Abstract
Purpose
Relationships between follicular fluid levels of IL-6 with ovarian response and clinical pregnancy were evaluated in IVF-ET cycles.
Methods
Follicular fluid was collected from ovarian follicles ≥ 14 mm, pooled for each patient, and IL-6 levels were assessed using ELISA (n = 68). Relationships between IL-6 levels and IVF cycle parameters were assessed using nonparametric tests, and between IL-6 levels and clinical pregnancy using multivariable logistic regression analyses.
Results
Significant positive correlations were observed between IL-6 with age (p = 0.035), and IL-6 with estradiol on the day of hCG (p = 0.011). On adjusted analyses, IVF cycles in patients with IL-6 levels <4.0 pg/ml (median value for the group) demonstrated an almost 4-fold increase in likelihood for clinical pregnancy (p = 0.045).
Conclusions
Lower follicular fluid IL-6 levels in IVF patients are associated with increased likelihood of clinical pregnancy. We hypothesize that endometrial receptivity is a likely target for any deleterious influences of elevated IL-6 levels.
Keywords: IL-6, Follicular fluid, Clinical pregnancy, IVF
Introduction
Advances in our understanding of the immune system have provided new insights into critical roles of immune mediators in reproductive biology. Cytokines consist mainly of smaller, water-soluble proteins and glycoproteins, which based on their functions, cellular origin and targets, are classified as lymphokines, interleukins, and chemokines [1]. In the context of reproductive physiology, cytokines are known to modulate ovarian function, gonadal steroid secretion, corpus luteum function, embryo development and implantation [1–6].
Interleukin 6 (IL-6) was first identified as a T-cell-derived cytokine that promotes B-cell differentiation and antibody production [7–9]. Many studies since have shown IL-6 to be a pleiotrophic cytokine with multiple cellular effects ranging from growth promotion, growth inhibition and cell differentiation to inflammation, hematopoiesis, neuronal function and osteoclastogenesis. Pathogenic implications of IL-6 are described for several diseases ranging from autoimmune disorders to malignancies [8, 9]. Effects of IL-6 are mediated via a unique receptor system which consists of two functional proteins; IL-6 receptor (IL-6R) and gp130 [10–12]. A soluble form of the IL-6 receptor (IL-6 sR) has been found in human serum [10] and urine [12]; IL-6 and IL-6 sR complexes exhibit an agonistic effect on cells expressing the IL-6 signal transducer gp130 on their surface [11, 13].
IL-6 expression is described within the human granulosa cells of the Graafian follicle [14–18], in the human corpus luteum and ovarian theca cells [19, 20], the endometrium [5, 21], and in the pre-implantation embryo [22]. Measurements of serum and follicular fluid (FF) levels of IL-6 in women undergoing in vitro fertilization (IVF) have been shown previously to be associated with etiology of infertility, stimulation protocol, fertilization rates and pregnancy outcome [7, 23–26], and increased FF levels of IL-6 are described in the context of ovarian hyperstimulation syndrome [27].
In a cross-sectional study of infertile women undergoing IVF, we herein report on the association between FF levels of IL-6 and the outcome of fresh embryo transfer (ET) IVF cycles. Our data suggest that high FF IL-6 levels are detrimental to IVF success; given that FF-IL6 levels did not exhibit any correlation with oocyte yield, nor with embryo parameters, we speculate that endometrial receptivity rather than ovarian response may be the site of detrimental influences of high FF IL-6.
