Abstract
Purpose
Recurrent pregnancy loss (RPL) could be caused by insufficient progesterone in the luteal phase of menstruation and early pregnancy. Progesterone plays a critical role in oocyte maturation, embryo implantation and placenta maintenance in early gestation. This study was set out to investigate the association between polymorphisms of the progesterone receptor (PGR) gene and idiopathic RPL.
Methods
One hundred twenty-one women with a history of idiopathic recurrent pregnancy loss (RPL) and 179 control subjects were enrolled into the study. Six tag SNPs and two functional SNPs [PROGINS (rs1042838), +331 C/T (rs10895068)] of the progesterone receptor gene were genotyped.
Results
We found that the allele and genotype frequencies of the functional SNP [PROGINS (rs1042838)] were both significantly higher in patients with idiopathic RPL than in the control subjects (both P values = 0.006). In addition, the C-C haplotype, which consists of rs590688C > G and rs11224592T > C, is associated with a decreased risk of RPL (p = 0.004).
Conclusion
PROGINS polymorphism confers susceptibility to idiopathic recurrent pregnancy loss in Taiwanese Han women.
Keywords: Progesterone receptor, PROGINS, Recurrent pregnancy loss, Tag SNP, Polymorphism
Introduction
The occurrence of repeated pregnancy loss (RPL) affects about 1–5% of couples [1]. RPL is a multifactorial disorder and various factors have been implicated, including uterine anomaly, chromosomal abnormalities, endocrine dysfunction, thrombophilia, immune disorders, lifestyle factors and maternal infections. However, the exact underlying causes remain undetermined in up to 50% of cases [2].
Progesterone plays a crucial role in the regulation of female reproduction, including regulating the menstrual cycle, maintaining implantation, promoting uterine growth and suppressing uterine contraction [3, 4]. The major physiological effect of progesterone is mediated by progesterone receptors (PGR) or by changing the isoforms ratio and/or the expression level of the PGR [5]. In a PGR knockout mice model, male and female embryos homozygous for the PGR mutation developed normally to adulthood. However, the adult female PGR mutant displayed significant defects in all reproductive tissues, including an inability to ovulate, uterine hyperplasia and inflammation, severely limited mammary gland development, and an inability to exhibit sexual behavior [6]. During the past two decades, mifepristone (RU486, progesterone antagonist) has been widely used to terminate early pregnancy by competitively blocking the PGR [7]. All of the above evidence supports the crucial role of progesterone and PGRs in maintaining early pregnancy in the human being.
To date, three reports on different ethnicities have been published to explore the association between the PGR polymorphism and recurrent miscarriage, but the study designs were different and the results were inconsistent [8–10]. Considering the important role of PGR in regulating human early pregnancy, but the contradictory results of the genetic association studies, this study was conducted, for the first time, using tag SNPs and haplotype analysis to investigate the association between polymorphisms of PGR and idiopathic RPL. We finally found that the functional SNP of PGR (PROGINS) was associated with a higher risk of RPL, and a C-C haplotype of PGR was associated with a decreased risk of RPL.
Materials and methods
The present study was approved by the Institutional Review Board of National Cheng Kung University Hospital. A total of 121 women who had experienced at least two consecutive spontaneous abortions were recruited. All subjects had undergone a comprehensive examination, including a detailed history, a physical examination, chromosome analysis of peripheral blood lymphocytes, and trans-vaginal three-dimensional ultrasound or hysterosalpingography (to detect uterine anomalies and endometrial defects). They were also checked for 75-gram oral glucose tolerance, thyroid functions (T3, T4, and TSH), thrombophilia factors including anti-cardiolipin anbibodies (IgG,, IgM and β-glycoprotein), lupus anticoagulant, anti-thrombin III, protein S, protein C, and endocrinology profiles on day 3 of the menstrual cycle [follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), and testosterone (T)]. Women with any identifiable cause of spontaneous abortion were excluded from the study. We also recruited a total of 179 multiparous women as control subjects. The control subjects had conceived naturally without a history of early spontaneous abortion or other pregnancy complications.
