 patients had a cycle of IMSI after a previous cycle of ICSI. Therefore, a cycle to cycle comparison of embryo quality after computerised selection of spermatozoa was possible. Ovarian stimulation protocols and clinical characteristics were not significantly different between the two cycles. Moreover, fertilisation rates did not differ between cycles (Table 2). However, we noted a significant increase in embryo quality between the two groups (Fig. 4). In group A (ICSI cycle), a total of 58 oocytes fertilised and produced embryos. Of these, 35 (60.3%) were regarded grade A on the day of transfer (Table 2). In group B, 55 oocytes fertilised. Forty-six of these formed grade I embryos on the day of transfer (83.6%, Table 2). During IMSI cycles, we were always able to select grade I embryos with no fragmentation for transfer. Three pregnancies were obtained after IMSI in this patient group.
Table 2.
Data for cycle-matched IMSI trial
| ICSI cycle | Subsequent IMSI cycle | Significance (P value) a | |
|---|---|---|---|
| Patients | 8 | 8 | 1 |
| Mean age (years±sd) | 34.9 ± 2.8 | 34.9 ± 2.8 | 1 |
| Number of mature oocytes retrieved (mean±sd/patient) | 74 (9.3 ± 2.8) | 78 (9.8 ±2.7) | 0.72 |
| Number of fertilised oocytes (mean±sd/patient %) | 58 (79.4 ± 14.0%) | 55 (70.1 ± 13.1%) | 0.19 |
| Number of grade A embryos (% of total) | 35 (60.3%) | 46 (83.6%) | 0.009 |
| Number of transfers | 8 | 8 | 1 |
| Number of embryos transferred | 24 | 24 | 1 |
| Number of grade I embryos transferred (Mean±sd per patient) | 21 (2.6 ± 0.7) | 24 (3.0 ± 0) | 0.12 |
| Number of clinical pregnancies (% pregnancies/transfer) | 0 | 3 (37.5%) | 0.1 |
| Number of fhb’sb (Implantation rate %) | 0 | 5 (20.8%) | 0.03 |
| Pregnancies to term | n/a | 3 | 0 |
| Live births | n/a | 5 | 0 |
Data is presented as actual figures. Percentages are presented in parentheses where necessary. Mean±sd is presented where necessary. Significance is calculated with Students t-test for means±sd or 2-tailed z-test to examine the differences between proportions. aP-values <0.05 are considered significant. N/A not applicable. bFoetal heart beats
Fig. 4.
Improvement in embryo quality after IMSI. a Embryos selected for transfer from a patient in a standard ICSI cycle. The patient did not achieve pregnancy. b Embryos selected for transfer in the same patient after an IMSI cycle. Here, embryo quality is clearly improved and the patient achieved a singleton pregnancy
We extended these results into a prospective, randomised trial to test whether IMSI improved results over standard ICSI procedures. A group of 250 patients were randomised after informed consent into two groups; group A performed standard ICSI whereas group B had IMSI. All patients had ovarian hyperstimulation cycles initiated with the same protocol. After cancellations for severe hyperstimulation or poor response, 110 patients were treated by standard ICSI techniques (Group A, Table 3) and 122 patients with IMSI (Group B, Table 6). Table 3 shows the characteristics of these patients.
Table 3.