Material and methods
The study was approved by the Institutional Review Board of Montefiore Medical Center and participants provided written consent. Sixty-eight infertile women undergoing IVF were enrolled between March 2005–December 2007 [28]. Suppression of the endogenous luteinizing hormone surge was done with either GnRH agonist (Leuprolide Acetate [Lupron®; TAP Pharmaceuticals North Chicago, IL, USA]) or antagonist (Ganirelix Acetate [Antagon TM, Organon Inc. West Orange, NJ]). Controlled ovarian hyperstimulation (COH) was initiated with injectable gonadotropins, and the starting gonadotropin dose was selected on the basis of age, early follicular FSH and estradiol (E2) levels and the number of antral follicles. Individualized step-down or step-up protocols were instituted and serial monitoring of ovarian response was assessed by transvaginal ultrasound and serum E2 assays. Nuclear maturation was triggered with 10, 000 IU hCG intramuscularly when 2 or more follicles >17 mm were achieved. Transvaginal ultrasound guided oocyte retrieval was performed 34 h following the hCG injection. Retrieval of oocytes was followed by insemination by IVF or ICSI as per clinical practice at our center. Fertilization was evaluated 12 to 20 h after insemination. The presence of two pronuclei confirmed normal fertilization. Embryo transfers were performed on Day 3 or Day 5 post-insemination using an Echotip (COOK, OB/GYN) or SureView (Smiths Medical, UK) catheter. Luteal support was provided with intramuscular injections of progesterone in oil (50 mg daily). Positive serum hCG tested 12 days after embryo transfer was considered as evidence of implantation. Clinical pregnancy (CP) was defined as intrauterine gestational sac visible on transvaginal ultrasound. Luteal support with progesterone (P) was continued until documentation of the fetal cardiac activity, and subsequently tapered off.
At the time of oocyte retrieval, FF was collected from follicles ≥ 14 mm; FF for each patient was pooled, centrifuged at 3000 g for 15 min and the supernatant was stored at −80°C until assayed. The IL-6 (pg/ml) assay utilized is a commercially available ELISA kit (ALPCO Diagnostics, Salem, NH, USA, sensitivity <2 pg/mL, intra-assay CV 6.2% and inter-assay CV 7.9%). Details on patient demographics (age, race [Caucasian, Black, other race], BMI and etiology for infertility, ovarian reserve status as reflected by early follicular phase [days 1–3] serum FSH and E2 levels), IVF cycle parameters (COH and suppression protocol, dose of gonadotropins used, duration of COH (days), peak serum E2 (pg/ml) on the day of hCG administration, number of eggs retrieved (n), fertilization rate (%), number of embryos transferred and CP following embryo transfer were obtained from medical records. Implantation rate (IR) was calculated as number of gestational sacs divided by the number of embryos transferred × 100.
Statistical analysis
Continuous data are reported as mean and standard deviation (SD) for data demonstrating normal distribution and as median and inter-quartile range [IQR] for skewed data; categorical data are presented as percentage (%). Data for IL-6 and for peak E2 were log transformed for parametric analyses. Relationships between FF IL-6 levels and patient characteristics and with IVF cycle parameters were assessed using Spearman correlation, and Mann Whitney U Rank sum test for skewed data and Pearson’s correlation and Student’s T test for normally distributed data. Quartiles of FF IL-6 levels were computed; the proportion of patients achieving CP and implantation rates across the quartiles of FF IL-6 distribution were assessed by Kruskall Wallis Rank Test. Step wise multivariable logistic regression analyses were conducted to assess the relationship between FF IL-6 levels and CP following IVF; the final statistical model included FF IL-6<4.0 pg/ml as the independent variable of interest and, duration of COH (days) and number of embryos transferred as adjustment variables . The likelihood of CP is presented as odds ratio (OR) ± 95% confidence interval (95% CI). Goodness of fit for the multivariable model was assessed by Hosmer-Lemeshow goodness of fit test [29]. STATA IC 10 (StataCorp, College Station, TX USA) was used for statistical analyses and p < 0.05 was considered to be statistically significant.
Results
Details on the cohort are previously published [28]. Aliquots of FF were available for 68 of the originally enrolled 84 infertile women undergoing IVF. Mean values (SD) for age, BMI and early follicular FSH were 34.35 (4.77 years), 25.36 (6.14 kg/m2) and 8.28 (2.52 mIU/ml) respectively. Information on race was available for 61/68 women; 65% of the participants were of Caucasian race; 13% identified themselves as Black and the remainder as belonging of ‘other race’.