We explored the tag SNPs of the PGR gene on the web site (www.hapmap.org). A total of 7 HapMap tag SNPs (rs545835, rs495997, rs503362, rs508533, rs560291, rs590688, rs11224592) for population CHB (Chinese Hans in Beijing) were selected to encompass the entire haplotype block structure of the PGR gene using the algorithm Tagger pair-wise Tagging (Filter: minor allele frequency≧0.05, r2 ≧0.80). We also included 2 functional PGR polymorphisms [a missense SNP in exon 4 (rs1042838) of PROGINS and +331 G/A (rs10895068) in the PGR gene promoter] into the genotyping panel. Genomic DNA was extracted from the peripheral blood lymphocytes using a Puregene DNA isolation kit (Gentra, Minneapolis, MN, USA). These SNPs were detected by primer extension analysis using end-point TaqMan assays (Applied Biosystems, Warrington, UK) in 96-well arrays, and the genotypes were subsequently read on a 7900 Sequence Detector (Applied Biosystems). Reactions were carried out using the standard conditions supplied by the company.
Tests for association with single markers and haplotypes between RPL patients and normal controls were performed using a χ2 test or Fisher’s exact test. A p value of <0.05 was considered statistically significant. Tests for haplotype association with habitual abortion were performed using SNPAlyze 7.0.Pro software (DYNACOM Co., Ltd., Yokohama, Japan), and statistically significant p values were corrected for multiple testing by the permutation test. The LD coefficient (D’) between each pair of SNPs was analyzed by Haploview 4.1 (Daley Lab at the Broad Institute, Cambridge, USA).
Results
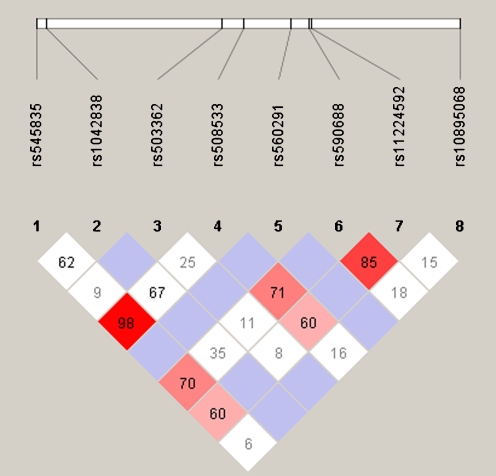
The patients and controls were Taiwanese Han women and their ages were 30.95 ± 5.04 (mean ± standard deviation) and 29.76 ± 4.87 years, respectively. The genotypic and allelic frequencies of the PGRs in the women with RPL and the control subjects are shown in Table 1. One SNP (rs495997) that was not in Hardy-Weinberg equilibrium was excluded. The risk allelic (A allele) and genotypic frequency of the PROGINS polymorphism showed significant differences between patients and controls (both p values = 0.006). After setting the threshold of the LD coefficient [(D’) ≥ 0.85], we selected two loci (rs590688 and rs11224592) of PGR for haplotypic analysis (Fig. 1) and found the C-C haplotype was significantly different between patients and controls (p = 0.004)(Table 2).
Table 1.
Allele and genotype frequencies of PGR polymorphisms
| SNP | Allele frequency | Genotype frequency | ||||||
|---|---|---|---|---|---|---|---|---|
| Allele | Case (n = 242) | Control (n = 358) | P value | Genotype | Case (n = 121) | Control (n = 179) | P value | |
| rs545835 | G | 185 (76.4%) | 275 (76.8%) | 0.92 | GG | 69 (57%) | 108 (60.3%) | 0.57 |
| A | 57 (23.6%) | 83 (23.2%) | GA | 47 (38.9%) | 59 (33%) | |||
| AA | 5 (4.1%) | 12 (6.7%) | ||||||
| rs1042838 (V660L) (PROGINS) | G | 237 (97.9%) | 358 (100%) | 0.006 | GG | 116 (95.9%) | 179 (100%) | 0.006 |
| T | 5 (2.1%) | 0 (0%) | GT | 5 (4.1%) | 0 (0%) | |||
| TT | 0 (0%) | 0 (0%) | ||||||
| rs503362 | G | 238 (98.3%) | 351 (98%) | 0.79 | GG | 117 (96.7%) | 173 (96.6%) | 0.98 |
| C | 4 (1.7%) | 7 (2%) | GC | 4 (3.3%) | 5 (2.8%) | |||
| CC | 0 (0%) | 1 (0.6%) | ||||||
| rs508533 | G | 190 (78.5%) | 290 (81%) | 0.45 | GG | 73 (60.3%) | 122 (68.2%) | 0.16 |
| T | 52 (21.5%) | 68 (19%) | GT | 44 (36.4%) | 46 (25.7%) | |||
| TT | 4 (3.3%) | 11 (6.1%) | ||||||
| rs560291 | G | 241 (99.6%) | 358 (100%) | 0.22 | GG | 120 (99.2%) | 179 (100%) | 0.22 |
| T | 1 (0.4%) | 0 (0%) | GT | 1 (0.8%) | 0 (0%) | |||
| TT | 0 (0%) | 0 (0%) | ||||||
| rs590688 | C | 192 (79.3%) | 291 (81.3%) | 0.50 | CC | 75 (62%) | 123 (68.7%) | 0.19 |