Data for ICSI and IMSI trial
| Group A (ICSI) | Group B (IMSI) | Significance (P value) a | |
|---|---|---|---|
| Patients | 125 | 125 | 1 |
| Cycles started | 125 | 125 | 1 |
| Mean age (years ± sd) | 34.2 ± 4.0 | 33.6 ± 4.5 | 0.27 |
| Number of oocyte retrievals | 110 | 122 | N/A |
| Number of mature oocytes inseminated (mean ± sd/cycle) | 1211 (12.4 ± 4.0) | 1384 (11.1 ± 6.0) | 0.06 |
| Number of sperm analysed (number selected) | 1211 (1211) | 5126 (1384) | 0 |
| Number of oocytes fertilised (fertilisation rate) | 798 (65.9%) | 941 (68.0%) | 0.72 |
| Number of embryos (% development) | 790 (98.9%) | 935 (99.4%) | 0.43 |
| Number of transfers | 110 | 122 | N/A |
| Number of transferred embryos (Mean ± sd) | 324 (2.8 ± 1.3) | 355 (2.9 ± 1.3) | 0.56 |
| Number of grade I embryos transferred (% total) | 214 (66.0%) | 350 (98.6%) | 0 |
| Number of clinical pregnancies (% pregnancies/transfer) | 44 (40.0%) | 80 (65.6%) | 0 |
| Number of fhb’sa (Implantation rate %) | 48 (14.8%) | 86 (24.2%) | 0.003 |
| Number of pregnancies to term | 44 | 79 | 0.76 |
| Live births | 45 | 84 | 0.5 |
Data is presented as actual figures. Percentages are presented in parentheses where necessary. Mean±sd is presented where necessary. Significance is calculated with Students t-test for means±sd or 2-tailed z-test to examine the differences between proportions. aP-values <0.05 are considered significant. N/A not applicable. bFoetal heart beats
During computer-enhanced morphological selection of spermatozoa for ICSI, sperm were initially captured for morphological assessment using standard ICSI methodology. Enhanced morphological assessment of these spermatozoa led to the further rejection of 73% of these spermatozoa, otherwise considered suitable for ICSI procedures. In total, 1384/5126 of spermatozoa that were selected by standard ICSI procedures were considered suitable for microinjection after computerised assessment (Table 3).
In the 122 group B patients selected for IMSI, a total of 1384 metaphase II oocytes were injected with spermatozoa selected after computer-enhanced analysis (Table 3). Nine hundred and forty-one oocytes (68.0%) were observed to be fertilised 16–17 h after injection. A total of 798/1211 mature oocytes were fertilised (65.9%) in the 110 control patients (Group A, Table 3). The rate of fertilisation was not significantly different between normal ICSI procedures and after computer-enhanced sperm analysis (Table 3). Neither did the percentage of embryo development differ between normal ICSI procedures and after computer-enhanced sperm selection techniques. Interestingly however, the number of morphologically grade I embryos was increased after computer-enhanced sperm selection. Here, a total of 350 grade I embryos were obtained (98.6% of transferred embryos), in contrast to the 66.0% of grade I embryos obtained after normal ICSI procedures in the trial. All patients undergoing computer-enhanced sperm selection were characterised by the transfer of 100% grade I embryos, in contrast to controls. Moreover, an increased number of grade I embryos were available for cryopreservation after IMSI procedures than the classical ICSI technique.
The pregnancy rate of patients undergoing IMSI was also significantly increased with respect to the ICSI controls. Of the 122 patients in which IMSI was performed, 80 achieved pregnancy (65.6%). Forty-four of the 110 patients in which normal ICSI was performed achieved pregnancy (40.0%). The implantation rate of embryos created with IMSI procedures was also greater than that of standard ICSI techniques. Of the 355 embryos replaced after IMSI, 86 embryos implanted (24.2%). However, once pregnancy was established, we noted no difference in the proportion of pregnancies arriving to term (Table 3).
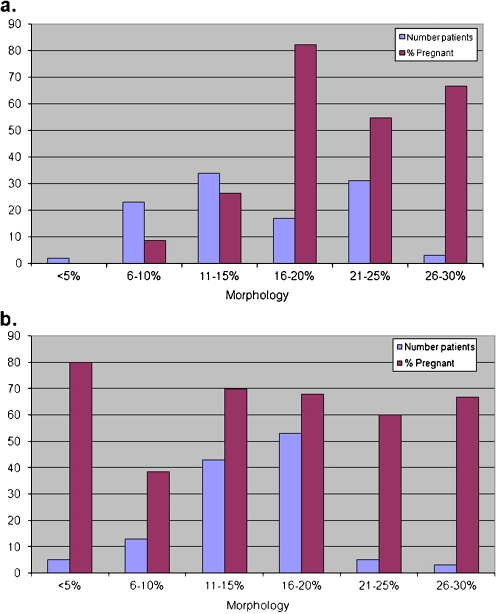
We compared the initial morphology of semen samples with pregnancy rates in the group of patients in which normal ICSI was performed and the group of patients selected for IMSI. Semen morphology in patients selected for ICSI followed a normal distribution with sperm morphology of 14.3 ± 5.6%. In these patients, a correlation of r = 0.85 existed between pregnancy rate and sperm morphology (Fig. 5). IMSI patients were characterised by a completely different correlation between semen morphology and pregnancy rates. Here, no correlation was observed. The semen of patients selected for IMSI was characterised by a normal distribution with morphology of 15.1 ± 6.2%, not significantly different to that of group A patients (p = 0.30, Fig. 5). However, the correlation between sperm morphology and pregnancy rate was r = −0.015 (Fig. 5).