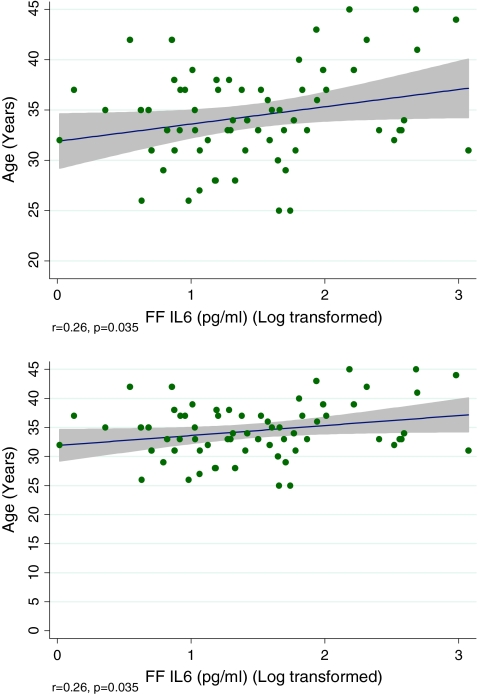
The median value of FF IL-6 for the cohort was 4.0 pg/ml (2.71–6.76). Although higher FF IL-6 levels were noted in Blacks (n = 8) compared to the non-Blacks (log transformed value 1.84 [0.61] versus 1.44 [0.68]), this difference was not of statistical significance (p = 0.123). No relationship was observed between FF IL-6 levels and infertility diagnoses (data not shown). Statistically significant positive correlation was observed between FF IL-6 level with advancing age (Fig. 1) and with peak E2 level (Fig. 2). While additional linear correlation was observed between the number of mature (metaphase II) oocytes retrieved and FF IL-6 level, this relationship was not of statistical significance (r = 0.19, p = 0.131). Fertilization rate was unrelated to FF IL-6 levels (Table 1).
Fig. 1.
Increasing FF IL-6 noted with advancing age; shaded area identifies 95% CI for the data
Fig. 2.
Positive correlation between FF IL-6 and serum estradiol level on the day of hCG administration in IVF cycles; shaded area identifies 95% CI for the data
Table 1.
Patient characteristics and cycle parameters according to follicular fluid level of IL-6
| Parameter | Group 1 (IL-6 < 4 pg/ml) n = 38 | Group II (IL-6 ≥ 4 pg/ml) n = 38 | P value |
|---|---|---|---|
| Age (years) | 33.58 (4.28) | 35.20 (5.09) | 0.161a |
| BMI (kg/m2) | 25.93 (5.04) | 26.13 (7.47) | 0.475a |
| Race | 19/28 (68) | 20/32 (62) | 0.664c |
| Whiteb | |||
| FSH d(mIU/ml) | 7.28 (2.94) | 6.54 (2.15) | 0.255a |
| GnRH Agonist Use | 53% | 41% | 0.455c |
| # Ampules | 34.5 (24.5–50) | 34 (25.5–46) | 0.923e |
| Peak E2f (pg/ml) | 1804.5 (1303–2506) | 2109.5 (1577–2039) | 0.300e |
| Day of hCG | 11.79 (1.82) | 11.37 (1.41) | 0.302a |
| # Eggs Retrieved | 11.85 (6.60) | 12.44 (5.81) | 0.440a |
| FF IL-6 (pg/ml) | 2.67 (0.76) | 8.39 (4.48) | <0.001a |
| Fertilization Rate (%) | 65.09 (28.60) | 57.84 (25.59) | 0.297a |
| # Embryos Transferred (n) | 2.06 (0.98) | 2.36 (1.19) | 0.298a |
| CP n (%) | 13 (38) | 8 (23) | 0.189c |
Continuous data are presented as median (interquartile range) or mean (standard deviation). Categorical data presented as %
a Student t test
b Information on race was missing for some participants in either group.