| G | 50 (20.7%) | 67 (18.7.%) | GC | 42 (34.7%) | 45 (25.2%) | |||
| GG | 4 (3.3%) | 11 (6.1%) | ||||||
| rs11224592 | T | 196 (81%) | 282 (78.8%) | 0.51 | TT | 79 (65.3%) | 113 (63.1%) | 0.70 |
| C | 46 (19%) | 76 (21.2%) | TC | 38 (31.4%) | 56 (31.3%) | |||
| CC | 4 (3.3%) | 10 (5.6%) | ||||||
| rs10895068 (+331 C/T) | C | 242 (100%) | 356 (99.4%) | 0.24 | CC | 121 (100%) | 177 (98.9%) | 0.24 |
| T | 0 (0%) | 2 (0.6%) | CT | 0 (0%) | 2 (1.1%) | |||
| TT | 0 (0%) | 0 (0%) | ||||||
Fig. 1.
Haplotype block and tag SNPs of the PGR gene from Haploview. The D’ values in each box are shown, and the haplotype block composed by rs590688 and rs11224592 shows high linkage disequilibrium (D’ = 0.85)
Table 2.
Haplotype analysis of PGR (rs590688 and rs11224592)
| SNP marker | Haplotype | Frequency | Global P-value1 | P-value2 | |
|---|---|---|---|---|---|
| Cases | Controls | ||||
| rs590688 | CT | 0.789 | 0.764 | 0.029 | 0.473 |
| rs11224592 | GC | 0.186 | 0.164 | 0.480 | |
| CC | 0.004 | 0.049 | 0.004 | ||
| GT | 0.021 | 0.023 | 0.813 | ||
1 Global P-value: Global Permutation P-value
2P-value: Permutation P-value
Discussion
The human PGR gene is transcripted from two alternative promoters and translated into two different zinc-finger proteins: PR-A and PR-B. PR-B is a potent transcriptional activator and contributes to the proliferative effects of estrogen, whereas PRA, the shorter isoform, is necessary to oppose the effects of both PR-B and the estrogen receptor [11]. The action of progesterone is mediated by the PGR and non-genomic action. Binding of progesterone to its receptor results in a complex activation cascade, which starts from conformational changes, protein phosphorylation, dissociation from heat shock protein, and dimmer formation, and finally to nuclear transport of the active protein-progesterone complex. The hormone receptor-complexes bind to specific DNA sequences and act as transcriptional factors for target genes [7]. In the endometrium of patients with idiopathic RPL, diminished PGR immunostainng was noted in the intermediate trophoblast cells [12] and the cytoplasmic PGR protein level was significantly lower compared to normal controls, while the serum progesterone level did not show a significant difference [13].
PROGINS was first described as an Alu element (306 bp) in intron 7, but it also included a missense SNP in exon 4 (V660L, rs1042838) and a silence SNP in exon 5. Together, these 3 polymorphisms form a complex referred to as PROGINS [14]. These three polymorphisms are in complete linkage disequilibrium and their effects cannot be distinguished using genetic epidemiology [15]. PROGINS variants act as risk-modulating factors in several progesterone-dependent neoplasms, such as breast cancer, ovarian cancer and endometrial cancer [14, 16]. The functional consequences of PROGINS variants were demonstrated by increasing transcription activity for mutated transcripts[17] and reducing the response to progesterone by affecting gene expression and mRNA stability [18]. The V600L substitution is located in the hinge region of the receptor, a link between the DNA binding domain and the ligand binding domain. The amino acid substitution in exon 4 (V660L) leads to differences in PGR phosphorylation and degradation in the two PGR variants upon ligand binding, subsequently affecting the hormone-receptor binding complex and transcription of downstream genes; this phenomenon is attributed to the steric effect of the non-synonymous change of the PGR variants [18]. These genetic variations may cause alteration in biological function in PGRs and can potentially contribute to an individual’s susceptibility to the development of pregnancy loss.