Fig. 5.
Correlation between semen sample sperm morphology and pregnancy rates. a Standard ICSI cycles. The semen samples are characterised by a normal distribution with mean 14.3% normal morphology and standard deviation 5.6%. A correlation of r=0.85 exists between increasing morphology and pregnancy. b Correlation between semen morphology and pregnancy after IMSI. The samples here are characterised by a normal distribution with mean 15.1% and standard deviation 6.2%. The correlation between incidence of pregnancies and initial semen morphology is 0.015
Discussion
The development of intracytoplasmic sperm injection marked an important advance in the field of assisted reproduction. One major criticism of the ICSI technique has however been that operator-assisted fertilisation of oocytes with semen samples otherwise considered infertile could produce an increase in birth defects and cause dramatic consequences as babies produced with this technique become adults, although this is yet to be proven [26–28]. In fact, a slight increase in birth defects is noted after ICSI, and the rate of spontaneous pregnancy loss remains higher than that of traditional IVF [26–28]. Although it is not certain that these differences are due to the technique per se, one criticism of ICSI is that the optics used for visualisation of gametes do not provide a high enough resolution to permit the visualisation of small defects and morphological abnormalities in the human spermatozoa. Oocytes and preimplantation embryos are large cells whose morphological features are distinguishable using optics usually applied in the laboratory of assisted reproduction, and in fact the selection of this material based on morphological assessment has been widely studied [22, 27–29]. The morphology of human spermatozoa has also been widely studied [29], but until recently it has been difficult to correlate morphological features of individual spermatozoa with outcome. In fact, apart from a small amount of works [8, 30, 31], it is generally assumed that the quality of spermatozoa has little correlation on the outcome after assisted reproduction and that the selection of spermatozoa for assisted reproduction can be achieved by general techniques such as swim-up or gradients.
The technique of MSOME has revealed small morphological abnormalities and other defects in human spermatozoa otherwise selected for ICSI. These defects have not been previously observed, and in fact MSOME has been suggested to provide sperm selection criteria stricter than standard Tygerberg assessment [14]. In this work, we prepare spermatozoa for assisted reproduction and examine the morphology of spermatozoa otherwise selected for standard ICSI procedures using computer-enhanced imaging techniques. Our data indicates that 64.8% of these spermatozoa would not have been suitable for ICSI procedures. We analyse whether there is a correlation between morphological features revealed by MSOME and the presence of fragmented DNA by TUNEL assay. We suggest that a correlation exists between the presence of large vacuoles in spermatozoa and DNA fragmentation. In a preliminary study, we compare cycles of ICSI with and without MSOME in a small series of patients. Although percentages of fertilisation and embryo development are equivalent, embryos created after MSOME appear to be of higher quality, and more pregnancies are achieved. We confirm these findings in a prospective, randomised trial. Our data further demonstrates that the correlation between the initial semen morphology and outcome after ICSI is eliminated with MSOME. These data suggest that, during the standard ICSI procedure, high quality spermatozoa are not preferentially selected as previously thought, and therefore the probability of achieving pregnancy is correlated with semen morphology. After IMSI, the highest quality spermatozoa are always selected and therefore the correlation disappears.
The analysis and selection of spermatozoa for ICSI using MSOME can improve results in ART cycles through an increase in the number of grade A embryos formed and a decrease in the level of fragmentation in these embryos. Physiologically, this may occur through a reduction in the percentage of abnormal sperm with fragmented DNA injected into oocytes. Although little published data shows a correlation between morphological defects and physiological abnormalities, our direct comparisons of sperm morphology and DNA fragmentation suggest that these morphological abnormalities indicate poor sperm physiology. It is not known whether natural insemination or traditional IVF techniques prevent the fertilisation of oocytes with such morphologically abnormal sperm, although it is assumed that the zona pellucida provides a natural barrier to contact between abnormal spermatozoa and the oocyte [32]. However, we also suggest that the quality of embryos produced after IMSI is improved over ICSI. Interestingly, an increase in embryo fragmentation has been observed in ICSI cycles as compared to IVF, although no effect on reproductive outcome was noted [33].
Acknowledgements
A grant from Merck-Serono was provided to Dr Coppola for this work. We thank Vincenzo Monfrecola for his assistance.