c chisquare test
d Early follicular phase FSH level in the cycle preceding IVF
e Mann Whitney U test
f Serum E2 on day of hCG administration
Clinical pregnancy was seen in 21 of 68 patients (30.88 %). The distribution of infertility diagnoses was comparable between cycles attaining CP and failed cycles. Likelihood for CP was unrelated to infertility etiology (p > 0.05, data not shown). COH protocol was comparable for the two groups (attaining CP versus not pregnant following embryo transfer) as shown in Table 1. In the majority of cycles (81%), embryo transfer was undertaken on D3 whereas the remaining underwent D5 embryo transfer. Cycle success was unrelated to the day of embryo transfer (p > 0.05, data not shown). Lower FF IL-6 levels, albeit insignificantly so, were noted in patients achieving CP following fresh embryo transfer (median 3.65 [2.67–5.71] versus 4.49 [2.75–7.29], p = 0.422). Although declining CP rates were observed across quartiles of FF IL-6 (35, 41%, 29% and 18% respectively from lowest to the highest quartile, this relationship was not statistically significant (p = 0.498).
Patients with FF IL-6 level < median for the cohort (4.0 pg/ml) were twice as likely to achieve CP compared to those with higher FF IL-6 levels (OR 2.01, 95% CI 0.70-5.76), although this relationship was not of statistical significance (p = 0.193). IR demonstrated a similar declining trend with higher IR in those with IL-6 < 4.0 pg/ml compared to those with higher FF IL-6 levels (24.0 ± 35% versus 11.4 ± 18%, p = 0.197). Table 1 presents participant characteristics and IVF cycle parameters by FF IL-6 level categorized by the group median (<4 versus ≥ 4 pg/ml).
Using stepwise multivariable logistic regression analyses, FF IL-6 level <4.0 pg/ml was identified as an independent predictor of CP following ET. The multivariable model included duration of COH, FF IL-6<median (4.0 pg/ml) and the number of embryos transferred. After adjusting for duration of COH ≥ 12 days and the number of embryos transfered, IVF cycles in patients with FF IL-6 levels less than the median for the cohort (<4.0 pg/ml) were almost four times more likely to result in CP compared to cycles with higher FF IL-6 levels (p = 0.045, Table 2). Duration of COH of 12 or more days was associated with 83% decreased likelihood for CP (p = 0.009, Table 2). Although each additional embryo transferred almost doubled the likelihood of CP (Table 2), this relationship was not of statistical significance on adjusted analyses. The model demonstrated 76% power in predicting CP following IVF.
Table 2.
Determinants of likelihood for clinical pregnancy following IVF. The magnitude of association is presented as odds ratio (OR) and 95% Confidence Interval (95% CI)
| Parameter | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|
| COH> = 12 days | 0.21(0.07–0.458) | 0.003 | 0.17 (0.04–0 .64) | 0.009 |
| # Embryos Transferred | 1.75 (1.04–2.95) | 0.034 | 2.10 (0.90–3.10) | 0.104 |
| FFIL-6 < 4.0 pg/ml | 2.01(0.70–5.76) | 0.193 | 3.98(1.03–15.39) | 0.045 |
Discussion
Our data imply detrimental effects of elevated FF IL-6 levels on the outcome of IVF, and are in contrast to findings reported by Bedaiwy et al [26]. In a larger cross-sectional cohort of 112 infertile women undergoing IVF, the authors report on cytokine profiles in pooled FF samples (methodology similar to ours) and identified significantly higher FF IL-6 levels in patients achieving pregnancy (17.38 ± 14.30 in those achieving CP versus 6.36 ± 6.20 pg/ml in the non-pregnant group; values are reported as mean±SD) following IVF. It is noteworthy that the statistical approach focused exclusively on univariate analyses. While the reported patient characteristics (i.e. age, ovarian reserve status and COH parameters) are comparable to our patient population, marked differences in the FF IL-6 concentrations are obvious in those achieving CP in these two study populations (mean ± SD values were 4.86 ± 4.30 pg/ml in our patients compared to 17.38 ± 14.30 in the study by Bedaiwy et al); FF IL-6 levels in the non-pregnant groups of the two cohorts are comparable (5.89 ± 4.32 compared to 6.36 ± 6.20 pg/ml respectively). FF samples in the present study were frozen and thawed, which theoretically may have contributed to the low levels of IL-6 in our population, but this should have equally impacted on assay values across the entire cohort and thus cannot explain the discrepant lower values in our patients achieving CP. While these two studies are opposed in their findings relating FF IL-6 levels to IVF cycle outcome, it is worth noting that elevated FF levels of IL-12 (24.45 ± 20.15 in the non-pregnant versus 3.38 ± 10.6 pg/ml in those achieving pregnancy) were prognostic of cycle failure in the study by Bedaiwy et al. This finding is consistent with our data suggestive of detrimental influences of FF cytokines on reproductive success following IVF. Interestingly, higher IL-6 levels have been described in the FF compared to the serum [18], although biological rationale for such a differential remains unclear.