PGR polymorphisms were reported to be associated with adverse reproductive outcomes, including unexplained infertility [19], repeated IVF implantation failure [20] and unexplained RPL [8–10], although the results were not consistent. Three articles have been published to explore the association between the PGR polymorphism and recurrent miscarriage [8–10]. Two of these three articles were based on only specific loci of the PGR. By using single strand conformation polymorphism (SSCP) analysis and further sequencing, Schweikert A. et al. (2004) reported that three SNPs in exon 1 (G1031CSer344Thr), exon 4 (G1978TLeu660Val) and exon 5 (C2310THis770His) of PGR genes were linked to PROGINS (Alu-Insertion) and were associated with an increased risk of repeated miscarriages [8]. However, the finding reported by Kurz et al. (2001) and Aruna et al. (2010) for Australian and Indian populations did not support the association between PROGINS Alu-Insertion and idiopathic repeated spontaneous abortions [9, 10]. There are great differences in the allele and genotype frequencies of PROGINS (V660L, rs1042838) among different ethnical populations. The minor allele (T allele) is rare in Asians and Africans [2% in Chinese Hans (HapMap-CHB); 0% in Japanese (HapMap-JPT); 0% in Africans (HapMap-YRI)], but much more frequent in Europeans (21% in HapMap-CEU). The genotype frequencies of GG/GT/TT are 0.65/0.28/0.07 in Europeans, but 0.98/0.02/0.00 in Chinese Hans. Nevertheless, our study (from Taiwan, Asia) and a German study showed PROGINS (660 L) variants were strongly associated with idiopathic RPL [8], suggesting this genetic alteration would impair reproductive function and contribute to miscarriage regardless of the ethnic differences.
Conclusions
In this report, we showed a significant association of PROGINS with RPL in Taiwanese Han women by using tagging SNPs to capture the genetic information within the PGR gene block. The fact that the PROGINS allele occurs more frequently in RPL patients indicates that PROGINS may be a risk modifier for miscarriage. Further studies should use a larger sample size and establish the contribution of the PGR polymorphism to the risk for RPL. Considering that PROGINS occurs in only 4.1% of Taiwanese Han female patients, the etiology of idiopathic RPL is extremely heterogeneous and multiple factors are implicated in the pathogenesis of recurrent miscarriage.
Acknowledgements
This study was supported by an intramural grant from National Cheng-Kung University Hospital (NCKH-9702035).
Financial support This study was supported by an intramural grant from National Cheng-Kung University Hospital (NCKH-9702035).
Footnotes
Capsule
In this study, we used tagging SNPs to capture the genetic information within the PGR gene block and showed a significant association of PROGINS with idiopathic recurrent pregnancy loss in Taiwanese Han women.