Footnotes
Capsule
The use of computer assisted sperm selection improves results after ICSI due to the deselection of physiologically abnormal spermatozoa.
References
- 1.Palermo G, Joris H, Devroey P, Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 2.Palermo GD, Cohen J, Alikani M, Adler A, Rosenwaks Z. Intracytoplasmic sperm injection: a novel treatment for all forms of male factor infertility. Fertil Steril. 1995;63:1231–40. doi: 10.1016/s0015-0282(16)57603-1. [DOI] [PubMed] [Google Scholar]
- 3.Lim AS, Tsakok MF. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265–71. doi: 10.1016/S0015-0282(97)81513-0. [DOI] [PubMed] [Google Scholar]
- 4.Sherins RJ, Thorsell LP, Dorfmann A, Dennison-Lagos L, Calvo LP, Krysa L, Coulam CB, Schulman JD. Intracytoplasmic sperm injection facilitates fertilization even in the most severe forms of male infertility: pregnancy outcome correlates with maternal age and number of eggs available. Fertil Steril. 1995;64:369–75. doi: 10.1016/s0015-0282(16)57737-1. [DOI] [PubMed] [Google Scholar]
- 5.Sauer MV. The impact of age on reproductive potential: lessons learned from oocyte donation. Maturitas. 1998;30:221–5. doi: 10.1016/S0378-5122(98)00077-2. [DOI] [PubMed] [Google Scholar]
- 6.Kini S, Morrell D, Thong KJ, Kopakaki A, Hillier S, Irvine DS. Lack of impact of semen quality on fertilization in assisted conception. Scott Med J. 2010;55:20–3. doi: 10.1258/rsmsmj.55.1.20. [DOI] [PubMed] [Google Scholar]
- 7.Campbell AJ, Irvine DS. Male infertility and intracytoplasmic sperm injection (ICSI) Br Med Bull. 2000;56:616–29. doi: 10.1258/0007142001903427. [DOI] [PubMed] [Google Scholar]
- 8.Ménézo Y, Dale B. Paternal contribution to successful embryogenesis. Hum Reprod. 1995;10:1326–8. doi: 10.1093/humrep/10.6.1326. [DOI] [PubMed] [Google Scholar]
- 9.Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. J Androl. 2002;23:1–8. doi: 10.1002/j.1939-4640.2002.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 10.Berkovitz A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod. 2006;21:1787–90. doi: 10.1093/humrep/del049. [DOI] [PubMed] [Google Scholar]
- 11.Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, Bartoov B. How to improve IVF-ICSI outcome by sperm selection. Reprod Biomed Online. 2006;12:634–8. doi: 10.1016/S1472-6483(10)61191-1. [DOI] [PubMed] [Google Scholar]
- 12.Cassuto NG, Bouret D, Plouchart JM, Jellad S, Vanderzwalmen P, Balet R, Larue L, Barak Y. A new real-time morphology classification for human spermatozoa: a link for fertilization and improved embryo quality. Fertil Steril. 2009;92:1616–25. doi: 10.1016/j.fertnstert.2008.08.088. [DOI] [PubMed] [Google Scholar]
- 13.Chelli MH, Albert M, Ray PF, Guthauser B, Izard V, Hammoud I, Selva J, Vialard F. Can intracytoplasmic morphologically selected sperm injection be used to select normal-sized sperm heads in infertile patients with macrocephalic sperm head syndrome? Fertil Steril. 2010;93:1347. doi: 10.1016/j.fertnstert.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira JB, Massaro FC, Mauri AL, Petersen CG, Nicoletti AP, Baruffi RL, Franco JG., Jr Motile sperm organelle morphology examination is stricter than Tygerberg criteria. Reprod Biomed Online. 2009;18:320–6. doi: 10.1016/S1472-6483(10)60088-0. [DOI] [PubMed] [Google Scholar]
- 15.Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, Bartoov B. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod. 2005;20:185–90. doi: 10.1093/humrep/deh545. [DOI] [PubMed] [Google Scholar]
- 16.Bartoov B, Berkovitz A, Eltes F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N Engl J Med. 2001;345:1067–8. doi: 10.1056/NEJM200110043451416. [DOI] [PubMed] [Google Scholar]
- 17.Nadalini M, Tarozzi N, Distratis V, Scaravelli G, Borini A. Impact of intracytoplasmic morphologically selected sperm injection on assisted reproduction outcome: a review. Reprod Biomed Online. 2009;19(Suppl 3):45–55. doi: 10.1016/S1472-6483(10)60283-0. [DOI] [PubMed] [Google Scholar]