Others have reported lower FF concentrations of another member of the IL family, IL-1, in IVF cycles resulting in CP [30, 31]. Higher IL-6 levels are previously reported in women with endometriosis [21] and our findings are thus consistent with published data. We were unable to identify negative influences of FF IL-6 on parameters of folliculogenesis (i.e. ovarian response as reflected by required dose of gonadotropins, duration of COH or the number of oocytes retrieved); in fact, a linear and statistically significant correlation was observed between FF IL-6 levels and E2 on the day of hCG administration. This latter observation is of special interest given the observed linear and positive correlation observed between FF IL-6 and advancing age. While these counterintuitive observations are difficult to explain given the study design, if higher FF IL-6 levels play a causative role in ovarian steroidogenesis, as has been previously suggested, these observations may partly explain the hyperestrogenemic milieu of reproductive aging, as has been previously described [32]. FF levels of IL-6 were unrelated to COH protocol or fertilization rates and in this context our findings are consistent with published data [33, 34]. Given our study design where FF samples were pooled for each patient, we cannot relate FF IL-6 with individual oocytes within each follicle; the linear relationship observed between FF IL-6 and yield of mature oocytes however, is supportive of previously published work by Kawasaki et al [25]; these authors described higher IL-6 levels in fluid from follicles yielding mature oocytes.
Our data identify adverse influences of an intra-follicular inflammatory milieu on successful CP following fresh embryo transfer; given the lack of observed detriment on parameters of COH nor on fertilization, endometrial receptivity may be identified as a likely target for deleterious influences of elevated FF IL-6 levels, as suggested by the observed decline in rates of CP and implantation with increasing FF IL-6 levels. This latter conjecture is plausible given that IL-6 shares gp130 signaling protein in the receptor complex with leukemia inhibitory factor (LIF), a recognized marker of endometrial receptivity [35]. IL-6 immunoreactivity is described in both proliferative and secretory endometrium, and endometrial IL-6 synthesis is elevated at the time of implantation [22, 36]. However, aberrant expression of IL-6 may be associated with the failed implantation, a mechanism suggested to explain contraceptive efficacy of intrauterine contraceptive devices [37]. Inflammatory cytokines including IL-6 are implicated in processes of luteolysis in non-human models [38]; while luteal insufficiency may be a possible sequel of exposure to higher IL-6 levels, thereby impairing endometrial receptivity, this mechanism cannot be rationalized as contributory to reduced implantation and CP rates, given the universal progesterone supplementation to all patients undergoing IVF. Higher E2 levels (that were observed to positively correlate with FF IL-6 levels) have previously been suggested as being detrimental to endometrial receptivity [39]. Given the positive correlation between FF IL-6 and peak E2 levels observed in our cohort, higher E2 levels may be considered as an alternative explanation for the observed detriment associating higher FF IL-6 levels with reduced likelihood for CP. In the absence of data on endometrial markers of receptivity in our patient cohort, we can only hypothesize that luteal endometrium may be the target site for detrimental influences of elevated FF IL-6 levels. These hypotheses merit targeted testing in future studies and if substantiated, may partly explain the reproductive compromise associated with minimal to mild cases of endometriosis.