References
- 1.Baek KH, Lee EJ, Kim YS. Recurrent pregnancy loss: the key potential mechanisms. Trends Mol Med. 2007;13:310–7. doi: 10.1016/j.molmed.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Li TC, Makris M, Tomsu M, Tuckerman E, Laird S. Recurrent miscarriage: aetiology, management and prognosis. Hum Reprod Update. 2002;8:463–81. doi: 10.1093/humupd/8.5.463. [DOI] [PubMed] [Google Scholar]
- 3.Clarke CL, Sutherland RL. Progestin regulation of cellular proliferation. Endocr Rev. 1990;11:266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- 4.Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–19. doi: 10.1210/er.18.4.502. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen BM, Schittone SA, Richer JK, Horwitz KB. Progesterone-independent effects of human progesterone receptors (PRs) in estrogen receptor-positive breast cancer: PR isoform-specific gene regulation and tumor biology. Mol Endocrinol. 2005;19:574–87. doi: 10.1210/me.2004-0287. [DOI] [PubMed] [Google Scholar]
- 6.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–78. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 7.Spitz IM, Bardin CW. Mifepristone (RU 486)—a modulator of progestin and glucocorticoid action. N Engl J Med. 1993;329:404–12. doi: 10.1056/NEJM199308053290607. [DOI] [PubMed] [Google Scholar]
- 8.Schweikert A, Rau T, Berkholz A, Allera A, Daufeldt S, Wildt L. Association of progesterone receptor polymorphism with recurrent abortions. Eur J Obstet Gynecol Reprod Biol. 2004;113:67–72. doi: 10.1016/j.ejogrb.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Kurz C, Tempfer CB, Boecskoer S, Unfried G, Nagele F, Hefler LA. The PROGINS progesterone receptor gene polymorphism and idiopathic recurrent miscarriage. J Soc Gynecol Investig. 2001;8:295–8. doi: 10.1016/S1071-5576(01)00123-X. [DOI] [PubMed] [Google Scholar]
- 10.Aruna M, Nagaraja T, Andal S, Tarakeswari S, Sirisha PV, Reddy AG, Thangaraj K, Singh L, Reddy BM. Role of progesterone receptor polymorphisms in the recurrent spontaneous abortions: Indian case. PLoS One. 2010;5:e8712. doi: 10.1371/journal.pone.0008712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conneely OM, Jericevic BM, Lydon JP. Progesterone receptors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2003;8:205–14. doi: 10.1023/A:1025952924864. [DOI] [PubMed] [Google Scholar]
- 12.Hickman TN, Shih LM, Zacur HA, Kurman RJ, Diener-West M, Gearhart JD. Decreased progesterone receptor expression in the intermediate trophoblastic cells of spontaneous abortions. Fertil Steril. 2002;77:1001–5. doi: 10.1016/S0015-0282(02)02953-9. [DOI] [PubMed] [Google Scholar]
- 13.Carranza-Lira S, Blanquet J, Tserotas K, Calzada L. Endometrial progesterone and estradiol receptors in patients with recurrent early pregnancy loss of unknown etiology–preliminary report. Med Sci Monit. 2000;6:759–62. [PubMed] [Google Scholar]
- 14.Romano A, Lindsey PJ, Fischer DC, Delvoux B, Paulussen AD, Janssen RG, Kieback DG. Two functionally relevant polymorphisms in the human progesterone receptor gene (+331 G/A and progins) and the predisposition for breast and/or ovarian cancer. Gynecol Oncol. 2006;101:287–95. doi: 10.1016/j.ygyno.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 15.Rowe SM, Coughlan SJ, McKenna NJ, Garrett E, Kieback DG, Carney DN. Headon DR.Ovarian carcinoma-associated TaqI restriction fragment length polymorphism in intron G of the progesterone receptor gene is due to an Alu sequence insertion. Cancer Res. 1995;55:2743–5. [PubMed] [Google Scholar]
- 16.Vivo I, Huggins GS, Hankinson SE, et al. A functional polymorphism in the promoter of the progesterone receptor gene associated with endometrial cancer risk. Proc Natl Acad Sci U S A. 2002;99:12263–8. doi: 10.1073/pnas.192172299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agoulnik IU, Tong XW, Fischer DC, Körner K, Atkinson NE, Edwards DP, Headon DR, Weigel NL, Kieback DG. A germline variation in the progesterone receptor gene increases transcriptional activity and may modify ovarian cancer risk. J Clin Endocrinol Metab. 2004;89:6340–7. doi: 10.1210/jc.2004-0114. [DOI] [PubMed] [Google Scholar]
- 18.Romano A, Delvoux B, Fischer DC, Groothuis P. The PROGINS polymorphism of the human progesterone receptor diminishes the response to progesterone. J Mol Endocrinol. 2007;38:331–50. doi: 10.1677/jme.1.02170. [DOI] [PubMed] [Google Scholar]
- 19.Pisarska MD, Carson SA, Casson PR, Tong X, Buster JE, et al. A mutated progesterone receptor allele is more prevalent in unexplained infertility. Fertil Steril. 2003;80:651–653. doi: 10.1016/S0015-0282(03)00755-6. [DOI] [PubMed] [Google Scholar]
- 20.Cramer DW, Hornstein MD, McShane P, Powers RD, Lescault PJ, et al. Human progesterone receptor polymorphisms and implantation failure during in vitro fertilization. Am J Obstet Gynecol. 2003;189:1085–1092. doi: 10.1067/S0002-9378(03)00517-9. [DOI] [PubMed] [Google Scholar]