- 18.Bartoov B, Berkovitz A, Eltes F, Kogosovsky A, Yagoda A, Lederman H, Artzi S, Gross M, Barak Y. Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertil Steril. 2003;80:1413–9. doi: 10.1016/j.fertnstert.2003.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Hazout A, Dumont-Hassan M, Junca AM, Cohen Bacrie P, Tesarik J. High-magnification ICSI overcomes paternal effect resistant to conventional ICSI. Reprod Biomed Online. 2006;12:19–25. doi: 10.1016/S1472-6483(10)60975-3. [DOI] [PubMed] [Google Scholar]
- 20.Antinori M, Licata E, Dani G, Cerusico F, Versaci C, d’Angelo D, Antinori S. Intracytoplasmic morphologically selected sperm injection: a prospective randomized trial. Reprod Biomed Online. 2008;16:835–41. doi: 10.1016/S1472-6483(10)60150-2. [DOI] [PubMed] [Google Scholar]
- 21.Kacem O, Sifer C, Barraud-Lange V, Ducot B, Ziegler D, Poirot C, Wolf J. Sperm nuclear vacuoles, as assessed by motile sperm organellar morphological examination, are mostly of acrosomal origin. Reprod Biomed Online. 2010;20:132–137. doi: 10.1016/j.rbmo.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–831. doi: 10.1016/j.fertnstert.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 23.Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, François Guerin J. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87:93–100. doi: 10.1016/j.fertnstert.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 24.Meseguer M, Santiso R, Garrido N, García-Herrero S, Remohí J, Fernandez JL. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2010;(in press). [DOI] [PubMed]
- 25.Placido G, Wilding M, Strina I, Alviggi E, Alviggi C, Mollo A, Varicchio MT, Tolino A, Schiattarella C, Dale B. High outcome predictability after IVF using a combined score for zygote and embryo morphology and growth rate. Hum Reprod. 2002;17:2402–9. doi: 10.1093/humrep/17.9.2402. [DOI] [PubMed] [Google Scholar]
- 26.Wen SW, Leader A, White RR, Léveillé MC, Wilkie V, Zhou J, Walker MC. A comprehensive assessment of outcomes in pregnancies conceived by in vitro fertilization/intracytoplasmic sperm injection. Eur J Obstet Gynecol Reprod Biol. 2010;150:160–5. doi: 10.1016/j.ejogrb.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 27.Hindryckx A, Peeraer K, Debrock S, Legius E, Zegher F, Francois I, Vanderschueren D, Demyttenaere K, Rijkers A, D’Hooghe T. Has the Prevalence of Congenital Abnormalities after Intracytoplasmic Sperm Injection Increased? The Leuven Data 1994 –2000 and a Review of the Literature. Gynecol Obstet Invest. 2010;70:11–22. doi: 10.1159/000279323. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig AK, Hansen A, Katalinic A, Sutcliffe AG, Diedrich K, Ludwig M. Thyen Ute. Assessment of vision and hearing in children conceived spontaneously and by ICSI: a prospective controlled, single-blinded follow-up study. Reprod Biomed Online. 2010;20:391–397. doi: 10.1016/j.rbmo.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Wilding M, Matteo L, D'Andretti S, Montanaro N, Capobianco C, Dale B. An oocyte score for use in assisted reproduction. J Assist Reprod Genet. 2007;24:350–8. doi: 10.1007/s10815-007-9143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ubaldi F, Rienzi L. Morphological selection of gametes. Placenta. 2008;29(Suppl B):115–20. doi: 10.1016/j.placenta.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update. 2010;16:65–79. doi: 10.1093/humupd/dmp027. [DOI] [PubMed] [Google Scholar]
- 32.Sathananthan AH. Functional competence of abnormal spermatozoa. Baillieres Clin Obstet Gynaecol. 1994;8:141–56. doi: 10.1016/S0950-3552(05)80029-X. [DOI] [PubMed] [Google Scholar]
- 33.Frattarelli JL, Leondires MP, Miller BT, Segars JH. Intracytoplasmic sperm injection increases embryo fragmentation without affecting clinical outcome. J Assist Reprod Genet. 2000;17:207–12. doi: 10.1023/A:1009439800398. [DOI] [PMC free article] [PubMed] [Google Scholar]