Our findings identify prolonged COH during IVF as a negative predictor of cycle success. We specifically observed significant reduction in CP following embryo transfer when duration of COH approached or extended beyond 12 days. Consistent with our observation, extended duration of COH has previously been suggested to portend poor IVF success [40, 41].
Our study is limited by several factors such as small sample size, its retrospective and cross-sectional design, pooling and freeze/thaw of FF for individual patients, and lack of information regarding markers of endometrial receptivity in the cohort. The predominant Caucasian representation (65%) does not allow meaningful assessment of racial variability in FF IL-6 levels. Explanation regarding the observed positive correlation between FF IL-6 and peak E2 levels remains elusive at this time. The statistical methodology pursued, i.e. adjusted analyses, as well as consistency of observed decline in implantation rates and in CP with FF IL-6 > 4 pg/ml add credence to our observations.
In summary, our findings identify detrimental implications of elevated FF IL-6 levels on the outcome of IVF. While future studies may elucidate mechanistic aspects to this observed relationship, given the absence of any detriment on parameters of folliculogenesis, we hypothesize that increased FF IL-6 levels may adversely influence endometrial receptivity. This assumption merits further exploration in future studies.
Footnotes
Capsule
Relationship between follicular fluid IL-6 levels and likelihood of clinical pregnancy following IVF.
References
- 1.Adashi EY. The potential relevance of cytokines to ovarian physiology: the emerging role of resident ovarian cells of the white blood cell series. Endocr Rev. 1990;11:454–464. doi: 10.1210/edrv-11-3-454. [DOI] [PubMed] [Google Scholar]
- 2.Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27:14–23. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 4.Kyama CM, Debrock S, Mwenda JM, D'Hooghe TM. Potential involvement of the immune system in the development of endometriosis. Reprod Biol Endocrinol. 2003;1:123. doi: 10.1186/1477-7827-1-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayisli UA, Guzeloglu-Kayisli O. Arici : Endocrine-immune interactions in human endometrium. Ann N Y Acad Sci. 2004;1034:50–63. doi: 10.1196/annals.1335.005. [DOI] [PubMed] [Google Scholar]
- 6.Spangelo BL, Judd AM, Call GB, Zumwalt J, Gorospe WC. Role of the cytokines in the hypothalamic-pituitary-adrenal and gonadal axes. Neuroimmunomodulation. 1995;2:299–312. doi: 10.1159/000097209. [DOI] [PubMed] [Google Scholar]
- 7.Hammadeh ME, Ertan AK, Zeppezauer M, Baltes S, Georg T, Rosenbaum P, Schmidt W. Immunoglobulins and cytokines level in follicular fluid in relation to etiology of infertility and their relevance to IVF outcome. Am J Reprod Immunol. 2002;47:82–90. doi: 10.1034/j.1600-0897.2002.1o024.x. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto T. Interleukin-6: from basic science to medicine–40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 10.Honda M, Yamamoto S, Cheng M, Yasukawa K, Suzuki H, Saito T, Osugi Y, Tokunaga T, Kishimoto T. Human soluble IL-6 receptor: its detection and enhanced release by HIV infection. J Immunol. 1992;148:2175–2180. [PubMed] [Google Scholar]
- 11.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 12.Novick D, Engelmann H, Wallach D, Rubinstein M. Soluble cytokine receptors are present in normal human urine. J Exp Med. 1989;170:1409–1414. doi: 10.1084/jem.170.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackiewicz A, Schooltink H, Heinrich PC, Rose-John S. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. J Immunol. 1992;149:2021–2027. [PubMed] [Google Scholar]
- 14.Buyalos RP, Watson JM, Martinez-Maza O. Detection of interleukin-6 in human follicular fluid. Fertil Steril. 1992;57:1230–1234. [PubMed] [Google Scholar]
- 15.Machelon V, Emilie D, Lefevre A, Nome F, Durand-Gasselin I, Testart J. Interleukin-6 biosynthesis in human preovulatory follicles: some of its potential roles at ovulation. J Clin Endocrinol Metab. 1994;79:633–642. doi: 10.1210/jc.79.2.633. [DOI] [PubMed] [Google Scholar]
- 16.Gorospe WC, Spangelo BL. Interleukin-6 production by rat granulosa cells in vitro: effects of cytokines, follicle-stimulating hormone, and cyclic 3', 5'-adenosine monophosphate. Biol Reprod. 1993;48:538–543. doi: 10.1095/biolreprod48.3.538. [DOI] [PubMed] [Google Scholar]
- 17.Gorospe WC, Hughes FM., Jr Spangelo BL: Interleukin-6: effects on and production by rat granulosa cells in vitro. Endocrinology. 1992;130:1750–1752. doi: 10.1210/en.130.3.1750. [DOI] [PubMed] [Google Scholar]
- 18.Salmassi A, Lu S, Hedderich J, Oettinghaus C, Jonat W, Mettler L. Interaction of interleukin-6 on human granulosa cell steroid secretion. J Endocrinol. 2001;170:471–478. doi: 10.1677/joe.0.1700471. [DOI] [PubMed] [Google Scholar]
- 19.Breard E, Benhaim A, Feral C, Leymarie P. Rabbit ovarian production of interleukin-6 and its potential effects on gonadotropin-induced progesterone secretion in granulosa and theca cells. J Endocrinol. 1998;159:479–487. doi: 10.1677/joe.0.1590479. [DOI] [PubMed] [Google Scholar]
- 20.Richards JS, Liu Z, Shimada M. Immune-like mechanisms in ovulation. Trends Endocrinol Metab. 2008;19:191–196. doi: 10.1016/j.tem.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81:1118–1122. doi: 10.1210/jc.81.3.1118. [DOI] [PubMed] [Google Scholar]
- 22.Tabibzadeh S, Kong QF, Babaknia A, May LT. Progressive rise in the expression of interleukin-6 in human endometrium during menstrual cycle is initiated during the implantation window. Hum Reprod. 1995;10:2793–2799. doi: 10.1093/oxfordjournals.humrep.a135793. [DOI] [PubMed] [Google Scholar]
- 23.Hammadeh ME, Braemert B, Baltes S, Georg T, Rosenbaum P, Schmidt W. Relationship between ovarian stimulation regimen and cytokine concentration in follicular fluid and their effect on fertilization and pregnancy outcome of patients undergoing ICSI program. Am J Reprod Immunol. 2000;43:12–20. doi: 10.1111/j.8755-8920.2000.430103.x. [DOI] [PubMed] [Google Scholar]
- 24.Souter I, Huang A, Martinez-Maza O, Breen EC, Decherney AH, Chaudhuri G, Nathan L. Serum levels of soluble vascular cell adhesion molecule-1, tumor necrosis factor-alpha, and interleukin-6 in in vitro fertilization cycles. Fertil Steril. 2009;91:2012–2019. doi: 10.1016/j.fertnstert.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki F, Kawano Y. Kosay Hasan Z, Narahara H, Miyakawa I: The clinical role of interleukin-6 and interleukin-6 soluble receptor in human follicular fluids. Clin Exp Med. 2003;3:27–31. doi: 10.1007/s102380300012. [DOI] [PubMed] [Google Scholar]
- 26.Bedaiwy M, Shahin AY, AbulHassan AM, Goldberg JM, Sharma RK, Agarwal A, Falcone R. Differential expression of follicular fluid cytokines: relationship to subsequent pregnancy in IVF cycles. Reprod Biomed Online. 2007;15:321–325. doi: 10.1016/S1472-6483(10)60346-X. [DOI] [PubMed] [Google Scholar]
- 27.Artini PG, Monti M, Fasciani A, Battaglia C, D'Ambrogio G, Genazzani AR. Vascular endothelial growth factor, interleukin-6 and interleukin-2 in serum and follicular fluid of patients with ovarian hyperstimulation syndrome. Eur J Obstet Gynecol Reprod Biol. 2002;101:169–174. doi: 10.1016/S0301-2115(01)00568-1. [DOI] [PubMed] [Google Scholar]
- 28.Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94:1314–1319. doi: 10.1016/j.fertnstert.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosmer DW, Hosmer T, Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 965;16:965–980. doi: 10.1002/(SICI)1097-0258(19970515)16:9<965::AID-SIM509>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 30.Mendoza C, Ruiz-Requena E, Ortega E, Cremades N, Martinez F, Bernabeu R, Greco E, Tesarik J. Follicular fluid markers of oocyte developmental potential. Hum Reprod. 2002;17:1017–1022. doi: 10.1093/humrep/17.4.1017. [DOI] [PubMed] [Google Scholar]
- 31.Baka S, Malamitsi-Puchner A. Novel follicular fluid factors influencing oocyte developmental potential in IVF: a review. Reprod Biomed Online. 2006;12:500–506. doi: 10.1016/S1472-6483(10)62005-6. [DOI] [PubMed] [Google Scholar]
- 32.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–1501. doi: 10.1210/jc.81.4.1495. [DOI] [PubMed] [Google Scholar]
- 33.Ficicioglu C, Kumbak B, Akcin O, Attar R, Yildirim G, Yesildaglar N. Comparison of follicular fluid and serum cytokine concentrations in women undergoing assisted reproductive treatment with GnRH agonist long and antagonist protocols. Gynecol Endocrinol. 2010;26:181–186. doi: 10.3109/09513590903215557. [DOI] [PubMed] [Google Scholar]
- 34.Asimakopoulos B, Abu-Hassan D, Metzen E, Al-Hasani S, Diedrich K, Nikolettos N. The levels of steroid hormones and cytokines in individual follicles are not associated with the fertilization outcome after intracytoplasmic sperm injection. Fertil Steril. 2008;90:60–64. doi: 10.1016/j.fertnstert.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 35.Pennica D, Shaw KJ, Swanson TA, Moore MW, Shelton DL, Zioncheck KA, Rosenthal A, Taga T, Paoni NF, Wood WI. Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J Biol Chem. 1995;270:10915–10922. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- 36.Makkar G, Ng EH, Yeung WS, Ho PC. Reduced expression of interleukin-11 and interleukin-6 in the periimplantation endometrium of excessive ovarian responders during in vitro fertilization treatment. J Clin Endocrinol Metab. 2006;91:3181–3188. doi: 10.1210/jc.2006-0180. [DOI] [PubMed] [Google Scholar]
- 37.Ammala M, Nyman T, Strengell L, Rutanen EM. Effect of intrauterine contraceptive devices on cytokine messenger ribonucleic acid expression in the human endometrium. Fertil Steril. 1995;63:773–778. doi: 10.1016/s0015-0282(16)57480-9. [DOI] [PubMed] [Google Scholar]
- 38.Kucharski J, Jana B. Immuno-endocrine mechanisms connected with the creation of corpora lutea persistent in animal ovaries. Pol J Vet Sci. 2005;8:255–259. [PubMed] [Google Scholar]
- 39.Valbuena D, Jasper M, Remohi J, Pellicer A, Simon C. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14(Suppl 2):107–111. doi: 10.1093/humrep/14.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 40.Bar-Hava I, Yoeli R, Yulzari-Roll V, Ashkenazi J, Shalev J, Orvieto R. Controlled ovarian hyperstimulation: does prolonged stimulation justify cancellation of in vitro fertilization cycles? Gynecol Endocrinol. 2005;21:232–234. doi: 10.1080/09513590500282331. [DOI] [PubMed] [Google Scholar]
- 41.Chuang M, Zapantis A, Taylor M, Jindal S, Neal-Perry G, Lieman H, Polotsky A: Gonadotropin stimulation of 13 days or longer is associated with decreased ART success. J Assist Reprod Genetics 2010;September 7 (Epub ahead of print). [DOI] [PMC free article] [PubMed]